Abstract
Gene therapy of human cancer using genetically engineered lymphocytes is dependent on the identification of highly reactive T-cell receptors (TCRs) with antitumor activity. We immunized transgenic mice and also conducted high-throughput screening of human lymphocytes to generate TCRs highly reactive to melanoma/melanocyte antigens. Genes encoding these TCRs were engineered into retroviral vectors and used to transduce autologous peripheral lymphocytes administered to 36 patients with metastatic melanoma. Transduced patient lymphocytes were CD45RA− and CD45RO+ after ex vivo expansion. After infusion, the persisting cells displayed a CD45RA+ and CD45RO− phenotype. Gene-engineered cells persisted at high levels in the blood of all patients 1 month after treatment, responding patients with higher ex vivo antitumor reactivity than nonresponders. Objective cancer regressions were seen in 30% and 19% of patients who received the human or mouse TCR, respectively. However, patients exhibited destruction of normal melanocytes in the skin, eye, and ear, and sometimes required local steroid administration to treat uveitis and hearing loss. Thus, T cells expressing highly reactive TCRs mediate cancer regression in humans and target rare cognate–antigen-containing cells throughout the body, a finding with important implications for the gene therapy of cancer. This trial was registered at www.ClinicalTrials.gov as NCI-07-C-0174 and NCI-07-C-0175.
Introduction
Tumor-associated antigens have been identified on a wide variety of human cancers. Many of these antigens are normal, nonmutated self-proteins selectively expressed or overexpressed on cancers.1 Antigens such as MART-1 and gp100 are expressed on melanomas and normal melanocytes in the skin, eye, and ear.2,3 Other cancer-associated antigens such as carcinoembryonic antigen (CEA), Her2/neu, and Muc-1 are expressed at low levels on some normal tissues, whereas antigens such as NY-ESO-1 and the MAGE family of proteins are expressed on fetal tissue and the adult testes but not on other normal adult tissues.4 The presence of these normal proteins during fetal development leads to central tolerance based on negative selection in the thymus of lymphocyte clones bearing high-affinity self-reactive T-cell receptors (TCRs). Occasionally, lymphocytes bearing high-affinity TCR escape thymic deletion; and in these instances, mechanisms of peripheral tolerance can suppress their activity.5
Cell transfer therapies have emerged as a tool to overcome the limitations imposed by both central and peripheral tolerance.6–9 Transfer of antitumor T cells to lymphodepleted mice can mediate the rejection of large, vascularized tumors,10 and the administration of naturally occurring antimelanoma tumor-infiltrating lymphocytes (TILs) can mediate objective cancer regressions in 51% to 72% of lymphodepleted patients with metastatic melanoma.7,8,11
A major obstacle to the widespread application of cell transfer therapies is the difficulty in identifying human T cells with antitumor recognition. Only approximately half of melanomas reproducibly give rise to antitumor TILs,12 and other cancer types only rarely contain identifiable tumor-reactive lymphocytes. An alternative to finding these natural tumor-reactive cells for every patient is the transfer to normal lymphocytes of tumor-reactive TCR genes recognizing shared tumor antigens.
In a prior study, we identified a TCR (MSGV1AIB, here referred to as DMF4) that recognized the MART-1 melanoma/melanocyte antigen cloned from the TILs of a resected melanoma lesion.13 We treated 31 patients with autologous peripheral blood lymphocytes (PBLs) transduced with genes encoding this receptor (17 were previously reported),14 and 4 patients (13%) experienced an objective regression of metastatic melanoma. None of the 31 patients exhibited skin rash or normal melanocyte toxicity in the eye or ear. The DMF4 receptor had only moderate ability to recognize limiting amounts of antigen, and we hypothesized that a more highly reactive TCR might be clinically more effective in recognizing malignant cells that expressed the target antigen.
We thus generated a high-avidity TCR from a human T cell that recognized the MART-1:27-35 epitope (here referred to as DMF5).15 The gp100:154-162 epitope from the gp100 melanoma-melanocyte antigen is the most highly expressed peptide from this protein, displayed on the cell surface. Attempts to generate a high-avidity human TCR against this epitope were unsuccessful. We were able, however, to generate a highly avid TCR against this epitope in human leukocyte antigen-A2 (HLA-A2) transgenic mice. These anti–MART-1 and anti-gp100 TCRs were used to treat patients with metastatic melanoma.
Methods
Patients
Thirty-six patients with metastatic melanoma were treated at the Surgery Branch, National Cancer Institute, between July 2007 and March 2008, in protocols approved by the Institutional Review Board and Food and Drug Administration, 20 with TCR recognizing the HLA-A*02–restricted melanoma antigen MART-1 (DMF5)15 and 16 with TCR recognizing the HLA-A*02–restricted melanoma antigen gp100(154).16 All patients gave informed consent for treatment in accordance with the Declaration of Helsinki. Patients were HLA-A*02+, 18 years of age or older, had measurable metastatic melanoma, and Eastern Cooperative Oncology Group status 0 or 1. All patients had progressed after prior treatment with interleukin-2 (IL-2)/Aldesleukin and had tumors that expressed the appropriate antigen (MART-1/Melan-A or gp100/HMB45). Contraindications were as follows: concurrent major medical illnesses, any form of primary or secondary immunodeficiency, severe hypersensitivity to any of the agents used in this study, contraindications for high-dose IL-2 administration, systemic steroid treatment within 30 days before treatment, and untreated intracranial metastases more than 1.0 cm in diameter.
TCR recognizing shared melanoma antigens
The generation of the DMF5 TCR has been previously described.15 The patient from whom the MART-1–reactive TCR was derived experienced vitiligo but no eye or ear toxicity. To generate a high-avidity TCR against the gp100:154-162 epitope, HLA-A*0201 mice were immunized twice with the human gp100:154-162 peptide and the I-Ab-binding synthetic T helper peptide representing residues 128 to 140 of the hepatitis B virus core protein emulsified in incomplete Freund adjuvant. Seven days after the second immunization, splenocytes were stimulated in vitro with equal numbers of irradiated, lipopolysaccharide-activated, A2.1 transgenic mouse splenocytes pulsed with 0.01 μg/mL human gp100:154-162 peptide and 10 μg/mL human β2-microglobulin in media containing 5 IU IL-2. Eight days after a third stimulation, T cells were cloned by limiting dilution in presence of irradiated T2 cells pulsed with 0.01 μg/mL human gp100:154-162 peptide and irradiated C57Bl/6 splenocytes. 5′ RACE TCR isolation and RNA electroporation into donor PBLs were as previously described.15 The gp100(154) TCR conferring the highest antitumor avidity to donor PBLs functioned independently of CD4 or CD8 coreceptor and was selected for clinical use.
Retroviral gene therapy vectors
pMSGV1 is derived from pMSGV murine stem cell virus long terminal repeat containing an extended gag region and Kozak sequence.13 Vector pMSGV1 gp100(154)-AIB was produced by linking the TCR-α via an internal ribosome entry site (IRES) element followed by insertion of the TCR-β chain. Vector pMSGV DMF5 furin 2-A was generated by introducing DMF5 TCR-α cDNA15 followed by a furin T2A cleavage sequence and DMF5 TCR-β. Clinical grade cGMP-quality retroviral supernatants were produced by the National Gene Vector Laboratories at Indiana University. Patient PBLs were stimulated with anti-CD3 monoclonal antibody (mAb) OKT-3 2 days before transduction using retronectin (Takara Bio Inc)–coated plates, per the manufacturer's recommendations.
Preparation of PBLs transduced with TCR
A total of 5 to 10 × 108 patient PBLs were obtained by leukapheresis and stimulated in vitro at 106/mL with 50 ng/mL anti-CD3 mAb OKT-3, in complete AIMV media (Invitrogen) supplemented with 5% human serum (Surgery Branch, National Cancer Institute), and 300 IU IL-2 (TCR media). Two days later, TCR-encoding retroviral supernatant was rapid-thawed, diluted 1:1 in TCR media, and added to plates that had been coated overnight with 10 μg/mL retronectin (Takara Bio Inc). Supernatant was spin-loaded onto plates by centrifuging 2 hours at 2000g at 32°C. The stimulated PBLs were washed and resuspended at 0.25 to 0.5 × 106/mL in TCR media, and 1 to 2 × 106 PBLs were added per well to the retrovirus-loaded plates. Plates were spun at 1000g at 32°C for 10 minutes and incubated overnight at 37°C, 5% CO2. The next day (day 3), PBLs were transferred to newly prepared retroviral-coated 6-well plates as on day 2. The following day, transduced PBLs were washed, resuspended in fresh TCR media, and transferred to flasks at 37°C, 5% CO2. On days 9 to 12, cells were expanded or not an additional 9 to 14 days in 6000 IU IL-2 with 50 ng/mL anti-CD3 mAb OKT-3 and 100-fold excess 5 Gy irradiated allogeneic PBL feeder cells. Before treatment, TCR-transduced PBLs from all patients were evaluated for expression of the appropriate TCR by tetramer staining and flow cytometric analysis, and cell function was evaluated by overnight coculture with cognate antigen-bearing target cells (1 × 105:1 × 105) and enzyme-linked immunosorbent assay (ELISA) measurement (Pierce Endogen) of interferon-γ (IFN-γ) produced in the culture supernatant. Treatment cells were washed in saline before infusion into patients intravenously.
Clinical protocol
The clinical trial registration numbers and approved registry names are as follows: NCI-07-C-0175, Phase II Study of Metastatic Melanoma Using Lymphodepleting Conditioning Followed by Infusion of Anti-MART-1 F5 TCR-Gene Engineered Lymphocytes; NCI-07-C-0174, Phase II Study of Metastatic Melanoma Using Lymphodepleting Conditioning Followed by Infusion of Anti-gp100:154-162 TCR-Gene Engineered Lymphocytes.
Before receiving treatment with transduced PBLs, patients were transiently lymphoablated using a nonmyeloablative lymphodepleting regimen as previously described,8 by intravenous administration of cyclophosphamide 60 mg/kg for 2 days followed by fludarabine 25 mg/m2 for 5 days. One day after completion of their lymphodepleting regimen, patients received transduced lymphocytes infused intravenously followed by high-dose (720 000 U/kg) IL-2 (Aldesleukin; Chiron Corp) every 8 hours to tolerance. Five DMF5 patients (patients 7-11), and 4 gp100(154) patients (patients 7-10) received TCR-transduced cells on days 10 to 12 after stimulation. The remaining 15 DMF5 patients and 12 gp100(154) patients received a larger number of TCR-transduced cells, which were grown for an additional 9 to 14 days after a second OKT-3 stimulation.
Patients received baseline computed tomography (CT) and/or magnetic resonance imaging before treatment and underwent pretreatment and posttreatment ocular and audiology examinations. Tumor size was evaluated monthly by CT, magnetic resonance imaging, or documented with photography for cutaneous/subcutaneous lesions. Tumor measurements and patient responses were determined according to Response Evaluation Criteria in Solid Tumors (RECIST).17 Samples of patient PBLs and serum were taken after transduced PBL infusion. Serum cytokine levels were measured by ELISA assay (Pierce Endogen).
Evaluation of cell activity and persistence
Skin and tumor biopsies were obtained and immunohistochemically stained for the presence of CD4 and CD8 T cells. In one patient with uveitis, ocular fluid was evaluated for the presence of transduced antitumor T cells by flow cytometry. Cell activity was evaluated by coculturing patient PBLs with cognate antigen on T2 target cells, or HLA-matched and mismatched melanomas mel526, mel624 (HLA-A*02+), or mel888 and mel938 (non-HLA-A*02). IL-2 and IFN-γ were measured by ELISA (Pierce Endogen), ELISPOT (reagents from Mabtech Inc, Millipore Corp, Invitrogen, BD Biosciences PharMingen, and Kirkegaard & Perry), or intracellular staining (mAbs from eBiosciences) and flow cytometry. Lysis was evaluated by target cell 51chromium-release assay. Transferred cell persistence in blood was followed by tetramer staining with HLA-A2/MART-1:27-35 or HLA-A2/gp100:154-162 tetramer (Beckman Coulter Immunotech).
Statistical significance was evaluated using the paired t test.
Results
DMF5 and gp100(154) are highly reactive TCRs that confer melanoma tumor reactivity to donor PBLs
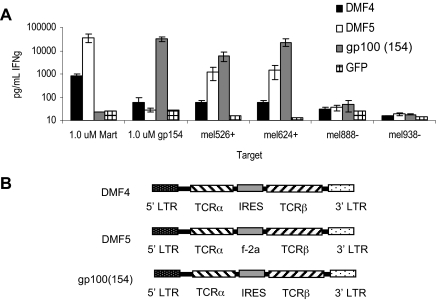
We have used 2 highly reactive TCRs capable of recognizing the MART-1 or gp100 melanocyte antigens overexpressed on melanomas. To overcome the problem of central deletional tolerance of lymphocytes expressing high-affinity antitumor TCRs, we raised a highly reactive TCR called gp100(154) against the human melanocyte gp100:154-162 epitope by immunizing HLA-A*0201 transgenic mice with this peptide that differs from the mouse sequence by a single amino acid. In addition, extensive screening of more than 600 clones in TILs from multiple patients revealed a lymphocyte clone called DMF5 with far greater reactivity than the previously identified DMF4 against the MART-1:27-35 peptide epitope.15 The TCR genes from the mouse cells and from the human DMF5 clone were isolated, and equivalent amounts of RNA were generated in vitro and transferred into both Jurkat and donor PBLs. After transferring the same amount of exogenous TCR (evaluated by CD3 surface expression in Jurkat cells),15 the higher-avidity DMF5 and gp100(154) TCR conferred higher reactivity to donor PBLs than the previously identified DMF4 in recognizing tumor antigen (Figure 1A). The genes encoding the alpha and beta chains from these 2 receptors, gp100(154) and DMF5, were each cloned into bicistronic gamma-retroviral vectors using an IRES or a furin 2-A picornavirus-like cleavage sequence, respectively, to drive expression of the second gene (Figure 1B). These retroviral vectors were used to transduce normal human peripheral lymphocytes (Figure 2). It has been demonstrated that DMF4 binds tetramer weakly, underrepresenting the amount of surface TCR,14,15 a result also observed here (Figure 2A). Using similarly prepared retroviral supernatants in the same donor cell transduction procedure, DMF4 on the surface of transduced CD8+ PBLs only bound 2% of MART-1 tetramer and transduced CD4+ cells bound no tetramer. In contrast, 30% to 60% of both CD4+ and CD8+ lymphocytes transduced with the improved gene constructs encoding the highly reactive DMF5 or gp100(154) TCR bound tetramer efficiently (Figure 2A). These new TCR constructs conferred high expression of TCR, which was coreceptor independent on donor PBLs. A comparison of the functional reactivity of donor lymphocytes transduced with the respective gamma-retroviruses encoding these DMF4, DMF5, or gp100(154) TCR are shown in Figure 2B and C. Cells expressing either the highly reactive DMF5 or gp100(154) TCR recognized 100-fold lower peptide concentrations, produced more IFN-γ, and lysed melanoma targets more effectively than those expressing DMF4.
Figure 1.
Tumor-reactive DMF5 or gp100(154) alpha and beta TCR chain RNA electroporated into PBLs confer high reactivity to melanoma tumor antigens. (A) Ten-day anti-CD3–stimulated donor PBLs were electroporated with in vitro–transcribed RNA encoding paired DMF4, DMF5, or gp100(154) TCR alpha and beta chains, or GFP control. Cells were cocultured for 18 hours with T2 cells pulsed with peptide, HLA-A*02+ melanomas mel624+ or mel526+, or HLA-A*02− melanomas mel888− or mel938−. IFN-γ in the supernatant was detected by ELISA. (B) Structure of the MSGV-based γ-retroviral vectors DMF4 and gp100(154), incorporating an IRES and DMF5 with a furin 2A ribosomal skip sequence, allowing for dual gene expression.
Figure 2.
DMF5 and gp100(154) TCR retroviral constructs conferred greater antitumor reactivity to donor PBLs than the original DMF4 receptor. (A) Donor PBLs were stimulated with anti-CD3 mAb OKT-3 and separated into CD4 and CD8 populations before retroviral transduction with DMF4, DMF5, or gp100(154) TCR constructs. TCR expression was analyzed 7 days later by tetramer staining and flow cytometry. (B) Donor PBLs transduced with retroviral TCR constructs were cocultured with T2 cells pulsed with MART-1:27-35 or gp100:154-162 peptide, and IFN-γ secretion was measured by ELISA. (C) Transduced PBLs were cocultured with mel624+ melanomas, and tumor target lysis was evaluated by 51Cr-release assay. Cells did not lyse HLA-mismatched tumors (data not shown).
Administration of TCR-transduced autologous PBLs to patients with metastatic melanoma
To investigate the in vivo activity of autologous cells transduced with these highly reactive TCRs, 36 patients with heavily pretreated, progressive metastatic melanoma received transduced cells (20 patients with DMF5 and 16 with gp100(154)), after a lymphodepleting preparative regimen to deplete endogenous circulating lymphocytes.8 Patient demographics and treatment details are presented in Tables 1 and 2. Intravenous IL-2 was administered to patients starting 8 hours after adoptive cell transfer and continued every 8 hours for up to 3 days. All of the patients were refractory to prior treatment with IL-2, and 42% and 33% had progressed through prior chemotherapy and radiotherapy, respectively.
Table 1.
DMF5 patient cell treatment characteristics
| Patient no. | Age, y/sex | Prior treatment* | Sites of disease† | No. of cells × 109 | Percentage CD4/8 | Percentage Tet | Percentage IC IFN-γ‡ | ELISPOT |
Doses IL-2 | Toxicity¶ (skin/eye/ear) | Tumor response, mo** | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2§ | IFN-γ‖ | |||||||||||
| 1 | 36/F | R,S,I | bo, li, lu | 22.2 | 17/83 | 73 | 52 | 95 | 173 | 10 | 1/2/3 | NR |
| 2 | 43/F | S,I | lu | 10.5 | 15/85 | 46 | 37 | 37 | 166 | 5 | 2/2/3 | PR (17+) |
| 3 | 60/F | S,I | bo, ln, sc | 6.5 | 7/93 | 56 | 40 | 1 | 56 | 3 | 0/0/0 | NR |
| 4 | 38/M | S,C,I | eye, sc | 12.0 | 22/78 | 65 | 38 | 24 | > 112 | 8 | 2/2/1 | NR |
| 5 | 47/F | R,S,I | sc, cu | 23.3 | 37/63 | 61 | 21 | 91 | 53 | 5 | 1,1††/2,2††/0,3†† | PR (17+) |
| 6 | 57/M | R,S,C,I | ln, bo, sc, lu | 17.6 | 27/73 | 69 | 29 | 38 | 52 | 7 | 1/0/0 | NR |
| 7 | 33/M | S,I | ln, li, lu | 1.5 | 11/89 | 33 | 19 | 68 | 132 | 10 | 1/0/0 | NR |
| 8 | 46/M | S,I | ln, br, lu | 5.7 | 32/68 | 49 | 17 | 26 | 65 | 7 | 0/2/0 | PR (16+) |
| 9 | 54/M | S,I | ln, sp, sc | 3.8 | 28/72 | 69 | 28 | 860 | 164 | 5 | 1/0/0 | PR (9) |
| 10 | 35/M | R,S,I | ln, lu, sc | 2.0 | 61/39 | 48 | 7 | 1200 | 32 | 11 | 1/2/0 | NR |
| 11 | 60/F | R,S,I | br, sc, lu | 3.0 | 27/73 | 60 | 19 | 310 | 152 | 15 | 1/0/0 | NR |
| 12 | 49/F | S,I | ln, ki, li, lu, sp | 4.8 | 2/98 | 85 | 61 | 66 | 34 | 4 | 0/0/0 | NR |
| 13 | 54/M | R,S,I | In, sc | 38.0 | 6/94 | 91 | 70 | 2250 | > 292 | 9 | 0/2/3 | PR (4) |
| 14 | 24/F | R,S,I | ln, sc | 80.0 | 10/90 | 75 | 64 | 4767 | > 349 | 6 | 1/1/3 | NR |
| 15 | 56/M | R,S,I | ip, li, lu | 30.5 | 13/87 | 95 | 72 | 990 | > 191 | 9 | 1/1/1 | NR |
| 16 | 54/M | S,C,I | ln, sc | 29.1 | 4/96 | 89 | 54 | > 270 | > 253 | 7 | 0/0/0 | NR |
| 17 | 37/M | S,I | adr, ln, lar, sc | 9.9 | 2/98 | 77 | 50 | 83 | 27 | 10 | 0/0/0 | NR |
| 18 | 56/F | S,I | ln, sc | 48.2 | 21/79 | 92 | 68 | 1410 | > 157 | 3 | 1/2/3 | PR (3) |
| 19 | 31/F | R,S,C,I | br, sc | 73.7 | 13/60 | 92 | ND | 166 | > 332 | 8 | 2/2/3 | NR |
| 20 | 56/M | S,C,I | adr, ln, lu, im, panc | 107.0 | 33/66 | 91 | ND | 1500 | > 376 | 11 | 1/0/3 | NR |
ND indicates not done.
R indicates radiation therapy; S, surgery; C, chemotherapy; and I, immunotherapy (high-dose IL-2).
ln indicates lymph node; li, liver; lu, lung; sp, spleen; sc, subcutaneous; br, brain; bo, bone; ki, kidney; ip, intraperitoneal; adr, adrenal; lar, larynx; im, intramuscular; and panc, pancreas.
Treatment cell intracellular IFN-γ detected by flow cytometry after 18-hour coculture with mel526+ tumor cells (gated on CD3+).
Number of positive IL-2 ELISPOTs per 100 000 infusion PBMCs after 4-hour coculture with mel624+ tumor cells.
Number of positive IFN-γ ELISPOTs per 10 000 infusion PBMCs after 4-hour coculture with mel624+ tumor cells.
Skin toxicity: grade 1, erythema; grade 2, desquamation < 50%; grade 3, desquamation > 50%. Eye toxicity (uveitis): grade 1, asymptomatic; grade 2, anterior, steroid eye drops; grade 3, pan uveitis, surgery. Ear (hearing): grade 1, hearing loss 15 to 25 dB at 2 frequencies; grade 2, > 25 dB at 2 frequencies; grade 3, > 25 dB at 3 frequencies (per CTCAE, Version 3.0).
PR indicates partial responder and NR, nonresponder (all by RECIST criteria); and +, ongoing. Values in parentheses are response duration (in months) after treatment.
Second treatment.
Table 2.
gp100(154) patient cell treatment characteristics
| Patient no. | Age, y/sex | Prior treatment* | Sites of disease† | No. of cells × 109 | Percentage CD4/8 | Percentage Tet | ELISPOT |
Doses IL-2 | Toxicity‖ (skin/eye/ear) | Tumor response, mo¶ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2‡ | IFN-γ§ | ||||||||||
| 1 | 31/F | S,C,I | ln, ip, li | 3.7 | 35/65 | 60 | 7100 | > 137 | 5 | 1/0/0 | NR |
| 2 | 32/M | S,C,I | ln, im, sc | 10.0 | 36/54 | 78 | 5300 | > 362 | 5 | 2/0/0 | NR |
| 3 | 54/M | S,I | sc | 9.8 | 13/82 | 87 | 2590 | > 302 | 4 | 1/0/0 | NR |
| 4 | 50/M | S,I | ln, gb | 4.6 | 11/78 | 97 | 12 050 | > 409 | 2 | 1/0/0 | NR |
| 5 | 49/F | S,I | ln, lu, sc | 9.9 | 21/80 | 91 | 11 683 | > 451 | 2 | 2/0/0 | NR |
| 6* | 36/F | R,S,C,I | ln, bo, li, sp | 5.8 | 6/89 | 77 | 2370 | > 336 | 5 | 1/0/0 | NR |
| 7 | 60/M | S,I | In, bo, li, lu, sc | 1.8 | 40/56 | 83 | 6117 | > 213 | 4 | 1/0/0 | NR |
| 8 | 50/F | S,C,I | st, li, lu, int, sc | 19.4 | 44/53 | 55 | 2900 | 294 | 5 | 2/2/0 | NR |
| 9 | 25/M | S,I | ln, bo, ip | 2.3 | 44/56 | 84 | ND | 46 | 9 | 0/0/0 | NR |
| 10 | 40/F | S,C,I | br, ln, im | 2.7 | 24/69 | 59 | 6700 | > 445 | 10 | 1/0/0 | NR |
| 11** | 50/M | R,S,C,I | br, ln | 68.8 | 5/96 | 97 | 8167 | > 495 | 8 | 1/2/3 | CR (14+) |
| 12 | 62/M | S,C,I | lu, ln | 46.5 | 20/82 | 85 | 14 583 | > 526 | 7 | 1/2/2 | NR |
| 13** | 44/F | R,S,C,I | li, ln | 54.0 | 2/97 | 90 | 8700 | > 339 | 13 | 1/2/1 | NR |
| 14 | 51/F | S,I | ln, li, lu, sp | 110.0 | 11/88 | 92 | 11 817 | > 421 | 6 | 1/0/2 | PR (4) |
| 15 | 33/M | S,I | ln | 94.1 | 16/78 | 89 | 6833 | > 389 | 6 | 2/0/0 | NR |
| 16 | 41/M | S,I | ln | 39.1 | 18/79 | 90 | 10 967 | > 485 | 7 | 1/0/1 | PR (3) |
ND indicates not done.
R indicates radiation therapy; S, surgery; C, chemotherapy; and I, immunotherapy (high-dose IL-2).
ln indicates lymph node; li, liver; lu, lung; br, brain; sp, spleen; sc, subcutaneous; bo, bone; ki, kidney; ip, intraperitoneal; adr, adrenal; lar, larynx; im, intramuscular; panc, pancreas; st, stomach; int, intestine; gb, gallbladder.
Number of positive IL-2 ELISPOTs per 100 000 infusion PBMCs after 4-hour coculture with mel624+ tumor cells.
Number of positive IFN-γ ELISPOTs per 10 000 infusion PBMCs after 4-hour coculture with mel624+ tumor cells.
Skin toxicity: grade 1, erythema; grade 2, desquamation < 50%; grade 3, desquamation > 50%. Eye toxicity (uveitis): grade 1, asymptomatic; grade 2, anterior, steroid eye drops; grade 3, pan uveitis, surgery. Ear (hearing): grade 1, hearing loss 15 to 25 dB at 2 frequencies; grade 2, > 25 dB at 2 frequencies; grade 3, > 25 dB at 3 frequencies (per CTCAE, Version 3.0).
CR indicates complete responder; PR, partial responder; NR, nonresponder (all by RECIST criteria); and +, ongoing. Values in parentheses are response duration in months after treatment.
Prior treatment with DMF4 TCR.
Based on tetramer staining, the mean transduction efficiencies for cells administered to these 36 patients were 71% and 82% for DMF5 and gp100(154) TCR, respectively (Tables 1–2). All treatment cells showed high levels of specific reactivity against cognate antigen-bearing tumor targets as assessed by intracellular cytokine staining and ELISPOT analysis (both IFN-γ and IL-2; Tables 1–2) and for IFN-γ release (Table 3) and target cell lysis (data not shown).
Table 3.
Interferon-γ production by TCR-transduced infusion cells
| Patient no. | Melanoma cell line |
T2 cells + μM peptide* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None | A2− |
A2+ |
Negative |
Positive |
|||||||
| 888 | 938 | 526 | 624 | None | 1.0 | 1.0 | 0.1 | 0.01 | 0.001 | ||
| DMF5 | |||||||||||
| 1 | 0 | 6 | 49 | 62 300 | 111 200 | 102 | 236 | 132 400 | 56 700 | 12 330 | 1139 |
| 2† | 7 | 133 | 175 | 16 800 | 99 400 | 159 | 141 | 117 400 | 53 700 | 6910 | 1182 |
| 3 | 0 | 125 | 163 | 3830 | 27 700 | 37 | 64 | 50 500 | 15 000 | 984 | 241 |
| 4 | 0 | 1 | 0 | 983 | 148 400 | 7 | 4 | 45 000 | 11 760 | 798 | 139 |
| 5† | 0 | 2 | 3 | 7760 | 180 800 | 20 | 21 | 34 000 | 17 000 | 1076 | 298 |
| 6 | 1 | 3 | 4 | 3020 | 2980 | 13 | 17 | 9580 | 8880 | 4810 | 960 |
| 7 | 13 | 16 | 41 | 8420 | 39 700 | 102 | 134 | 50 400 | 32 200 | 11 950 | 1556 |
| 8† | 744 | 663 | 1320 | 37 500 | 88 000 | 1299 | 1254 | 113 100 | 14 510 | > 16 460 | > 3278 |
| 9† | 0 | 1 | 6 | 7440 | 13 400 | 176 | 270 | 68 000 | 54 800 | 14 290 | > 1649 |
| 10 | 51 | 80 | 155 | 102 300 | 119 200 | 527 | 857 | 93 400 | 104 800 | > 5226 | 2005 |
| 11 | 17 | 8 | 77 | 6460 | 26 700 | 221 | 321 | 42 300 | 36 200 | 12 860 | 1576 |
| 12 | 0 | 0 | 8 | 1300 | 19 700 | 24 | 190 | 16 150 | 4720 | 338 | 124 |
| 13† | 0 | 6 | 0 | 3120 | 117 900 | 78 | 156 | 137 900 | 41 900 | 1750 | 345 |
| 14 | 0 | 0 | 31 | > 131 600 | 426 600 | 84 | 352 | > 283 400 | 38 800 | 7950 | 956 |
| 15 | 5 | 91 | 152 | 9400 | 47 200 | 54 | 295 | 69 400 | 71 400 | > 1575 | 271 |
| 16 | NA | 40 | 15 | 5410 | 17 525 | NA | 247 | 52 650 | 20 225 | 6370 | 1830 |
| 17 | 0 | 11 | 8 | 7100 | 63 200 | 46 | 394 | 70 200 | 3260 | 118 | 73 |
| 18† | 42 | 56 | 226 | 71 800 | 328 900 | 651 | 1400 | > 283 400 | 62 800 | 11 450 | 47 510 |
| 19 | 2 | 34 | 82 | > 142 700 | 275 590 | 829 | 1155 | 329 600 | > 184 200 | 7100 | > 2470 |
| 20 | NA | 17 | 9 | 9995 | 24 900 | NA | 81 | 22 750 | 6710 | 1560 | 590 |
| gp100(154) | |||||||||||
| 1 | 46 | 25 | 32 | 14 460 | 74 900 | 282 | 275 | 7540 | 9100 | 4210 | 3900 |
| 2 | 0 | 26 | 42 | 88 300 | 199 400 | 46 | 37 | 2080 | 40 900 | > 19 200 | 1620 |
| 3 | 15 | 80 | 185 | 90 500 | 209 800 | 87 | 163 | 15 490 | 14 870 | 8970 | 10 040 |
| 4 | 0 | 94 | 101 | 90 300 | 138 800 | 87 | 80 | 3000 | 70 500 | > 19 770 | 829 |
| 5 | 22 | 0 | 15 | 40 000 | 287 800 | 253 | 43 | 142 400 | 114 500 | 11 540 | 4540 |
| 6 | 0 | 38 | 31 | 83 400 | 163 200 | 35 | 57 | 14 160 | 11 750 | 7160 | 6640 |
| 7 | 23 | 169 | 82 | 4670 | 18 350 | 23 | 212 | 10 700 | 7370 | 6060 | 1100 |
| 8 | 166 | 102 | 120 | 98 600 | 179 400 | 87 | 777 | 59 200 | 55 600 | 16 070 | 5050 |
| 9 | 2 | 36 | 23 | 14 420 | 91 800 | 96 | 32 | 39 600 | 52 500 | 11 890 | 2440 |
| 10 | 200 | 20 | 245 | 13 640 | 148 800 | 21 | 22 | 64 300 | 115 900 | > 30 880 | 4140 |
| 11† | 71 | 0 | 3 | 141 700 | 143 600 | 22 | 265 | 161 900 | 58 700 | 4850 | 790 |
| 12 | 87 | 10 | 48 | 14 720 | 164 100 | 8 | 210 | 115 700 | 69 500 | 12 500 | 849 |
| 13 | NA | 14 | 30 | 102 600 | 172 800 | NA | 30 | 130 900 | 59 800 | 8295 | 651 |
| 14† | NA | 314 | 151 | 62 750 | 85 400 | NA | 142 | 135 700 | 58 700 | 11 360 | 4390 |
| 15 | NA | 43 | 0 | 2455 | 21 300 | NA | 149 | 7420 | 3855 | 2360 | 974 |
| 16† | NA | 86 | 113 | 23 050 | 110 350 | NA | 50 | 58 800 | 225 500 | 3840 | 1436 |
IFN-γ (pg/mL) measured by ELISA, 1 × 105:1 × 105 effector:target cell 18-hour coculture.
NA indicates not available.
Peptides, MART-1:27-35 and gp100:154-162 (positive, cognate peptide; negative, nonspecific peptide).
Responding patient.
Adoptively transferred highly reactive antitumor cells persisted at high levels in patients
In prior trials of patients receiving unmodified TILs, in vivo cell persistence of the transferred cells highly correlated with antitumor response.18 We thus evaluated the persistence of the human DMF5 and the murine gp100(154) TCR-transduced cells using tetramer binding and ELISPOT assays. All patients had measurable levels (≥ 1%) of tetramer-positive T cells in their circulation at 1 month after treatment (Figure 3A-B). There was no difference in the persistence of cells bearing the human DMF5 TCR (22% ± 6%) or the murine gp100(154) TCR (22% ± 5%; P = .4; Figure 3B). To measure functional recognition of tumor cells, posttreatment PBLs were cocultured with major histocompatibility complex–matched or mismatched melanomas, and cell reactivity was assessed using ELISPOT and intracellular fluorescence-activated cell sorter (FACS) assays (Figure 3). IFN-γ ELISPOT assay showed persistence (> 20 specific spots/100 000 cells) of tumor-reactive transduced cells in 11 of 20 DMF5 and 7 of 16 gp100(154) patients (Figure 3B). Similarly, 11 and 7 of the DMF5 and gp100(154) patients, respectively, exhibited persistence of active cells via IL-2 ELISPOT assays of PBLs at one month after treatment (Figure 3B). At this same 1-month time point, specific intracellular IFN-γ staining was seen in 12 of the 20 DMF5 patients (Figure 3A). Thus, as determined in multiple assays, both murine- and human-derived TCR gene–modified cells persisted in the circulation at 1 month in the majority of patients. Comparing between responding and nonresponding patients, responding patients all had highly persistent tumor-reactive cells at more than or equal to 10% tetramer-positive T cells in the blood 1 month after treatment. However, some nonresponding patients also had high levels of active persistent cells, suggesting that persistence may be necessary, but not sufficient, to cause tumor regression in patients.
Figure 3.
TCR-transduced cells from responding patients persisted and showed antitumor activity ex vivo. Blood samples were taken from patients' cells before and after TCR-transduced cell infusion. PBMCs were evaluated for persistence of infused cells in peripheral blood after treatment by specific tetramer staining and were also used directly in coculture assays with mel624 tumors (MART1+, gp100+, HLA-A2+). Antitumor activity was evaluated by IFN-γ and IL-2 ELISPOT, and also by intracellular staining for IFN-γ production. (A) Persistence and activity of DMF5 patient treatment cells before, 2 weeks after, and 1 month after infusion. Responding (PR) and nonresponding (NR) patients are represented by solid and broken lines, respectively. (B) Comparison of PBMCs from patients treated with either DMF5 or gp100(154) TCR-transduced cells. Tetramer staining and ELISPOT analysis of IFN-γ and IL-2 production from nonresponding (NR) and responding (PR) patients at 1 month after treatment. All samples had less than 10 ELISPOTs (per 100 000 PBMCs) and less than 1% IFN-γ–positive cells, respectively, against the HLA-A*02− mel888 tumor (data not shown). Patients with objective clinical responses (PR) had higher numbers of antitumor IFN-γ (P = .02) and IL-2 (P = .02) secreting cells than nonresponders (NR).
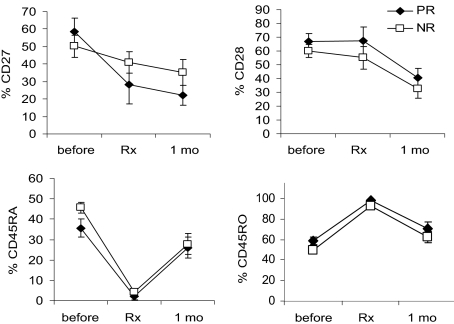
Adoptively transferred T-cell phenotype reverts from CD45RA−, CD45RO+ to CD45RA+, CD45RO− in vivo
We evaluated the phenotype of the DMF5 MART-1–reactive TCR-treated patients' cells before, during, and after treatment by flow cytometry for the costimulatory molecules CD27 and CD28 and the cell differentiation markers CD45RA and CD45RO. Before TCR gene modification, on average (± SEM), patients' peripheral T lymphocytes consisted of 53% plus or minus 5% CD27+, 62% plus or minus 4% CD28+, 42% plus or minus 3% CD45RA+, and 53% plus or minus 3% CD45RO+ cells (Figure 4). After expansion ex vivo, the tetramer-positive infusion cells displayed less CD27 (36% ± 6%), similar CD28 (59% ± 6%), almost complete loss of CD45RA (3.5% ± 1.2%), and a gain of CD45RO to 94% plus or minus 2% (Figure 4). Evaluating tetramer-positive cells in the blood 1 month after treatment showed levels of CD27 similar to infusion (30% ± 5%), reduced levels of CD28 (36% ± 5%), and intriguingly, levels of CD45RA increased and CD45RO decreased, reverting back to levels similar to those seen in the pretreatment PBL samples (27% ± 4% CD45RA+, 66% ± 4% CD45RO+; Figure 4). This suggests that either the few tetramer-positive CD45RA+ cells present at infusion persisted and expanded, or, in agreement with a previous published clinical report of a patient receiving a gene-marked allogeneic cell transfer to treat leukemia,19 cells that had been CD45RA− and CD45RO+ reverted to CD45RA+ and CD45RO− in vivo. There were no substantial differences in the cell phenotype between responding and nonresponding patients.
Figure 4.
Phenotype of patient treatment cells before and after infusion. DMF5 patient PBMCs were stained by tetramers for TCR-recognizing MART1:27-35, and by mAb for CD3 and the activation and differentiation markers CD27, CD28, CD45RA, and CD45RO. Cell phenotype was evaluated by flow cytometry, gated on CD3+ cells before treatment, and on CD3+tetramer+ cells for infusion (Rx) and 1 month after treatment (1 mo) samples. Error bars indicate mean ± SEM. Responding patients (PR) are represented by solid symbols, and nonresponding patients (NR) by open symbols.
Clinical course of patients receiving TCR-transduced cells: reactivity against normal tissues
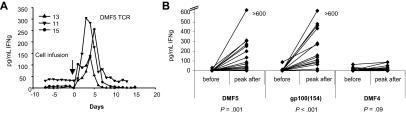
The clinical course of patients receiving the highly tumor-reactive DMF5 and gp100(154) TCR-transduced cells were quite different from that of our prior study with the DMF4 receptor.14 In the current trial, increased levels of IFN-γ were detected in the serum of patients, peaking around day 3 to 5 after treatment (Figure 5A). The mean peak IFN-γ serum values for patients receiving the improved DMF5 and gp100(154) gene constructs were 128 pg/mL and 210 pg/mL, respectively, compared with 14 pg/mL for the previous DMF4 patients (P = .03; Figure 5B). As IFN-γ is an effector cytokine produced by activated T lymphocytes and patients remained depleted of their endogenous lymphocytes during the first week after the preparative lymphodepletion, it is presumed that the transferred cells were the source of this cytokine production.
Figure 5.
Patients treated with the highly reactive DMF5 or gp100(154), but not DMF4 TCR, had increased serum IFN-γ levels after treatment. Patient IFN-γ serum levels were measured daily before, during, and after cell infusion by ELISA. (A) Serum IFN-γ levels increased from baseline to a peak at 3 to 6 days after cell infusion (3 representative DMF5 patients are shown). (B) Peak IFN-γ levels in serum after treatment for patients treated with DMF5, gp100(154), and DMF4 TCR-transduced cells. Only patients treated with the highly reactive DMF5 or gp100(154) TCR demonstrated increased IFN-γ in serum (P = .001 and P < .001, respectively, compared with P = .09 for DMF4 TCR).
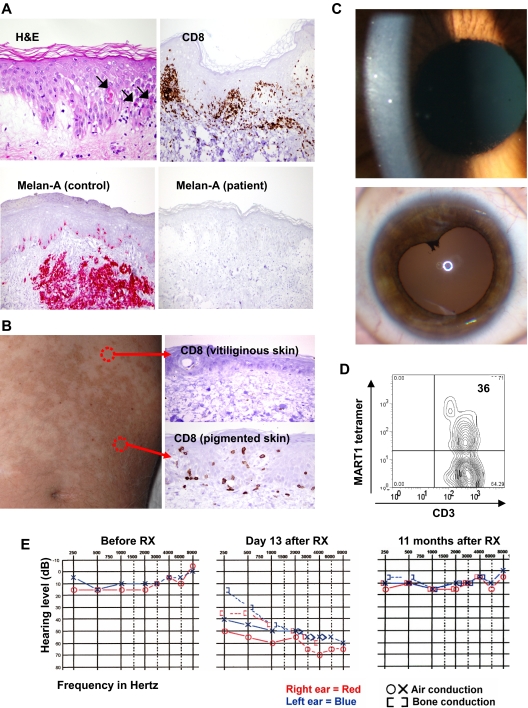
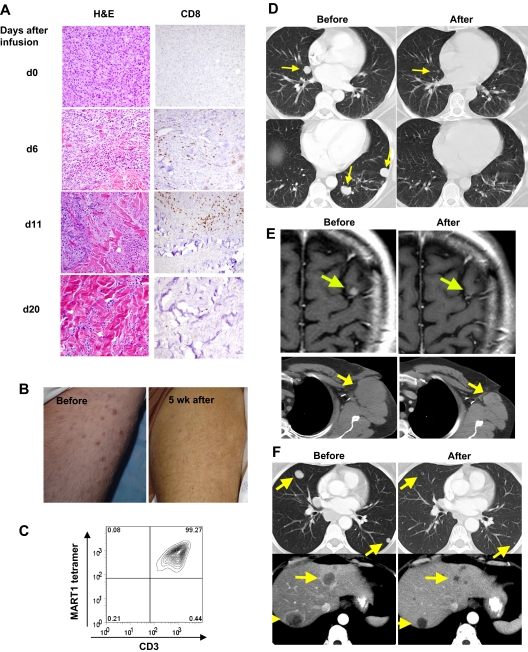
At the time of this cytokine surge, 29 of the 36 patients in the current trial exhibited a widespread erythematous skin rash that on biopsy showed prominent epidermal spongiosis and necrotic epidermal keratinocytes, with a dense infiltrate of CD3+ T lymphocytes (predominantly CD8+; Figure 6A). Surprisingly, there was destruction of epidermal melanocytes in all biopsies performed on 14 DMF5 patients and 13 gp100(154) patients, starting as early as day 5 after treatment (Figure 6A), although rare remaining melanocytes were sometimes observed around hair follicles. This loss of melanocytes coincided with the dermal and epidermal infiltration of lymphocytes as well as evidence of vitiligo in patients on later follow-up. This rash gradually subsided over several days without treatment in all patients. To elucidate the factors inciting the rash and the lymphocyte infiltration into skin, we studied 2 patients who presented with preexisting patchy vitiligo. These 2 patients developed a rash only in pigmented and not in vitiliginous skin (Figure 6B). Biopsies after treatment revealed diffuse infiltrates of CD8+ cells in areas of pigmented skin with little or no lymphocytic infiltrate in vitiliginous skin (Figure 6B). These findings, along with the evidence of melanocyte destruction at a time when endogenous lymphocytes have been depleted, strongly suggested that the epidermal melanocytes were the targets of immune attack by the gene-engineered cells.
Figure 6.
Tissue trafficking and target cell destruction in patients after infusion of TCR-transduced cells. (A) Biopsy of inflamed skin from DMF5 patient 2, day 5 after treatment, demonstrating spongiotic vesicles and necrotic/dyskeratotic keratinocytes (arrows), stained with hematoxylin and eosin, and immunohistochemically stained for CD8+ cells or the Melan-A antibody recognizing MART-1 antigen. (Bottom left) Positive control staining for the anti-MART-1 Melan-A antibody showing normal epidermal melanocytes and a subcutaneous melanoma deposit (original magnification ×20). (B) One week after treatment, biopsies from gp100(154) patient with preexistent patchy vitiligo, demonstrating CD8+ cellular infiltrate into the epidermis of pigmented, but not vitiliginous skin (original magnification ×20). (C) Slit-lamp ophthalmologic evaluation of DMF5 patient 5 eye, 2 weeks after treatment, demonstrating cloudy cellular anterior chamber infiltrate (above) and (below) with induced iris dilation 6 months after TCR treatment and steroid eye drop administration, demonstrating posterior synechiae (asymptomatic). (D) Cells present in ocular fluid from panel C (top), analyzed directly by staining and flow cytometry. (E) Audiologic examination of DMF5 patient 2 before TCR treatment, day 13 after treatment showing hearing loss, and 11 months after treatment, showing hearing recovery after intratympanic steroid treatment.
Because melanocytic cells expressing MART-1 and gp100 exist in the eye, all patients underwent ophthalmologic examination before and after treatment. None of the patients in the prior DMF4 TCR trial developed uveitis (cellular infiltrate into the eye). In contrast, 11 of 20 patients (55%) receiving the more reactive DMF5 TCR cells and 4 of 16 (25%) receiving the gp100(154) TCR cells developed an anterior uveitis, 2 of which were asymptomatic and 13 required the transient administration of steroid eye drops (Tables 1, 2; Figure 6C). Two patients developed synechiae of the iris that were asymptomatic (Figure 6C). In all patients, ocular findings reverted to normal. Sampling of the eye anterior chamber fluid in one patient with uveitis who received the DMF5-transduced cells revealed a predominance of MART-1 tetramer-positive CD3+ T cells by FACS analysis (Figure 6D), indicating trafficking of gene-modified cells into the eye.
Melanocytic cells also exist in the striae vascularis of the inner ear. Although none of the patients in the prior DMF4 trial exhibited hearing loss, audiometric examinations revealed evidence of hearing loss in 10 of 20 of the DMF5 patients starting approximately 1 week after treatment (Table 1; Figure 6E), 7 of whom received intratympanic steroid injections. All patients improved, and the grade 3 hearing losses resolved or improved to a grade 1 or 2 with the exception of 2 patients who died of progressive metastatic melanoma before they were retested. Five of the 16 gp100(154) patients developed mild hearing loss (Table 2), only one of whom required treatment and continues to have mild residual changes. Nine of the 36 patients experienced inner ear–related dizziness that responded to treatment. This constellation of symptoms is similar to that observed in patients with Vogt-Koyanagi-Harada disease,20 thought to result from autoimmune destruction of melanocytes located in the eye, the inner ear, skin, and hair. There were no off-target autoimmune effects observed in any patients.
DMF4 and DMF5 TCRs recognize the same MART-1:27-35 epitope and were derived from the same melanoma patient.15 The improved ability of transduced cells expressing the DMF5 or gp100(154) TCR to recognize target antigens in the skin, eye, and ear and to persist in patients at levels up to 80% of circulating peripheral T lymphocytes for several months appears the result of the higher reactivity conferred by these improved TCR constructs compared with the prior DMF4 TCR.
Antitumor impact of the TCR-transduced cells
One patient who received DMF5-transduced cells underwent a series of sequential biopsies of subcutaneous metastatic lesions before and after treatment. Beginning at approximately 5 days after treatment, the biopsies revealed an increasing infiltration of CD8+ T lymphocytes throughout the tumor that coincided with the progressive necrosis and partial regression of his tumors (Figure 7A-B). Growth of lymphocytes from resected lesions at days 9, 15, and 26 revealed more than 97% MART-1 tetramer-positive lymphocytes (Figure 7C). Although initially the majority of this patient's disease regressed, numerous new lesions developed after 2 months; and as such, this patient was a nonresponder.
Figure 7.
Highly reactive transferred cells traffic to and destroy melanoma tumors in patients. (A) Sequential biopsies of subcutaneous tumors from DMF5 patient 4 before (d0) and after treatment, stained with hematoxylin and eosin (left), or anti-CD8 (right). Original magnification ×20. (B) DMF5 patient 4 thigh covered with multiple subcutaneous melanoma lesions before treatment, and after partial tumor regression 5 weeks after treatment. (C) Flow cytometry of TILs grown from DMF5 patient 4 subcutaneous tumor resected 4 weeks after treatment. (D-F) Before treatment and after treatment CT scans of (D) DMF5 patient 2 lungs, (E) DMF5 patient 8 brain (top) and axilla (bottom), and (F) gp100(154) patient 14 lungs (top) and liver (bottom). Arrows represent location of melanoma metastases.
Six of the 20 patients (30%) treated with DMF5 TCR and 3 of 16 (19%) treated with gp100(154) TCR experienced an objective antitumor response, as defined by RECIST criteria17 (Tables 1–2). Tumors regressed in multiple organs, including the brain, lung, liver, lymph nodes, and subcutaneous sites (Figure 7D-F). There were no treatment-related deaths, and all patients recovered from treatment.
In analyzing the 36 patients, there was no correlation between the number of cells administered and the likelihood of a clinical response, with some responding patients receiving a log fewer cells than others (Table 1). There was also no correlation between clinical response and the duration the cells were grown ex vivo, or whether they received one or 2 OKT-3 stimulations. There was a correlation with clinical response and the persistence of administered cells at one month as assessed by ELISPOT analysis of both specific IFN-γ and IL-2 release (both P = .02; Figure 3B). Although all responding patients had more than or equal to 10% tetramer-positive cells persisting in blood at 1 month, this parameter did not correlate with response (P = .4; Figure 3B).
Discussion
The ability of genetically modified normal peripheral lymphocytes bearing highly avid TCR to destroy isolated antigen-expressing cells, such as individual melanocytes in the ear, the basal layer of the epidermis, and in immunoprivileged sites such as the eye, as well as melanoma metastases in visceral organs and the brain, has important implications for the development of cancer immunotherapy. This approach bypasses the need to identify and isolate antitumor effector cells from each patient. These gene-modified cells targeted only specific shared differentiation antigens and thus these findings validate the use of differentiation antigens as targets of cancer immunotherapy. It is of interest that cells expressing the murine gp100(154) TCR persisted at the same levels as those expressing the human DMF5 TCR, and also mediated cancer regression, suggesting that an immune reaction against the mouse TCR sequences may not be a limiting factor in TCR gene therapy in humans. The ability to use HLA-transgenic mice to raise TCRs against human cancer antigens represents a valuable approach to bypass the tolerance of patients to self-antigens and thus enable the generation of highly reactive antitumor TCRs.21
It was surprising that the percentage of PBLs staining positive for tetramer was always higher than the proportion of cells displaying antitumor activity by ELISPOT or intracellular cytokine staining (Tables 1–2; Figure 3). Part of this difference may be accounted for by the inclusion of all mononuclear cells in patient PBL functional assays, compared with the ability to gate on the CD3+ T lymphocyte population by flow cytometry. However, this difference is small compared with the logs of difference between tetramer-positive cells by FACS (approaching 100% in treatment samples and up to 80% in posttreatment PBLs), and in ELISPOT results (generally < 1% of cells). It is also possible that, although tetramer staining identifies all lymphocytes expressing the tumor-reactive TCR, not all TCR+ cells have equal antitumor function. As T lymphocytes can have an antitumor effector phenotype, they can also be anergic or have a suppressive phenotype, such as regulatory T cells. In the case of these latter phenotypes, antitumor functional response could be diminished or abrogated. Another potential explanation is the different sensitivities of direct tetramer staining for flow cytometry vs ELISPOT or intracellular cytokine staining.
The recognition of normal quiescent cells expressing the targeted cancer antigen raises obvious questions concerning the toxicity of this gene therapy approach. These results paralleled our findings in a murine melanoma model, which showed an association of ocular toxicity and antitumor activity using gp100 as the target antigen,22 although clinically the local application of steroids attenuated normal tissue toxicity for patients treated with these melanocyte-specific TCRs. These findings emphasize the importance of the targeted tumor antigen. It may be highly effective to target differentiation antigens on cancers that arise in nonessential organs, such as the prostate, ovary, breast, and thyroid. Other cancers have higher expression of differentiation antigens, such as CEA and Muc-1, than are expressed in normal tissues, and this may create a therapeutic window to be exploited. The cancer-testes class of antigens not expressed on normal adult tissues may be ideal targets for this approach. In any case, the risks versus benefits of infusing highly reactive self-antigen specific TCRs must be carefully considered.
Although the low numbers in these clinical trials preclude statistical conclusions, the 30% and 19% objective cancer response rates seen with the DMF5 and gp100(154) TCR, respectively, were lower than the 51% to 72% response rates seen with the use of autologous TILs screened and grown individually from each patient's resected tumor.7,8,11 Anterior uveitis and ototoxicity were seen in 6 of 93 (6.5%) and only one of 93 (1.1%) melanoma patients treated with TILs, respectively, in contrast with the 41.7% incidence of both toxicities seen in the current trial (P < .001), sometimes in the absence of tumor regression, implying that the shared melanoma/melanocyte antigens may not be the predominant targets of therapeutic TILs. Although many TILs do recognize the MART-1 and gp100 antigens, T-cell clones have been isolated from many clinically effective TILs that recognize mutated or unidentified antigens as well, and these may also be responsible for the antitumor effects of TIL transfer. Alternatively, differences in homing molecules or other phenotypic differences between blood and tumor-derived lymphocytes may account for this finding.
Several patients exhibited melanocyte toxicity without experiencing tumor rejection. Treating patients with low (DMF4) and high (DMF5) avidity TCR recognizing the same MART-1 antigen resulted in 13% (4 of 34) and 30% (6 of 20) objective tumor responses, respectively, suggesting that increased TCR avidity may improve tumor rejection. However, because of the small sample numbers, it is possible that the avidity of TCR in vivo is not a major determinant of tumor rejection. It is probable that factors, such as lymphocyte homing to normal vs tumor tissue, heterogeneity of antigen expression by tumor, T-cell exhaustion, suppression in the tumor microenvironment, or the need for polyvalent T-cell populations, may all need to be considered to improve tumor rejection in patients. Murine tumor models predict that intensifying the lymphodepletion before cell transfer and administering a vaccine to stimulate the transferred cells in vivo can improve the antitumor efficacy of this approach,10,23 and we are currently implementing a clinical trial combining the DMF5 and gp100(154) TCR, with increased patient immunodepletion, followed by administration of a peptide vaccine.
Adoptive cell transfer therapy using gene-modified lymphocytes has also been shown to be effective in treating malignancies other than melanoma. In preclinical models, lymphocytes have been genetically modified to express costimulatory molecules designed to increase antitumor activity and T-cell survival, such as CD80 or 4-1BB ligand, or to abrogate the effects of inhibitory signals.24 Genetically modified human lymphocytes expressing chimeric antigen receptors (CARs),25 combining the antigen recognition of an antibody with T-cell signaling motifs, were able to eradicate human B-cell lymphoma by targeting CD19,26 and human prostate tumors by targeting ErbB2/Her227 in xenogeneic SCID mouse models. Using Epstein-Barr virus–specific human T cells transduced with CARs recognizing the Hodgkin lymphoma–specific CD30 antigen has also been shown to be effective in a xenogeneic mouse model.28 Clinically, Epstein-Barr virus–specific cells expressing CARs that recognize the neuroblastoma tumor-specific diasialoganglioside GD2 have also recently been used to successfully treat patients with neuroblastoma.29
In an attempt to expand this TCR therapy to treat cancers other than just melanoma, we have now cloned the genes encoding high-avidity TCR that recognize a variety of antigenic epitopes, such as NY-ESO-1:157-165,30 p53:264-272,21,31 and CEA:691-699,32 expressed on many common epithelial cancers.9 To target tumors in a non–major histocompatibility complex-restricted fashion, we have also engineered lymphocytes to express chimeric receptors incorporating the antigen combining site of anti-Her2 or anti-CD19 mAb,26 which may target tumor cells overexpressing these determinants on the cell surface.
Possible toxicities resulting from the expression of tumor-associated antigens on normal tissues need to be considered in the application of this approach. Our results, however, support the hypothesis that the administration, to cancer patients, of T cells transduced with highly reactive human or murine TCRs can mediate in vivo destruction of tissues that express the target antigen and suggest that cell transfer therapies can be a valuable adjunct to the treatment of patients with metastatic cancer.
Acknowledgments
The authors thank Dr Yangbing Zhao, Dr Cyrille Cohen, and Zhili Zheng for their work in constructing the clinical retroviral vectors; Dr Ken Cornetta and the Indiana University Vector Production Facility for providing the cGMP retroviral supernatants; Takara Bio Inc, Otsu, Japan for providing retronectin; Dr Franz Smith, who conducted assays on patient infusion samples; and the nursing staff on the 3NW ward and the Intensive Care Unit in the Clinical Center, National Institutes of Health who provided these patients with outstanding care.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.A.J. prepared the DMF5 patient treatment cells, conducted the laboratory experiments and analysis, and wrote the paper; R.A.M. prepared the gp100(154) patient treatment cells and conducted experiments; M.E.D., A.P.C., and J.R.W. prepared the patient treatment cells; S.L.S. performed experiments; L.C. and N.P.R. identified and isolated the gp100(154) TCR; J.C.Y., M.S.H., U.S.K., R.E.R., R.M.S., J.L.D., A.M., R.T.R., and S.A.R. were physicians and D.A.N. was a Research Nurse on the clinical protocols; R.A.M. constructed the retroviral TCR vectors; C.-C.R.L. conducted immunohistologic staining of patient biopsies; R.J.B. conducted patient ocular evaluations; H.K., C.C.B., S.F.R., and C.V.W. conducted patient auditory evaluations; C.M.L. provided support in clinical protocol preparation and reporting; S.A.R. supervised the clinical trials and contributed to data analysis and writing of the paper; R.J.B. provided information regarding patient ophthalmologic evaluations; H.K, C.C.B., S.F.R., and C.V.W. provided information regarding patient otolaryngologic evaluations; and L.A.J. and S.A.R. analyzed the data, to which all authors have access.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for L.A.J. is Division of Neurosurgery, Department of Surgery, Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC.
Correspondence: Steven A. Rosenberg, Surgery Branch, National Cancer Institute, Building 10CRC, Hatfield Clinical Research Center, Bethesda, MD, 20892; e-mail SAR@nih.gov.
References
- 1.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 5.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 6.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LA, Heemskerk B, Powell DJ, Jr, et al. Gene transfer of tumor-reactive TCR confers both high-avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Cohen CJ, Peng PD, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marktel S, Magnani Z, Ciceri F, et al. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101:1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- 20.Fang W, Yang P. Vogt-Koyanagi-Harada syndrome. Curr Eye Res. 2008;33:517–523. doi: 10.1080/02713680802233968. [DOI] [PubMed] [Google Scholar]
- 21.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer DC, Chan CC, Gattinoni L, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy: how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 25.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 27.Pinthus JH, Waks T, Malina V, et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J Clin Invest. 2004;114:1774–1781. doi: 10.1172/JCI22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen CJ, Zheng Z, Bray R, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkhurst M, Joo J, Riley JP, et al. Characterization of genetically modified T cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]