Summary
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of T cell dysregulation caused by defective Fas-mediated apoptosis. Patients with ALPS can develop a myriad of clinical manifestations including lymphadenopathy, hepatosplenomegaly, autoimmunity and increased rates of malignancy. ALPS may be more common that originally thought, and testing for ALPS should be considered in patients with unexplained lymphadenopathy, hepatosplenomegaly, and/or autoimmunity. As the pathophysiology of ALPS is better characterized, a number of targeted therapies are in preclinical development and clinical trials with promising early results. This review describes the clinical and laboratory manifestations found in ALPS patients, as well as the molecular basis for the disease and new advances in treatment.
Keywords: cytopenia, autoimmune haemolytic anaemia, autoimmune neutropenia, lymphoproliferative disease, immune thrombocytopenic purpura
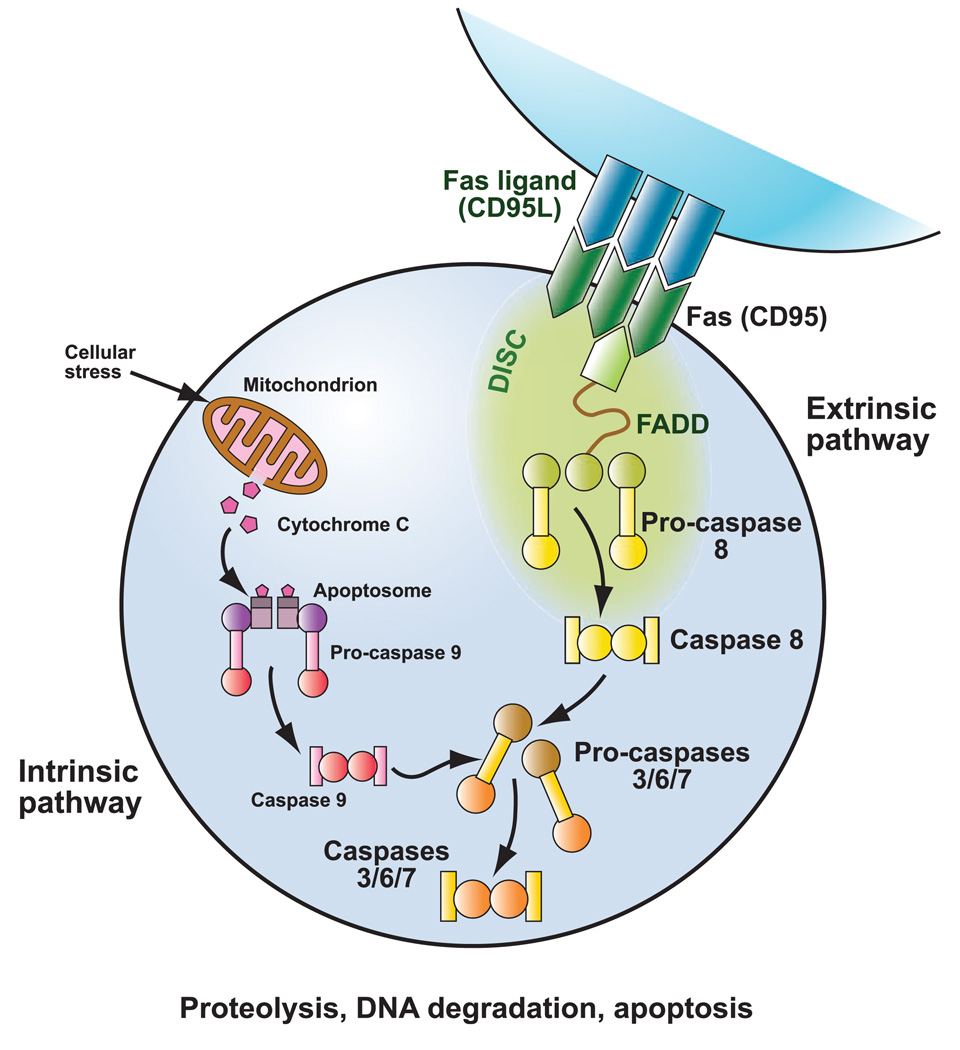
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of disrupted lymphocyte homeostasis caused by defective Fas-mediated apoptosis (Fig 1). As part of the normal down-regulation of the immune response, activated T lymphocytes upregulate expression of Fas, while activated B and T lymphocytes upregulate expression of Fas ligand (Nagata & Golstein, 1995). Fas and Fas ligand interact through the Fas-activating death domain (FADD) to trigger the caspase cascade, leading to proteolysis, DNA degradation, and apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic lymphoproliferation, autoimmune manifestations, and a propensity to develop malignancies (Bleesing, 2002).
Fig 1.
Fas apoptotic pathway. Patients with ALPS have defective Fas-mediated apoptosis. During downregulation of the immune response, activated B and T lymphocytes upregulate expression of Fas ligand, and activated T lymphocytes up-regulate expression of Fas. Fas ligand and Fas interact, activating the intracellular Fas-associated death domain (FADD) and triggering the caspase cascade with subsequent proteolysis, DNA degradation, and apoptosis. Apoptotic signalling mediated by Fas is part of the extrinsic pathway as it is activated through engangement of cell surface death receptors. The intrinsic apoptotic pathway is activated by cellular stressors, leading to alterations in mitochondrial membrane permeability and release of apoptosis-inducing substances. © Sue Seif, MA (used with permission).
ALPS is a rare disorder first characterized in the 1990s in a cohort of patients with chronic lymphoproliferation and an increased number of a T cell population termed ‘double negative T cells’ (DNTs; cell phenotype CD4−/CD8−, CD3+, TCRαβ+) (Sneller et al, 1992). DNTs typically represent a small subset of T cells (<1%) in peripheral blood in unaffected individuals (Bleesing et al, 2002). These patients had clinical features resembling two mouse models of autoimmunity, lpr and gld. Lpr and gld mice were later shown to have defective Fas-mediated apoptosis with homozygous mutations in the FAS and FASLG genes, respectively (Watanabe-Fukunaga et al, 1992; Takahashi et al, 1994). Patients with lymphoproliferation and autoimmunity were subsequently confirmed to have similar genetic defects to the lpr and gld mice and were classified as having ALPS (Fisher et al, 1995; Rieux-Laucat et al, 1995).
Clinical manifestations
Under the original classification system proposed by the National Institute of Health ALPS (NIH-ALPS) group, in order for a patient to be diagnosed with ALPS, they must meet three mandatory criteria: (1) clinically identifiable, non-malignant, chronic lymphoproliferation; (2) elevated DNTs; and, (3) in vitro evidence of defective Fas-mediated apoptosis (Table Ia) (Bleesing, 2002). Supportive but non-diagnostic evidence for ALPS includes genetic mutations in the Fas pathway (FAS, FASLG or CASP10) and/or systemic auto-immunity (Rieux-Laucat et al, 2003). However, these criteria may need modification, as recent reports have identified patients who have ALPS by the ‘gold standard’ in vitro apoptosis assay but do not meet all three diagnostic criteria (Holzelova et al, 2004).
Table I.
Diagnostic criteria.
| (a) Original diagnostic criteria (Bleesing et al, 2000) |
| 1. Chronic non-malignant lymphoproliferation |
| 2. Elevated peripheral blood DNTs |
| 3. Defective in vitro Fas-mediated apoptosis |
| Supporting: genetic mutations; autoimmunity |
| Diagnosis: Must meet all three criteria |
| (b) New Diagnostic Criteria (Seif et al, 2008) |
| Major criteria |
| 1. Chronic non-malignant lymphoproliferation |
| (a) >6 months |
| (b) Splenomegaly and/or lymphadenopathy of ≥2 nodal groups |
| 2. Marked elevation in peripheral blood DNTs ≥5%* |
| 3. Defective in vitro Fas-mediated apoptosis |
| 4. Identifiable genetic mutation (FAS, FASLG, CASP10, NRAS): germline or somatic |
| Minor criteria |
| 1. Autoimmune cytopenias |
| (a) Thrombocytopenia, neutropenia and/or haemolytic anaemia, and |
| (b) Proven to be immune-mediated by identifiable autoantibody (e.g. DAT) or response to immunosuppressive medication |
| 2. Moderate elevation in DNTs* |
| (a) Elevated DNTs in spleen or lymph node biopsy, and/or |
| (b) Peripheral blood DNTs >2 S.D. of testing labs mean and <5% |
| 3. Elevated serum IgG |
| 4. Elevated serum IL-10 |
| 5. Elevated serum vitamin B12 |
| 6. Elevated plasma Fas ligand level |
| Diagnosis: three major criteria or two major plus two minor criteria |
DNT, double negative T cells; DAT, direct antiglobulin test.
One criterion must be elevated DNTs.
Lymphoproliferation is the most common clinical manifestation in patients with ALPS and can manifest as lymphadenopathy, hepatomegaly, or splenomegaly (van der Werff ten Bosch, 2003). To be diagnosed with ALPS, lymphoproliferation must be chronic (>6 months) and, if limited to adenopathy, must affect at least two distinct nodal groups (Jackson & Puck, 1999). Lymphadenopathy and splenomegaly are very common in ALPS patients, as over 80% of patients have lymphadenopathy and over 85% of patients have splenomegaly (Jackson & Puck, 1999; Straus et al, 1999). Hepatomegaly is found in approximately 45% of patients (Bleesing, 2003). The majority of patients present with lymphoproliferation at a young age (median 11·5 months) (Bleesing, 2003). Nevertheless, patients have been described who did not develop symptoms until after adolescence (Bleesing, 2002; Wei & Cowie, 2007). Lymphoproliferation frequently improves with age (typically in later adolescence) and may wax and wane in severity.
Autoimmunity is the second most common clinical manifestation found in ALPS patients. Systemic autoimmunity is the disease manifestation most frequently requiring medical intervention(s). Autoimmune destruction of blood cells is the most common presentation of autoimmunity in ALPS, affecting over 70% of patients (Straus et al, 1999). This destruction can be of erythrocytes (autoimmune haemolytic anaemia), platelets (immune thrombocytopenia) or neutrophils (autoimmune neutropenia). Many patients have destruction in multiple cell lines. Autoimmune cytopenias can range from mild disease that is only identified by laboratory testing to chronic severe disease, requiring immunosuppressive medications (Bleesing, 2002). In many patients, autoimmunity fluctuates and may flare with systemic insult. As with lymphoproliferation, autoimmune manifestations may improve with age.
Other autoimmune manifestations are found less frequently, including autoimmune disease of the kidney (nephritis), liver (hepatitis), joint (arthritis), eye (uveitis), neurological system (autoimmune cerebellar syndrome) and gut (colitis) (Sneller et al, 1997). Rashes, especially urticarial, are reported to be common; however, the true incidence is unknown (Bleesing et al, 2000; Auricchio et al, 2005; Worth et al, 2006). Immune-mediated pulmonary fibrosis, occasionally with bronchiolitis obliterans/organizing pneumonia (BOOP), is being found at increased frequency as patients with ALPS are now followed into adulthood (Jackson et al, 1999). As with systemic lupus erythematosis (SLE), almost any organ system can be affected in ALPS patients. Accordingly, ALPS patients may be erroneously diagnosed as having lupus or mixed connective tissue disease. Autoimmune manifestations usually present in early childhood, and may occur months to years after lymphoproliferation (Bleesing et al, 2000).
Patients with ALPS have an increased risk of developing malignancies (Straus et al, 1999). The exact risk is unknown but hypothesized to be 10–20% (Straus et al, 2001). Most commonly, patients develop lymphoma (non-Hodgkin or Hodgkin), but leukaemias, and a number of solid tumours (thyroid, breast, and liver carcinoma) have been described (Jackson et al, 1999). An increased risk of cancer has been reported in unaffected family members of patients with ALPS (Straus et al, 2001). These family members may inherit the same genetic mutations but fail to develop an overt ALPS phenotype. The increased risk for malignancy in persons with germline mutations in the Fas apoptotic pathway (FAS, FASLG, CASP10) is understandable, as somatic mutations in these genes are found in high prevalence in lymphomas in the general population (Poppema et al, 2004).
Patients with ALPS do not typically develop constitutional symptoms, and with few exceptions, most ALPS patients do not have an increased risk for infection. A subset of patients with ALPS has co-morbid common variable immunodeficiency (5–10%) (Campagnoli et al, 2006). ALPS patients are also reported to have an increased risk of post-splenectomy pneumococcal sepsis, even with appropriate vaccination and antibiotic prophylaxis (Sneller et al, 1997). Splenectomy should be avoided in ALPS patients whenever possible. ALPS patients with autoimmune neutropenia can typically mount a neutrophil response in the setting of infection and are not at increased risk of invasive infection (Capsoni et al, 2005). On the other hand, the immunosuppressive medications often required to treat ALPS do increase infectious risk.
Laboratory and radiological findings
Until recently, in order for a patient to be diagnosed with ALPS, he or she must fulfil two mandatory laboratory diagnostic criteria: (1) elevated DNTs; and (2) defective in vitro Fas-mediated apoptosis. DNTs are normally present in low numbers (<1%) in the peripheral blood and lymphoid tissue (lymph nodes and spleen) (Bleesing et al, 2001a). Elevation of DNTs in peripheral blood and lymphoid tissue is a hallmark of ALPS and was originally thought to be pathognomic (Rao et al, 2007). Patients with other autoimmune diseases, including SLE and immune thrombocytopenic purpura have since been reported to have mild elevations in DNTs (Dianzani et al, 1997; Dean et al, 2002). Marked elevations of DNTs (>5%), however, are only described in ALPS patients. Of note, severely lymphopenic patients may have falsely negative elevations in DNTs, as the low total lymphocyte counts may render quantifying any subset by flow cytometry inaccurate.
DNTs are assessed by flow cytometry in specialized laboratories. It is important to know the normal value for a particular laboratory, as the percentage of DNTs in normal patients can vary between laboratories based on gating strategies (Teachey et al, 2005). Measurement of the α/β-T cell receptor (TCRα/β) in addition to CD4, CD8, and CD3, as natural killer (NK) cells, NK/T cells, and γ/δ-TCR-expressing double negative T cells can have the immunophenotype, CD3+/CD4−/CD8−. Patients with ALPS also may have elevations of other lymphocyte subsets, including CD5+ B cells, CD 8+ T cells, γ/δ-DNTs, CD57+ T cells, and HLA-DR+ T cells (Bleesing et al, 2001a).
The elevated DNTs in ALPS patients were originally postulated to be merely an epiphenomenon of the disease; however, the elevated DNTs in ALPS patients may drive abnormal B cell activity and subsequent autoimmunity (Ohga et al, 2002). The origin of peripheral blood DNTs is debated, but a recent study suggests they may be a subset of thymic-derived regulatory T (Treg) cells (Fischer et al, 2005). Another theory is that the DNTs are CD8+ T cells that have lost CD8 expression, however, recent evidence suggests that may not be the case (Bleesing et al, 2001b; Marlies et al, 2007).
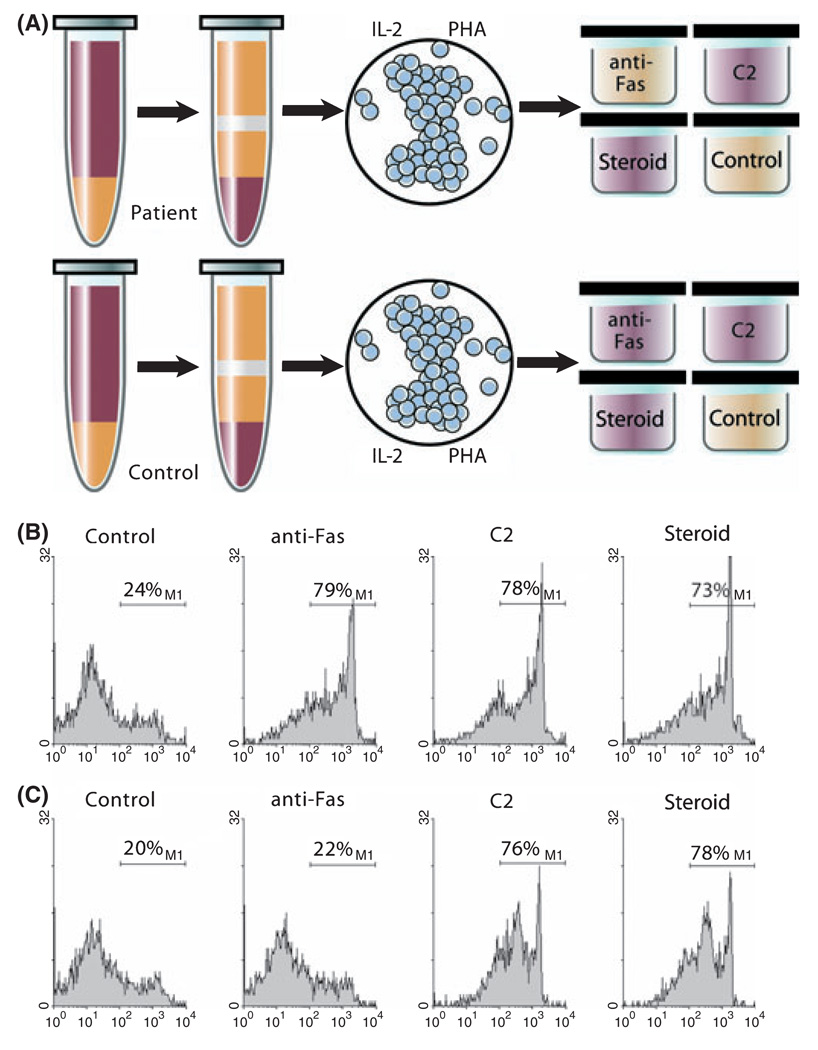
The second laboratory criterion for the diagnosis of ALPS is defective in vitro Fas-mediated apoptosis (Fig 2). Peripheral blood mononuclear cells are isolated from a patient, and T cells are activated with mitogen and expanded with interleukin-2 (IL-2) in culture for approximately 28 d (Fisher et al, 1995). In normal individuals, mitogen activation and expansion results in upregulation and priming of the Fas apoptotic pathway. When normal T cells are then exposed to anti-Fas immunoglobulin M (IgM) monoclonal antibody in vitro, they undergo rapid cell death and apoptosis (Teachey et al, 2005). Because patients with ALPS have a defect in this pathway, their cells do not die after exposure to anti-Fas IgM monoclonal antibody (Sneller et al, 2003). This laboratory test is labour-intensive and expensive to perform, requiring multiple controls, including positive and negative controls from both the patient and a normal control to be analysed in tandem. Accordingly, it is only performed in a handful of research laboratories in the world.
Fig 2.
ALPS apoptosis assay. Peripheral blood mononuclear cells are isolated from a patient, and T cells are activated with mitogen (phytohae-magglutinin, PHA) and expanded with IL-2 in culture for approximately 28 d (Fisher et al, 1995). In normal individuals, mitogen activation and expansion results in upregulation and priming of the Fas apoptotic pathway. When normal T cells are exposed to anti-Fas IgM monoclonal antibody in vitro, they undergo rapid cell death and apoptosis (Teachey et al, 2005). Because patients with ALPS have a defect in this pathway, the cells do not die after exposure to anti-Fas IgM monoclonal antibody (Sneller et al, 2003). Dexamethasone and ceramide (C2) are used as positive controls. (A) Red wells depict cell death after treatment with anti-fas IgM, C2, or dexamethasone. Yellow wells depict lack of cell death with similar treatment. (B) Normal control demonstrating cell death with anti-Fas IgM, ceramide, and dexamethasone as depicted by histogram for 7-Aminoactinomycin D. (C) Prototypical patient with ALPS demonstrating cell death with exposure to ceramide and dexamethasone, but no increased death over baseline with exposure to anti-Fas monoclonal antibody. Data collected under Institutional Review Board approved protocol. © Sue Seif, MA (used with permission).
Because DNTs do not survive in culture, the apoptosis assay only identifies Fas defects in surviving T cells, specifically CD4+ and CD8+ T cells. Thus, only patients with germline mutations will have abnormal apoptosis assays. Recently, a number of patients with ALPS have been demonstrated to have acquired somatic mutations in FAS in the DNT compartment (Holzelova et al, 2004). These patients have elevated DNTs but have normal in vitro apoptosis and do not meet the old diagnostic criteria for ALPS. To establish diagnosis, patients need to have mutations identified in flow cytometry-sorted DNTs as compared to other lymphocyte populations (Holzelova et al, 2004). Only somatic mutations in the FAS gene have been described with the ALPS phenotype. It is hypothetically plausible that somatic mutations in other genes causative for ALPS (CASP10, FASLG) may be involved as well. Finally, patients with germline mutations in FASLG have a normal ‘apoptosis’ assay, as their defect is in the ligand for Fas and is, as such, unaffected by the anti-Fas antibody in the assay.
Other diseases can have defective Fas-mediated apoptosis, including multiple sclerosis, and type I diabetes mellitus (Comi et al, 2000; DeFranco et al, 2001). These disorders typically only have a mild T cell apoptotic defect, usually distinguishable from ALPS by the degree of apoptotic hindrance. Patients with SLE can also have defective Fas-mediated apoptosis (Cheng et al, 1994). Unlike ALPS patients, where the underlying defect is intrinsic to the Fas apoptotic pathway, patients with SLE may have excess soluble Fas in their serum which binds to the Fas receptor, blocking apoptosis (Cheng et al, 1994). Because SLE and ALPS patients can have a similar phenotype, with elevated DNTs, autoantibodies, and defective Fas-mediated apoptosis in vitro, it can be difficult to distinguish the two diseases. As mentioned, patients with ALPS usually have marked elevations in DNTs; whereas patients with SLE have mild elevations. A patient with DNTs >5% is unlikely to have SLE. Patients with mild elevation of DNTs and clinical concern for SLE or ALPS should undergo genetic testing for ALPS-associated mutations.
Other laboratory abnormalities commonly found in ALPS include B and T cell lymphocytosis, positive autoantibodies (DAT, anti-platelet, and anti-neutrophil), positive anti-nuclear antibodies, and polyclonal hypergammaglobulinemia (Bleesing, 2003). Most patients have elevated IgG levels, but they can also have elevations in IgA or IgM. Nevertheless, a small subset of patient with ALPS (<10%) have hypogammaglobulinaemia, and a subset of those patients have common variable immunodeficiency (Jackson et al, 1999; Campagnoli et al, 2006). Patients with ALPS frequently have marked elevated serum levels of IL-10, elevated serum vitamin B12, and elevated serum Fas ligand (Sneller et al, 1997; Bowen et al, 2008; Magerus-Chatinet et al, 2009).
Because patients with ALPS can have massive lymphadenopathy and hepatosplenomegaly, imaging studies are frequently obtained to evaluate for malignancy. Unfortunately, no imaging modality can accurately distinguish benign from malignant lymphoproliferation, including fluorodeoxyglucose positron emission tomography (FDG-PET) because ALPS is FDG-PET avid (Rao et al, 2006).
Histopathology may help distinguish ALPS from malignant diseases, infections, and other lymphoproliferative syndromes. Lymph nodes demonstrate marked paracortical expansion of T cells. Many of these T cells are DNTs, and these are typically negative for CD45RO. Many of the paracortical lymphocytes express perforin, TIA-1, and CD57, and are CD25 negative (Lim et al, 1998). Other features commonly found include follicular hyperplasia, plasmacytosis, and prominent vascularity of the interfollicular areas (Lim et al, 1998; Jackson & Puck, 1999). Frequently, the T cells demonstrate a high proliferation index with a large number of mitoses and elevated expression of Ki-67 (Bleesing et al, 2000).
Genetics
Approximately 70% of patients with ALPS have an identifiable genetic mutation (Table II) (Rieux-Laucat et al, 2003). Almost all of these mutations have been identified in genes associated with the Fas apoptotic pathway. ALPS is classified into sub-types based on underlying genetic mutations. Patients with ALPS, type I, have germline mutations in the gene that encodes Fas protein (FAS; type Ia), the gene that encodes Fas ligand protein (FASLG), or somatic mutations in FAS (type 1s) (Holzelova et al, 2004). Patients with mutations in genes in the caspase family are classified as ALPS, type II (mutations in the gene coding for caspase 10, CASP10) (Rieux-Laucat et al, 2003). Patients with no identifiable genetic mutation (20–30% of patients) are classified as ALPS, type III (Rieux-Laucat et al, 2003).
Table II.
ALPS classification (Bleesing et al, 2000; van der Werff ten Bosch, 2003; Holzelova et al, 2004; Oliveira et al, 2007).
| Type | Mutation |
|---|---|
| 1a | Germ-line in FAS (TNFRSF6) |
| 1b | Germ-line in FASLG |
| 1s | Somatic mutation in FAS |
| II | Germ-line in CASP10 |
| III | No identifiable mutation |
| IV | Germ-line in NRAS |
Mutations in the genes most commonly associated with ALPS (FAS, FASLG and CASP10) are usually inherited in an autosomal dominant fashion; however, cases with mutations in both alleles have been reported (Rieux-Laucat et al, 2003). Frequently, the mutated protein functions as a dominant-negative, inhibiting the function of the wild-type allele (Rieux-Laucat et al, 2003). Most of these genes have variable penetrance. Patients with ALPS will often have family members with the same genetic alterations with no clinical phenotype, a very mild phenotype such as mild splenomegaly or borderline cytopenias, or only with increased rates of malignancy (Straus et al, 1999, 2001). Early data suggested there may be a genotype/phenotype correlation in ALPS patients based on the type of mutation in FAS (Jackson et al, 1999; Rieux-Laucat et al, 1999; Vaishnaw et al, 1999). Mutations in the extracellular domain of FAS were found to be associated with low penetrance and mutations in the death domain were shown to be associated with high penetrance. Nevertheless, more recent data suggests rather that penetrance is not related to the type of mutation and is probably dictated by secondary genetic and environmental modifiers (Fuchs et al, 2009). Based on the inability to predict penetrance based on genetic mutations, genetic counselling is warranted for all unaffected family members who inherit a mutation in FAS.
Patients with mutations in CASP8 were originally considered to have ALPS because caspase 8 and caspase 10 have similar functions in the caspase cascade, and patients with CASP8 mutations present with lymphadenopathy and defective Fas-mediated apoptosis (Chun et al, 2002). Patients with ALPS primarily have apoptotic defects in T lymphocytes, although some patients with ALPS also have mild apoptotic defects in B lymphocytes (Rieux-Laucat et al, 2003). Patients with CASP8 mutations have profound apoptotic defects in B and T lymphocytes, as well as NK cells (Chun et al, 2002). In addition, patients with CASP8 mutations are predisposed to mucocutaneous infections with herpes virus (Chun et al, 2002). Thus, patients with CASP8 mutations are now considered to have a distinct disease.
Until recently, all of the genetic mutations associated with ALPS were found in genes that impair the extrinsic (Fas-mediated) apoptotic pathway. One patient has been described with an ALPS-like syndrome with an associated gain of function mutation in NRAS (Oliveira et al, 2007). This patient had elevated DNTs, normal Fas-mediated apoptosis, but abnormal lymphocyte apoptosis with IL-2 withdrawal in vitro. This is the first report of an ALPS-like phenotype caused by abnormal intrinsic pathway apoptosis.
Differential diagnosis and testing patients with autoimmunity
Patients with ALPS can present with a heterogeneous phenotype, but most frequently have lymphadenopathy, organomegaly, and autoimmunity. This constellation of clinical findings can be found in a number of malignant, infectious, autoimmune, and rheumatological conditions. In addition, patients with other lymphoproliferative disorders, such as Castleman disease, Rosai-Dorfman disease, X-linked lymphoproliferative disease, Dianzani Autoimmune Lymphoproliferative Disease, and Kikuchi-Fujimoto disease can present with clinical features similar to ALPS. At initial presentation, most patients should undergo tissue biopsy (bone marrow and/or lymph node) to distinguish ALPS from malignancy, other lymphoproliferative disorders, and infectious processes. Patients with common variable immunodeficiency (CVID) can also present with lymphadenopathy and autoimmune disease. As a subset of patients with ALPS have co-morbid CVID, distinguishing between the two diseases can be difficult. More specific testing for ALPS, including DNT analysis, apoptosis assays, and genetic testing, can be helpful in distinguishing the conditions.
Evans syndrome, defined by autoimmune destruction of at least two haematological cell types, (Evans et al, 1951) can also have a similar presentation to ALPS. Recently, a small single institution trial showed that a significant percentage of children with Evans syndrome have ALPS (Teachey et al, 2005). This study was confirmed in a multi-institutional paediatric observational study (Seif et al, 2008). Hypergammaglobulinaemia was found to be a strong predictor for ALPS in patients with Evans syndrome and elevated DNTs. Four patients in the study had autoimmunity, markedly elevated DNTs (>5%), and defective in vitro apoptosis without clinically identifiable lymphoproliferation. These reports suggest that ALPS may be more common than previously anticipated, and the threshold for screening for ALPS with DNTs should be low in patients with unexplained autoimmune disease or lymphoproliferation. Arguably, any patient with a combination of autoimmunity and lymphoproliferation should be tested for ALPS. Also, any patient with either multi-lineage autoimmune cytopenias and/or single lineage autoimmune cytopenias and either hypergammaglobulinemia or autoimmune disease of a second organ system should be tested.
Treatment
Some patients with ALPS require no treatment. Many patients, however, require medications primarily directed toward autoimmune manifestations, particularly autoimmune cytopenias. Patients usually respond to short bursts of immunosuppressive medications, including corticosteroids (Bleesing et al, 2000). Occasionally, patients with severe cytopenias need more aggressive immunosuppresion. Unlike many non-ALPS patients who have immune-mediated cytopenias, the cytopenias in patients with ALPS typically do not respond to intravenous immunoglobulin G; however, a small subset of patients with ALPS do respond (Bleesing et al, 2000).
After corticosteroids, the immunosuppressant that is the most studied in ALPS patients is mycophenolate mofetil (MMF, Cellcept). MMF inactivates inosine monophosphate, a key enzyme in purine synthesis, resulting in inhibition of proliferating T and B lymphocytes (Izeradjene et al, 2001; Rao et al, 2005). Over 30 ALPS patients have been treated with MMF with over 80% of patients demonstrating a measurable improvement in autoimmune disease (Rao et al, 2005, 2009a; Kossiva et al, 2006). Nevertheless, these patients did not have improvement in lymphoproliferation or normalization of DNTs. Also, many of these patients had only partial responses. MMF is a well-tolerated medication with relatively few side effects. Patients can develop diarrhoea and cytopenias, mostly neutropenia. Cytopenias are idiosyncratic and thus patients on MMF should be followed closely (Nogueras et al, 2005).
Sirolimus (rapamycin), a mammalian target of rapamycin (mTOR) inhibitor, has also been studied extensively in ALPS. Sirolimus was first isolated from Streptomyces hygroscopicus bacteria in the soil of Easter Island (Rapa Nui). Sirolimus has been in clinical use for over 20 years and its toxicities are well-described; (Hartford & Ratain, 2007) it is approved by the U.S. Federal Drug Administration for use in patients undergoing solid organ transplantation (Abdel-Karim & Giles, 2008). There has been recent interest in mTOR inhibitors in both malignant and non-malignant lymphoid diseases, including leukaemia, rheumatoid arthritis, SLE, and lymphomas (Brown et al, 2003; Fernandez et al, 2006; Teachey et al, 2006a; Smith, 2007; Bruyn et al, 2008). It was hypothesized that mTOR inhibitors would be beneficial in patients with ALPS for three compelling reasons: (i) mTOR inhibitors induce cell death and apoptosis in abnormal lymphocytes; (ii) mTOR inhibitors, unlike most other immunosuppressive medications, increase peripheral blood regulatory T cells (Tregs); and, (iii) mTOR inhibitors are safe and well-tolerated (Teachey et al, 2006b). Tregs are a subset of T lymphocytes that suppress the activation of the immune system, and increasing Treg numbers may improve autoimmune diseases (Brusko et al, 2008).
Sirolimus was extremely effective in mouse models of ALPS, with better efficacy than conventional therapies, including MMF (Teachey et al, 2006b). Sirolimus also had marked activity with documented complete responses in children with refractory autoimmunity and ALPS (Janić et al, 2009; Teachey et al, 2009). Many of these children had failed multiple immunosuppressive medications, including MMF. Patients not only had resolution of autoimmunity, but they also had resolution of lymphadenopathy and splenomegaly. The majority of patients had normalization of DNTs. No other agent used to treat ALPS has been shown to normalize DNTs consistently, including corticosteroids.
Common toxicities found in patients taking sirolimus include hypercholesterolemia, hypertension, and mucositis (Hartford & Ratain, 2007). Sirolimus requires therapeutic drug monitoring to maximize effect and avoid toxicity, and patient compliance is therefore important. Sirolimus is metabolized by cytochrome P450 isoenzyme CYP3A4, and certain drugs and herbal medications (e.g. imidazoles, macrolides, cyclosporine, St. John’s Wort) as well as grapefruit juice can significantly affect serum drug levels (Hartford & Ratain, 2007).
Chronic exposure to any immunosuppressive agent bears a theoretical risk for development of secondary malignancies because the immune system is involved in tumour surveillance. Patients taking immunosuppressive agents after solid organ transplantation have an increased risk of developing secondary lymphoma (Bakker et al, 2007). In vitro data evaluating immunosuppressive drugs for mutagenesis in human lymphocyte cultures demonstrated that tacrolimus and MMF are strongly mutagenic even at low doses; cyclosporine is strongly mutagenic but only at high doses; and sirolimus is not mutagenic unless given at very high concentrations and then is only weakly mutagenic (Oliveira et al, 2004). As patients with ALPS have a high risk of developing secondary malignancies, chronic exposure to mutagens is problematic. Sirolimus is an effective anti-neoplastic agent with efficacy in leukaemia and lymphoma (Brown et al, 2003; Nepomuceno et al, 2003). Recently, a series of patients were described who developed Kaposi sarcoma (KS) while taking MMF or cyclosporine for immune suppression after renal transplant, all of whom achieved complete resolution of their KS by discontinuing these agents and changing to sirolimus (Stallone et al, 2005). Thus, unlike other immunosuppressive agents, which may increase risk of developing secondary cancers, sirolimus may decrease this risk, making it a potentially more attractive immunosuppressive agent for ALPS patients.
Data is limited on the use of other immunosuppressants and chemotherapeutic agents in patients with ALPS. Anecdotal reports and small series have described responses to a number of medications, including cyclosporine, vincristine, mercaptopurine, and methotrexate (Drappa et al, 1996; Sneller et al, 1997; Heelan et al, 2002; Rao et al, 2005; Sobota et al, 2009). Based on these reports, these agents have been recommended as third line in ALPS treatment algorithms (Rao et al, 2009b).
In addition to sirolimus, a number of other targeted therapies are undergoing preclinical testing and clinical trials. Pyrimethamine and sulfadoxine were shown to reduce lymphoproliferation and autoimmune cytopenias in a small series of patients with ALPS; however, this combination failed to show a response in a larger clinical trial (van der Werff Ten Bosch et al, 2002; Rao et al, 2007). Nevertheless, pyrimethamine and sulfadoxine may be useful for some patients. Targeting the Notch signalling pathway was beneficial in preclinical models of ALPS (Teachey et al, 2008). Arsenic and histone deacetylase (HDAC) inhibitors were also effective in preclinical models of ALPS (Bobe et al, 2006; Dowdell et al, 2006). Interestingly, the efficacy of HDAC inhibitors and arsenic in other autoimmune diseases were found to be caused by increasing Tregs(Hernandez-Castro et al, 2009; Saouaf et al, 2009).
Rituximab, an anti-CD20 monoclonal chimeric antibody, is being used increasingly in patients with a number of autoimmune conditions, including ALPS; however, a percentage of ALPS patients are predisposed to develop CVID, and a number of reports have shown that rituximab may increase that risk in ALPS patients (Cooper et al, 2009; Rao et al, 2009b). While rituximab is clearly an effective agent in patients with autoimmune cytopenias, the risk of clinically significant hypogammaglobulinemia may be disporportionately high in patients with underlying immunodeficiency, including ALPS. Thus, until further clinical trials are completed in ALPS patients, its use should be reserved for patients who fail all other therapies.
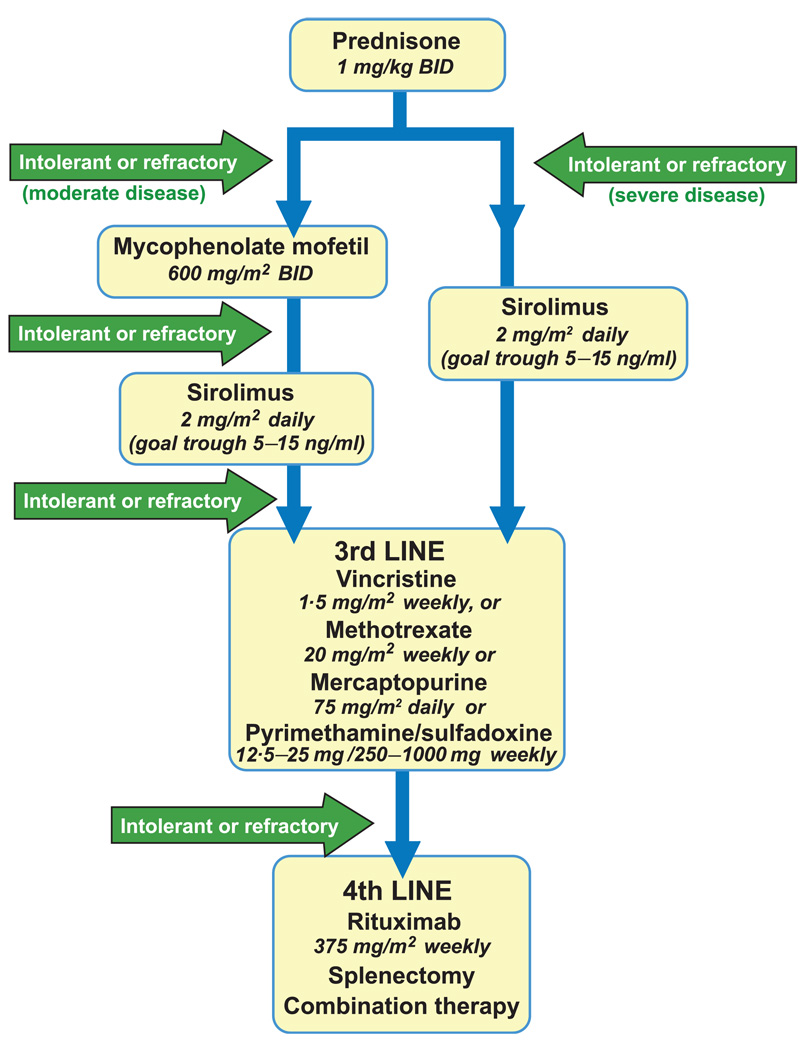
Historically, a significant number of patients with ALPS have undergone splenectomy either to alleviate non-antibody-mediated or antibody-mediated destruction of haematological cell types; however, haematological improvement after splenectomy is variable, and patients with ALPS have an increased risk of developing post-splenectomy sepsis despite vaccination and antibiotic prophylaxis (Bleesing et al, 2000). Thus, except in the case of uncontrollable hypersplenism, splenectomy should be avoided in ALPS patients. Based on all of these data, a novel treatment algorithm for autoimmunity in ALPS patients is presented in Fig 3. This algorithm is very similar to a published algorithm from the NIH-ALPS group, but updated based on the recently published data that demonstrated efficacy of sirolimus in ALPS patients (Teachey et al 2009, Rao et al, 2009b). Doses for the different agents are included in Fig 3.
Fig 3.
Treatment of autoimmune cytopenias in ALPS patients. Front-line therapy for autoimmune cytopenias in patients with ALPS is corticosteroids. For patients with disease refractory to steroids, for patients who cannot tolerate steroids, or for patients who need long-term immunosuppression, alternative immunosuppressive agents are required. Mycophenolate mofetil (MMF) and sirolimus are the most studied and most effective agents in patients with ALPS and autoimmune disease (Rao et al, 2005; Janić et al, 2009; Teachey et al, 2009). For patients with moderate disease, MMF is a well-tolerated medication that should be considered as second-line therapy after steroids. For patients with severe disease, sirolimus may be indicated, as it has established efficacy in MMF-refractory patients (Teachey et al, 2009). Sirolimus should also be considered for patients with moderate disease who fail MMF. Sirolimus requires therapeutic drug monitoring. For patients who are intolerant of or refractory to MMF and/or sirolimus, other agents include vincristine, methotrexate, mercaptopurine and rituximab. Combination therapy with sirolimus and methotrexate or mercaptopurine and methotrexate may be required in the most severe cases.
The role of hematopoietic stem cell transplant in patients with ALPS is not clear. A number of patients with ALPS have been transplanted successfully with both matched and unrelated donors (Sleight et al, 1998; Cohen et al, 2007; Kahwash et al, 2007). Matched related donors should be tested to ensure they do not carry an ALPS-associated mutation. Less toxic reduced-intensity conditioning (RIC) protocols are being used more frequently for autoimmune, rheumatological, and immunodeficiency disorders. As RIC-transplants are safer than traditional myeloablative approaches, stem cell transplantation is now a more attractive option for ALPS patients than it was a few years ago. Nevertheless, as the majority of patients are treatable with single agent therapy and with the development of more effective targeted agents, stem cell transplantation should be reserved for those patients with highly refractory disease.
Redefining the diagnostic criteria for ALPS
In order for a patient to be diagnosed with ALPS, he or she must meet 3 mandatory diagnostic criteria (Table Ia). The current criteria rely heavily on the ‘gold-standard’ in vitro evidence of defective Fas-mediated apoptosis. Unfortunately, this assay does not identify patients with ALPS types 1b or 1s, which may represent a significant number of patients with ALPS (Holzelova et al, 2004). The assay is time-consuming and costly, requiring T cells to be maintained in culture for weeks. The resources required for this test render it unlikely to be routinely available in clinical laboratories; therefore, it remains a ‘research’ test that is only performed in a few highly specialized labs, often requiring patients and treating physicians to wait long periods of time to establish a diagnosis. In addition, there can be false positives with this assay as other diseases that mimic ALPS can have defective Fas-mediated apoptosis (Teachey et al, 2005). Finally, immunosuppressive medications can affect mitogen stimulation of T cells, making results in patients who are on treatment at the time of testing unreliable. While a number of other autoimmune and rheumatological diseases can have mild elevations in DNTs, and these patients should undergo a more through evaluation, marked (>5%) elevations are virtually pathognomonic for ALPS. Thus, patients with a clinical phenotype consistent with ALPS and marked elevations in DNTs may not need specialized apoptosis testing. The current definition does not incorporate available genetic data as diagnostic criteria and excludes other clinical and laboratory features strongly associated with ALPS, including hypergammaglobulinaemia, elevated serum IL-10, elevated serum vitamin B12, and elevated serum Fas ligand (Bowen et al, 2008; Magerus-Chatinet et al, 2009). The current definition also does not include autoimmune disease. Autoimmune cytopenias are a clear predictor of ALPS (Teachey et al, 2005; Seif et al, 2008). Other autoimmune disease may be helpful in making a diagnosis of ALPS; however, this is not established. ALPS can be effectively diagnosed without performing the labour-intensive apoptosis assay in a subset of patients, and the resources required for this assay should be applied towards patients with borderline diagnostic criteria and clinical findings (Magerus-Chatinet et al, 2009).
Based on these concerns with the current diagnostic criteria, a new diagnostic algorithm has been proposed (Table Ib) (Seif et al, 2008). This algorithm expands the current definition of ALPS to allow detection of ALPS types 1s and 1b and permits an experienced physician to make the diagnosis of ALPS in certain patients without the need for ‘research-testing’. The apoptosis assay remains useful for patients with equivocal laboratory findings and a clinical picture consistent with ALPS.
Conclusion
In conclusion, ALPS is a rare syndrome caused by defective lymphocyte apoptosis. Patients can present with a wide range of symptoms, including lymphoproliferation, autoimmunity, and increased propensity to malignancy. The diagnosis should be considered in any child with unexplained lymphadenopathy, organomegaly, or autoimmune cytopenias. Newer treatment modalities targeting the abnormal cells in ALPS patients may lead to a new era of improved disease outcome. These patients need coordinated care from physicians with haematology, immunology, and genetic expertise.
Acknowledgements
This work was supported by grants from the United States Immunodeficiency Network (USIDNET, N01-A1-30070) (DTT), a Foerderer-Murray Award (DTT), the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (DTT), Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award (DTT) and the Sanford Chair and Weinberg Fund of the Children’s Hospital of Philadelphia (SAG).
Footnotes
No author has competing financial interests to declare.
References
- Abdel-Karim IA, Giles FJ. Mammalian target of rapamycin as a target in hematological malignancies. Current Problems in Cancer. 2008;32:161–177. doi: 10.1016/j.currproblcancer.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Auricchio L, Vitiello L, Adriani M, Ferri P, Chiocchetti A, Pettinato G, Racioppi L, Maiuri L, Dianzani U, Pignata C. Cutaneous manifestations as presenting sign of autoimmune lymphoproliferative syndrome in childhood. Dermatology. 2005;210:336–340. doi: 10.1159/000084762. [DOI] [PubMed] [Google Scholar]
- Bakker NA, van Imhoff GW, Verschuuren EA, van Son WJ. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transplant International. 2007;20:207–218. doi: 10.1111/j.1432-2277.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ. Autoimmune lymphoproliferative syndrome: a genetic disorder of abnormal lymphocyte apoptosis. Immunology and Allergy Clinics of North America. 2002;22:339–349. doi: 10.1016/s0031-3955(05)70272-8. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ. Autoimmune lymphoproliferative syndrome (ALPS) Current Pharmaceutical Design. 2003;9:265–278. doi: 10.2174/1381612033392107. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Straus SE, Fleisher TA. Autoimmune lymphoproliferative syndrome. A human disorder of abnormal lymphocyte survival. Pediatric Clinics of North America. 2000;47:1291–1310. doi: 10.1016/s0031-3955(05)70272-8. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Brown MR, Straus SE, Dale JK, Siegel RM, Johnson M, Lenardo MJ, Puck JM, Fleisher TA. Immunophenotypic profiles in families with autoimmune lymphoproliferative syndrome. Blood. 2001a;98:2466–2473. doi: 10.1182/blood.v98.8.2466. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Brown MR, Dale JK, Straus SE, Lenardo MJ, Puck JM, Atkinson TP, Fleisher TA. TcR-alpha/beta(+) CD4(−)CD8(−) T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clinical Immunology. 2001b;100:314–324. doi: 10.1006/clim.2001.5069. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Brown MR, Novicio C, Guarraia D, Dale JK, Straus SE, Fleisher TA. A composite picture of TcR alpha/ beta(+) CD4(−)CD8(−) T Cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clinical Immunology. 2002;104:21–30. doi: 10.1006/clim.2002.5225. [DOI] [PubMed] [Google Scholar]
- Bobe P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. Arsenic trioxide: a promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood. 2006;108:3967–3975. doi: 10.1182/blood-2006-04-020610. [DOI] [PubMed] [Google Scholar]
- Bowen R, Dale J, Brown M, Drake S, Moura T, Dowdell K, Remaley A, Fleisher T, Horton G, Nexo E, Strauss S, Rao VK. Significantly elevated vitamin B12 levels in autoimmune lymphoproliferative syndrome (ALPS), a rare lymphoproliferative disorder with apoptosis defect. Blood. 2008;112:4898. (ASH Annual Meeting Abstracts) [Google Scholar]
- Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunological Reviews. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Bruyn GA, Tate G, Caeiro F, Maldonado-Cocco J, Westhovens R, Tannenbaum H, Bell M, Forre O, Bjorneboe O, Tak PP, Abeywickrama KH, Bernhardt P, van Riel PL. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebocontrolled, parallel-group, proof-of-concept study. Annals of the Rheumatic Diseases. 2008;67:1090–1095. doi: 10.1136/ard.2007.078808. [DOI] [PubMed] [Google Scholar]
- Campagnoli MF, Garbarini L, Quarello P, Garelli E, Carando A, Baravalle V, Doria A, Biava A, Chiocchetti A, Rosolen A, Dufour C, Dianzani U, Ramenghi U. The broad spectrum of autoimmunelymphoproliferative disease:molecular bases, clinical features and long-term follow-up in 31 patients. Haematologica. 2006;91:538–541. [PubMed] [Google Scholar]
- Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Research & Therapy. 2005;7:208–214. doi: 10.1186/ar1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Sebire NJ, Harvey J, Gaspar HB, Cathy C, Jones A, Rao K, Cubitt D, Amrolia PJ, Davies EG, Veys P. Successful treatment of lymphoproliferative disease complicating primary immunodeficiency/immunodysregulatory disorders with reduced-intensity allogeneic stem-cell transplantation. Blood. 2007;110:2209–2214. doi: 10.1182/blood-2006-12-062174. [DOI] [PubMed] [Google Scholar]
- Comi C, Leone M, Bonissoni S, DeFranco S, Bottarel F, Mezzatesta C, Chiocchetti A, Perla F, Monaco F, Dianzani U. Defective T cell fas function in patients with multiple sclerosis. Neurology. 2000;55:921–927. doi: 10.1212/wnl.55.7.921. [DOI] [PubMed] [Google Scholar]
- Cooper N, Davies EG, Thrasher AJ. Repeated courses of rituximab for autoimmune cytopenias may precipitate profound hypogammaglobulinaemia requiring replacement intravenous immunoglobulin. British Journal of Haematology. 2009;146:120–122. doi: 10.1111/j.1365-2141.2009.07715.x. [DOI] [PubMed] [Google Scholar]
- Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4− CD8− (double negative) T cells in patients with systemic lupus erythematosus: production of IL-4. Lupus. 2002;11:501–507. doi: 10.1191/0961203302lu234oa. [DOI] [PubMed] [Google Scholar]
- DeFranco S, Bonissoni S, Cerutti F, Bona G, Bottarel F, Cadario F, Brusco A, Loffredo G, Rabbone I, Corrias A, Pignata C, Ramenghi U, Dianzani U. Defective function of Fas in patients with type 1 diabetes associated with other autoimmune diseases. Diabetes. 2001;50:483–488. doi: 10.2337/diabetes.50.3.483. [DOI] [PubMed] [Google Scholar]
- Dianzani U, Bragardo M, DiFranco D, Alliaudi C, Scagni P, Buonfiglio D, Redoglia V, Bonissoni S, Correra A, Dianzani I, Ramenghi U. Deficiency of the Fas apoptosis pathway without Fas gene mutations in pediatric patients with autoimmu-nity/lymphoproliferation. Blood. 1997;89:2871–2879. [PubMed] [Google Scholar]
- Dowdell KC, Pesnicak L, Hoffmann V, Steadman K, M R, Rao VK, Straus SE. Valproic acid (VPA), a histone deacetylase (HDAC) inhibitor, diminishes lymphoproliferation in the Fas deficient MRL/lpr−/− murine model of autoimmune lymphoproliferative syndrome (ALPS) Blood (ASH Annual Meeting Abstracts) 2006;108:2497. doi: 10.1016/j.exphem.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. New England Journal of Medicine. 1996;335:1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- Evans RS, Takahashi K, Duane RT, Payne R, Liu C. Primary thrombocytopenic purpura and acquired hemolytic anemia; evidence for a common etiology. AMA Archives of Internal Medicine. 1951;87:48–65. doi: 10.1001/archinte.1951.03810010058005. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8− double-negative regulatory T cells. Blood. 2005;105:2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Fuchs H, Posovszky C, Lahr G, van der Werff ten Bosch J, Boehler T, Debatin KM. Residual CD95-pathway function in children with autoimmune lymphoproliferative syndrome is independent from clinical state and genotype of CD95 mutation. Pediatric Research. 2009;65:163–168. doi: 10.1203/PDR.0b013e318191f7e4. [DOI] [PubMed] [Google Scholar]
- Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clinical Pharmacology and Therapeutics. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- Heelan BT, Tormey V, Amlot P, Payne E, Mehta A, Webster AD. Effect of anti-CD20 (rituximab) on resistant thrombocytopenia in autoimmune lymphoproliferative syndrome. British Journal of Haematology. 2002;118:1078–1081. doi: 10.1046/j.1365-2141.2002.03753.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Castro B, Doniz-Padilla LM, Salgado-Bustamante M, Rocha D, Ortiz-Perez MD, Jimenez-Capdeville ME, Portales-Perez DP, Quintanar-Stephano A, Gonzalez-Amaro R. Effect of arsenic on regulatory T cells. Journal of Clinical Immunology. 2009;29:461–469. doi: 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- Holzelova E, Vonarbourg C, Stolzenberg MC, Arkwright PD, Selz F, Prieur AM, Blanche S, Bartunkova J, Vilmer E, Fischer A, Le Deist F, Rieux-Laucat F. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. New England Journal of Medicine. 2004;351:1409–1418. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- Izeradjene K, Quemeneur L, Michallet MC, Bonnefoy-Berard N, Revillard JP. Mycophenolate mofetil interferes with interferon gamma production in T-cell activation models. Transplantation Proceedings. 2001;33:2110–2111. doi: 10.1016/s0041-1345(01)01965-0. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Puck JM. Autoimmune lymphoproliferative syndrome, a disorder of apoptosis. Current Opinion in Pediatrics. 1999;11:521–527. doi: 10.1097/00008480-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, Dale JK, Fleisher TA, Middelton LA, Sneller MC, Lenardo MJ, Straus SE, Puck JM. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. American Journal of Human Genetics. 1999;64:1002–1014. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janić MD, Brasanac CD, Janković JS, Dokmanović BL, Krstovski RN, Kragulijac Kurtović JN. Rapid regression of lymphadenopathy upon rapamycin treatment in a child with autoimmune lymphoproliferative syndrome. Pediatric Blood & Cancer. 2009;53:1117–1119. doi: 10.1002/pbc.22151. [DOI] [PubMed] [Google Scholar]
- Kahwash SB, Fung B, Savelli S, Bleesing JJ, Qualman SJ. Autoimmune lymphoproliferative syndrome (ALPS): a case with congenital onset. Pediatric and Developmental Pathology. 2007;10:315–319. doi: 10.2350/06-06-0105.1. [DOI] [PubMed] [Google Scholar]
- Kossiva L, Theodoridou M, Mostrou G, Vrachnou E, Le Deist F, Rieux-Laucat F, Kanariou MG. Mycophenolate mofetil as an alternate immunosuppressor for autoimmune lymphoproliferative syndrome. Journal of Pediatric Hematology/Oncology. 2006;28:824–826. doi: 10.1097/MPH.0b013e31802d7503. [DOI] [PubMed] [Google Scholar]
- Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, Sneller MC, Puck JM, Lenardo MJ, Elenitoba-Johnson KS, Lin AY, Raffeld M, Jaffe ES. Pathological findings in human autoimmune lymphoproliferative syndrome. American Journal of Pathology. 1998;153:1541–1550. doi: 10.1016/S0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, Arkwright PD, Bader-Meunier B, Barbot J, Blanche S, Casanova JL, Debre M, Ferster A, Fieschi C, Florkin B, Galambrun C, Hermine O, Lambotte O, Solary E, Thomas C, Le Deist F, Picard C, Fischer A, Rieux-Laucat F. FAS-L, IL-10, and double-negative CD4- CD8- TCR alpha/ beta+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–3030. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- Marlies A, Udo G, Juergen B, Bernd S, Herrmann M, Haas JP. The expanded double negative T cell populations of a patient with ALPS are not clonally related to CD4+ or to CD8+ T cells. Autoimmunity. 2007;40:299–301. doi: 10.1080/08916930701356473. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Research. 2003;63:4472–4480. [PubMed] [Google Scholar]
- Nogueras F, Espinosa MD, Mansilla A, Torres JT, Cabrera MA, Martin-Vivaldi R. Mycophenolate mofetil-induced neutropenia in liver transplantation. Transplantation Proceedings. 2005;37:1509–1511. doi: 10.1016/j.transproceed.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Ohga S, Nomura A, Takahata Y, Ihara K, Takada H, Wakiguchi H, Kudo Y, Hara T. Dominant expression of interleukin 10 but not interferon gamma in CD4(−)CD8(−)alphabetaT cells of autoimmune lymphoproliferative syndrome. British Journal of Haematology. 2002;119:535–538. doi: 10.1046/j.1365-2141.2002.03848.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, Danner RL, Barb J, Munson PJ, Puck JM, Dale J, Straus SE, Fleisher TA, Lenardo MJ. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira VD, Zankl H, Rath T. Mutagenic and cytotoxic effects of immunosuppressive drugs on human lymphocyte cultures. Experimental and Clinical Transplantation. 2004;2:273–279. [PubMed] [Google Scholar]
- Poppema S, Maggio E, van den Berg A. Development of lymphoma in Autoimmune Lymphoproliferative Syndrome (ALPS) and its relationship to Fas gene mutations. Leukaemia & Lymphoma. 2004;45:423–431. doi: 10.1080/10428190310001593166. [DOI] [PubMed] [Google Scholar]
- Rao VK, Carrasquillo JA, Dale JK, Bacharach SL, Whatley M, Dugan F, Tretler J, Fleisher T, Puck JM, Wilson W, Jaffe ES, Avila N, Chen CC, Straus SE. Fluorodeoxyglucose positron emission tomography (FDG-PET) for monitoring lymphadenopathy in the autoimmune lymphoproliferative syndrome (ALPS) American Journal of Hematology. 2006;81:81–85. doi: 10.1002/ajh.20523. [DOI] [PubMed] [Google Scholar]
- Rao VK, Dugan F, Dale JK, Davis J, Tretler J, Hurley JK, Fleisher T, Puck J, Straus SE. Use of mycophenolate mofetil for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome. British Journal of Haematology. 2005;129:534–538. doi: 10.1111/j.1365-2141.2005.05496.x. [DOI] [PubMed] [Google Scholar]
- Rao VK, Dowdell KC, Dale JK, Dugan F, Pesnicak L, Bi LL, Hoffmann V, Penzak S, Avila NA, Fleisher TA, Puck JM, Straus SE. Pyrimethamine treatment does not ameliorate lymphoproliferation or autoimmune disease in MRL/lpr−/− mice or in patients with autoimmune lymphoproliferative syndrome. American Journal of Hematology. 2007;82:1049–1055. doi: 10.1002/ajh.21007. [DOI] [PubMed] [Google Scholar]
- Rao VK, Price S, Perkins K, Aldridge P, Tretler J, Davis J, Dowdell K, Niemela JE, Brown M, Fleisher T. Use of mycophenolate mofetil in children with chronic, refractory immune cytopenias associated with autoimmune lymphoproliferative syndrome (ALPS) Pediatric Blood & Cancer. 2009a;52:697. doi: 10.1002/pbc.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VK, Price S, Perkins K, Aldridge P, Tretler J, Davis J, Dale JK, Gill F, Hartman KR, Stork LC, Gnarra DJ, Krishnamurti L, Newburger PE, Puck J, Fleisher T. Use of rituximab for refractory cytopenias associated with autoimmune lymphoproliferative syndrome (ALPS) Pediatric Blood & Cancer. 2009b;52:847–852. doi: 10.1002/pbc.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux-Laucat F, Blachere S, Danielan S, De Villartay JP, Oleastro M, Solary E, Bader-Meunier B, Arkwright P, Pondare C, Bernaudin F, Chapel H, Nielsen S, Berrah M, Fischer A, Le Deist F. Lymphoproliferative syndrome with autoimmunity: a possible genetic basis for dominant expression of the clinical manifestations. Blood. 1999;94:2575–2582. [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Fischer A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death and Differentiation. 2003;10:124–133. doi: 10.1038/sj.cdd.4401190. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Saouaf SJ, Li B, Zhang G, Shen Y, Furuuchi N, Hancock WW, Greene MI. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Experimental and Molecular Pathology. 2009;87:99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif A, Manno C, Grupp SA, Teachey DT. Testing patients with Evans syndrome for the Autoimmune Lymphoproliferative Syndrome (ALPS): results of a large multi-institutional clinical trial (ASPHO supplement) Pediatric Blood & Cancer. 2008;50:S22–S23. [Google Scholar]
- Sleight BJ, Prasad VS, DeLaat C, Steele P, Ballard E, Arceci RJ, Sidman CL. Correction of autoimmune lymphoproliferative syndrome by bone marrow transplantation. Bone Marrow Transplantation. 1998;22:375–380. doi: 10.1038/sj.bmt.1701306. [DOI] [PubMed] [Google Scholar]
- Smith SM. Clinical development of mTOR inhibitors: a focus on lymphoma. Reviews on Recent Clinical Trials. 2007;2:103–110. doi: 10.2174/157488707780599366. [DOI] [PubMed] [Google Scholar]
- Sneller MC, Straus SE, Jaffe ES, Jaffe JS, Fleisher TA, Stetler-Stevenson M, Strober W. A novel lymphoproliferative/ autoimmune syndrome resembling murine lpr/gld disease. Journal of Clinical Investigation. 1992;90:334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, Lim MS, Jaffe ES, Puck JM, Lenardo MJ, Straus SE. Clincial, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- Sneller MC, Dale JK, Straus SE. Autoimmune lymphoproliferative syndrome. Current Opinion in Rheumatology. 2003;15:417–421. doi: 10.1097/00002281-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Sobota A, Neufeld EJ, Lapsia S, Bennett CM. Response to mercaptopurine for refractory autoimmune cytopenias in children. Pediatric Blood & Cancer. 2009;52:80–84. doi: 10.1002/pbc.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. New England Journal of Medicine. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Straus SE, Sneller M, Lenardo MJ, Puck JM, Strober W. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Annals of Internal Medicine. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, Fischer RE, Jackson CM, Lin AY, Baumler C, Siegert E, Marx A, Vaishnaw AK, Grodzicky T, Fleisher TA, Lenardo MJ. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, McMann JM, Sullivan KE, Travis SF, Grupp SA. Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS) Blood. 2005;105:2443–2448. doi: 10.1182/blood-2004-09-3542. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, Houghton PJ, Brown VI, Grupp SA. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006a;107:1149–1155. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2006b;108:1965–1971. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, Choi JK, Fish JD, Hall J, Reid GS, Ryan T, Sheen C, Zweidler-McKay P, Grupp SA. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111:705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, Manno C, Rappaport E, Schwabe D, Sheen C, Sullivan KE, Zhuang H, Wechsler DS, Grupp SA. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. British Journal of Haematology. 2009;145:101–106. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnaw AK, Orlinick JR, Chu JL, Krammer PH, Chao MV, Elkon KB. The molecular basis for apoptotic defects in patients with CD95 (Fas/Apo-1) mutations. Journal of Clinical Investigation. 1999;103:355–363. doi: 10.1172/JCI5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff ten Bosch J. Autoimmune lymphoproliferative syndrome: etiology, diagnosis, and management. Paediatric Drugs. 2003;5:185–193. doi: 10.2165/00128072-200305030-00005. [DOI] [PubMed] [Google Scholar]
- van der Werff Ten Bosch J, Schotte P, Ferster A, Azzi N, Boehler T, Laurey G, Arola M, Demanet C, Beyaert R, Thielemans K, Otten J. Reversion of autoimmune lymphoproliferative syndrome with an antimalarial drug: preliminary results of a clinical cohort study and molecular observations. British Journal of Haematology. 2002;117:176–188. doi: 10.1046/j.1365-2141.2002.03357.x. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wei A, Cowie T. Rituximab responsive immune thrombocytopenic purpura in an adult with underlying autoimmune lymphoproliferative syndrome due to a splice-site mutation (IVS7+2 T>C) affecting the Fas gene. European Journal of Haematology. 2007;79:363–366. doi: 10.1111/j.1600-0609.2007.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. British Journal of Haematology. 2006;133:124–140. doi: 10.1111/j.1365-2141.2006.05993.x. [DOI] [PubMed] [Google Scholar]