Summary
Olfactory signals influence social interactions in a variety of species [1, 2]. In mammals, pheromones and other social cues can promote mating or aggression behaviors, can communicate information about social hierarchies, genetic identity and health status, and can contribute to associative learning [1–5]. However, the molecular, cellular and neural mechanisms underlying many olfactory-mediated social interactions remain poorly understood. Here, we report that a specialized olfactory subsystem that includes olfactory sensory neurons (OSNs) expressing the receptor guanylyl cyclase GC-D, the cyclic nucleotide-gated channel subunit CNGA3 and the carbonic anhydrase isoform CAII (GC-D+ OSNs) [6–11] is required for the acquisition of socially transmitted food preferences (STFPs) in mice. Using electrophysiological recordings from gene-targeted mice, we show that GC-D+ OSNs are highly sensitive to the volatile semiochemical carbon disulfide (CS2), a component of rodent breath and a known social signal mediating the acquisition of STFPs [12–14]. Responses to sub-micromolar concentrations of CS2 in the main olfactory epithelium or in identified GC-D+ OSNs are absent in mice lacking CNGA3 or CAII and drastically reduced in mice lacking GC-D. Mice in which GC-D+ OSN transduction mechanisms have been disrupted fail to acquire STFPs from either live or surrogate demonstrator mice and do not exhibit neuronal activation of the ventral subiculum of the hippocampus, a brain region implicated in STFP retrieval [15]. Our findings indicate that GC-D+ OSNs detect chemosignals that facilitate food-related social interactions.
Keywords: GC-D, semiochemical, CS2, CNGA3, carbonic anhydrase, social communication, odor, mouse
Results and Discussion
The olfactory system contains several subsystems, each of which can be distinguished by distinct sensory neuron subpopulations that express unique complements of chemosensory receptors, make discrete neural connections to the olfactory forebrain, and can respond to a plethora of diverse stimuli [16]. GC-D+ OSNs and their olfactory bulb targets, the necklace glomeruli (Figure 1A), comprise one subsystem of the main olfactory system [11, 16]. GC-D+ OSNs use a cGMP-mediated signaling cascade to transduce a small group of chemostimuli: urine [10], a rich source of social signals for mice; the natriuretic peptide hormones uroguanylin and guanylin [10]; and the gas carbon dioxide (CO2) [9]. We reasoned that the GC-D/necklace subsystem was an ideal candidate to mediate chemosignals related to the acquisition of socially transmitted food preferences (STFPs), which require the pairing of a food odor with a social stimulus such as CS2. First, GC-D+ OSNs respond to diverse stimuli implicated as semiochemicals [9, 10]. Second, carbonic anhydrases may play either a direct or indirect role in the metabolism of CS2 [17–19]. Third, the necklace glomeruli are innervated by more than one sensory neuron type and exhibit extensive connections with other main olfactory bulb glomeruli [20], suggesting that they could integrate diverse chemostimuli such as semiochemicals and food odors. Therefore, we sought to determine whether the GC-D/necklace subsystem plays an essential olfactory role in the detection of CS2 and the acquisition of STFPs.
Figure 1.
MOE responses to sub-micromolar concentrations of CS2 require signaling components expressed in GC-D+ OSNs. (A) Schematic illustrating several key regions of the mouse olfactory system. GG, Grueneberg ganglion; VNO, vomeronasal organ (apical and basal regions); MOE, main olfactory epithelium; MOB, main olfactory bulb; NG, necklace glomeruli (blue spheres); AOB, accessory olfactory bulb (anterior and posterior regions). Blowout is a confocal micrograph showing a single GC-D+ OSN (white, labeled with antisera to PDE2) in the MOE amongst numerous DAPI (blue)-stained nuclei of other neurons, supporting cells and progenitor cells. See Figure 2A for schematic of an OSN. Scale bar, 20 µm. (B) Examples of local field potentials generated in the MOE of C57BL/6J (B6), Gucy2d−/−, Car2n or Cnga3−/− mice, with 500-ms pulses of 1 µM guanylin (G), 1 µM uroguanylin (UG), 0.4 µM CS2 or 13.3 µM CS2. (C) Mean EOG responses to different concentrations of CS2 from MOE of B6, Gucy2d+/−, Gucy2d−/−, Car2n or Cnga3−/− mice (n ≥ 3 each). LSD: *p < 0.05, **p < 0.01, ***p < 0.0001; ns, not significant. Responses were concentration dependent (ANOVA: F = 25.47, p < 0.0001). Number of independent recordings in parentheses. Data are expressed as means ± s.e.m. (D) Mean EOG responses to G or UG (1 µM each) from MOE of B6, Gucy2d+/−, Gucy2d−/−, Car2n or Cnga3−/− mice (n ≥ 3 each). Responses to G and UG were indistinguishable from each other in Gucy2d+/− and Car2n mice (LSD: p = 0.09 – 0.96) but absent in Gucy2d−/− and Cnga3−/− mice (LSD: p < 0.0001). Number of independent recordings in parentheses. Data are expressed as means ± s.e.m. (E) Slice preparation of Grueneberg ganglion [41] from Omp-EGFP mouse showing sensory cells (green). Scale bar, 25 µm. (F) Ca2+ response of a single Grueneberg ganglion cell to 1 mM CS2 and 60 mM KCl. Responses are representative of the analysis of eight Grueneberg ganglion slices, each from a different Omp-EGFP mouse.
GC-D+ OSNs respond to CS2 at sub-micromolar concentrations
We first used field potential recordings (electroolfactograms, or EOGs) to characterize CS2 responses in the main olfactory epithelium (MOE) of mice. EOG recordings from the MOE of C57BL/6 (B6) mice routinely showed responses to CS2 at concentrations as low as 0.13 µM (Figure 1B,C). Dose-response measurements confirmed that CS2 is a potent olfactory stimulus and that these responses are concentration-dependent (Figure 1C). CS2 concentrations of ~ 1 ppm (13 µM) are found in rat breath and can elicit the formation of an STFP when paired with a food odor [12]. Thus, the MOE can easily respond to CS2 at behaviorally relevant concentrations.
Next, we sought to determine if proteins previously implicated in the transduction of chemosignals by GC-D+ OSNs are required for MOE responses to CS2. GC-D appears to serve as both receptor and effector enzyme for the peptide stimuli uroguanylin and guanylin [10, 21]. Alternatively, CAII promotes the hydration of CO2 [9], producing bicarbonate ion that may act to stimulate GC-D enzymatic activity [22, 23]. CAII may also play a role in the metabolism of CS2 or its oxidation product, carbonyl sulfide [18, 24] (carbonyl sulfide may also function as a social cue in STFP formation [12]). We measured EOG responses in the MOE of mice lacking GC-D (Gucy2d-Mapt-lacZ−/− mice [10], hereafter referred to as Gucy2d−/− mice) or CAII (B6.D2-Car2n [25] mice, hereafter referred to as Car2n mice; Figures 1B, 1C and 1D). Robust EOG responses to uroguanylin or guanylin (1 µM each) were present in Gucy2d+/− and Car2n mice but absent in Gucy2d−/− mice (Figures 1B and 1D and [10]). Drastically reduced CS2 responses were observed in Gucy2d−/− mice (Figures 1B and 1D), suggesting that while GC-D plays a role in CS2 transduction, it is not required to maintain minimal responses. In contrast, EOG responses to 0.4 µM CS2 were absent in Car2n mice (Figures 1B and 1D), suggesting that MOE responses to sub-micromolar concentrations of CS2 require CAII. The cGMP-gated channel subunit CNGA3 is thought to be the final common target of both branches of the chemotransduction cascade in GC-D+ OSNs [9, 10]. Cnga3−/− mice [26] show no EOG responses to either peptide (Figure 1E, and [10]) or to CS2 concentrations below 13.3 µM (Figures 1B and 1D). Higher CS2 concentrations have a perceptible odor that could activate canonical OSNs (Supplementary Figure S1), likely accounting for residual responses in Car2n and Cnga3−/− mice to 13.3 µM CS2. In contrast, even 1 mM CS2 failed to elicit responses in Grueneberg ganglion neurons (Figures 1E and 1F), a group of chemosensory neurons in the anterior nasal cavity that express CNGA3 but not GC-D [27, 28]. Together, these data indicate that CAII, CNGA3 and GC-D play critical roles in the MOE responses to CS2 and suggest that GC-D+ OSNs are the sole chemosensors for sub-micromolar concentrations of this semiochemical.
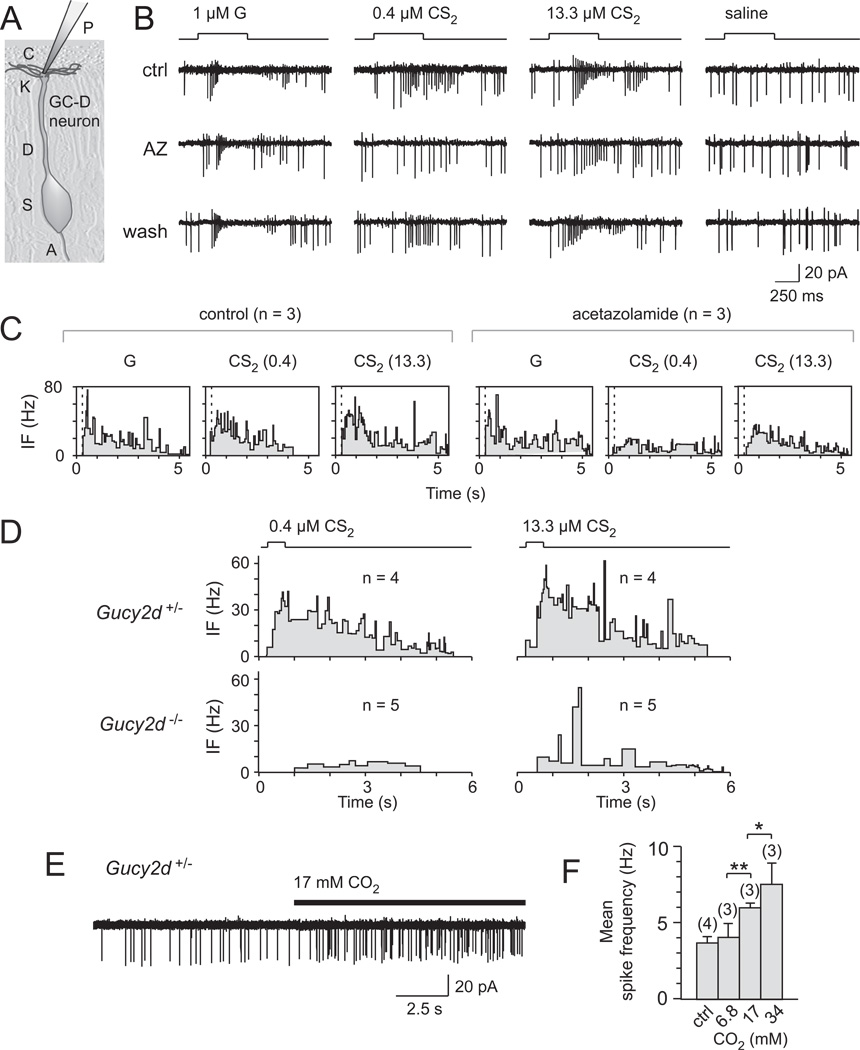
To directly demonstrate that GC-D+ OSNs are activated by CS2, we next performed cell-attached patch clamp recordings from β-galactosidase-expressing (β-gal+) dendritic knobs in Gucy2d+/− mice. These mice express a tau-β-gal fusion protein from the targeted allele under the control of the Gucy2d promoter [10]. Extracellular loose patch clamp recordings can be obtained from the dendritic knobs of identified GC-D+ OSNs in these mice as the cells can be visualized using a fluorescent β-gal substrate (Figure 2A; [10]). Both guanylin peptides (1 µM each) and CS2 (0.4 or 13.3 µM), but not saline, elicited a transient increase in action potential firing in β-gal+ OSNs from Gucy2d+/− mice (Figures 2B and 2D and data not shown; [10]). CS2-induced spike frequencies were concentration-dependent. Responses to CS2, but not guanylin, were significantly reduced in the presence of the CA inhibitor acetazolamide (AZ; 1 mM; Figures 2B, 2C). Consistent with the results of the EOG recordings, CS2 responses were strongly reduced but not fully abolished in β-gal+ OSNs from Gucy2d−/− mice (Figure 2D). Together with the EOG results (Figure 1) and our previous report [10], these data indicate that GC-D+ OSNs are exquisitely sensitive chemosensors, responding to sub-micromolar levels of CS2 and to picomolar concentrations of guanylin family peptides (EC50 = 60 - 770 pM [10]). In contrast, GC-D+ OSNs are at least 10,000-fold less sensitive to CO2 (spike threshold > 6.8 mM (Figures 2E and 2F); knob Ca2+ responses = 6.4 mM [22]; behavior threshold = ~ 15 mM [9]). The high degree of sensitivity to CS2 and guanylin peptides is strikingly reminiscent of that seen for other semiochemical-responsive OSNs and VSNs [16].
Figure 2.
GC-D+ OSNs are highly sensitive CS2 detectors. (A) Schematic of patch clamp recording from dendritic knobs of identified (i.e., β-galactosidase-expressing) GC-D+ OSNs. P, patch electrode; C, cilia; K, knob; D, dendrite; S, soma; A, axon. (B) Stimulus-evoked discharges recorded from an individual dendritic knob of a ®-gal-expressing OSN of Gucy2d+/− mice (continuous recording) in response to guanylin (G, 1 ⎧M), CS2 (0.4 ⎧M or 13.3 ⎧M), or saline before, during or after treatment with 1 mM acetazolamide (AZ; n=3). CS2 produced a profound, concentration-dependent excitation in these cells (ANOVA: F = 13.4, p = 0.002). (C) Mean instantaneous firing frequencies before (area under the curve (AUC): guanylin, 73.5 ± 4.9 Hz·s; 0.4 µM CS2, 83.2 ± 5.2 Hz·s; 13.3 µM CS2, 100.8 ± 3.0 Hz·s) or after AZ treatment (AUC: guanylin, 83.8 ± 10.0 Hz·s; 0.4 µM CS2, 56.7 ± 6.1 Hz·s; 13.3 µM CS2, 74.8 ± 1.3 Hz·s). The CS2 response magnitude was significantly reduced after AZ treatment (guanylin: LSD: P=0.05; CS2: LSD: P < 0.0001). (D) Mean instantaneous firing frequencies of CS2-dependent action potentials in β-gal-expressing OSNs of Gucy2d+/− (AUC: 0.4 µM CS2, 70.8 ± 10.8 Hz·s; 13.3 µM CS2, 94.4 ± 18.5 Hz·s) and Gucy2d−/− mice (AUC: 0.4 µM CS2, 16.3 ± 4.1 Hz·s; 13.3 µM CS2, 35.8 ± 14.8 Hz·s; LSD: P < 0.0001). No increase in firing frequency was observed upon stimulation with 0.4 µM CS2 in Gucy2d−/− mice (LSD: p = 0.76). Number of cells indicated in the figure. (E) Stimulus-evoked discharges recorded from dendritic knob of β-gal-expressing OSN from a Gucy2d+/− mouse in response to 17.4 mM CO2. (F) Mean spike frequency in response to CO2 stimulation of β-gal-expressing OSNs from Gucy2d+/− mice. Number of independent recordings in parentheses. Experiments were performed in at least three mice. Data are expressed as means ± SD. LSD: *p ≤ 0.05, **p < 0.01.
Mice lacking CNGA3, GC-D or CAII function fail to acquire socially transmitted food preferences
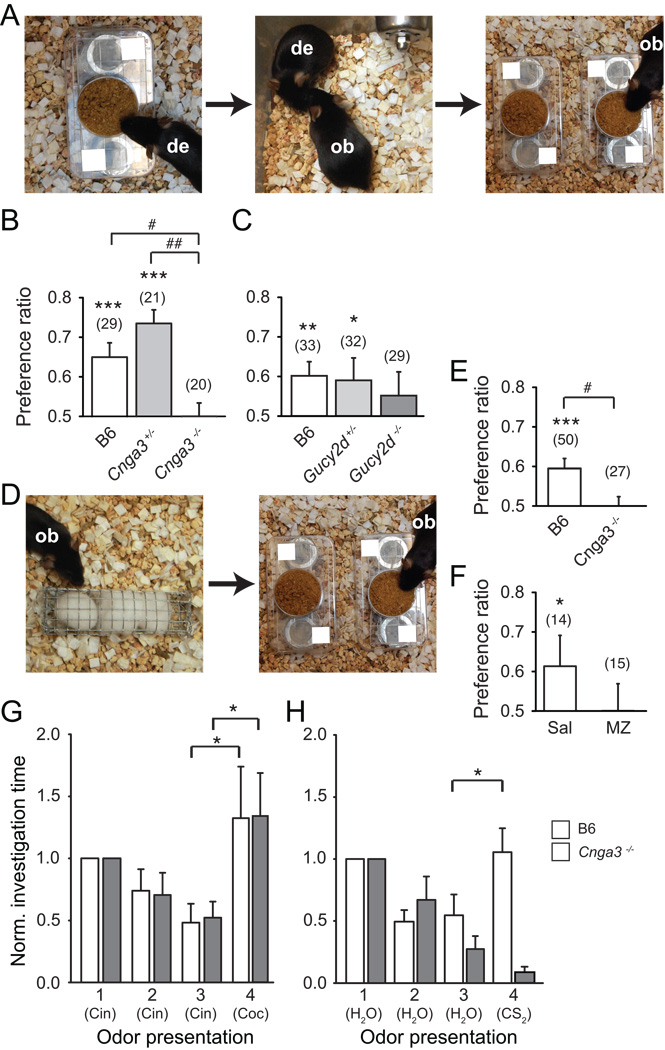
The acquisition of STFPs requires both a social cue (e.g., breath of a conspecific, or the semiochemical CS2) and a food odor [12–14, 29]. We modified a bioassay used for assaying STFPs in rodents (Figure 3A; [13, 14, 30, 31]) to test whether GC-D+ OSNs are required for the formation of an STFP. First, group housed “observer” mice (B6, Cnga3+/−, Cnga3−/−, Gucy2d+/− or Gucy2d−/−) were introduced to a food odor by a live “demonstrator” mouse that had eaten that food (standard mouse chow with either 1% cinnamon or 2% cocoa added) for the previous hour. Following a 1 hr interaction with the demonstrator, observers were separated and provided with a choice of cinnamon- or cocoa-odorized food. The amount of each food consumed was measured after 60 min. B6 and Cnga3+/− (Figure 3B) observers show a preference for the demonstrated food (see figure legend for preference ratios and statistics). In parallel experiments, both B6 and Gucy2d+/− (Figure 3C) observers also showed a preference for the demonstrated food. In contrast, neither Cnga3−/− nor Gucy2d−/− mice showed a preference for the demonstrated food (Figures 3B and 3C). The most robust effect of the genotype was seen in Cnga3−/− mice, which showed significantly lower PRs than either B6 (p = 0.03) or Cnga3+/− (p = 0.002) mice (one-way ANOVA of ranks with Mann-Whitney U test). Together, these results indicate that CNGA3 and GC-D, both of which are required for normal sensitivity to sub-micromolar CS2-sensitivity of GC-D+ OSNs, are necessary for the acquisition of STFPs.
Figure 3.
Mice deficient in GC-D+ OSN-mediated CS2 responses fail to acquire socially transmitted food preferences. (A) In the STFP bioassay, a live demonstrator mouse (de) eats odored food (left) and is then exposed to one or more observer mice (ob; middle). Each observer mouse is then given a choice of food with the demonstrated odor or a novel odor (right). (B) Preference ratios (PR) for B6, Cnga3+/− and Cnga3−/− mice in the STFP bioassay. [PR: demonstrated food consumed (g) / total food consumed (g); PR of 0.50 indicates no preference]. PR (B6) = 0.65 ± 0.04; PR (Cnga3+/−) = 0.74 ± 0.03; PR (Cnga3−/−) = 0.50 ± 0.03; Z test: z (B6) = 4.18, p < 0.0001; z (Cnga3+/−) = 6.84, p < 0.0001; z (Cnga3−/−) = 0.03, p = 0.49. ***, p < 0.0001 (z score); #, p < 0.05 (Mann Whitney U). Parentheses, number of mice. (C) Preference ratios for B6, Gucy2d+/− and Gucy2d−/− mice in the STFP bioassay. PR (B6) = 0.60 ± 0.04; PR (Gucy2d+/−) = 0.59 ± 0.06; PR (Gucy2d−/−) = 0.55 ± 0.06; z (B6) = 2.84, p = 0.003; z (Gucy2d+/−) = 1.6, p = 0.05; z (Gucy2d−/−) = 0.87; p = 0.19. **, p < 0.01, *, p < 0.05 (z score). Parentheses, number of mice. (D) In the CS2 bioassay, a live observer mouse (ob) is exposed to a surrogate demonstrator treated with 1 ppm (13 µM) CS2 and a food odor (left). Each observer mouse is then given a choice of food with the demonstrated odor or a novel odor (right). (E) Preference ratios for B6 and Cnga3−/− mice in the CS2 bioassay. PR (B6) = 0.59 ± 0.02; z = 3.7, p = 0.001; PR (Cnga3−/−) = 0.50 ± 0.02; z = -0.08, p = 0.47. ***p < 0.001 (z score); #, p < 0.05 (Mann Whitney U). Parentheses, number of mice. (F) Preference ratios of B6 mice treated with intranasal application of saline or methazolamide (MZ). PR (MZ) = 0.49 ± 0.07; z = 0.95, p = 0.17; PR (saline) = 0.61 ± 0.08; z = 1.68, p = 0.05. *, p < 0.05 (z score). (G) Performance of B6 (white) and Cnga3−/− (gray) mice in an olfactory habituation/dishabituation task with two food odors. Numbers indicate odor presentation order. Cin, cinnamon (1% in water); Coc, cocoa (2% in water). One-way repeated measures ANOVA: F = 3.20, p = 0.04 (B6); F = 4.2, p = 0.01 (Cnga3−/−); *, p < 0.05 (SNK post-hoc). (H) Performance of B6 and Cnga3−/− mice (gray) in an olfactory habituation/dishabituation task with water and 13 µM CS2. Numbers indicate odor presentation order. One-way repeated measures ANOVA: F = 2.33, p = 0.004 (B6); F = 2.97, p < 0.001 (Cnga3−/−); *, p < 0.05 (SNK post-hoc).
Rodents are able to form food preferences to odorized food in the absence of a live demonstrator when CS2 is presented as the sole social stimulus [12]. Furthermore, CS2 enhances preference for, and ingestion of, food by mice [32]. We next asked if mice with disruptions in GC-D+ transduction mechanisms would fail to display a preference for odorized food demonstrated with CS2. First, Cnga3−/− observer mice were presented with a cotton surrogate instead of a live demonstrator (Figure 3D). Surrogates were first treated with powdered food odorized with the demonstrated odor (1% cinnamon or 2% cocoa) along with 13.3 µM CS2. Observer mice were then given a choice to consume cinnamon- or cocoa-odorized food. B6 mice preferred to consume food odorized with the demonstrated odor, while Cnga3−/− mice showed no preference (Figure 3E). Observers did not form a preference from surrogate demonstrators treated with a food odor alone (data not shown; PR = 0.45 ± 0.08; z = −0.63, p = 0.25), consistent with the observation that activation of the GC-D/necklace subsystem requires external stimuli, not components of the observer’s breath [33]. Next, we used the same behavioral paradigm to test the role of CA activity in the acquisition of CS2-mediated food preferences. B6 mice pretreated with an intranasal application of the CA inhibitor methazolamide (MZ; 10 mM) failed to form a preference (Figure 3F). In contrast, saline treated mice preferred the demonstrated food (Figure 3F). The ability of mice to acquire olfactory-mediated STFPs is impaired after pharmacological or genetic disruption of each of three transduction proteins (CAII, GC-D and CNGA3) expressed in GC-D+ OSNs and required for electrophysiological responses to sub-micromolar concentrations of CS2. Although we cannot fully exclude the possibility that all three proteins act somewhere in the central nervous system to influence this specific behavior, we conclude that the acquisition of STFPs requires functional GC-D+ OSNs.
The acquisition of an STFP is not a purely sensory phenomenon, but is a learned association that requires memory formation. To control for deficits in short-term memory that might prevent the acquisition of an STFP, B6 and Cnga3−/− mice were tested in a standard habituation/dishabituation assay (Figure 3G). Mice were presented with cotton swabs odorized with cinnamon for three 1 min trials, each trial separated by a 2 min interval. During the fourth trial, the swab was changed to a cocoa odor. Both B6 and Cnga3−/− mice showed decreasing investigation time with each presentation of the cinnamon odor (habituation) followed by increased investigation time of the novel cocoa odor (dishabituation), indicating that the failure of Cnga3−/− mice to acquire an STFP does not result either from a deficit in short term memory or the inability to detect food odors such as cocoa or cinnamon. However, in a similar experiment using three habituation trials of water and a final trial with 13.3 µM CS2 (Figure 3H), only B6 mice exhibited dishabituation upon presentation of 13 µM CS2 (SNK, p = 0.02); exploration time of Cnga3−/− mice was not significantly different than the last water presentation (SNK, p = 0.31). Thus, Cnga3−/−, but not B6 mice, fail to differentiate CS2 from water in an olfactory task.
Rodents that acquire an STFP show increased immunoreactivity for the immediate early gene product c-Fos in the ventral subiculum of the hippocampus, but not in dorsal subiculum or entorhinal cortex [15] (Figure 4A). Using random subsets of mice tested in Figure 3B, we found that B6 but not Cnga3−/− observer mice showed increased c-Fos immunoreactivity in the ventral subiculum after acquiring an STFP (Figures 4B and 4C; demonstrated odor: cinnamon; presented foods flavored with cocoa or cinnamon). Both B6 and Cnga3−/− mice demonstrated an irrelevant odor (ginger; presented foods odored with cocoa or cinnamon) failed to show an increase in c-Fos immunoreactivity in the ventral subiculum (Figures 4B and 4C). No significant differences were seen between genotypes or demonstrated odor in either dorsal subiculum or entorhinal cortex (Figures 4D and 4E; and see Figure S2 in Supplementary Materials). Thus, Cnga3−/− mice do not exhibit central nervous system correlates of STFP retrieval, a result consistent with their failure to acquire an STFP.
Figure 4.
CNS correlate of food preference learning is absent in Cnga3−/− mice. (A) Schematic of the mouse brain (one hemisphere, coronal section) indicating areas of ventral subiculum (magenta box), dorsal subiculum (yellow box) and entorhinal cortex (green box) analyzed for c-Fos immunoreactivity in H-K. (B) Representative c-Fos immunohistochemistry in hippocampus ventral subiculum of B6 (top) and Cnga3−/− (bottom) observer mice demonstrated an irrelevant (ginger, left) or relevant (cinnamon, right) odor. All mice were then given a choice of cocoa- or cinnamon-flavored food. Scale bar, 100 um. See Figure S2 in Supplementary data for representative images of dorsal subiculum and entorhinal cortex. (C−E) Mean counts (per mm2) of c-Fos immunoreactive (c-Fos +) cells in ventral subiculum (I), dorsal subiculum (J) and entorhinal cortex (K) of B6 (white; n=6) and Cnga3−/− (gray; n=5) mice demonstrated ginger or cinnamon odors. Two-way ANOVAs revealed no significant effects of genotype or demonstrated odor for either dorsal subiculum (F = 0.580; p = 0.635) or entorhinal cortex (F = 0.624; p = 0.609). There was a significant effect of genotype in ventral subiculum (F = 6.74; p = 0.002). SNK post-hoc tests revealed significant differences between B6 mice demonstrated cinnamon vs. ginger (*, p = 0.05) and between B6 and Cnga3−/− mice demonstrated cinnamon (*, p = 0.02) but not ginger (p = 0.34). (F) Summary schematic. Food odors activate subsets of canonical OSNs in the MOE, while CS2 stimulates GC-D+ OSNs. Afferent information from these two olfactory subsystems is integrated in the olfactory CNS, perhaps as early as the necklace glomeruli in the MOB. An association is formed, which is manifest as a preference for the food paired with CS2.
Conclusions
We conclude that GC-D+ OSNs and the GC-D/necklace olfactory subsystem mediate the detection of social chemostimuli necessary for the formation of STFPs. Rodents and other animals make use of a diverse repertoire of chemical cues to communicate with conspecifics [34]. While some of these chemostimuli may elicit innate behaviors, it is likely that most only have their full meaning in the context of additional sensory cues or previously learned associations. By linking a specific olfactory subsystem to both the expression of an established social learning behavior and to the detection of a chemostimulus that can elicit that behavior (Figure 4F), we highlight the possibility that the subsystem structure of the mammalian main olfactory system may help mammals associate chemostimuli with other sensory cues in a meaningful and organized way. The GC-D/necklace subsystem may be particularly well suited for putting olfactory cues in context. Each necklace glomerulus is innervated by more than one OSN population: GC-D+ OSNs and GC-D-negative OSNs [20]. The heterogeneous sensory innervation of necklace glomeruli is strikingly different from the homogeneous sensory innervation of other MOB glomeruli [35, 36], and suggests that necklace glomeruli could be capable of integrating multiple sensory inputs. Furthermore, extensive connections with other MOB glomeruli via interneurons [20] provide the opportunity to further integrate social stimuli such as CS2 with more general odor stimuli. In this way, the olfactory system could facilitate the initial association of social and food cues that is necessary for STFP formation (Figure 4F). Indeed, the olfactory bulb (or its equivalent in invertebrates) has been implicated as an important site of plasticity with odor-dependent associative learning in several species [37–39]. STFPs are likely critical to survival in the wild, allowing conspecifics to make rapid decisions about whether a particular food is safe to eat and if a food source is nearby. We have speculated that guanylin peptides, which can also activate GC-D+ OSNs, may similarly serve as social signals to communicate information about food [10, 11]. Levels of uroguanylin secreted in urine rise postprandially [40], providing an opportunity for this chemostimulus to convey information that food has been safely eaten and digested. In this light, the GC-D/necklace olfactory subsystem is ideally suited to integrate information about the presence and safety of food.
Highlights
A mammalian olfactory subsystem is essential for a type of social learning.
A food-related social stimulus, CS2, activates specialized olfactory neurons.
Mice with impaired CS2 responses don’t acquire socially transmitted food preferences.
Supplementary Material
Acknowledgements
We thank G. Schoenbaum for helpful comments on the manuscript. These studies were supported by the NIDCD (DC005633 to SDM; DC006603 to K.R.K.) and the DFG (SFB 530/A7 to FZ). T.L.-Z. is a Lichtenberg Professor of the Volkswagen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data
Supplemental Data include supplemental experimental procedures, one supplemental table and two supplemental figures and are available with this article online at
References
- 1.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- 2.Cheal ML, Sprott RL. Social olfaction: a review of the role of olfaction in a variety of animal behaviors. Psychol Rep. 1971;29:195–243. doi: 10.2466/pr0.1971.29.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo D, Lin W, Salcedo E, Yamazaki K, Beauchamp G. Odortypes and MHC peptides: Complementary chemosignals of MHC haplotype? Trends Neurosci. 2006;29:604–609. doi: 10.1016/j.tins.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Kavaliers M, Choleris E, Pfaff DW. Genes, odours and the recognition of parasitized individuals by rodents. Trends Parasitol. 2005;21:423–429. doi: 10.1016/j.pt.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Leon M. The neurobiology of filial learning. Annu Rev Psychol. 1992;43:377–398. doi: 10.1146/annurev.ps.43.020192.002113. [DOI] [PubMed] [Google Scholar]
- 6.Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 10.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zufall F, Munger SD. Receptor guanylyl cyclases in mammalian olfactory function. Mol Cell Biochem. 2010;334:191–197. doi: 10.1007/s11010-009-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galef BG, Jr, Mason JR, Preti G, Bean NJ. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 1988;42:119–124. doi: 10.1016/0031-9384(88)90285-5. [DOI] [PubMed] [Google Scholar]
- 13.Galef BG, Jr, Wigmore SW, Kennett DJ. A failure to find socially mediated taste aversion learning in Norway rats (R. norvegicus) J Comp Psychol. 1983;97:358–363. [PubMed] [Google Scholar]
- 14.Posadas-Andrews A, Roper TJ. Social transmission of food preference in adult rats. Anim Behav. 1983;31:265–271. [Google Scholar]
- 15.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 17.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Goldberg L. A critical review of the literature on carbon disulfide toxicity. Crit Rev Toxicol. 1983;11:169–278. doi: 10.3109/10408448309128255. [DOI] [PubMed] [Google Scholar]
- 18.Haritos VS, Dojchinov G. Carbonic anhydrase metabolism is a key factor in the toxicity of CO2 and COS but not CS2 toward the flour beetle Tribolium castaneum [Coleoptera: Tenebrionidae] Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:139–147. doi: 10.1016/j.cca.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Dalvi RR, Neal RA. Metabolism in vivo of carbon disulfide to carbonyl sulfide and carbon dioxide in the rat. Biochem Pharmacol. 1978;27:1608–1609. doi: 10.1016/0006-2952(78)90494-x. [DOI] [PubMed] [Google Scholar]
- 20.Cockerham RE, Puche AC, Munger SD. Heterogeneous sensory innervation and extensive intrabulbar connections of olfactory necklace glomeruli. PLoS ONE. 2009;4:e4657. doi: 10.1371/journal.pone.0004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci U S A. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chengelis CP, Neal RA. Hepatic carbonyl sulfide metabolism. Biochem Biophys Res Commun. 1979;90:993–999. doi: 10.1016/0006-291x(79)91925-9. [DOI] [PubMed] [Google Scholar]
- 25.Lewis SE, Erickson RP, Barnett LB, Venta PJ, Tashian RE. N-ethyl-N-nitrosourea-induced null mutation at the mouse Car-2 locus: an animal model for human carbonic anhydrase II deficiency syndrome. Proc Natl Acad Sci U S A. 1988;85:1962–1966. doi: 10.1073/pnas.85.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, Biel M. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci. 2005;46:1516–1524. doi: 10.1167/iovs.04-1503. [DOI] [PubMed] [Google Scholar]
- 27.Liu CY, Fraser SE, Koos DS. Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. J Comp Neurol. 2009;516:36–48. doi: 10.1002/cne.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischer J, Mamasuew K, Breer H. Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem Cell Biol. 2009;131:75–88. doi: 10.1007/s00418-008-0514-8. [DOI] [PubMed] [Google Scholar]
- 29.Galef BG, Jr, Kennett DJ. Different mechanisms for social transmission of diet preference in rat pups of different ages. Devel Psychobiol. 1987;20:209–215. doi: 10.1002/dev.420200209. [DOI] [PubMed] [Google Scholar]
- 30.Valsecchi P, Galef BG., Jr Social influences of the food preferences of house mice (Mus musculus) Int J Comp Psychol. 1989;2:245–256. [Google Scholar]
- 31.Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bean NJ, Galef BG, Jr, Mason JR. The effect of carbon disulphide on food consumption by house mice. J Wildl Manage. 1988;52:502–507. [Google Scholar]
- 33.Cockerham RE, Margolis FL, Munger SD. Afferent activity to necklace glomeruli is dependent on external stimuli. BMC Res Notes. 2009;2:31. doi: 10.1186/1756-0500-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyatt T. Pheromones and animal behaviour: communication by smell and taste. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 35.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 36.Spehr M, Munger SD. Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem. 2009;109:1570–1583. doi: 10.1111/j.1471-4159.2009.06085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- 38.Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- 39.Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Forte LR., Jr Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104:137–162. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Schmid A, Pyrski M, Biel M, Leinders-Zufall T, Zufall F. Grueneberg ganglion neurons are finely tuned cold sensors. J Neurosci. 2010;30:7563–7568. doi: 10.1523/JNEUROSCI.0608-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.