Abstract
Purpose
To cross-validate two recent noninvasive elastographic techniques, Ultrasound-based Transient Elastography (UTE) and Magnetic Resonance Elastography (MRE). As potential alternatives to liver biopsy, UTE and MRE are undergoing clinical investigations for liver fibrosis diagnosis and liver disease management around the world. These two techniques use tissue stiffness as a marker for disease state and it is important to do a cross-validation study of both elastographic techniques to determine the consistency with which the two techniques can measure the mechanical properties of materials.
Materials and Methods
In this paper, 19 well-characterized phantoms with a range of stiffness values were measured by two clinical devices (a Fibroscan and a MRE system based respectively on the UTE and MRE techniques) successively with the operators double-blinded.
Results
Statistical analysis showed that the correlation coefficient was r2=0.93 between MRE and UTE, and there was no evidence of a systematic difference between them within the range of stiffnesses examined.
Conclusion
These two noninvasive methods, MRE and UTE, provide clinicians with important new options for improving patient care regarding liver diseases in terms of the diagnosis, prognosis, and monitoring of fibrosis progression, as well for evaluating the efficacy of treatment.
Keywords: Fibrosis diagnosis, Phantoms, Elastography, Ultrasound-based Elastography, Magnetic Resonance Elastography, Cross-validation
1. Introduction
Liver biopsy is usually the most specific test to assess the nature and severity of liver diseases. However approximately a quarter of patients feel pain in the right upper quadrant or right shoulder after percutaneous liver biopsy, and it can cause more serious complications like bleeding and peritonitis due to its invasive nature (1). Two noninvasive quantitative techniques, Magnetic Resonance Elastography (MRE) and Ultrasound-based Transient Elastography (UTE) have emerged into clinical practice for diagnosing hepatic fibrosis and are now undergoing clinical investigations around the world (2–11). A study with 35 normal volunteers and 50 patients with chronic liver diseases showed that MRE had a specificity of 99% and sensitivity of 98% to detect all grades of liver fibrosis using a shear modulus threshold of 2.9 kPa, and a specificity of 85% and sensitivity of 86% to detect significant fibrosis (>F2) using a shear modulus threshold of 4.89 kPa (10). A study of 327 patients with chronic hepatitis C concluded that a Young’s modulus threshold of 8.7 kPa (shear modulus of 2.9 kPa) allowed correct diagnosis of significant fibrosis (>F2) with an area under ROC of 0.79 (11). Motivated by the success of both techniques in liver disease diagnosis, they have been studied to compare their specificity and sensitivity for diagnosing fibrosis, as well as to highlight the advantages and disadvantages of each technique, such as their performance on obese patients and those with ascites (2, 9, 12). Bensamoun et al (2) found a mean shear stiffness measured with the MRE and UTE techniques equal to 1.95 ± 0.06 kPa and 1.79 ± 0.30 kPa, respectively on the liver of 10 healthy subjects. No significant difference between the liver stiffness estimates measured with both techniques was shown. However, the focus of these previous studies has centred on comparing the performance of these two techniques when imaging in vivo, which lacks the control necessary to assess the agreement of the fundamental mechanical properties measured by the two techniques. The goal of this study was to directly compare the stiffness values measured by MRE and UTE on a set of standardized phantoms.
2. Material and Methods
Phantom Construction
Nineteen MRI- and ultrasound- compatible B-gel and polymer standardized homogeneous phantoms with a range of stiffnesses were made and used in the lab for this study (13–15). The stiffness range was designed to cover the range of normal and cirrhotic liver stiffness shown in previous studies (10, 11, 16). Copolymer-in-oil phantoms were formed from a mixture of Styrene-Ethylene/Butylene-Styrene (SEBS) copolymer, mineral oil and silica powder (15). The copolymer concentration varied from 3 to 8.5% of the oil mass. Silica powder was used as acoustic scattering particles while the elasticity of each phantom was controlled by the copolymer concentration. B-gel phantoms were composed of water, agar gelatine, bovine gelatine (B-gel) and carbonate particles for echogenicity. The B-gel concentration was varied from 5 to 17.5% to change the stiffness. The composition of the phantoms is shown in Table 1. All of the phantoms were made to be cylinders, and their size was about 10 cm in height and 11 cm in diameter.
Table 1.
Composition of the copolymer-in-oil and B-gel phantoms used in the study.
| Phantom # |
B-Gel % |
Agar % |
Water mL |
Carbonate Particles % |
Oil mL |
Copolymer % |
Silica % |
|---|---|---|---|---|---|---|---|
| Ph1 | NONE | NONE | NONE | NONE | 1000 | 3 | 1 |
| Ph2 | NONE | NONE | NONE | NONE | 1000 | 4 | 1 |
| Ph3 | NONE | NONE | NONE | NONE | 1000 | 5 | 1 |
| Ph4 | NONE | NONE | NONE | NONE | 1000 | 5.5 | 1 |
| Ph5 | NONE | NONE | NONE | NONE | 1000 | 6 | 1 |
| Ph6 | NONE | NONE | NONE | NONE | 1000 | 7.5 | 1 |
| Ph7 | NONE | NONE | NONE | NONE | 1000 | 8.5 | 1 |
| Ph8 | 5 | 1 | 1000 | 0.1 | NONE | NONE | NONE |
| Ph9 | 5.5 | 1 | 1000 | 0.1 | NONE | NONE | NONE |
| Ph10 | 6 | 1 | 1000 | 0.1 – 0.2 | NONE | NONE | NONE |
| Ph11 | 7 | 1 | 1000 | 0.1 – 0.2 | NONE | NONE | NONE |
| Ph12 | 7.5 | 1 | 1000 | 0.1 – 0.2 | NONE | NONE | NONE |
| Ph13 | 8 | 1 | 1000 | 0.2 | NONE | NONE | NONE |
| Ph14 | 8.5 | 1 | 1000 | NONE | NONE | NONE | NONE |
| Ph15 | 9 | 1 | 1000 | 0.2 | NONE | NONE | NONE |
| Ph16 | 10 | 1 | 1000 | 0.1 – 0.2 | NONE | NONE | NONE |
| Ph17 | 11 | 1 | 1000 | 0.1 – 0.2 | NONE | NONE | NONE |
| Ph18 | 12 | 1 | 1000 | NONE | NONE | NONE | NONE |
| Ph19 | 17.5 | 1 | 1000 | NONE | NONE | NONE | NONE |
Elasticity Properties
Elasticity properties such as the shear modulus μ and Young’s modulus E describe the mechanical response of a medium under shear stress and longitudinal stress, respectively. Young’s modulus is the ratio between longitudinal stress and longitudinal strain, and shear modulus is the ratio between shear stress and shear strain. Since the Poisson’s ratio ν for most soft tissues is very close to that of an incompressible liquid (ν = 0.5), the shear modulus and Young’s modulus in tissue differ by a scaling factor of 3:
| [1] |
For a homogeneous, isotropic and linearly elastic medium, the shear modulus is a simple function of the speed of shear wave propagation in the medium:
| [2] |
where Vs is the speed of the shear wave and ρ is the density of the material (assumed to be 1.0 g/cm3 for soft tissue). Both MRE and UTE measure shear wave speed to calculate the shear modulus and Young’s modulus of an object (6, 7).
Transient Elastography
UTE uses a single ultrasound transducer in A-mode to measure the displacements induced in a medium by the propagation of a shear wave. The shear wave is produced by a transient mechanical vibration emitted at the same location as the ultrasound transducer (Figure 1). The applied vibration is one cycle of a 50-Hz sinusoidal wave. The ultrasound acquisitions were performed using a Fibroscan system (Echosens, Paris, France) using a measurement protocol similar to (7). The displacements induced by the shear wave in the medium were calculated by cross-correlating the successive ultrasound lines acquired with the high-frame-rate ultrasound system. A spatial-temporal strain map was then computed from the recorded displacements. The shear wave speed was calculated based on the slope of the wave front visualized in the strain map (7). In UTE, the value of the shear wave speed is influenced by diffraction and coupling effects (17). These diffraction and coupling effects can be due to the finite dimension of the source of vibration as well as the proximity of the source. In this last case, the shear wave speed may be overestimated by 50% in the immediate vicinity of the source. These effects were compensated for using the simplified Green’s function to deduce the true shear wave speed of the medium from the displacement field even in the near field (17, 18). A look-up table giving the measured shear wave speed as a function of the true shear wave speed was calculated. The true shear wave speed was deduced from a theoretical displacement field calculated from the convolution of the analytical impulse response of a circular piston on the axis of the excitation source with the low-frequency vibration applied to the medium. The diffraction impulse response (DIR) is determined from the simplified Green’s function (17):
| [3] |
where R is the radius of the excitation source, the z-axis is the direction of observation, ρ is the mass density, Vs is the shear wave speed, and a is the amplitude of the vibration applied in the z direction.
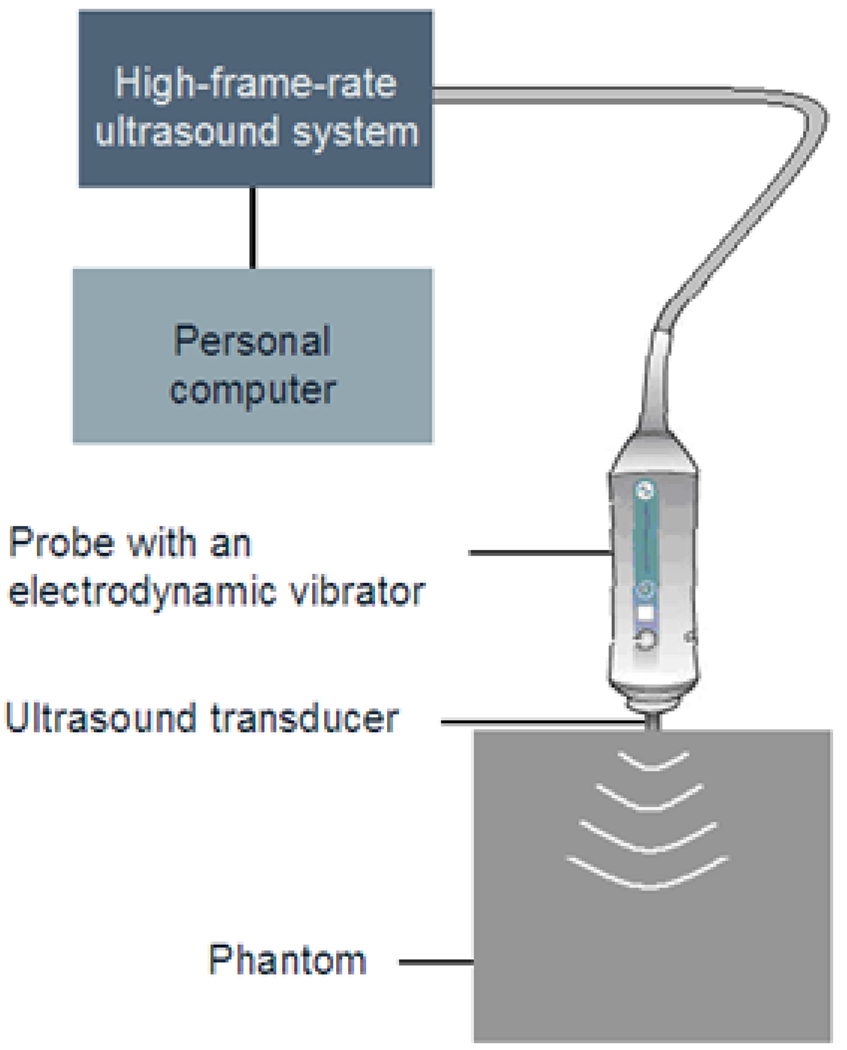
Figure 1.
Schematic diagram of the transient elastography system. The UTE system is composed of an ultrasound probe (3.5 MHz) and a vibrator located on the probe. The vibrator sends a low-frequency (50 Hz) transient wave inside the phantom. The ultrasound probe is used as an emitter-receptor to follow the shear wave propagation induced by the vibration.
Magnetic Resonance Elastography
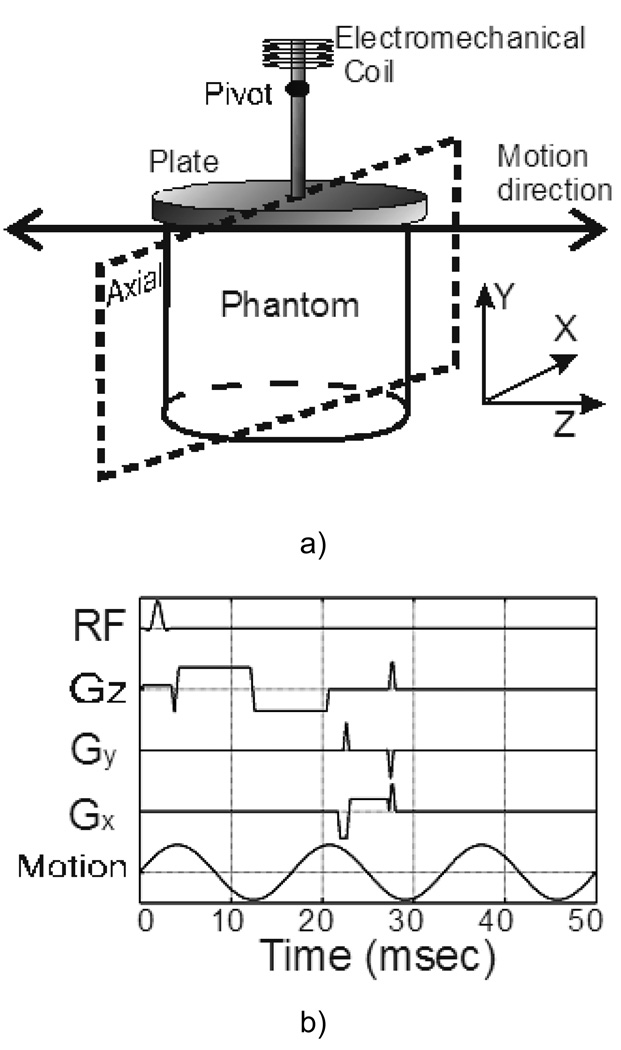
MRE uses a phase-contrast MRI acquisition strategy to acquire images (called phase or wave images) of steady-state shear wave propagation in an object that is induced by an external mechanical driver. These wave images are then processed using an inversion algorithm to calculate a stiffness map of the object, called an elastogram (6). In this study, an electromechanical shear driver was used to apply continuous 60-Hz sinusoidal mechanical vibrations to the surface of each phantom along the z-axis of the scanner (Figure 2a). The electromechanical coil of the driver was connected with a rigid rod to a plate placed on the top of the phantom. A pivot in the rod between the coil and the plate was used to convert the mechanical motion of the coil to shear motion of the plate. Single-slice MRE data were acquired in an axial plane through the middle of the phantom and the driver as indicated in Figure 2a. A motion-encoding gradient (MEG) was inserted in the z direction of a gradient echo sequence to encode the mechanical motion in each phantom (Figure 2b). The MEG was composed of a pair of bipolar trapezoidal gradients with a total duration of 16.67 ms and an amplitude of 17.6 mT/m. The displacement of the phantom can be calculated from the phase images obtained from the MR acquisitions using the following formula reflecting the phase sensitivity of a rectangular MEG to sinusoidal motion (6):
| [4] |
where ϕ is the phase of the reconstructed MR images, r is the mean position of the nuclear spins, N is the number of gradient pairs, T is the period of the mechanical motion and the MEG, γ is the gyromagnetic ratio of the nuclear spins, ζ⃗0 is the displacement vector of the spins, k is the shear wave vector and θ is the initial phase offset between the MEG and the mechanical motion. By repeating the acquisition while adjusting the phase relationship between the MEG and the motion, multiple images (or phase offsets) showing the wave propagation throughout each phantom can be obtained. The local shear wave speed was calculated for these wave images using a local frequency estimation (LFE) algorithm (19), and the stiffness was calculated based on Eq. (2). MRE acquisitions were performed on a 1.5-T MRI scanner (GE, Milwaukee, Wisconsin, USA) using a protocol similar to (10), with the following parameters: matrix = 256×64, TR = 50 ms, TE = 26.5 ms, slice thickness = 10 mm, FOV = 140 × 140 mm, BW = ±16 kHz, flip angle = 30°, and 4 phase offsets.
Figure 2.
Schematic diagram of the MRE setup (a) and the motion-encoding pulse sequence (b). The electromechanical coil vibrates continuously at 60 Hz and the motion is converted into Z-direction shear motion of a plate which is in contact with the surface of the phantom. The pulse sequence is a gradient echo sequence with a pair of bipolar trapezoidal motion-encoding gradients with a total duration of 16.67 ms and an amplitude of 17.6 mT/m applied along the Z-direction. The imaging plane is axial (X-Y plane), and the other MRE imaging parameters are as follows: acquisition matrix = 256 × 64, TR = 50 ms, TE = 26.5 ms, slice thickness = 10 mm, FOV = 140 × 140 mm, BW = ± 16 kHz, flip angle = 30°, and number of phase offsets = 4.
Statistics
The statistical analysis of Bland & Altman was used (20, 21) to evaluate the agreement between UTE and MRE measurement methods by studying the dependence of the mean of the two techniques and the standard deviation of the difference between the two techniques across the spectrum of phantoms studied. Linear regression was performed to assess the correlation between the two techniques.
One of the authors performed the MRE exams while another author performed the UTE exams. Both operators were blinded to the results from the other technique during the measurements. For each individual phantom, MRE and UTE were performed successively. UTE reported the mean stiffness and standard deviation among 10 valid measurements, and MRE reported the mean stiffness and standard deviation in a region of interest (ROI) in the MR elastogram.
3. Results
The shear modulus was measured successively by MRE and UTE in all 19 phantoms. Figure 3 shows an example of the data obtained from phantom #1 (1.1 kPa average of the MRE and UTE shear modulus estimates). Figure 3a shows an example MRE wave image for this phantom on the left, and the corresponding elastogram on the right. Figure 3b shows an example UTE spatio-temporal strain map for this phantom. The propagation of the transient wave front can be seen in the middle of the image, partly indicated by the dotted line. The slope of this line yields an estimate of the shear wave speed for the stiffness calculations. The shear moduli from all of the phantoms obtained by MRE and UTE are shown in Figure 4.
Figure 3.
Example data from phantom #1 with an average MRE and UTE shear modulus measurement of 1.1 kPa. The left image in (a) shows an example wave image from the MRE acquisition. The right image in (a) shows the corresponding elastogram. The spatio-temporal strain map in (b) from the UTE acquisition shows the transient wave propagation in the middle of the image, partially identified with the white dotted line.
Figure 4.
Compiled shear modulus (μ) results using MRE and UTE for all 19 phantoms. The error bars indicate ±1 standard deviation.
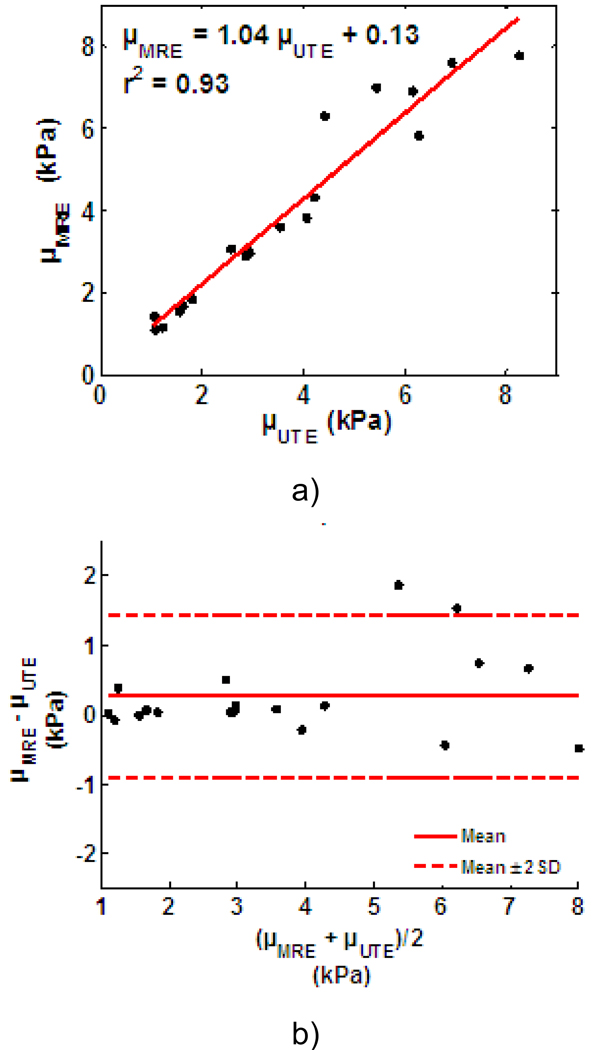
Linear regression analysis shows the stiffness measurements obtained using UTE and MRE are highly correlated with a correlation coefficient r2=0.93 (Figure 5a). The least-squares fit between them is μMRE = 1.04μUTE + 0.13. The results of the Bland-Altman analysis (20, 21) are shown in Figure 5b. The mean difference value (M=μMRE–μUTE) was 0.27 kPa, and the standard error (SD) was 0.58 kPa. The upper and lower limits of agreement (M±2SD) were 1.44 kPa and −0.91 kPa, respectively. The 95% confidence interval for the lower limit of agreement is −1.41 to −0.41 kPa. For the upper limit of agreement, the 95% confidence interval is 0.94 to 1.94 kPa.
Figure 5.
(a) Linear correlation analysis comparing MRE and UTE stiffness estimates for all 19 phantoms indicating a significant correlation between the two techniques. The Bland-Altman analysis of the same data is shown in (b). The x-axis is the average value of the MRE and UTE measurements, and the y-axis is the difference between them (μMRE–μUTE).
4. Discussion
The measurement difference between the two methods tends to be larger for stiff phantoms than for soft phantoms. This is because of the geometrical limitation of the stiff phantoms. An 8 kPa phantom, for example, has a shear wave speed of 2.8 m/s, and shear wavelength of 4.6 cm at 60 Hz, which is about half the size of the phantom. Because of the limited resolution of the LFE algorithm (19), such a large wavelength compared to the size of the phantom will decrease the accuracy of the local frequency estimation algorithm, hence the accuracy of the stiffness estimation of MRE. The propagation time from the UTE vibration source to the other side of the phantom for the 50-Hz transient shear wave is about 36 msec in this case, and the slope of the wave front in the temporal-spatial strain map is steep. This will reduce the accuracy of the stiffness measurements using UTE due to the limited temporal resolution. However, there was no evidence of a significant systematic difference between the two techniques across the range of stiffness values tested (Figure 5b).
In conclusion, detailed in vitro cross validation of MRE and UTE in well-characterized phantom materials has demonstrated excellent correlation in the measurement of shear modulus, and no evidence of systematic differences between the two techniques. The most significant differences between the two techniques in clinical practice are that MRE provides a spatial map of stiffness at different locations throughout the liver, while UTE provides an average stiffness estimate from a region of interest in the liver. However, by virtue of its ultrasound-based design, UTE is very portable compared to MRE. As potential alternatives to liver biopsy, these two noninvasive methods provide clinicians with important new options for improving patient care regarding liver diseases in terms of the diagnosis, prognosis, and monitoring of fibrosis progression, as well for evaluating the efficacy of treatment. Ongoing research will continue to evaluate the agreement between these two techniques in in vivo human studies.
Acknowledgement
We thank Stephen S. Cha for discussing the Bland-Altman analysis.
References
- 1.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 2.Bensamoun SF, Wang L, Robert L, et al. Measurement of liver stiffness with two imaging techniques: Magnetic resonance elastography and ultrasound elastometry. J Magn Reson Imaging. 2008;28:1287–1292. doi: 10.1002/jmri.21523. [DOI] [PubMed] [Google Scholar]
- 3.Huwart L, Salameh N, Ter Beek L, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 4.Malik R, Afdhal N. Stiffness and impedance: the new liver biomarkers. Clin Gastroenterol Hepatol. 2007;5:1144–1146. doi: 10.1016/j.cgh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Dresner MA, Rose G, Phillip H, et al. Magnetic resonance elastography of skeletal muscle. J Magn Reson Imaging. 2001;13:269–276. doi: 10.1002/1522-2586(200102)13:2<269::aid-jmri1039>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 7.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology. 2008;135:299–302. doi: 10.1053/j.gastro.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziol M, Christidis C, de Lédinghen V, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 12.Huwart L, Sempoux C, Salameh N, et al. Liver fibrosis: Noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 13.Madsen EL, Zagzebski JA, Banjavie RA, Jutila RE. Tissue mimicking materials for ultrasound phantoms. Med Phys. 1978;5:391–394. doi: 10.1118/1.594483. [DOI] [PubMed] [Google Scholar]
- 14.Madsen EL, Hobson MA, Shi HR, Varghese T, Frank GR. Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys Med Biol. 2005;50:5597–5618. doi: 10.1088/0031-9155/50/23/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oudry J, Bastard C, Miette V, Willinger R, Sandrin L. Copolymer-in-Oil Phantom Materials for Elastography. Ultrasound Med Biol. 2009;35:1185–1197. doi: 10.1016/j.ultrasmedbio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Sandrin L, Cassereau D, Fink M. The role of the coupling term in transient elastography. J Acoust Soc Am. 2004;115:73–83. doi: 10.1121/1.1635412. [DOI] [PubMed] [Google Scholar]
- 18.Aki K, Richards PG. Quantitative seismology. 2nd edition. Sausalito: University Science Book; 2002. p. 700. [Google Scholar]
- 19.Manduca A, Mahowald JL, Felmlee JP, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]