Abstract
Neocarzinostatin (1) biosynthesis is proposed to involve a vicinal diol intermediate. It is reported that NcsF2, one of two epoxide hydrolases encoded by the NCS gene cluster, catalyzes regiospecific addition of H2O to C-2 of both (R)- and (S)-styrene oxides to afford (R)- and (S)-1-phenyl-1,2-ethanediols, respectively, supporting its proposed role in 1 biosynthesis. (R)-1-Phenyl-1,2-ethanediol (87% yield and 99% ee) was obtained from (±)-styrene oxide hydrolysis by co-catalysis using NcsF2 and SgcF, the complementary epoxide hydrolase from the C-1027 biosynthetic pathway.
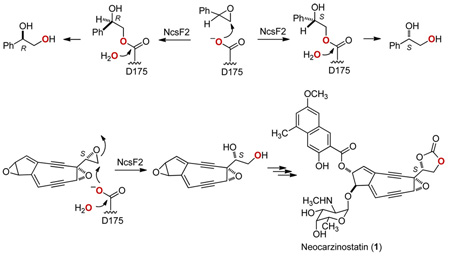
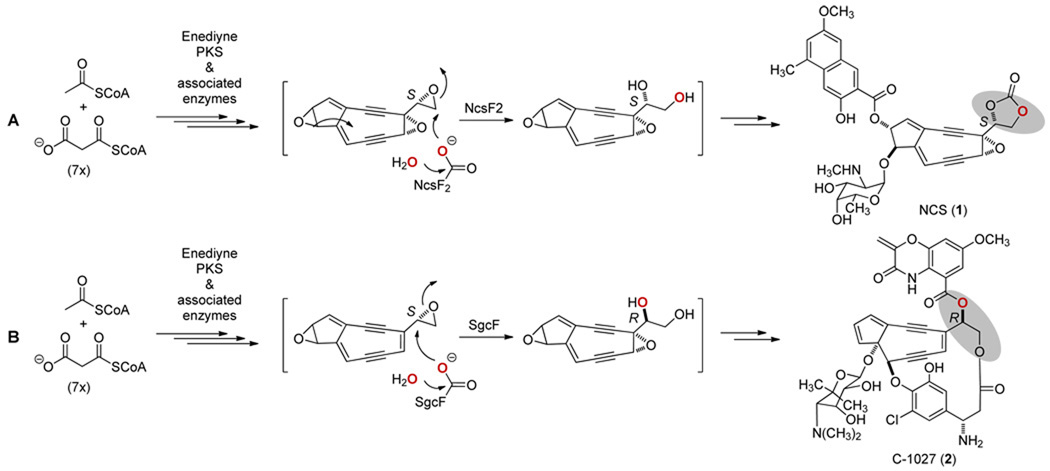
Neocarzinostatin (NCS) is an enediyne antitumor antibiotic produced by Streptomyces carzinostaticus ATCC 15944 as a chromoprotein complex consisting of an apoprotein and the NCS chromophore (1).1 Characterization of the NCS biosynthetic gene cluster from S. carzinostaticus led to a proposed convergent biosynthetic pathway in which the peripheral moieties are appended to an enediyne core vicinal diol intermediate to afford 1 (Figure 1A).2 The presumed (S)-vicinal diol is thought to arise from hydrolysis of an epoxide intermediate by one of two putative epoxide hydrolase (EH) enzymes encoded by the NCS gene cluster, NcsF1 or NcsF2.2 A similar convergent logic has been proposed for the C-1027 (2) biosynthetic pathway in Streptomyces globisporus (Figure 1B),3 from which an EH, SgcF, has been characterized using styrene oxide as a substrate mimic.4 SgcF was shown to catalyze an enantioconvergent process5 in which H2O attacks primarily at C-2 for (R)-styrene oxide and at the more hindered C-1 for (S)-styrene oxide (Figure 2A).4 Consequently the major product is always the (R)-vicinal diol, but the 37-fold higher specificity of SgcF for (S)-styrene oxide implicates an (S)-epoxide enediyne intermediate in C-1027 biosynthesis, consistent with the proposed pathway (Figure 1B).3,4 Here we now report that NcsF2, but not NcsF1, efficiently hydrolyzes styrene oxide. In contrast to SgcF, NcsF2 is regiospecific for C-2 such that hydrolysis of (R)- and (S)-styrene oxides yield (R)- and (S)-vicinal diol products, respectively (Figure 2B). Although hydrolysis of an (S)-epoxide enediyne intermediate would afford the expected (S)-vicinal diol (Figure 1A), the 44-fold preference of NcsF2 for (R)-styrene oxide is inconsistent with our biosynthetic proposal2 and may be an artifact of using styrene oxide as a substrate mimic. By exploiting the complementary activities of NcsF2 and SgcF, we finally demonstrated the preparation of (R)-1-phenyl-1,2-ethanediol from racemic styrene oxide with excellent chemical yield and enantiomeric excess (Figure 2C).
Figure 1.
Proposed biosynthetic pathways featuring (A) an NcsF2-catalyzed formation of an (S)-vicinal diol intermediate from an (S)-enediyne core epoxide precursor for NCS (1) and (B) an SgcF-catalyzed formation of a (R)-vivinal diol intermediate from an (S)-enediyne core epoxide precursor for C-1027 (2). The vicinal diol moieties in 1 and 2 concerned in this study are shaded.
Figure 2.
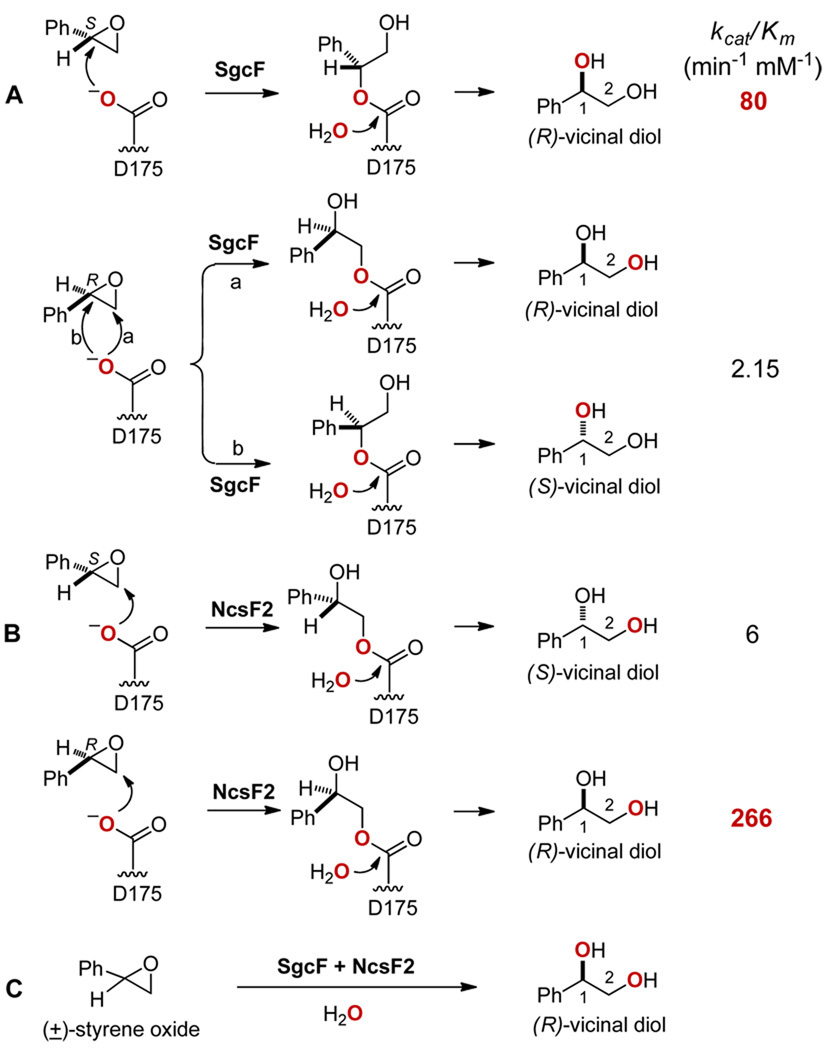
SgcF- and NcsF2-catalyzed hydrolysis of styrene oxide to 1-phenyl-1,2-ethanediol (vicinal diol): (A) SgcF-catalyzed regiospecific hydrolysis of (S)-styrene oxide to (R)-vicinal diol and regioselective hydrolysis of (R)-styrene oxide to both (R)- and (S)-vicinal diol with enantioselectivity (E = 37) for the (R)-styrene oxide; (B) NcsF2-catalyzed regiospecific hydrolysis of (S)- and (R)-styrene oxides to (S) and (R)-vicinal diols, respectively, with enantioselectivity (E = 44) for the (R)-styrene oxide; and (C) SgcF/NcsF2-catalyzed preparation of (R)-vicinal diol from racemic styrene oxide.
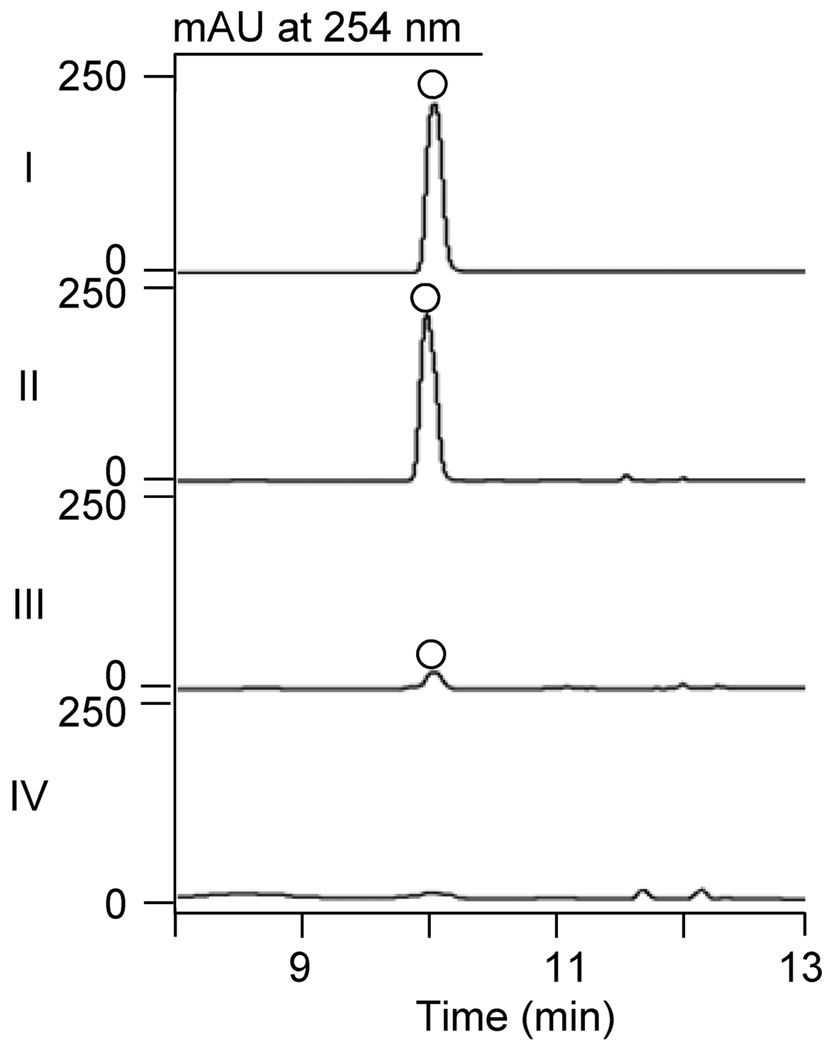
We have previously predicted two genes, ncsF1 and ncsF2, within the NCS biosynthetic gene cluster, to encode EHs on the basis of bioinformatics analysis.2 We first cloned, overproduced in E. coli, and purified both NcsF1 and NcsF2 to homogeneity (Figure S1).6 The EH activity of both enzymes towards racemic styrene oxide was then analyzed using HPLC.4,6 Incubation of NcsF2 (50 µM) with 2 mM styrene oxide produced a single peak corresponding to 1-phenyl-1,2-ethanediol, and, in contrast, NcsF1 produced only a very minor peak, indicating a dramatically less efficient enzyme (Figure 3). Careful inspection of the selected EH protein sequences reveals that, in contrast to SgcF and NcsF2, NcsF1 may lack key amino acid residues conserved among EHs (Figure S2). First, the acid residue of the nucleophile-His-acid catalytic triad has been replaced by Ala (Asp174-His361-Ala334) at its usual position after strand β7, but some EHs are known to harbor this residue after strand β6.7 Second, the two canonical EH Tyr residues that activate the epoxide oxygen – one of which is absolutely conserved7 – are missing in NcsF1 (Pro234 and His303), although we note that the adjacent Tyr304 may function equivalently. Thus, although NcsF1 is 53% and 59% identical to SgcF and NcsF2, respectively, its unusual sequence and relatively inefficient hydrolysis of styrene oxide suggest an alternative function for this enzyme in 1 biosynthesis, perhaps for hydrolysis of the internal epoxide of the NCS enediyne core precursor (Figure 1A).
Figure 3.
HPLC profiles for NcsF1- or NcsF2-catalyzed hydrolysis of racemic styrene oxide: (I)1- phenyl-1,2-ethanediol standard (○); (II) with 50 µM NcsF2; (III) with 50 µM NcsF1; and (IV) with 50 µM NcsF2 (boiled) as a negative control.
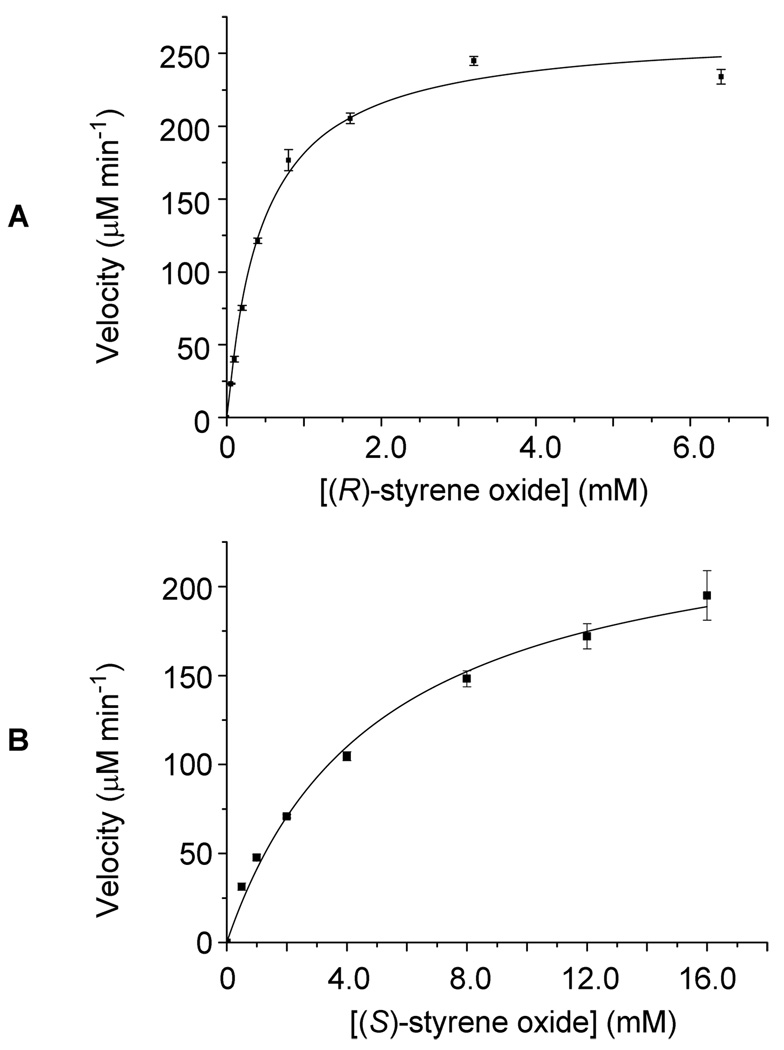
The observed activity of NcsF2 prompted further characterization of the enzyme. We then determined the steady-state kinetic parameters of NcsF2 towards (R)- and (S)-styrene oxides using a spectrophotometric assay6,8 to continuously monitor the formation of 1-phenyl-1,2-ethanediol. The Michaelis-Menten equation was fitted to plots of initial velocity versus substrate concentration (Figure 4) to yield kcat = 133 ± 4 min−1, KM = 0.5 ± 0.1 mM, and kcat/KM = 266 min−1 mM−1 for (R)-styrene oxide and kcat = 31 ± 2 min−1, KM = 5.0 ± 0.6 mM, and kcat/KM = 6 min−1 mM−1 for (S)-styrene oxide, respectively. Thus, NcsF2 is 44-fold more specific for (R)-styrene oxide (Figure 2B), in contrast to the 37-fold preference of SgcF for (S)-styrene oxide (Figure 2A).4 The opposite enantioselectivities of NcsF2 and SgcF were consistent with the respective vicinal diol stereochemistries of NCS and C-1027 (Figure 1), but only if NcsF2 was regioselective for C-2 and catalyzed stereochemical inversion of the preferred (R)-styrene oxide substrate to yield the (S)-vicinal diol.
Figure 4.
Kinetic analysis of NcsF2-catalyzed hydrolysis of (S)-and (R)-styrene oxide showing single substrate kinetic plots for (A) (R)-styrene oxide with 2 µM NcsF2 and (B) and (S)-styrene oxide with 8 µM NcsF2.
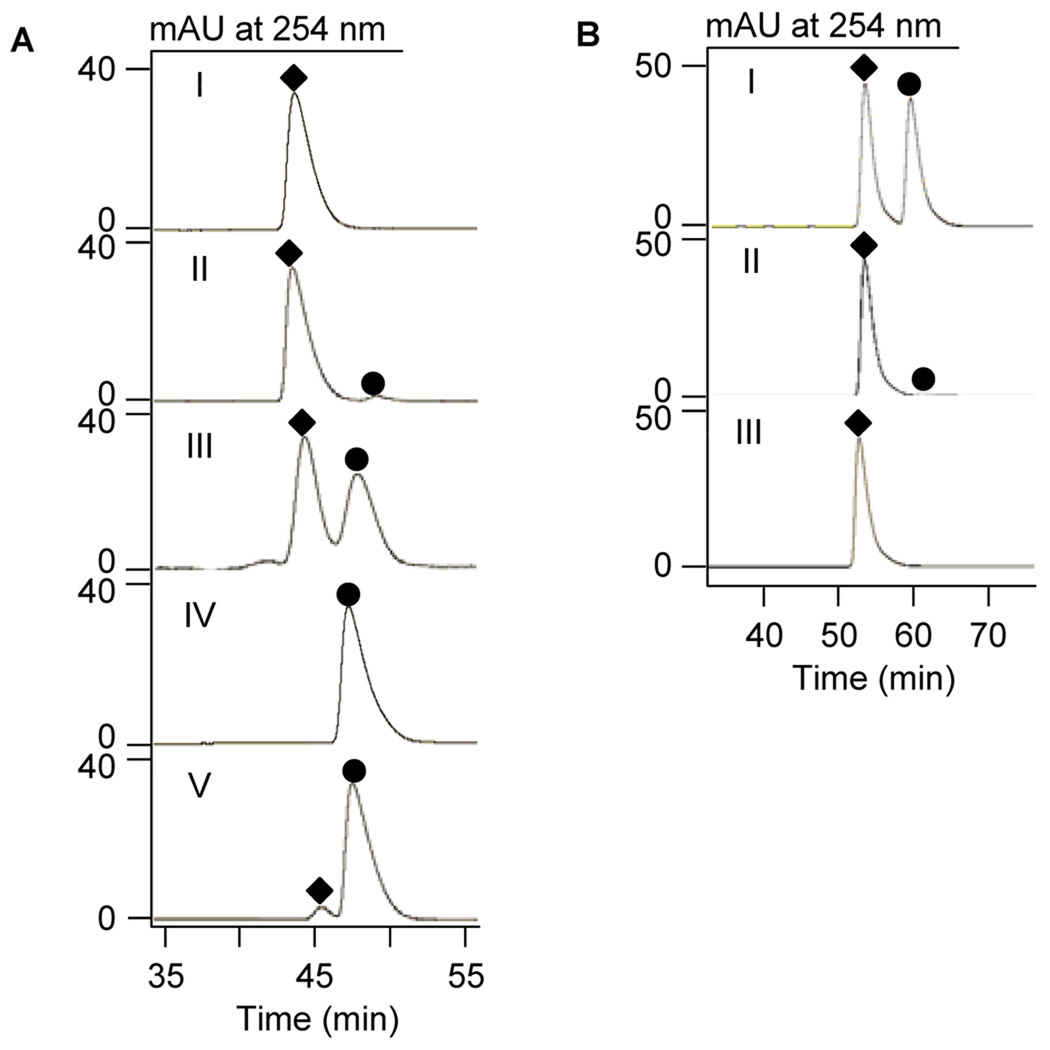
We therefore examined the stereochemistry of the NcsF2 reaction products using chiral HPLC (Figure 5),4,6 and surprisingly found no inversion of stereochemistry. Specifically, NcsF2 transformed (R)-styrene oxide to (R)-1-phenyl-1,2-ethanediol (Figure 5A, panel II, 98% ee) and (S)-styrene oxide to (S)- 1-phenyl-1,2-ethanediol (Figure 5A, panel V, 90% ee). Similarly, NcsF2 hydrolyzed the racemic substrate to afford racemic product (Figure 5A, panel III). This finding strongly suggested that, unlike SgcF (Figure 2A), NcsF2 catalyzes addition of water exclusively at C-2 for both enantiomers (Figure 2B).
Figure 5.
Chiral HPLC analysis for determination of stereochemistry of 1-phenyl-1,2-ethanediol resulting from NcsF2- or NcsF2/SgcF-catalyzed hydrolysis of styrene oxide.6,10 A: (I) (R)-1-phenyl-1,2-ethanediol standard; (II) NcsF2 with (R)-styrene oxide; (III) NcsF2 with (±)-styrene oxide; (IV) (S)-1-phenyl-1,2-ethanediol standard; and (V) NcsF2 with (S)-styrene oxide. B: (I) (±)-1- phenyl-1,2-ethanediol standard; (II) NscF2/SgcF with (R)-styrene oxide; and (III) (R)-1- phenyl-1,2-ethanediol standard. (♦), (R)-1- phenyl-1,2-ethanediol; (●), (S)-1-phenyl-1,2-ethanediol.
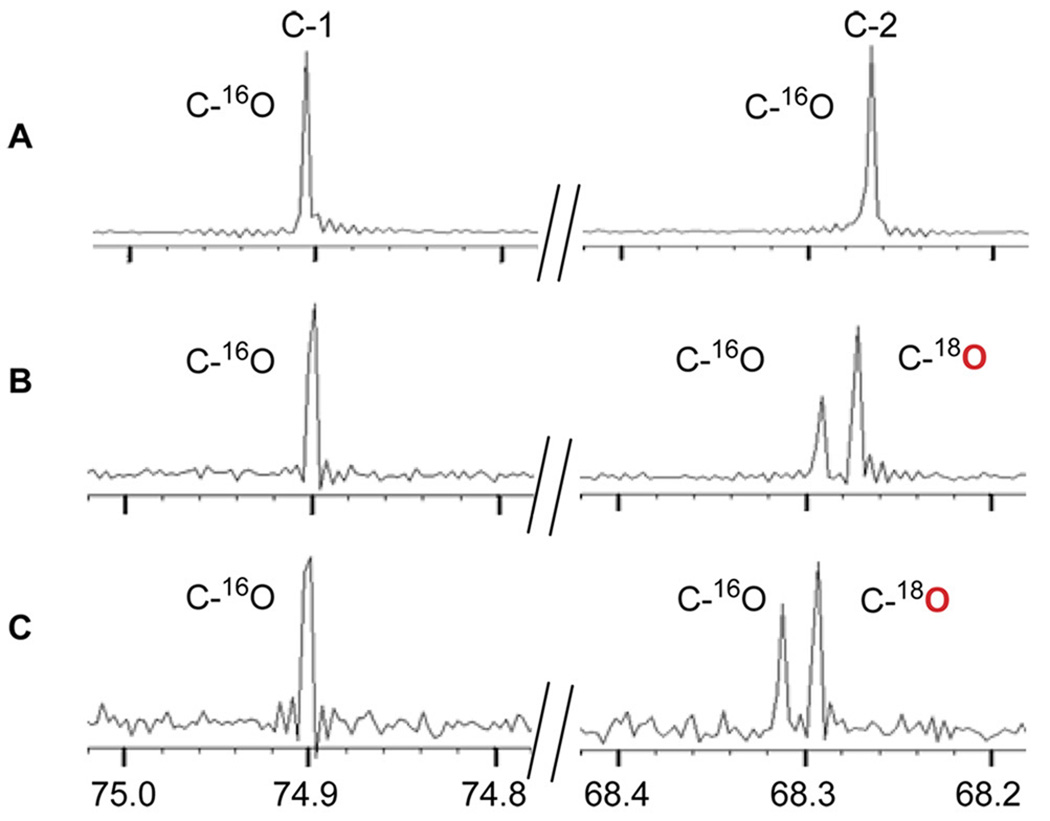
We next verified the C-2 regiospecificity of NcsF2 using an 18O labeling experiment.4,6 Hydrolysis of (R)- or (S)-styrene oxide by NcsF2 was performed in the presence of [18O]-H2O, and the products were analyzed by 13C-NMR. As expected, a 2.4 Hz chemical shift was observed exclusively at the C-2 position for the 1-phenyl-1,2-ethanediol product resulting from either the (R)- or (S)-styrene oxide substrate (Figure 6). In contrast to SgcF, which adds H2O to either C-1 or C-2 depending on the stereochemistry of the substrate (Figure 2A),4 NcsF2 is regiospecific for C-2 of styrene oxide (Figure 2B).
Figure 6.
13C NMR spectra for C-1 and C-2 of 1-phenyl-1,2-ethanediol produced by NcsF2-catalyzed hydrolysis of: (A) racemic styrene oxide in H2O; (B) (R)-styrene oxide in [18O]-H2O (~80% final concentration); and (C) (S)-styrene oxide in [18O]-H2O (~60% final concentration).
Finally, the complementary activities of NcsF2 and SgcF were exploited to prepare enantiopure (R)-1-phenyl-1,2-ethanediol from racemic styrene oxide (Figure 2C). For example, SgcF has 37-fold higher specificity for (S)-styrene oxide, which is transformed almost exclusively to (R)-1-phenyl-1,2-ethanediol while the slower-reacting (R)-styrene oxide produces a minor but significant amount of unwanted (S)-vicinal diol (Figure 2A). Conversely, NcsF2 preferentially hydrolyzes (R)-styrene oxide to afford the (R)-vicinal diol, but slower reacting (S)-styrene oxide yields unwanted (S)-vicinal diol product (Figure 2B). Thus, for a given enzyme, the ‘slow’ enantiomer that yields unwanted product is the substrate for the other enzyme, which rapidly generates the correct product. These complementary specificities and product stereochemistries predict that the two enzymes together should generate (R)-1-phenyl-1,2-ethanediol in high enantiomeric excess (Figure 2C). Indeed, we carried out the reaction with 3.5:1 ratio of SgcF:NcsF2 with 14 mM styrene oxide and observed (R)-1-phenyl-1,2-ethanediol in 87% yield with 99% ee (Figure 5B).6 Such systems employing complementary enzymes have previously been used to generate enantiomerically enriched products.9 For example, a potato EH and an engineered EH from Agrobacterium radiobacter AD1 together transformed racemic styrene oxide to (R)-1-phenyl-1,2-ethanediol with 98% ee after complete conversion.9(a) Interestingly, these cases resulted from deliberate efforts involving screening many enzymes to obtain a suitable biocatalytic process. In contrast, our results arose fortuitously from studies of enediyne biosynthesis and highlight the biocatalytic potential available to be harnessed from our ever-expanding knowledge of natural product biosynthetic machineries.
In conclusion, we have demonstrated that the NcsF2 enzyme is active as an epoxide hydrolase towards styrene oxide, an activity consistent with its proposed function in hydrolyzing a terminal epoxide enediyne intermediate. We further showed that NcsF2 regiospecificallly adds H2O to the less hindered C-2 position of styrene oxide such that the stereochemistry is retained in the vicinal diol product. Surprisingly, the enantioselectivity of NcsF2 for (R)-styrene oxide to (S)-styrene oxide (E = 44) is inconsistent with preferential production of an (S)-vicinal diol biosynthetic intermediate, as would be expected based on the structure of 1. However, NcsF2 remains our best candidate for this function, and its unexpected selectivity may reflect the inadequacy of styrene oxide as a substrate mimic. Moreover, the natural enediyne core epoxide precursor is likely a single enantiomer, in which case regioselective hydrolysis of an (S)-epoxide enediyne intermediate would produce the expected (S)-vicinal diol stereochemistry (Figure 1A).
Supplementary Material
Acknowledgment
We thank the Analytical Instrumentation Center of the School of Pharmacy, and Dr. Qiu Cui of the National Magnetic Resonance Facility at the University of Wisconsin-Madison for support in obtaining MS and NMR data, and Prof. W. Tang, School of Pharmacy, UW-Madison for providing access to chiral HPLC. This work is supported in part by NIH Grants CA78747 and CA113297. G. P. H. is the recipient of an NSERC (Canada) postdoctoral fellowship.
Footnotes
Supporting Information Available: Detailed experimental procedures, SDS-PAGE (Figure S1), and sequence alignment (Figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ishida N, Miyazaki K, Kumagai M, Rikimaru M. J. Antibiot. 1965;18:68–76. [PubMed] [Google Scholar]; (b) Edo K, Mizugaki M, Koide Y, Seto H, Furihata K, Otake N, Ishida N. Tetrahedron Lett. 1985;26:331–340. [Google Scholar]
- 2.Liu W, Nonaka K, Nie L, Zhang J, Christenson SD, Bae J, Van Lanen SG, Zazopoulos E, Farnet CM, Yang CF, Shen B. Chem. Biol. 2005;12:293–302. doi: 10.1016/j.chembiol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Christenson SD, Standage S, Shen B. Science. 2002;297:1170–1173. doi: 10.1126/science.1072110. [DOI] [PubMed] [Google Scholar]
- 4.Lin S, Horsman GP, Chen Y, Li W, Shen B. J. Am. Chem. Soc. 2009;131:16410–16417. doi: 10.1021/ja901242s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Faber K, Kroutil W. Tetrahedron Asymmetry. 2002;13:377–382. [Google Scholar]; (b) Hwang S, Choi CY, Lee EY. Biotechnol. Lett. 2008;30:1219–1225. doi: 10.1007/s10529-008-9668-7. [DOI] [PubMed] [Google Scholar]; (c) Steinreiber A, Faber K. Curr. Opin. Biotechnol. 2001;12:552–558. doi: 10.1016/s0958-1669(01)00262-2. [DOI] [PubMed] [Google Scholar]; (d) Bellucci G, Chiappe C, Cordoni A. Tetrahedron Asymmetry. 1996;7:197–202. [Google Scholar]; (e) Kroutil W, Mischitz M, Plachota P, Faber K. Tetrahedron Lett. 1996;37:8379–8382. [Google Scholar]
- 6.See Supporting Information for full experimental details.
- 7.Barth S, Fischer M, Schmid RD, Pleiss J. Proteins: Struct. Funct. Bioinform. 2004;55:846–855. doi: 10.1002/prot.20013. [DOI] [PubMed] [Google Scholar]
- 8.(a) Doderer K, Lutz-Wahl S, Hauer B, Schmid RD. Anal. Biochem. 2003;321:131–134. doi: 10.1016/s0003-2697(03)00399-3. [DOI] [PubMed] [Google Scholar]; (b) Mateo C, Archelas A, Furstoss R. Anal. Biochem. 2003;314:135–141. doi: 10.1016/s0003-2697(02)00646-2. [DOI] [PubMed] [Google Scholar]
- 9.(a) Cao L, Lee J, Chen JW, Wood TK. Biotechnol. Bioeng. 2006;94:522–529. doi: 10.1002/bit.20860. [DOI] [PubMed] [Google Scholar]; (b) Pedragosa-Moreau S, Archelas A, Furstoss R. J. Org. Chem. 1993;58:5533–5536. doi: 10.1021/jo960558i. [DOI] [PubMed] [Google Scholar]; (c) Manoj KM, Archelas A, Baratti J, Furstoss R. Tetrahedron. 2001;57:695–701. [Google Scholar]
- 10.The discrepency in retention times between A and B result from the use of a new OD-H column in the latter.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.