Abstract
Objective
To determine if suppressing Nogo-A, an axonal inhibitory protein, will promote functional recovery in a murine model of multiple sclerosis (MS).
Methods
A small interfering RNA was developed to specifically suppress Nogo-A (siRNA-NogoA). The siRNA-NogoA silencing effect was evaluated in vitro and in vivo via immunohistochemistry. The siRNA was administered intravenously in two models of experimental autoimmune encephalomyelitis (EAE). Axonal repair was measured by upregulation of GAP43. ELISA, flow cytometry and 3H-thymidine incorporation was used to determine immunological changes in myelin-specific T cells in mice with EAE.
Results
The siRNA-NogoA suppressed Nogo-A expression in vitro and in vivo. Systemic administration of siRNA-NogoA ameliorated EAE and promoted axonal repair as demonstrated by enhanced GAP43+ axons in the lesions. Myelin-specific T cell proliferation and cytokine production were unchanged in the siRNA-NogoA treated mice.
Interpretation
Silencing Nogo-A in EAE promotes functional recovery. The therapeutic benefit appears to be mediated by axonal growth and repair, and is not attributable to changes in the encephalitogenic capacity of the myelin-specific T cells. Silencing Nogo-A may be a therapeutic option for MS patients to prevent permanent functional deficits caused by immune-mediated axonal damage.
INTRODUCTION
Identifying molecules in the CNS that affects functional recovery following CNS injury is essential for the development of therapeutics for multiple sclerosis (MS) and other CNS diseases. Nogo-A is a member of the reticulon family of endoplasmic reticulum anchored proteins that share a common C-terminal region. Nogo-A, also referred to as RTN4, is a potent inhibitor of neurite outgrowth and plays a critical role in negatively regulating regeneration and plasticity in the adult CNS1-6. Nogo-A is expressed by oligodendrocytes and some neuronal subpopulations7-8. Two regions within Nogo-A are responsible for the inhibitory effect of neurite outgrowth, one within the N-terminal Nogo-A-specific region and a second in a 66-amino acid loop in the C-terminus, referred to as Nogo-662,4-5. Nogo-66 binds the Nogo receptor (NgR), which is also the receptor for two myelin-associated proteins, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp)9-11. Both MAG and OMgp inhibit axonal regeneration, suggesting that signaling through the NgR leads to inhibition of neurite outgrowth. In vivo administration of monoclonal antibodies specific for Nogo-A induces sprouting of Purkinje cells1 and enhances recovery following stroke6. Similarly, in vivo administration of Nogo receptor antagonist peptide or monoclonal antibody enhances functional recovery in rats with spinal cord transections3-4. Since axonal transection has been associated with permanent disability in MS12, suppressing Nogo-A expression may promote axonal regeneration in the CNS, enhancing functional recovery. Only a few studies have evaluated the role of Nogo-A in experimental autoimmune encephalomyelitis (EAE), a model for MS. Nogo-A-deficient mice develop less severe EAE13. This study also immunized mice with a Nogo-A peptide generating a high Nogo-A-specific antibody response prior to EAE induction, resulting in reduced incidence and severity of EAE. Interestingly, these mice had reduced inflammation, as well as a decrease in Th1 associated cytokines, suggesting that inhibiting Nogo-A may have anti-inflammatory properties. In another study, immunization with Nogo-A peptides and the subsequent generation of T cells and antibodies specific for Nogo-A appeared to be somewhat protective in EAE14.
Our laboratory has recently been using small interfering RNA (siRNA) to suppress particular proteins in mice with EAE15-18. We used a similar strategy in this study to inhibit Nogo-A. In MS and EAE, demyelination occurs due to an inflammatory response in the CNS, followed by axonal transaction, leading to permanent disability12,19-21. Since siRNA are extremely small and have a high degree of specificity, intravenous administration causes efficient suppression of their target genes. Since there is blood-brain barrier breakdown in EAE and MS, siRNA delivered intravenously should be able to access the site of injury. Since Nogo-A inhibits axonal elongation, silencing Nogo-A with a siRNA may provide a strategy to enhance functional recovery. This is the first study to demonstrate that inhibition of Nogo-A can improve the functional outcome in mice with established autoimmune demyelinating disease.
MATERIALS AND METHODS
Experimental Autoimmune Encephalomyelitis (EAE)
EAE was induced in C57BL/6 mice by subcutaneous immunization with 200 μg MOG35-55 peptide emulsified in CFA with intraperitoneal injection of 200 ng pertussis toxin on day 0 and 2, or in B10.PL mice by intraperitoneal injection of 107 MBP Ac1-11-specific T cell receptor transgenic splenocytes15-18.
Small Interfering RNA (siRNA)
The Nogo-A and nonsense (NS) siRNA (Dharmacon) target sequences were 5′-AAUGAUUCCGAGGCAGAUUAU-3′ for Nogo-A and 5′-AACGAACGAGUACCGUACACU-3′ for NS. For in vitro studies, the siRNA was transfected into N2A cells using TransIt TKO transfecting agent (Mirus) as recommended by the manufacturer. For in vivo studies, 2 mg/kg siRNA in PBS was injected into the tail vein.
Lymphocyte proliferation assays, ELISA and Flow Cytometry
The spleens of C57BL/6 mice were removed on day 17 or 41 post EAE induction. The splenocytes were cultured with MOG35-55 (2 μg/ml) for 72 hr and supernatants were collected and evaluated by ELISA to determine IFNγ and IL-17 production15-18. Infiltrating CNS lymphocytes were isolated from the CNS15 on day 17, cultured with MBP Ac1-11 overnight, and given PMA/ionomycin for 4 hr prior to analysis. Supernatant were evaluated by ELISA and the lymphocytes were stained for CD45, CD4, IFNγ and IL-17 for flow cytometric analysis18. For proliferation assays, splenocytes were cultured in quadruplicate with MOG35-55 or no antigen. After 72 hr, 3H-thymidine was added to the culture for an additional 18 hr and proliferation was determined15-18.
Immunohistochemistry
N2A cells were grown on cover slips, fixed with 4% paraformaldehyde and permeabilized with 0.05% saponin. The cells were stained with anti-Nogo-A (Santa Cruz) and Alexa Fluor 568 anti-rabbit IgG, and mounted on slides with media containing DAPI.
Mice were perfused with 4% paraformaldehyde, spinal cords removed, post-fixed for 2h and transferred to 0.2M phosphate buffer. Spinal cords were cryopreserved in 30% sucrose, frozen, and sequential longitudinal sections (10μm thickness) were cut, slide mounted and stored at −20°C. For immunofluorescence, sections were rinsed with PBS and incubated in 4%BSA/0.3%Tx-100/ PBS for 1 hr at room temp. Primary antibodies were applied for 20 h at room temp. Primary antibodies used were a mixture of rabbit anti-Nogo-A (1:2500; Santa Cruz) and rat anti-CD3 (1:100; BD Biosciences); rabbit anti-GAP43 (1: 2500; Millipore) and rat anti-CD3 (1:100); or mouse anti-adenomatous polyposis coli (APC; 1:500; Calbiochem). Sections were rinsed with PBS, incubated with Alexa Fluor 546 goat anti-rabbit 1:500 and Alexa Fluor 488 anti-rat or Alexa Fluor 568 goat anti-mouse (1:500; Molecular Probes) for 1 hr at room temp. Sections were rinsed with PBS and counterstained with DAPI .
Another set of slides were stained for neurofilament (NF; Boeringher Mannheim) and eriochrome cyanine (EC; Sigma). Sections were preincubated with 10% normal horse serum (NHS) followed by 1:2000 mouse anti-NF in 5% NHS overnight at 4°C and then secondary antibody in 5% NHS. The sections were treated with 6% hydrogen peroxide in methanol followed by Elite Avidin Biotin Conjugate (Vector) and visualized with DAB substrate. Slides were rinsed, treated with acetone, rinsed and immersed in EC solution for 30 min. The sections were differentiated in 5% Iron Alum and differentiated in borax ferricyanide. Sections were dehydrated, cleared and coverslipped.
Quantification of immunoreactivity was performed on images acquired using confocal microscopy (Zeiss 510 META Scanning Laser Confocal microscope) of sections double-labeled for Nogo-A and CD3 or Nogo-A and GAP43. Lesions were initially identified on sections using adjacent EC/NF labeled sections. Fluorescently-labeled sections were viewed by a blinded observer. CD3 immunoreactivity was used to locate white matter lesions. Images of Nogo-A and GAP43 immunoreactivity were collected within the lesion and lesion border. Images were converted to black and white, and Nogo-A and GAP43 immunoreactivity was quantified using a standardized sample box (0.001mm2) with a computerized image analysis system. A minimum of three lesions per animal were analyzed; lesion data for each animal were averaged and group means for immunoreactivity were calculated.
For quantification of T cells and lesion area, two longitudinal sections representing two distinct planes of each spinal cord were stained with rat anti-mouse CD4 plus rat anti-mouse CD8 (Santa Cruz), FluoroMyelin Green (Invitrogen) and DAPI to visualize the nuclei. Each section was photographed and analyzed in a blinded fashion using an Olympus EX41 microscope. Lesion was defined as the areas with a high density of nuclei, CD4 and CD8 positive cells, and loss of myelin. MicroSuite software was used to measure area of each lesion. CD4+ and CD8+ lymphocytes were counted throughout the entire length of each spinal cord by ImageJ (www.nih.gov).
RESULTS
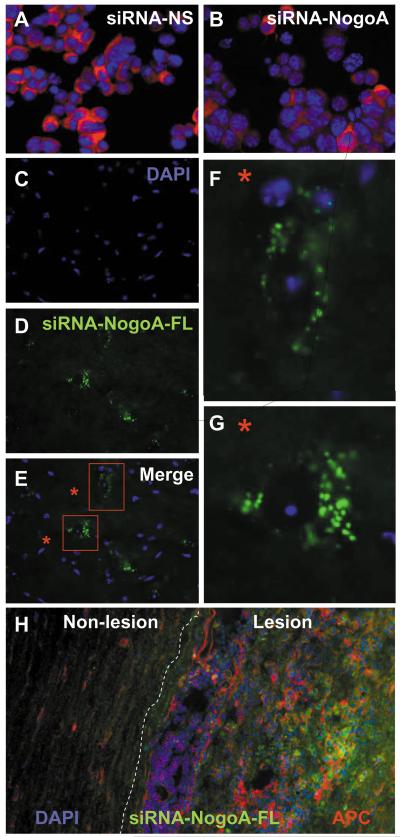
N2A cells, a neuroblastoma cell line, were transfected with a Nogo-A-specific siRNA (siRNA-NogoA) or a nonsense control siRNA (siRNA-NS, no significant sequence homology to other mouse genes). Immunocytochemistry demonstrated that Nogo-A expression was reduced by 75% in the siRNA-NogoA transfected cells (Figure 1A-B).
Figure 1. Development of Nogo-A-specific siRNA.
(A-B) N2A cells grown on coverslips were transfected with siRNA-NS (A) or siRNA-NogoA (B) using TransIT-TKO transfection reagent (Mirus) for 48 hr. The cells were stained with goat anti-mouse Nogo-A (Santa Cruz) followed by Alexa Fluor 568-conjugated rabbit anti-goat IgG (red; Molecular Probes) and mounted onto slides with Vectashield containing DAPI (blue; Vector Labs). (C-H) The siRNA-NogoA was conjugated to fluorescein and injected intravenously into C57BL/6 mice with EAE. The spinal cords were removed 48 hr later, fixed, frozen, sectioned longitudinally and stained with DAPI. DAPI (C), flourescein-siRNA-NogoA (D) and merged images (E-G) illustrate cytoplasmic localization of the siRNA within cells of the lumbar region of spinal cords. (H) Sections were also stained with anti-APC to illustrate colocalization of siRNA (green) with oligodendrocytes (red) in the lesions.
It had been demonstrated that siRNA could be administered intravenously and found in most organs, with one notable exception being the CNS22. Since the blood-brain barrier is compromised in mice with EAE, we hypothesized that siRNA could access active CNS lesions. To test this hypothesis, C57BL/6 mice were immunized with MOG35-55/CFA and administered fluorescein-labeled siRNA-NogoA intravenously after the onset of EAE. After 48 hr, the spinal cords were analyzed for the presence of fluorescein. Fluorescein was observed within the cytoplasm of cells in the lesions (Figure 1C-G), was rarely observed outside the areas of lesions, and frequently colocalized with oligodendrocytes (as illustrated by APC staining in red; Figure 1H). This data indicates that the blood-brain barrier breakdown in EAE was allowing the siRNA access to the CNS.
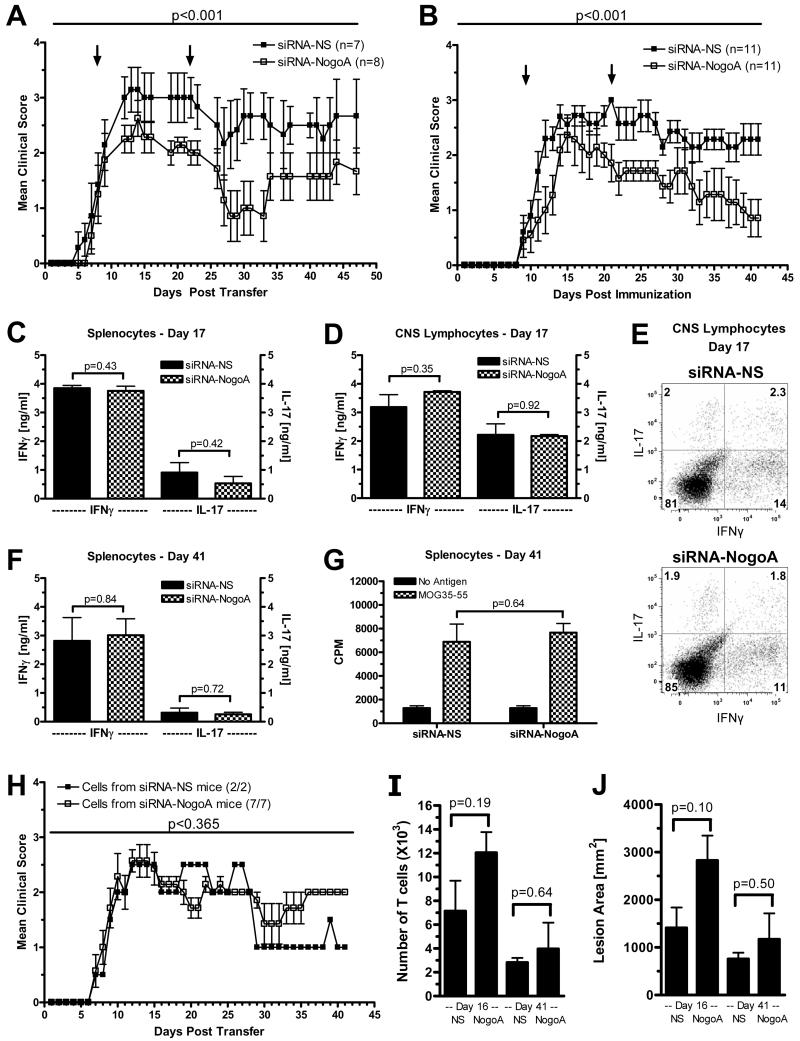
To determine if suppressing Nogo-A may have a clinical benefit in immune-mediated demyelinating disease, EAE was induced by adoptive transfer in B10.PL mice (Figure 2A) or immunization of C57BL/6 mice (Figure 2B and Table I). The siRNA was administered at disease onset and two weeks later, which was based on a previous observation that two systemic doses of siRNA has a significantly longer suppression period than one dose16. Within a week of the initial dose of siRNA, the siRNA-NogoA treated mice demonstrated lower clinical scores than the control mice. After the second dose of siRNA-NogoA, mice illustrated a clinical improvement which continued for the entire monitoring period for the C57BL/6 mice. Table I illustrates the clinical data from three independent experiments in C57BL/6 mice. In all three experiments, Mann-Whitney non-parametric analysis of the individual clinical scores of each mouse in the two groups resulted in a statistically significant improvement with siRNA-NogoA treatment. Analysis of the individual minimum and maximum clinical scores for each mouse was reduced in the siRNA-NogoA groups (Table I). In addition, the percentage of mice that recovered from their disease, defined as improving to a score of 0 or 1 by the end of the monitoring period, was dramatically higher in the siRNA-NogoA group compared to the control group. Mice given the siRNA specific for Nogo-A at the time of disease induction, in contrast to disease onset, had a similar disease course as control mice (data not shown), supporting the hypothesis that blood-brain barrier damage is necessary for the siRNA to gain access to the CNS.
Figure 2. Silencing Nogo-A ameliorates EAE.
(A) EAE was induced by adoptive transfer of MBP-specific T cell receptor transgenic splenocytes that were differentiated in vitro with MBP Ac1-11 (10 μg/ml) and IL-12 (0.5 ng/ml) into naïve B10.PL mice. siRNA-NogoA or siRNA-NS (2 mg/kg) was administered i.v. on days 8 (onset of disease) and 22. (B) EAE was induced in C57BL/6 mice by immunization with MOG35-55 emulsified in CFA. siRNA-NogoA or siRNA-NS were administered i.v. on days 9 (onset of disease) and 21. Mann-Whitney non-parametric analysis was used to generate p values. (C-G) Spleen and CNS were removed on day 17 and/or 41 post-immunization from C57BL/6 mice treated with siRNA. (C) IFNγ and IL-17 production measured by ELISA of splenocytes at day 17. (D) IFNγ and IL-17 production measured by ELISA of CNS lymphocytes at day 17. (E) Flow cytometric analysis of CNS lymphocytes for IFNγ and IL-17 expression. (F) IFNγ and IL-17 production measured by ELISA of splenocytes at day 41. (G) Lymphocyte proliferation was measured by 3H-thymidine incorporation in splenocytes on day 41. (H) Splenocytes from EAE-affected C57BL/6 mice treated with siRNA-NS or siRNA-NogoA were isolated and restimulated in vitro with MOG35-55 for 72 hr and transferred into naïve C57BL/6 mice. Mean clinical scores for each group (siRNA-NS n= 2; siRNA-NogoA n=7) is shown. Incidence of EAE was 100%. (I-J) Longitudinal sections of spinal cords from siRNA-NS and siRNA-NogoA treated mice were stained for CD4 and CD8, as well as myelin. The number of T cells (G) and lesion area (H) in two distinct longitudinal planes was determined at two time points, days 16 and 41, in a blinded manner for 3 mice per group.
Table I. Amelioration of EAE in C57BL/6 mice with siRNA-NogoA.
|
Group |
Number of mice |
Mean Day of EAE Onset |
Treatment Days |
Monitoring Period |
------- Mean Score1 ------- | Percent Recovery2 |
|
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | ||||||
| Experiment 1 | |||||||
| siRNA-NS | 8 | 12.75 | 14 & 28 | 49 days | 2.33±0.08 | 3.50±0.62 | 0% |
| siRNA-NogoA | 8 | 10.40 | 14 & 28 | 49 days3 | 1.25±0.25 | 2.63±0.46 | 25% |
| Experiment 2 | |||||||
| siRNA-NS | 11 | 11.80 | 9 &21 | 27 days | ND4 | ND | ND |
| siRNA-NogoA | 11 | 10.82 | 9 & 21 | 27 days3 | ND | ND | ND |
| Experiment 3 | |||||||
| siRNA-NS | 11 | 10.50 | 9 & 21 | 41 days | 2.00±0.235 | 3.14±0.145 | 14% |
| siRNA-NogoA | 11 | 12.64 | 9 & 21 | 41 days3 | 0.57±0.30 | 2.29±0.18 | 71% |
The mean±SEM of the highest clinical score (maximum) and lowest clinical score (minimum) of each mouse after the second siRNA treatment was determined.
The percentage of mice that recovered to a clinical score of 0 or 1 by the end of the monitoring period.
Using Mann-Whitney Analysis of the clinical scores, there was a statistically significant improvement (p<0.001) in the siRNA-NogoA treated mice compared to the siRNA-NS mice.
Not determined because experiment was terminated early for ex vivo experiments.
Using Mann-Whitney Analysis there was a statistical significance difference in mean clinical score minimum (p=0.007) and maximum (p=0.018).
To determine if the amelioration of disease in the siRNA-NogoA mice was a result of modulating the autoreactive T cells, the lymphocyte proliferative and cytokine responses were evaluated in the mice from the treatment experiments. There was no significant difference in antigen-specific production by splenocytes of the two cytokines most commonly associated with encephalitogenic T cells, IFNγ and IL-17, at day 17 or day 41 (Figure 2C and 2F). Lymphocytes isolated from the CNS on day 17 also had no difference in cytokine production by ELISA or flow cytometric analysis (Figure 2D and 2E), indicating that siRNA-NogoA was not affecting either the amount of cytokine produced or the number of cells expressing these cytokines. The anti-inflammatory cytokines, IL-4 and IL-10, were both undetectable in the splenocytes and CNS lymphocytes (data not shown). Antigen-specific proliferation was also not altered in the siRNA-NogoA treated mice (Figure 2G). Adoptive transfer of splenocytes reactivated in vitro with antigen from C57BL/6 siRNA-NogoA-treated mice transferred disease to naïve C57BL/6 recipients as effectively as splenocytes from siRNA-NS treated mice (Figure 2H), demonstrating that the encephalitogenic capacity of the myelin-specific cells had not been altered by suppressing Nogo-A.
Since we hypothesized that suppressing Nogo-A would have no effect on CNS inflammation or demyelination, we evaluated infiltrating T cells and lesion area. The number of T cells and lesion area was determined by staining T cells and myelin within the spinal cords of three mice from each treatment group at two time points, day 16 and day 41. Analysis of the number of infiltrating T cells and lesion area indicated that neither of these parameters was decreased in the siRNA-NogoA treated mice (Figure 2I-J). In fact, the number of T cells and lesion area was slightly higher in the siRNA-NogoA group at day 16, confirming that suppressing Nogo-A was not altering the inflammatory phase of the disease. As expected, the number of infiltrating T cells and lesion area was reduced by day 41 in both groups, and there was no statistically significant difference between the groups in either of these parameters even though there was a dramatic improvement in function at this time in the siRNA-NogoA treated mice (Figure 2B).
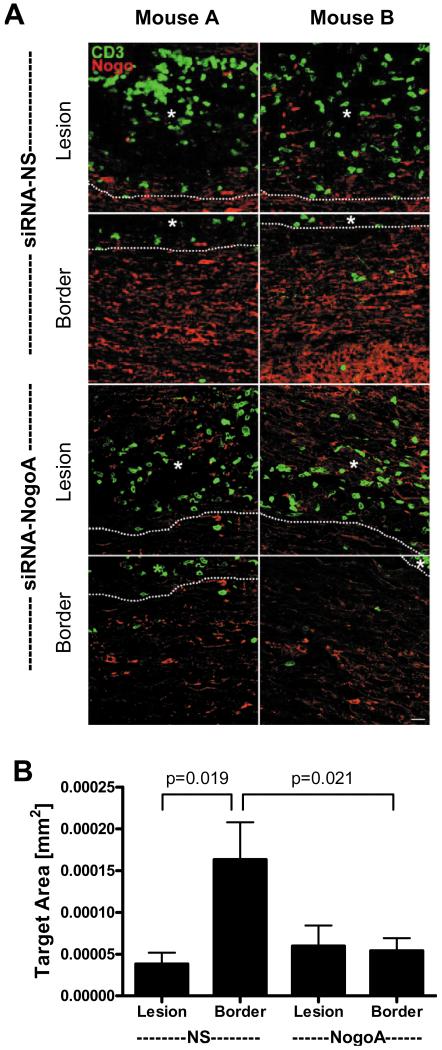
To confirm that the siRNA was suppressing Nogo-A expression, spinal cords of three mice per group were analyzed by immunohistochemistry for Nogo-A expression on day 16, one week after the first dose of siRNA. Using confocal microscopy, images of the lesions (≥3 lesions per mouse) and lesion borders were acquired. As seen in Figure 3A, Nogo-A expression was low in the lesions of the siRNA-NS mice compared to the lesion borders (Figure 3A, top four panels), not surprising since myelin and oligodendrocytes may be lost in the lesions. However, both the lesions and lesion borders have suppressed Nogo-A expression in the spinal cords from the mice that received siRNA-NogoA (Figure 3A, lower four panels), indicating that the siRNA reduced Nogo-A expression in the areas immediately surrounding the lesions. Quantification of the Nogo-A expression demonstrated that there was a significant reduction in Nogo-A levels in the lesion borders in the siRNA-NogoA treated mice compared to the siRNA-NS treated mice (Figure 3B).
Figure 3. Nogo-A expression is significantly decreased in the lesion borders in siRNA-NogoA treated mice.
Three C57BL/6 mice per group were perfused 7 days post-siRNA treatment (day 16 post immunization). (A) The spinal cords were sectioned longitudinally and stained for Nogo-A (red) and CD3 (green). Confocal microscopy was used to image lesions and lesion borders for each spinal cord. Two images of lesions and lesion borders from two different mice (mouse A and B) are shown for each treatment group. White dotted lines represent lesion border. (B) The amount of Nogo-A immunoreactivity was quantified using a standardized sample box (0.001mm2) with a computerized image analysis system. A minimum of three lesions per animal were analyzed; lesion data for each animal were averaged and group means for immunoreactivity were calculated and compared.
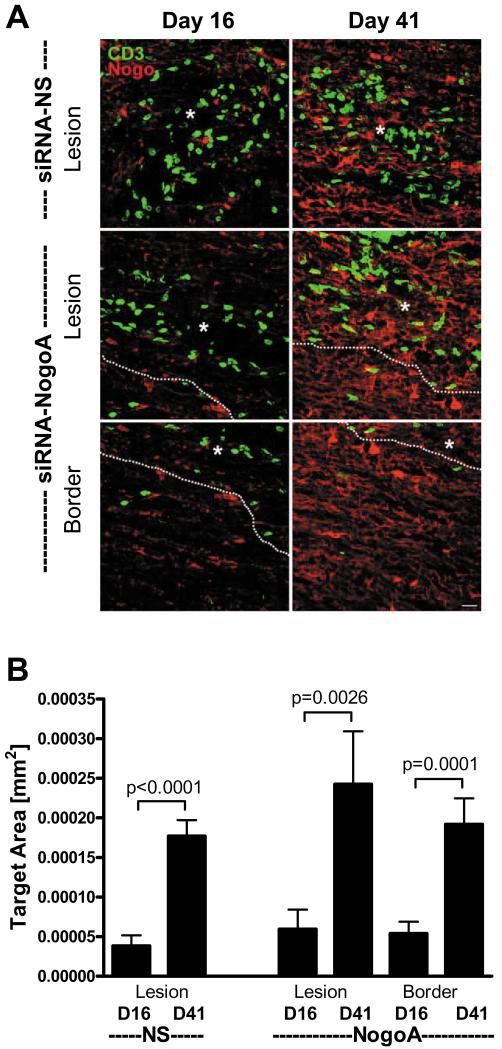
We evaluated whether Nogo-A expression normalized in the lesions during the course of disease. Both siRNA-NogoA and siRNA-NS treated mice had normal expression of Nogo-A by day 41 in the lesions (Figure 4A, top four panels). Since Nogo-A was also suppressed in the lesion borders of mice treated with the siRNA-NogoA, we evaluated Nogo-A expression at day 41 in these mice. Similar to the lesions, Nogo-A expression had returned to normal in the lesion borders (Figure 4A, lower two panels). Quantification of the Nogo-A expression illustrated a significant increase in Nogo-A from day 16 to day 41 (Figure 4B).
Figure 4. Nogo-A expression levels return to normal within three weeks of siRNA-NogoA treatment.
Three C57BL/6 mice per group were perfused on days 16 (7 days post-1st siRNA treatment) and 41 post-immunization (20 days post-2nd siRNA treatment). (A) The spinal cords were sectioned longitudinally and stained for Nogo-A (red) and CD3 (green). Confocal microscopy was used to image lesions and lesion borders (siRNA-NogoA only) for each spinal cord. Two images of lesions from two different mice (mouse A and B) are shown for each treatment group. White dotted lines represent lesion border. (B) The amount of Nogo-A immunoreactivity was quantified using a standardized sample box (0.001mm2) with a computerized image analysis system. A minimum of three lesions per animal were analyzed; lesion data for each animal were averaged and group means for immunoreactivity were calculated and compared.
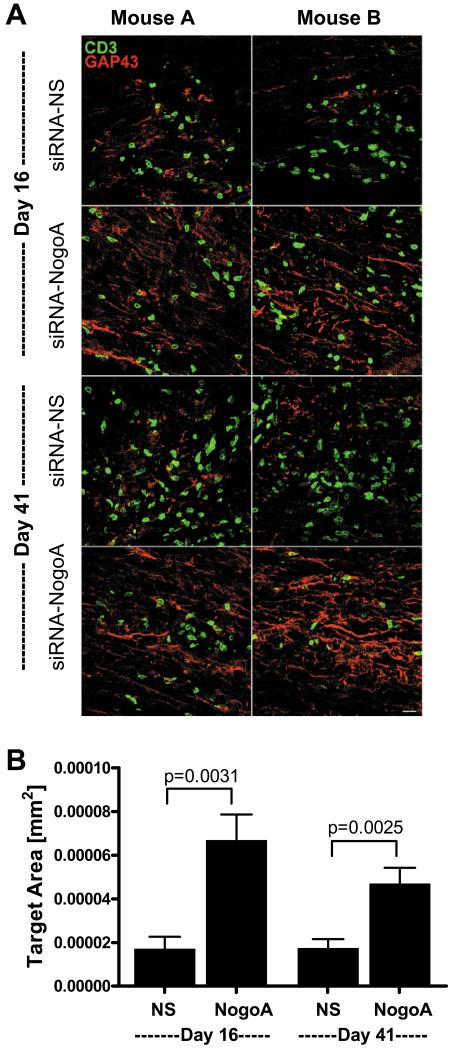
To determine if the functional recovery observed in EAE with the siRNA-NogoA treatment was due to axonal growth and/or sprouting, we analyzed GAP43 expression in the lesions and lesion borders. GAP43+ axons were rarely observed in the siRNA-NS treated spinal cords at day 16 or day 41 (Figure 5A). In contrast, GAP43+ axons were observed at day 16 and day 41 in the spinal cord lesions of mice treated with siRNA-NogoA (Figure 5A), indicating that axonal growth was occurring at the lesion sites in response to decreased Nogo-A levels. Quantification of the GAP43 expression demonstrated a statistically significant increase in GAP43 levels (Figure 5B). This data supports the hypothesis that inhibition of Nogo-A can promote neuronal repair and subsequent functional recovery in autoimmune demyelinating disease.
Figure 5. Silencing Nogo-A induces upregulation of GAP43.
Three C57BL/6 mice per group were perfused on days 16 (7 days post-1st siRNA treatment) and 41 post-immunization (20 days post-2nd siRNA treatment). (A) The spinal cords were sectioned longitudinally and stained for GAP43 (red) and CD3 (green). Confocal microscopy was used to image lesions for each spinal cord. Two images of lesions from two different mice (mouse A and B) are shown for each treatment group. White dotted lines represent lesion border. (B) The amount of GAP43 immunoreactivity was quantified using a standardized sample box (0.001mm2) with a computerized image analysis system. A minimum of three lesions per animal were analyzed; lesion data for each animal were averaged and group means for immunoreactivity were calculated and compared.
DISCUSSION
There are currently no therapeutic options for repairing axonal damage in MS. Although the disease is initially mediated by inflammation that damages myelin, the repeated inflammation and loss of myelin leaves axons vulnerable to damage that is irreparable, resulting in permanent functional deficits. Models of acute spinal cord injury have demonstrated that the Nogo-A pathway is amenable to therapeutic intervention that promotes axonal sprouting and elongation3-4. However, these studies have used monoclonal antibodies and peptide inhibitors which may be neutralized by the development of antibodies. This has been a common problem with the use of recombinant interferon-β for the treatment of relapsing-remitting MS, in which neutralizing antibodies diminish the therapeutic benefit of the agent23. Similarly strategies such as induction of a strong antibody response by immunization with Nogo-A peptide, as previously explored in EAE14, are not feasible in humans given the potential of generating encephalitogenic T cells. The goal of this study was to develop a therapeutic strategy that promotes functional recovery via axonal reparation with minimal adverse affects.
We have previously demonstrated that systemic administration of siRNA could be used to alter the encephalitogenic T cells and ameliorate EAE15-16,18. Although it had been shown that siRNA could access most tissues when administered systemically, the CNS was not accessible22. The lesions in MS are caused by blood-brain barrier breakdown initiated by inflammatory cells, and thus siRNA should be able to enter the site of CNS injury. We demonstrated that fluorescein-siRNA can enter the CNS in the lesion area and transfect cells, including oligodendrocytes. Since fluorescein is quite large relative to the siRNA, the number of fluorescein particles probably underrepresents the amount of siRNA that enter the CNS cells.
More importantly, we found that administration of a Nogo-A siRNA improved the functional capacity of mice with EAE which was associated with decreased Nogo-A expression. In addition, GAP43, a protein expressed by growing and sprouting axons, was significantly upregulated, suggesting that axonal repair may be responsible for the improved clinical outcome in the siRNA-NogoA treated mice. Since a previous study indicated that blocking Nogo-A with antibodies in EAE resulted in less CNS inflammation and found less Th1-associated cytokines13, we investigated whether silencing Nogo-A altered the phenotype of myelin-specific T cells. However, we found no change in T cell proliferation or cytokine production in the siRNA-NogoA treated mice. Transferred of myelin-specific T cells from the siRNA-NogoA and control mice into naïve recipient mice resulted in similar EAE courses, indicating that the encephalitogenic T cells had not been altered by the siRNA-NogoA treatment. A recent study analyzing NgR expression in human lymphocytes found that although B cells, T cells and monocytes express NgR1 in a regulated fashion, an inhibitory Nogo-A peptide failed to alter proliferation or cytokine production by human lymphocytes24. This study also found that myelin could regulate human T cell motility, but this study targeted NgR and not Nogo-A, one of several ligands of the NgR. Analysis of T cells and lesion area indicated that suppression of Nogo-A did not reduce T cell migration into the CNS or reduce lesion size. Thus, the data indicates that suppression of Nogo-A promoted functional recovery by promoting axonal repair.
This is the first study to demonstrate that siRNA directed toward axonal inhibitors expressed in the CNS is a promising therapeutic option for promoting functional recovery. Historically, 50% of MS patients with relapsing-remitting MS progressed to secondary-progressive MS within 10 years of diagnosis. The use of immunomodulatory therapies has slowed disease progression, but ultimately the majority of MS patients develop permanent functional deficits due to axonal damage. Thus, the development of therapies that may protect axons or promote axonal growth is critical for treating secondary progressive MS, as well as the 10% of MS patients with primary progressive MS.
ACKNOWLEDGMENTS
This work was supported by grants from the National Multiple Sclerosis Society (PP1028, JF2116 and RG3812) and NIH. AELR is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society. We thank Curtis Parnell for his excellent care of the animals used in this study.
REFERENCES
- 1.Buffo A, Zagrebelsky M, Huber AB, Skerra A, et al. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured Purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 3.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 4.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 5.Prinjha R, Moore SE, Vinson M, Blake S, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 6.Wiessner C, Bareyre FM, Allegrini PR, Mir AK, et al. Anti Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Ng CE, Tang BLl. Nogo-A expression in mouse central nervous system neurons. Neurosci Lett. 2002;328:257–260. doi: 10.1016/s0304-3940(02)00528-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Chun SJ, Treloar H, Vartanian T, et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associtated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 11.Wang KC, Koprivica V, Kim JA, Sivasankaran R, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 12.Trapp BD, Peterson J, Ransohoff RM, Rudick R, et al. Axonal transaction in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 13.Karnezis T, Mandemakers W, McQualter JL, Zheng B, et al. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nature Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- 14.Fontoura P, Ho PP, DeVoss J, Zheng B, et al. Immunity to the extracellular domain of Nogo-A modulates experimental autoimmune encephalomyelitis. J Immunol. 2004;173:6981–6992. doi: 10.4049/jimmunol.173.11.6981. [DOI] [PubMed] [Google Scholar]
- 15.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Gocke AR, Cravens PD, Ben L-H, Hussain RZ, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 17.Gocke AR, Hussain RZ, Ben L-H, Drew PD, et al. Transcription modulation of the immune response by PPARα agonists amerliorates autoimmune encephomyelitis. J Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Weiner J, Liu Y, Smith AJ, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onuki M, Ayers MM, Bernard CC, Orian JM. Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc Res Tech. 2001;52:731–739. doi: 10.1002/jemt.1057. [DOI] [PubMed] [Google Scholar]
- 20.Moore GR, McCarron RM, McFarlin DE, Raine CS. Chronic relapsing necrotizing encephalomyelitis produced by myelin basic protein in mice. Lab Invest. 1987;57:157–167. [PubMed] [Google Scholar]
- 21.Raine CS, Cross AH. Axonal dystrophy as a consequence of long-term demyelination. Lab Invest. 1989;60:714–725. [PubMed] [Google Scholar]
- 22.Braasch DA, Paroo Z, Constantinescu A, Ren G, et al. Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett. 2004;14:1139–1143. doi: 10.1016/j.bmcl.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 23.Rudick RA, Simonian NA, Alam JA, Campion M, et al. Multiple Sclerosis Collaborative Research Group (MSCRG) Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Neurology. 1998;50:1266–1272. doi: 10.1212/wnl.50.5.1266. [DOI] [PubMed] [Google Scholar]
- 24.Pool M, Niino M, Rambaldi I, Robson K, et al. Myelin regulates immune cell adhesion and motility. Exp Neurol. 2009;217:371–377. doi: 10.1016/j.expneurol.2009.03.014. [DOI] [PubMed] [Google Scholar]