Abstract
Necrotizing enterocolitis (NEC) is the most common severe gastrointestinal emergency that affects premature newborns. This disease often has a rapid onset with few, if any, antecedent signs that can be used to reliably predict its occurrence. Its rapid onset and progression to death, as well as its severe morbidity when the infant survives, begs for early diagnostic tools that may be used in determining those infants who would be at greatest risk for development of the disease, and for whom early preventative measures could be targeted. Although studies have suggested efficacy of several techniques such as breath hydrogen, inflammatory mediators in blood, urine or stool, and genetic markers, these all have drawbacks limiting their use. The application of newly developed “omic” approaches may provide biomarkers for early diagnosis and targeted prevention of this disease.
Necrotizing enterocolitis (NEC) is the most common severe gastrointestinal disease that predominantly afflicts premature infants in neonatal intensive care units (NICUs). Of 2500 annual cases reported in the US, 20–60% undergo surgery and 20–28% do not survive (1, 2). Progress in the prevention of NEC has been limited by difficulties in clearly defining the condition and by our inability to identify subsets of premature infants at highest risk of developing NEC (3). Part of the difficulty stems from the heterogeneity in what is termed NEC. In term and late preterm infants, what has been termed “NEC” has a greater association with predisposing factors such as low Apgar scores, chorioamnionitis, exchange transfusions, prolonged rupture of membranes, congenital heart disease, and neural tube defects (4). Spontaneous intestinal perforation frequently is not accompanied by significant intestinal necrosis, occurs earlier than NEC, and is associated with the combined use of glucocorticoids and indomethacin (5, 6). Here we will focus on “classic” NEC that is generally seen in premature infants born at less than 32 weeks of gestation and with a birth weight of less than 1500 gm. The increased susceptibility has been attributed to multifactorial etiologies including an immature mucosal barrier and barrier inflammatory response, genetic susceptibility, dysfunctional intestinal microecology, and idiosyncrasies related to aggressive feeding practices (7, 8).
Previously, the diagnosis of NEC was based on criteria first established by Bell et al in 1978, which are limited to the clinical and radiographic appearance of the infant (9). Newer criteria, such as the modified Bell system proposed by Walsh and Kleigman, have incorporated the original criteria with laboratory findings to more clearly classify NEC (10), aiding in diagnosis and therapy. Even more recently, the addition of gray-scale and color Doppler ultrasonography to the diagnostic arsenal has enhanced our ability to diagnose NEC, especially in cases with inconclusive plain film radiography or non-specific clinical presentations (11). However, all of the above diagnostic criteria and modalities are applicable only after the infant has developed signs or symptoms of the disease. But because of its delayed occurrence after birth and its highly fulminant nature, identifying prospective biomarkers specific for high NEC risk would offer opportunities for early intervention. In addition to being associated with NEC risk, such biomarkers should be non-invasive, easy to obtain, and inexpensive. Here we will first review several previously published methods (summarized in Table) and then discuss selected emerging technologies that may help us identify infants at risk for NEC and to prevent this devastating disease.
Table.
Biomarkers and mediators of necrotizing enterocolitis in neonates
| Pros | Cons | Comments | References | |
|---|---|---|---|---|
| Breath Hydrogen | Non-invasive Significantly higher excretion of H2 in infants with NEC Sensitivity is 86%, Specificity is 90% |
Technical difficulties in determining exhaled H2 Poor (33%) positive predictive value Other confounding factors include: Variations with feeds and with microbial colonization |
Non-invasive nature is appealing The cons outweigh the utility and feasibility |
12 |
|
Genomic Markers for Pro-inflammatory Responses e.g., IL-18 AA genotype, IL-4 mutant alleles, Single nucleotide polymorphisms of CD-14, TLR-4, CPS-1, and CARD15 |
Availability of highly sensitive and specific assay kits for evaluation for genetic markers offers some potential for predicting risk for NEC | Assays are expensive, time consuming and are not available for clinical practice. Furthermore, interaction with environmental factors influences sensitivity and specificity of these assays rendering genomic marker based prediction of NEC elusive at present | NEC is multi-factorial in origin. Most genetic marker assays may reflect secondary inflammatory responses (pathways common to other inflammatory diseases, e.g., sepsis) | 12, 14–17 |
| Molecular Fecal Microbiota Analysis | Detection of distortions in normal microbiota pattern, which might predispose infants to NEC, might lead to successful early treatment No requirement for extensive culturing (based on 16S rRNA analysis) |
Normal microbiota composition in preterm infants remains ill-defined. Fecal microbiota does not necessarily reflect microbiota at the site of disease |
Collection of a fecal sample is easy and non-invasive Specific molecular patterns unique to infants at risk for NEC have yet to be developed |
7, 8, 18–33 |
|
Inflammatory Mediators Endotoxin/LPS, PAF, TNF-α, cytokines, prostaglandins, leukotrienes, nitric oxide, CRP, fecal calprotectin, I-FABP, L-FABP |
Specific and sensitive assay kits for some mediators but not all are available Detection of differences in CRP, calprotectin, procalcitonin & I-FABP are of interest relevant to their use in clinical setting Use of urine or stool to test for inflammatory markers is attractive because of non-invasive nature of these tests |
Most of the studies with inflammatory mediators are not specific for NEC. Again the inflammatory pathways are common to other inflammatory disease processes such as in sepsis Clinical studies are limited |
Results of studies with respect to examining CRP, calprotectin, procalcitonin and I-FABP as inflammatory markers have yet to be confirmed using larger study samples Feasibility and cost of these tests in clinical settings has yet to be evaluated |
1, 34–44 |
|
Other Mediators Arginine, glutamine, sEGF, IGF-1 |
Specific tests are available for these mediators | None of these mediators have been directly liked to development of NEC | The multifactorial nature of NEC pathogenesis prevents these from being used as a sole diagnostic tool | 45–49 |
Abbreviations: CARD15, caspase recruitment domain 15; CPS-1, carbamoyl-phosphate synthetase 1; CRP, C-reactive protein; I-FABP, intestinal fatty acid binding protein; IGF=1, insulin-like growth factor 1; L-FABP, liver fatty acid binding protein; LPS, lipopolysaccharide; PAF, platelet activating factor; sEGF, salivary epidermal growth factor; TNF, tumor necrosis factor
PREVIOUSLY STUDIED TECHNIQUES
Breath hydrogen sampling
Microbial fermentation of unabsorbed carbohydrates results in production of hydrogen (H2) gas that, although partially utilized by H2 consuming intestinal microbes, is assimilated into the bloodstream and eventually exhaled. Previous studies have suggested that this technique could be utilized in the early detection of NEC (12). In one study, breath H2 excretion was determined in 122 neonates from birth to 1 month of age (12). The patients weighed less than 2000 g at birth and thus were at risk for developing NEC. Hydrogen excretion was normalized for the quality of the expired air by dividing the exhaled hydrogen pressure by the carbon dioxide (CO2) pressure of the gas sample. The mean (± SD) peak H2/CO2 ratio was greater in 7 infants who subsequently developed NEC (9.4 ± 2.7 ppm/mm Hg) than the 115 infants who did not (5.0 ± 3.5 ppm/mm Hg). Defining a positive test as one with a ratio value of ≥ to 8.0 ppm/mm Hg, the resulting screening test had a sensitivity of 86% and a specificity of 90%. The screening test yielded a 33% predictive value for a positive test and a 99% predictive value for a negative test. Increased H2 excretion occurred 8 to 28 hours before the onset of the earliest clinical signs of NEC. It was initially concluded that breath H2 excretion is a simple non-invasive test that may be useful in the management of the premature neonate at risk for the development of NEC. However, this test has not received acceptance largely because of technical difficulties in measuring the pressure of exhaled hydrogen, the large variations in food intake, the variability in the time it takes for bacterial colonization, and the poor predictive value for a positive test.
Genomic markers
Analyses of twin gestations support that intraventricular hemorrhage, necrotizing enterocolitis, and bronchopulmonary dysplasia are familial in origin (13). Given this genetic predisposition, finding a genetic marker that is sensitive, specific, and predictive could be a valuable adjunct to early identification and prevention of NEC. Because of the potential that inadequate innate immune responses to bacterial antigens in the intestinal flora may play a role in the development of NEC, single nucleotide polymorphisms (SNPs) of CD-14, TLR-4 (toll like receptor-4) and CARD15 (Caspase – recruitment domain 15) were analyzed in very low birth weight (VLBW) infants with and without NEC, but found not to be associated with increased risk (14). Another group of investigators, however, focused more specifically on the TLR-4 gene. This gene, responsible for part of the pro-inflammatory cascade, was studied in a murine model of NEC and its products were found to be present in higher levels in those animals at risk for developing NEC, and animals with a mutant TLR-4 gene had a decreased incidence of the disease (15). These findings suggested that gene products, not a SNP alone, might be of more prognostic utility. However, a SNP in the gene that encodes carbamoyl-phospate sythetase 1 (CPS1), the rate-limiting enzyme in the production of arginine, was found to be associated with an increased risk of NEC development (16).
The risk for NEC has also been associated with the frequency of the interleukin IL-18607 AA genotype (12). The frequency of the AA genotype is significantly higher in infants with stage III NEC compared to stages I and II (17). Thus, the presence of AA genotype may adversely affect the outcome of NEC through altered IL-18 levels, a cytokine that induces interferon –γ (IFN-γ) and amplifies T-helper cell type 1 (Th1) cytokine production and IL-8 accumulation (17). Very low birth weight infants with NEC were also shown to be less likely to possess the IL-4 receptor α-chain mutant allele compared to infants without NEC (17). The variant of IL-4 receptor gene is associated with enhanced transduction of IL-4 signals which shifts the development of lymphocytes to a more pronounced Th cell type 2 (Th2) state (17). It is speculated that the elevated number of Th2 cells in carriers of this genetic polymorphism is a protective factor against the development of NEC (17).
These findings suggest that NEC is mediated not by genetics alone, but by genetic factors in conjunction with host-bacteria interactions. Thus, the search for genetic markers appears to have some potential, but sensitivity and specificity for prediction remains elusive largely due to the interaction with environmental factors that also appear to play an important role in the pathogenesis of NEC.
Intestinal microbiota
Colonization of the gastrointestinal (GI) tract of the newborn with microorganisms from the extrauterine environment starts immediately after birth and is markedly affected by maternal and environmental microbial sources. The frequent use of antibiotics, type of feeding (human milk vs. formula), mode of delivery (vaginal vs. Caesarian section) and various manipulations in the NICU such as nursing in an incubator vs. under radiant warmers, have the potential to alter the intestinal microbiota (18–20). Recent technologic advances reveal that although the majority of microbes in the human GI tract cannot yet be cultured, powerful new molecular 16S rRNA based techniques now allow for a comprehensive analysis of microbiota (21, 22). The vast array of commensal microorganisms with their immense metabolic capabilities contributes significantly to host physiology. Microbiota contributions include vitamin synthesis, nutrient utilization and absorption, stimulation of immune responses to pathogens as well as tolerance to luminal antigens, and stimulation of Paneth cell peptide secretion, which in turn, promotes angiogenesis, growth, and an environment that facilitates colonization by commensals rather than opportunistic pathogenic microorganisms (23, 24). However, a different situation appears in the premature infant wherein microbes, although essential for normal intestinal growth and development, are also implicated in the development or exacerbation of NEC (7).
The large diversity of bacteria present in infants even before the development of NEC and the current failure to isolate/identify a known pathogen suggests that commensal microbes are usually bystanders that, under certain conditions, can amplify pathologic processes such as uncontrolled inflammation. A related hypothesis is that a particular microbiota composition, which might be normal in a full term infant, may be the culprit leading to the inappropriate inflammatory cascade producing NEC in preterm babies as opposed to the presence of particular pathogenic species (7, 8). There is older evidence, however, showing that specific changes can be observed in the intestinal flora preceding the development of NEC (25). Hence, it is apparent that the microbial ecology is a factor in the development of NEC, but a definitive causality has yet to be determined.
When infants with NEC were compared with control infants without NEC (matched for gestational age at birth and postmenstrual age), a study based on cultivating potential pathogens did not detect specific bacteria associated with disease (26). This observation does not exclude the possibility that sufficiently powered studies that utilize modern molecular microbiota analysis methods could identify pathogens in the future. In addition to specific pathogens, the colonization with a microbiota altered in its diversity or emergence of dominant commensal microbes that elucidate an inappropriate immune response may be of importance in the disease process (27). Recent studies using molecular techniques have provided initial insight (28). The use of 16S ribosomal RNA based approaches has greatly facilitated the study of GI tract microbial ecology because it circumvents the need for culturing (29). Powerful novel molecular approaches, including pyro-sequencing, now allow for an in-depth analysis reaching saturation that can detect sequence signatures for bacteria contributing only a small proportion to the overall microbiota (29).
These new molecular microbiota-typing techniques for the detection of otherwise non-cultivatable microbes offer new opportunities for delineating both specific NEC associated pathogens as well as intestinal micro-ecologic patterns that may be conducive to the pathogenesis of NEC. There have been arguments against the validity of using fecal microbiota as a surrogate temporal measure for the microbiota that is in close contact to the small and large intestinal mucosa. Preterm babies defecate infrequently and thus distortions in microbiota composition might not be detected in rectal swabs or stool specimens when inflammation is either starting or progressing at the intestinal mucosal surface.
Recently there has been an interest in altering the intestinal microbiota in order to manipulate disease. Studies of antibiotics (30), prebiotics (31) and probiotics (32, 33) have been carried out in the hopes of developing a prophylactic therapy for NEC. While there has been no conclusive evidence of definitive preventive strategies, much remains to be discovered in the realm of manipulation of the microflora.
Inflammatory mediators
Similar to sepsis and adult respiratory distress syndrome, the pathogenesis of NEC appears to involve a pathway that includes the endogenous production of inflammatory mediators involved in the development of intestinal injury (34). Endotoxin/lipopolysaccharide (LPS), platelet-activating factor (PAF), tumor necrosis factor alpha (TNF-α), IL-8, and other chemokines and cytokines, together with prostaglandins, leukotrienes, and nitric oxide, are thought to be involved in the final common pathway of NEC pathogenesis (1, 34, 35). Sera, tissue, stool, or other samples from early time points in the development of the disease may help delineate early inflammatory events that predispose an infant to NEC, thus providing an interventional opportunity. Here we will briefly review some of the inflammatory and other markers that have been and/or are still being evaluated for the early diagnosis of NEC.
In one study, concentrations of IL-1β, IL-6, endotoxin/LPS, and TNF-α were measured at the onset of clinical illness (35). Neonatal endotoxemia and release of proinflammatory cytokines were found to be important contributors to multiple organ failure and mortality. Endotoxemia was most severe at the onset of illness among the infants with NEC, suggesting that gut barrier failure plays an important role in adverse outcomes in the NICU (35).
In another study, association between serum C-reactive proteins (CRP) and NEC were evaluated (36). CRP levels were found to be abnormal in both stage II and stage III NEC. In infants with NEC, persistently elevated CRP after initiation of appropriate medical management suggested development of complications of NEC, which often required surgical intervention.
Another mediator, fecal calprotectin, has also been evaluated as a potential diagnostic tool for NEC. It is an established screening test used in diagnosing inflammatory bowel disease (37), and is now taking on an expanding role in disease monitoring and relapse detection (38). For NEC, levels of fecal calprotectin have been measured in stools of VLBW premature infants. One study showed that a fecal calprotectin level >2000 μg/g is a useful, but not an early marker of NEC and of other severe intestinal inflammatory conditions in VLBW infants (39). In another pilot study designed to determine whether it can be used to aid diagnosis of NEC in preterm infants (40), it was found that infants with NEC had greater fecal calprotectin concentrations at the time of diagnosis compared to matched controls (288.4 mg/L ± 49.1 vs. 98.0 mg/L ± 60.6, p = 0.0006). These studies were done primarily at the time of onset of NEC. In another study (41), fecal calprotectin levels of 14 VLBW infants (gestational age 23–30 weeks, birth weight ≤1,500 g) were serially measured in the first postnatal month. Fecal calprotectin levels significantly differed between ‘well’ and ‘sick’ infants (122.8 ± 98.9 vs. 380.4 ± 246.3 μg/g stool, p < 0.001). A fecal calprotectin level >350 μg/g stool was associated when signs of gastrointestinal injury, such as bloody stool or bowel perforation, were present. Levels decreased after initiation of treatments in sick infants who recovered. These studies concluded that fecal calprotectin levels may be a marker for early diagnosis and resolution of gastrointestinal illness in VLBW infants, but its utility for early diagnosis and assessment of resolution of NEC needs to be studied in a larger cohort of VLBW infants.
Fatty acid binding proteins, tissue-specific inflammatory markers usually found to be more elevated during periods of ischemia, have also been evaluated as potential markers for the diagnosis and staging of NEC. Specifically, in a recent study by Guthmann et al, plasma concentrations of both intestinal fatty acid binding protein (I-FABP) and liver fatty acid binding protein (L-FABP) were measured in healthy preterm infants and in preterm infants with NEC (42). I-FABP was found to be associated with advanced stages of NEC, while L-FABP was found to be significantly elevated in infants with only a suspicion of NEC (42), indicating that the latter may be a more sensitive marker for the early detection of the disease. Urinary I-FABP as a marker of intestinal injury was evaluated in another study (43). A value of 2 pg/nmol for the urinary I-FABP to creatinine ratio was capable of distinguishing the group of infants with NEC or intestinal necrosis from the other diagnoses. Given the ease and frequency with which urine can be collected without adverse consequences for the baby, it was concluded that the feasibility of urinary I-FABPs as a screening tool for NEC requires further evaluation in a prospective trial. Although these markers show some promise in diagnosis and classification of disease, much remains to be evaluated before their application in the clinical setting.
It is likely that the lack of diagnostic and therapeutic modalities for NEC is due to the lack of an overall view of the disease. Properly validated and calibrated mathematical models of inflammation and its pathologic consequences in NEC could be useful for predicting the physiologic and biologic response in infants suffering from the disease, but, as suggested recently, have not yet been fully developed (44).
Other mediators
Also associated with NEC are low concentrations of arginine and glutamine. Supplementation of each of these amino acids has been considered as a preventative strategy for the disease (45, 46). However, these therapies are likely to be non-specific for NEC and the deficiencies are likely caused by low intakes and high consumption during stress (47).
Finally, growth factors have also been assessed for use not only as diagnostic tools, but also as therapeutic interventions. For diagnostic purposes, a prospective analysis was performed in which levels of salivary derived epidermal growth factor (sEGF) were found to be significantly lower in preterm infants, and more rapid elevations of sEGF during the first weeks of life were associated with a greater incidence of NEC (48). For therapeutic purposes, another study evaluated levels of insulin-like growth factor 1 (IGF-1) and its effects on maturation of intestinal function in preterm infants (49). It was suspected that supplementation of IGF-1 in preterm formulas would speed the growth and development of the barrier and absorptive features of the gut (49), thereby potentially decreasing the likelihood of NEC development. However, supplementation was not found to be substantially beneficial, possibly due to synergy with other factors that were not supplemented in conjunction with IGF-1 (49). Nevertheless, these important observations suggest the feasibility of developing biomarkers for the early detection of NEC and encourage further investigation.
EMERGING BIOMARKER TECHNOLOGIES
New molecular techniques have recently emerged in their use to develop biomarkers for diseases such as prostate cancer (50), liver disease (51), infection in the amniotic fluid (52), and for the detection of retinopathy of prematurity (53). Even more promising is the finding that proteomic fingerprinting of amniotic fluid has proven to be more accurate than conventional gram stain and culturing in diagnosing intra-amniotic infection (54). Such successes using molecular techniques offer promise to the hope of finding a biomarker for detection, and possibly prevention, of NEC.
Proteomics
Proteomics is a rapidly growing science that focuses on the multitude of tasks assigned to proteins (54, 55). It relates to the structure, the functions, and the interactions of proteins including their identification and quantification (55). It also entails the study of impact of their interactions on biological functions. In VLBW infants susceptible to NEC, it is likely that on account of premature birth, the developing proteins are disharmonized and that either the signaling pathways between microbiota and the intestinal barrier or the trafficking of luminal matter through the intestinal barrier is altered in a manner that is conducive to the genesis of NEC (56).
Proteomic technologies involve several steps. Polyacrylamide (PAGE) and two dimensional gel (2D gel) electrophoresis methods of protein separation exploit the protein property that, in the presence of an electric field, the intrinsic charge of proteins impart a characteristic electrophoretic mobility to them permitting their separation and identification based on their molecular weights and net charge (57). Of these two, the method of 2D gel is considered a better option for high-resolution profiling of low abundance proteins in the quest for identifying biomarkers for NEC (58).
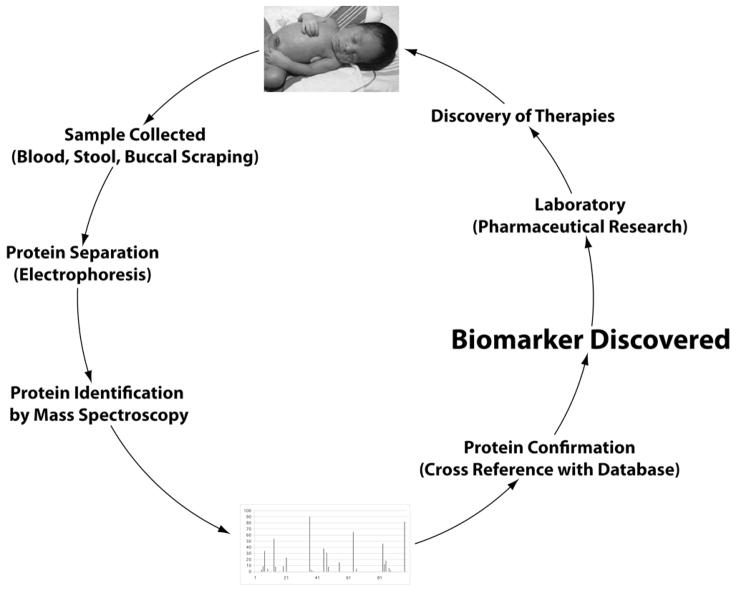
Mass spectrometry is another powerful technique emerging in the field of diagnostic biomarkers and identification of proteins involved in diseases (59). This technique involves introducing enough energy into a target molecule to cause its ionization and disintegration. The resulting fragments are then analyzed, based on the mass/charge ratio. Different types of mass spectrometry techniques, including surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF), and tandem mass spectroscopy (MS/MS), are available (57, 59). Two-D gel is often used in conjunction with mass spectrometry (55, 57). One such example is the detection of potential biomarkers characteristic of premature rupture of membranes in preterm deliveries (54). The figure illustrates how these techniques might be utilized in human babies.
Figure.
The biomarker discovery process
Transcriptomics
Transcriptomics is a global way of evaluating gene-expression patterns (60). It involves study of all messenger RNA molecules, or transcripts, and evaluation of changes in transcription initiation, processing, and degradation of proteins. The technique of polymerase chain reaction (PCR), based on transcriptomics, holds promise in discovering biomarkers in the area of NEC research in the near future. PCR is already being used to help identify previously un-cultivatable organisms (21, 29, 61). The collection of samples adequate for PCR is appealing as minute amounts of biological material are all that are required as they are amplified further during this process (60, 61). Samples can be obtained from almost any tissue such as skin, saliva, and buccal scrapings without undue extensive invasive tests or blood sampling.
These new methods are not, however, without their share of challenges. One of the most obvious is the need for proper instrumentation. Accurate and affordable equipment must be available for efficient application of these techniques. If a previously unknown gene is found to be differentially expressed in health vs. disease, it remains critical that the corresponding gene product be confirmed both phenotypically and at the protein level. Although the technology is available to perform this type of evaluation, the bottleneck in developing a good diagnostic platform derives from issues related to finding statistical significance and biological relevance of a differentially transcribed gene.
CONCLUSION
Emerging evidence suggests that major pathophysiologic contributions to the development of NEC originate from a defective interaction between intestinal microbes and the host’s response to these microbes. Newly developed technologies supported by initiatives such as the human microbiome project are likely to provide new information. “Omic-based” techniques for biomarker discovery in tissue samples obtained non-invasively appear particularly appealing for the development of novel point-of-care diagnostic markers, and are attractive for the development of effective therapies, such as nutritional supplements (growth factors, prebiotics, etc.), bacterial manipulations (probiotics, antibiotics), or even direct therapy with recombinant gene products to reduce or eliminate the inflammatory response.
Acknowledgments
No financial assistance was received to support this study
Abbreviations
- CRP
C-reactive protein
- GI
gastrointestinal
- I-FABP
intestinal fatty acid binding protein
- IGF-1
Insulin-like growth factor 1
- NEC
necrotizing enterocolitis
- SNP
single nucleotide polymorphism
- VLBW
very low birth weight
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 3.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Tallo E, Claure N, Bancalari E. Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol Neonate. 1997;71:292–298. doi: 10.1159/000244428. [DOI] [PubMed] [Google Scholar]
- 5.Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll BJ, Fanaroff AA, Ehrenkranz RA, Korones SB, Stevenson DK. Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 6.Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol. 2006;26:185–188. doi: 10.1038/sj.jp.7211439. [DOI] [PubMed] [Google Scholar]
- 7.Neu J. Neonatal Necrotizing enterocolitis: An update. Acta Paediatr Suppl. 2005;94:100–105. doi: 10.1111/j.1651-2227.2005.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 8.Neu J, Douglas-Escobar M, Lopez M. Microbes and the developing gastrointestinal tract. NCP- Nutr Clin Pract. 2007;22:174–182. doi: 10.1177/0115426507022002174. [DOI] [PubMed] [Google Scholar]
- 9.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, Taylor G, Gerstle JT. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics. 2007;27:285–305. doi: 10.1148/rg.272055098. [DOI] [PubMed] [Google Scholar]
- 12.Cheu HW, Brown DR, Rowe MI. Breath hydrogen excretion as a screening test for the early diagnosis of necrotizing enterocolitis. Am J Dis Child. 1989;143:156–159. doi: 10.1001/archpedi.1989.02150140042017. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 14.Szebeni B, Szekeres R, Rusai K, Vannay A, Veres G, Treszl A, Arato A, Tulassay T, Vasarhelyi B. Genetic polymorphisms of CD14, toll-like receptor 4, and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2006;42:27–31. doi: 10.1097/01.mpg.0000192246.47959.b2. [DOI] [PubMed] [Google Scholar]
- 15.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moonen RM, Paulussen AD, Souren NY, Kessels AG, Rubio-Gozalbo ME, Villamor E. Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res. 2007;62:188–190. doi: 10.1203/PDR.0b013e3180a0324e. [DOI] [PubMed] [Google Scholar]
- 17.Treszl A, Heninger E, Kalman A, Schuler A, Tulassay T, Vasarhelyi B. Lower prevalence of IL-4 receptor alpha-chain gene G variant in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg. 2003;38:1374–1378. doi: 10.1016/s0022-3468(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, Peterson EL, Joseph CL. Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol. 2005;115:1218–1224. doi: 10.1016/j.jaci.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Yan F, Polk DB. Commensal bacteria in the gut: learning who our friends are. Curr Opin Gastroenterol. 2004;20:565–571. doi: 10.1097/00001574-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Neu J. Perinatal and neonatal manipulation of the intestinal microbiome: a note of caution. Nutr Rev. 2007;65:282–285. doi: 10.1301/nr.2007.jun.282-285. [DOI] [PubMed] [Google Scholar]
- 21.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 22.Relman DA. The identification of uncultured microbial pathogens. J Infect Dis. 1993;168:1–8. doi: 10.1093/infdis/168.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 24.Hooper LV, Stappenbeck TS, Hong CV, Gordon J. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 25.Hoy C, Millar MR, MacKay P, Godwin PG, Langdale V, Levene MI. Quantitative changes in faecal microflora preceding necrotising enterocolitis in premature neonates. Arch Dis Child. 1990;65:1057–1059. doi: 10.1136/adc.65.10_spec_no.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter CS, Feuerhahn M, Bohnhorst B, Schlaud M, Ziesing S, von der Hardt H, Poets CF. Necrotising enterocolitis: is there a relationship to specific pathogens? Eur J Pediatr. 1999;158:67–70. doi: 10.1007/s004310051012. [DOI] [PubMed] [Google Scholar]
- 27.Alverdy JC. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83:461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 28.Butel MJ, Suau A, Campeotto F, Magne F, Aires J, Ferraris L, Kalach N, Leroux B, Dupont C. Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J Pediatr Gastroenterol Nutr. 2007;44:577–582. doi: 10.1097/MPG.0b013e3180406b20. [DOI] [PubMed] [Google Scholar]
- 29.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen Y-J, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim J-B, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bury RG, Tudehope D. Enteral antibiotics for preventing necrotising enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2000:CD000405. doi: 10.1002/14651858.CD000405. [DOI] [PubMed] [Google Scholar]
- 31.Danan C, Huret Y, Tessedre AC, Bensaada M, Szylit O, Butel MJ. Could oligosaccharide supplementation promote gut colonization with a beneficial flora in preterm infants? J Pediatr Gastroenterol Nutr. 2000;30:217–219. doi: 10.1097/00005176-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008:CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Embleton ND, Yates R. Probiotics and other preventative strategies for necrotising enterocolitis. Semin Fetal Neonatal Med. 2008;13:35–43. doi: 10.1016/j.siny.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25:329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R, Tepas J, Jr, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Pourcyrous M, Korones SB, Yang W, Boulden TF, Bada HS. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics. 2005;116:1064–1069. doi: 10.1542/peds.2004-1806. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283–1291. doi: 10.1007/s10350-008-9310-8. [DOI] [PubMed] [Google Scholar]
- 38.D’Inca R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, Oliva L, Sturniolo GC. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 39.Josefsson S, Bunn SK, Domellöf M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44:407–413. doi: 10.1097/MPG.0b013e3180320643. [DOI] [PubMed] [Google Scholar]
- 40.Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361:310–311. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94:267–271. doi: 10.1159/000151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guthmann F, Borchers T, Wolfrum C, Wustrack T, Bartholomaus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. 2002;239:227–234. [PubMed] [Google Scholar]
- 43.Derikx JP, Evennett NJ, Degraeuwe PL, Mulder TL, van Bijnen AA, van Heurn LW, Buurman WA, Heineman E. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007;56:1473–1475. doi: 10.1136/gut.2007.128934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upperman JS, Camerini V, Lugo B, Yotov I, Sullivan J, Rubin J, Clermont G, Zamora R, Ermentrout GB, Ford HR, Vodovotz Y. Mathematical modeling in necrotizing enterocolitis--a new look at an ongoing problem. J Pediatr Surg. 2007;42:445–453. doi: 10.1016/j.jpedsurg.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 45.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–431. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- 46.Tubman TR, Thompson SW, McGuire W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2008:CD001457. doi: 10.1002/14651858.CD001457.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr. 2000;137:785–793. doi: 10.1067/mpd.2000.109145. [DOI] [PubMed] [Google Scholar]
- 48.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007;150:358–363. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 49.Corpeleijn WE, van Vliet I, de Gast-Bakker DA, van der Schoor SR, Alles MS, Hoijer M, Tibboel D, van Goudoever JB. Effect of enteral IGF-1 supplementation on feeding tolerance, growth, and gut permeability in enterally fed premature neonates. J Pediatr Gastroenterol Nutr. 2008;46:184–190. doi: 10.1097/MPG.0b013e31815affec. [DOI] [PubMed] [Google Scholar]
- 50.Wagner M, Naik DN, Pothen A, Kasukurti S, Devineni RR, Adam BL, Semmes OJ, Wright GL. Computational protein biomarker prediction: a case study for prostate cancer. BMC Bioinformatics. 2004;5:26. doi: 10.1186/1471-2105-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poon TC, Hui AY, Chan HL, Ang IL, Chow SM, Wong N, Sung JJ. Prediction of liver fibrosis and cirrhosis in chronic hepatitis B infection by serum proteomic fingerprinting: a pilot study. Clin Chem. 2005;51:328–335. doi: 10.1373/clinchem.2004.041764. [DOI] [PubMed] [Google Scholar]
- 52.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, Roberts CT, Nagalla SR. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 53.Madan A, El-Ferzli G, Carlson SM, Whitin JC, Schilling J, Najmi A, Yu TT, Lau K, Dimmitt RA, Cohen HJ. A potential biomarker in the cord blood of preterm infants who develop retinopathy of prematurity. Pediatr Res. 2007;61:215–221. doi: 10.1203/pdr.0b013e31802d776d. [DOI] [PubMed] [Google Scholar]
- 54.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire L, Funai E, Norwitz ER, Paidas M, Copel JA, Weiner CP, Lockwood CJ, Buhimschi IA. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petricoin EF, Zoon KC, Kohn EC, Barrett JC, Liotta LA. Clinical proteomics: translating benchside promise into bedside reality. Nat Rev Drug Discov. 2002;1:683–695. doi: 10.1038/nrd891. [DOI] [PubMed] [Google Scholar]
- 56.Jiang P, Siggers JL, Ngai HH, Sit WH, Sangild PT, Wan JM. The small intestine proteome is changed in preterm pigs developing necrotizing enterocolitis in response to formula feeding. J Nutr. 2008;138:1895–1901. doi: 10.1093/jn/138.10.1895. [DOI] [PubMed] [Google Scholar]
- 57.Chertov O, Simpson JT, Biragyn A, Conrads TP, Veenstra TD, Fisher RJ. Enrichment of low-molecular-weight proteins from biofluids for biomarker discovery. Expert Rev Proteomics. 2005;2:139–145. doi: 10.1586/14789450.2.1.139. [DOI] [PubMed] [Google Scholar]
- 58.Tabuse Y, Nabetani T, Tsugita A. Proteomic analysis of protein expression profiles during Caenorhabditis elegans development using two-dimensional difference gel electrophoresis. Proteomics. 2005;5:2876–2891. doi: 10.1002/pmic.200401154. [DOI] [PubMed] [Google Scholar]
- 59.Park JS, Oh KJ, Norwitz ER, Han JS, Choi HJ, Seong HS, Kang YD, Park CW, Kim BJ, Jun JK, Syn HC. Identification of proteomic biomarkers of preeclampsia in amniotic fluid using SELDI-TOF mass spectrometry. Reprod Sci. 2008;15:457–468. doi: 10.1177/1933719108316909. [DOI] [PubMed] [Google Scholar]
- 60.Iacobas DA, Suadicani SO, Iacobas S, Chrisman C, Cohen MA, Spray DC, Scemes E. Gap junction and purinergic P2 receptor proteins as a functional unit: insights from transcriptomics. J Membr Biol. 2007;217:83–91. doi: 10.1007/s00232-007-9039-7. [DOI] [PubMed] [Google Scholar]
- 61.Mai V. Dietary modification of the intestinal microbiota. Nutr Rev. 2004;62:235–242. doi: 10.1301/nr2004.jun235-242. [DOI] [PubMed] [Google Scholar]