Abstract
Background
The prevalence and recurrence rates of postpartum depression (PPD) are 13% and 25%, respectively. Despite its detrimental impact on the mother - infant dyad’s health, there is a paucity of data in the literature regarding the efficacy of pharmacological treatment of PPD.
Objective
1)To review the literature on the use of antidepressants and hormonal supplements for the prevention and the treatment of PPD; 2) to provide the authors’ opinion regarding the current status of the pharmacological treatment of PPD, and 3) to discuss developments that are likely to be important in the future
Methods
An electronic search was performed by using PubMed, Medline, and PsychINFO. Inclusion criteria were: 1) empirical articles in peer-reviewed English language journals, 2) well validated measures of depression, and 3) uniform scoring system for depression among the sample.
Results/Conclusion
The electronic search yielded a total of 19 articles (12 on treatment and 7 on prevention of PPD) with the following study designs: 8 randomized clinical trials (6 usingplacebo control and 2 using active control groups), and 11 open label studies.The selection of the specific antidepressant for a woman with PPD should derive from a personalized risk-benefit analysis.
Keywords: postpartum depression, pharmacotherapy, prevention, treatment
1 INTRODUCTION
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV TR) [1] includes the term “with postpartum onset ”. This specifier defines a time criterion (the onset of episode must be within 4 weeks after birth). The “postpartum” specifier can be applied to the current or most recent episode of four diagnoses (major depressive disorder; major depressive, manic, or mixed episode in bipolar I or II disorder; or brief psychotic disorder). The International Classification of Diseases, 10th Edition (ICD-10)[2] includes “postpartum” or “postnatal depression NOS” among the mental and behavioral disorders associated with the puerperium (defined as beginning within 6 weeks after birth), not elsewhere classified. Gaynes et al [3] broadly defined perinatal depression (during pregnancy through one year postpartum) in their comprehensive review. From a public health perspective, women, families and communities suffer regardless of when the maternal episode begins. For the purposes of this review, we describe the treatment of major depressive disorder in women through the first year following birth, for which we used the term postpartum depression (PPD).
In the first meta-analysis, O’Hara and Swain [4] reported that approximately 13% of new mothers experience PPD in the first year after childbirth. The Agency for Healthcare Research and Quality (AHRQ) commissioned an evidence–based evaluation of 30 studies on perinatal depression, defined as minor or major depression occurring during pregnancy or within the first year after birth [3]. The authors found that the point prevalence of depression reached a peak in the third month after birth (12.9 %), then declined slightly to a range of 9.9% –10.6 % in the fourth through seventh month and 6.5% in the remaining five months of the first postpartum year [3]. In a cohort study conducted in Denmark the risk of postpartum depression was increased for five months after birth (RR, 95% CI) [2.8 (1.57–2.77)][5].
The differential diagnosis of early onset PPD includes the maternity blues, a transitional syndrome experienced by up to 80% of new mothers[6] and resolved by 10 days after birth. Postpartum psychosis occurs in 1–2/1000 mothers within the first 8 weeks after delivery and represents a psychiatric emergency due to the risk of infanticide and suicide[10,11]. PPD does not differ from depression at other periods during the childbearing years with respect to clinical presentation and duration of untreated episodes [7, 8]. However, aggressive obsessional thoughts occur more commonly in women who have PPD compared to those who have depression outside the one year postpartum time frame [9].
Epidemiological research has shown that women have an increased risk of major depressive disorder (MDD) during the childbearing years and reduced risk after menopause [10, 11]. Consistently, specific female lifecycle periods of hormonal fluctuation (i.e., menstrual cycle, pregnancy, postpartum, and perimenopause) are associated with depressive syndromes [12, 13]. In particular, during pregnancy, the brain is exposed to a 100-fold increase in ambient estradiol (E2) levels, which abruptly decrease in the first postpartum week [14]. With evidence of no significant difference in hormonal levels between depressed and non depressed postpartum women [15], researchers have suggested that the rapid change in gonadal steroid level, rather that the absolute level, is involved in the etiopathogenesis of PPD. This hypothesis was supported by the work of Bloch et al [16], who simulated the withdrawal of hormones after birth by inducing a hypogonadal state in women with leuprolide, adding back supraphysiologic doses of estradiol and progesterone for 8 weeks, and then withdrawing both steroids under double–blind conditions. Five of eight women with a history of PPD, compared to none of the eight women without a history of depression, developed mood symptoms. Depressive symptoms peaked in the withdrawal (postpartum simulation) phase.
The recurrence rate of PPD has been estimated to be as high as 25% [17]. Its detrimental impact on the mother - infant dyad’s health [18] [19] underlines the importance of prevention of both its occurrence (to block the immediate post-birth cascade of events which result in PPD primary prevention) and its persistence and worsening (secondary prevention). PPD provides an ideal opportunity for prevention because its onset is preceded by a clear marker (birth), there is a defined period of risk for onset, and a high-risk sample of mother can be identified. [7]
There is a paucity of data in the literature regarding the efficacy of pharmacological treatment of PPD. The goals of this paper are to: 1) review the literature on the use of antidepressants and hormonal supplements for the prevention and the treatment of PPD, 2) provide the authors’ opinion regarding the current status of the pharmacological treatment of PPD, and 3) discuss developments that are likely to be important in the future.
2 METHOD
An electronic search was performed by using PubMed, Medline, and PsychINFO. We identified articles whose titles contained the following key terms in various combinations of the following: depression, depressive illness, postpartum, postnatal, treatment, prevention, therapy, pharmacotherapy, antidepressant, the name of each antidepressant drug, hormonal therapy, estrogen, and progesterone. The search was not limited by any dates. Inclusion criteria were: 1) empirical articles in peer-reviewed English language journals, 2) well validated measures of depression, and 3) uniform scoring system for depression among the sample. Relevant reviews were examined. This review was based on methods and results as described in the selected published articles.
3 RESULTS
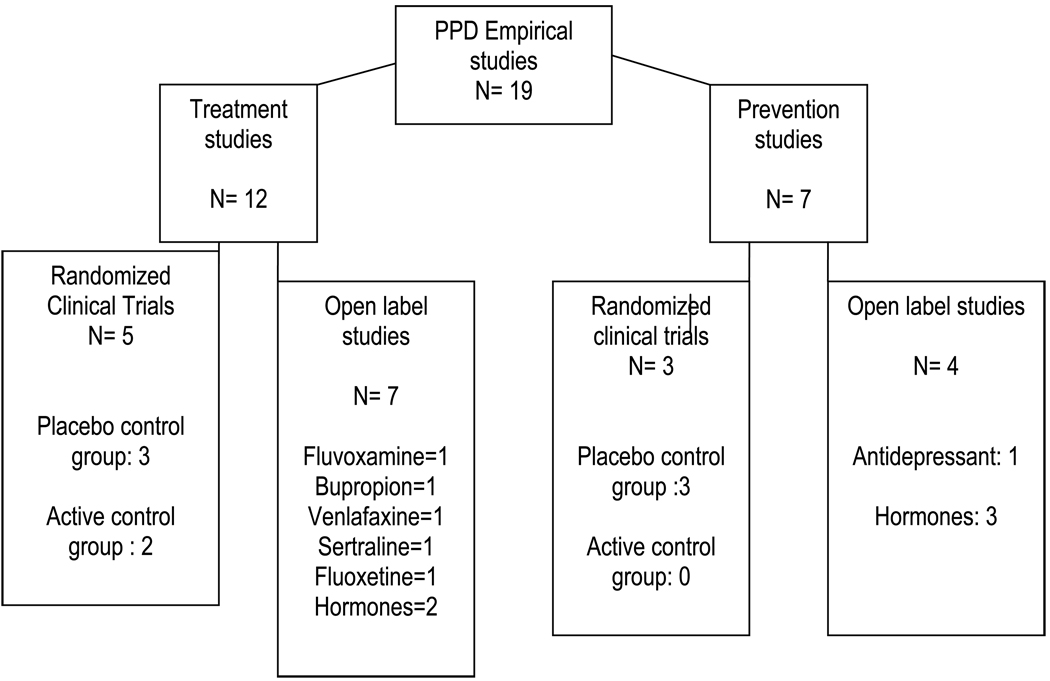
The electronic search yielded a total of 19 articles (12 on treatment and 7 on prevention of PPD) with the following study designs: 8 randomized clinical trials (6 using placebo controls and 2 using active control groups), and 11 open label studies (see Figure 1).
Fig. 1.
Number of studies meeting inclusion criteria
3.1 Prevention of PPD – Open-label studies (Table 1)
TABLE 1.
PPD PREVENTION – OPEN LABEL STUDIES
| Article | Sample Size | Intervention | Duration Treatmen t |
Measures | Outcome/Results |
|---|---|---|---|---|---|
| Dalton K., 1985 |
321 (100 in the treated group; 221 in the untreated group) Breastfeeding: NS |
Progesterone: IM injection (100 mg/day for seven days after birth) followed by suppositories (400 mg twice daily) for 2 months or until the first menstruation after birth. |
2 months (or until the first menstrual tion after delivery) |
NS |
Recurrence rate in the treated group was reduced (10%) as compared with of the untreated group (68%) Recurrence criteria: NS |
| Dalton K., 1989 |
202 (181 in the treated group; 21 in the untreated group) Breastfeeding : NS |
Progesterone: IM injection (100 mg/day for seven days after birth) followed by suppositories (400 mg twice daily) for two months or until the first menstruation after birth. # |
2 months (or until the first menstrual tion after delivery) |
NS | Recurrence rate in the treated group was reduced (7%) as compared with of the untreated group (67%) Recurrence criteria: NS |
| Wisner et al., 1994 |
23 (8 in the monitoring group; 15 in the monitoring + AD group). Breastfeeding women: Included |
Intervention group: PP monitoring (weekly phone contacts) + AD § Control group: PP monitoring alone |
3 months | IDD , clinical interview |
Rate of Recurrence: monitoring + AD= 6.7%; monitoring alone= 62.5%; p=.0086. Odds Ratio for recurrence when un-medicated= 19.2 (95% CI,1.5–1,179). Recurrence criteria: an episode that fulfilled DSM-III criteria by both clinical interview and self-report evaluation by IDD |
| Sichel et al., 1995 |
11 Breastfeeding women: NS |
Oral conjugated estrogen: dose begun at 10 mg/day and decreased over 4 weeks. Two women received IV estrogen for 2 days and then switched to oral |
DSM-III checklist |
None of the ten compliant women experienced relapse during the follow up period Relapse criteria : NS |
Abbreviations= M: intramuscular; IV: intravenous therapy; NS: not specified in the published article; ECT: electroconvulsive therapy; AD: antidepressant; PP: postpartum; IDD: The Inventory to Diagnose Depression;
Women in the study were advised to increase the dosage from up to six suppositories daily depending on the symptoms, but details on the taken dosage is not indicated;
Subjects received the antidepressants to which they had responded for past episodes and the first dose was received within 24 hours after birth [Type text]
All studies targeted the prevention of recurrence of PPD in women with at least one previous lifetime episode. No pharmacologic studies have been published on prevention of the initial episode of PPD after an index birth.
Wisner et al. [20] conducted the first study evaluating pharmacotherapy to prevent PPD in 23 asymptomatic women with at least one previous episode of PPD according to DSM III criteria. The subjects were recruited in a 3-month prospective, open-label controlled trial. Eight women elected to receive postpartum monitoring (weekly phone contact during the first 2 weeks after birth and further phone contacts or clinic visit depending on the clinical status) and 15 women chose postpartum monitoring plus antidepressant treatment. The women were compared for recurrence (defined as an episode that fulfilled DSM-III criteria by both clinical interview and self-report evaluation. Each subject received the antidepressant to which she had responded for past episodes and the first dose was received within 24 hours after birth. Medications included fluoxetine, N=4; nortriptyline, N=7; imipramine, N=2; and clomipramine, N=2. After 3 months, the rate of recurrence was significantly lower in women taking antidepressants compared to women receiving monitoring alone (respectively 6.7% and 62.5%, p=.0086), and the odds for recurrence when unmedicated was 19.2 (95% CI= 1.5–1,179), independent of treatment with antidepressant during pregnancy, history of nonpostpartum depressive episodes, and breastfeeding. The major limitations of this study were the lack of randomization and double-blind design and the small sample size. Ten out of the 23 women were taking antidepressants during pregnancy and the dosage was tapered off two weeks before the delivery; therefore, the unique contribution of antidepressant taken in the study in preventing PPD recurrence remains unknown.
Sichel et al. [21] conducted an open trial of intravenous and oral estrogen in 11 women with history of severe postpartum affective disorder. Immediately after delivery, oral estrogen was administered daily (initially 10 mg/day and tapered over 28 days to 0.625 mg/day; 2 subjects received intravenous estrogen for 2 days and then switched to oral). None of the 10 women who were compliant with treatment experienced postpartum relapse within the first postpartum year. The small sample size, lack of placebo control group and the fact that neither the subjects nor mood raters were blinded to treatment limit the results of the study, which has not been replicated.
Dalton [22] studied 100 women with previous postnatal episodes. They received intramuscular injection of progesterone (100 mg/day for 7 days after birth) followed by progesterone suppositories (400 mg twice daily) for 2 months or until the first menstrual period after birth. The recurrence rate of PPD was reduced (10%) compared with the untreated women (68%). In a subsequent study using the same drug regimen, Dalton [23] reported a recurrence rate of 7% among 181 women who received prophylactic progesterone. The comparison group of 21 untreated women had a recurrence rate of 67%. In both of these studies, the baseline depression severity and the criteria for PPD recurrence were not specified. The details of the intervention were not fully described in the latter paper [23] .
3.2 Prevention of PPD – Randomized Clinical Trials (Table 2)
TABLE 2.
PPD PREVENTION – RANDOMIZED CLINICAL TRIALS
| Article | Sample Size (N) | Intervention | Duration treatment |
Measures | Outcome/Results |
|---|---|---|---|---|---|
| Lawrie et al.,1998 (29) | 180 (90 in the PROG group, 90 in the placebo group). Breastfeeding women: Included |
Synthetic PROG: intramuscular injection of norethisterone enenthate (200 mg/day) within 48 hours after birth |
Single dose within 48 hours after birth. |
MADRS, EPDS |
Women in the PROG group were more likely to report minor/major depressive symptoms at week 6 but no at week 12. Relative risk to score MADR>9 = 2.56 (95% CI, 1.26–5.18). Relative risk to score EPDS>11 = 3.04 (95% CI, 1.52–6.08) |
| Wisner et al., 2001 (17) |
51 (26 in the NTP group, 25 in the placebo group). Breastfeeding women: Included |
NTP: dose begun at 20 mg within 24 hours after birth, tapered at week 17, discontinued at week 20. Mean serum level across the study= 83 ng/mL |
20 weeks | HAM-D | Recurrence rates in the NTP group and placebo group were 23.5% and 24% (p =1), respectively. Time to PPD recurrence ranged from 1 to 16 weeks and it did not differ between the 2 groups (p=.83). |
| Wisner et al.,2004 (25) | 22 (14 in the SER group, 8 in the placebo group) Breastfeeding women: Included |
SER: dose begun at 50 mg within 24 hours after birth, tapered at week 17, discontinued at week 20. Ranges of serum level across the study = 23–48 ng/mL |
20 weeks | HAM -D | Recurrence rates in the SER group and placebo group were 7% and 50% (p=0.04), respectively. Time to recurrence was longer in the intervention group compared to the placebo group (p=0.02). |
Abbreviations: PROG: progesterone; NTP= nortriptyline; SER: sertraline; PPD: postpartum depression; EPDS = Edinburgh Postnatal Depression Scale; HAM-D: Hamilton Depression Scale ; MADRS: Montgomery-Åsberg Depression Rating Scale.
Wisner et al [17] tested the efficacy of the tricyclic antidepressant nortriptyline for the prevention of PPD recurrence in a double-blind randomized placebo-controlled clinical trial. The authors reported data from 51 women with at least one lifetime episode of major depression with postpartum onset. The subjects were recruited during pregnancy and randomly assigned to receive nortriptyline (N=26) or placebo (N=25) immediately after birth. For the first postpartum week, the dose was increased daily as follows (mg): 20,30,40,50,50,60,70 and continued at 75 mg/day through day 21, when it was adjusted based on the serum level from day 14. The drug was provided for 17 weeks to cover the high-risk period for PPD as defined by Kendell et al [24] of 3 months, or 14 weeks. The drug was tapered starting at week 17 at a rate of 33% per week and was discontinued at 20 weeks postpartum. The mean serum drug level for nortriptyline (83 ng/mL) across the study period was within the usual therapeutic range for this drug (50 –150 ng/ml). The rate of recurrence of PPD did not differ between the two groups (23.5%=nortriptyline; 24%= placebo). The time to PPD recurrence ranged from 1 to 16 weeks and it also did not differ between the two groups. Contrary to the results of the previous open-label study conducted by the same author [20], the efficacy of nortriptyline in the prevention of PPD recurrence was not confirmed by this randomized controlled study. As the authors pointed out, this difference may be due to the fact that women are more likely to respond to the treatment to which they have previously responded. The major limitation of this study is the small sample size.
In a second randomized clinical trial with the same protocol, Wisner et al [25] demonstrated that sertraline conferred additional preventive efficacy beyond that of placebo (7% in the sertraline group versus 50% in the placebo group; p=.04). The authors reported data from 22 women (sertraline, N=14, placebo, N=8). The dosing protocol began with 25 mg/day of sertraline.for 4 days, increased to 50 mg/day through week 4, then to 75 mg/day during weeks 5–17. At study week 17 the dose was tapered across 3 weeks, and treatment was discontinued at week 20. Serum levels were determined to assess compliance. Interestingly, after sertraline was withdrawn (at week 20), 2 of the 9 women who completed the trial experienced PPD recurrence. This observation suggested that the vulnerability to experience PPD persists beyond the period of drug therapy and pharmacological treatment should be continued for more than 17 weeks. The authors recommended a minimum treatment period of 26 weeks, or about 6 months, which is consistent with American Psychiatric Association guidelines for the duration of treatment for a single episode of depression [26]. The major limitation of this study is the small sample size.
The positive results of this study are in contrast to the authors’ results with the tricyclic antidepressant nortriptyline as the preventive agent. Consistently, serotonin selective reuptake inhibitors are effective compared to placebo for premenstrual dysphoric disorder, but non-serotonergic tricyclics are not. A possible explanation of this selective efficacy may be that serotonin selective reuptake inhibitors (but not tricyclics) act to increase levels of neurosteroids [27]. Neuroactive steroids are synthesized from cholesterol and act directly in the brain. Some neuroactive steroids, such as allopregnanolone, produce behavioral effects that are similar to those caused by benzodiazepine drugs, including anticonvulsant and anxiolytic actions. They alter neuronal excitability by binding to allosteric sites on neurotransmitter-gated ion channels such as the GABA-mediated chloride ion channel [28]. Allopregnanolone and progesterone (which has both neuoractive and standard steroid effects) show a significant increase across pregnancy, with the highest levels at term. Rapid withdrawal, such as that which occurs at birth, could produce a marked increase in anxiety, activation, and excitability in vulnerable women in the postpartum period [16].
Synthetic progesterone has also been investigated for prophylactic efficacy for depression in the post-birth period [29]. The efficacy of norethisterone enanthate was assessed in a double-blind placebo-controlled randomized clinical trial involving 180 women using non-hormonal contraception (progesterone, N=90; placebo, N=90). Women were randomly assigned to receive either a single dose of norethisterone enanthate (200 mg) or normal saline placebo by intramuscular injection within 2 days after birth. The mean depression scores were significantly higher in the progestogen-treated compared to the placebo-treated women at 6 weeks postpartum. However, no significant difference in depressive symptomatology was evident at one week or three months postpartum. This study should be considered of considerable interest due to the quality of the methodology.
3.3 Treatment of PPD – Open-label studies and Case Reports (Table 3)
TABLE 3.
PPD TREATMENT – OPEN STUDIES AND CASE REPORTS
| Article | Sample size (N) | Depression Baseline |
Intervention | Duration Treatment | Measures | Outcome/Results |
|---|---|---|---|---|---|---|
| Roy et al., 1993 (30) |
4 Breastfeeding women: NS |
Case 1: HAM=30 Case 2:HAM =26 Case 3: HAM= 25 Case 4: HAM= 22 |
FLUO: 20 mg/day | NS (at least 3 weeks) |
HAM-D, BDI, CGI-S |
All cases recovered. Recovery criteria: HAM-D ≤7 |
| Stowe et al., 1995 (31) |
26 Breastfeeding women: Included |
Mean (SD) SIGH-D= 22 (3) Mean (SD) BDI=24 (7) Mean (SD) EPDS= 20 (3) |
SER: dose begun at 50 mg/day for the first 2 weeks, adjusted up to a maximum of 200 mg/day. Mean (SD) dose in the study 108 (37) mg/day |
8 weeks | EPDS, SIGH-D, CGI |
Response rate and remission rates by week 8 were 95.2% and 66.6%, respectively. Response criteria: >50% reduction from baseline SIGH-D scores. Recovery criteria: SIGH-D < 7 or CGI = 1 |
| Ahokas et al.,1998 (35) |
2 Breastfeeding women: NS |
Case 1: MADRS=43 Case 2: MADRS=39 |
Sublingual estradiol: dose begun at 4 mg/day for the first week and decreased to 3 mg/day for the second week for one case; the other case continued with 4 mg/day for the second week |
2 weeks | MADRS | Both cases recovered by week 2 Recovery criteria: MADRS ≤ 7 |
| Ahokas et al.,2001(36) |
23 Breastfeeding women: Included |
Mean (SD) MADRS tot score= 40.7 (2.8) |
Sublingual physiologic 17β- estradiol : mean daily dose for the first week 3.9 mg and thereafter 4.8 mg |
8 weeks | MADRS | Recovery rate at week 1 and week 8 were 39.1% and 100%, respectively. Recovery criteria: MADRS ≤ 7 |
| Suri et al.,2001 (33) |
6 Breastfeeding: NS |
Inclusion criteria included HAM D≥17 and EPDS≥12 |
FLUVO: dose begun at 50 mg/day, was titrated to 150 mg/day over the first 2 weeks |
8 weeks | HAM-D, EPDS | Remission rate at week 8 was 67%. The greatest degree of improvement occurred between weeks 2 and 3 (p= 0.003). Remission criteria: HAM-D≤7 |
| Cohen et al., 2001 (32) |
15 Breastfeeding: Excluded |
Mean (SD) HAM-D tot score= 26.13 (5.15). Mean(SD) Kellner anxiety subscale score=18.8 (3.8) |
VENLA immediate release: dose begun at 37.5 mg/day up to a maximum of 225 mg/day. Mean dose across the study:162.5mg/day (range 75–225 mg/day) |
8 weeks | HAM -D, KSQ, CGI |
Significant improvement in depressive and anxiety symptoms occurred by week 2. Remission rate by end point=80% Remission criteria: HAM-D ≤7, or CGI ≤2 |
| Nonacs RM et al., 2004(34) |
8 Breastfeeding: Included |
Median HAM- D= 20.5 (range 15– 38). Median Kellner depression subscore=17.5 (range 11–33). Media Kellner anxiety subscore= 15 (range 3–22) |
BUPRO sustained release: dose begun at 150 mg/day, adjusted up to a maximum of 400 mg/day. Median dose across the study:262.5 mg (range 37.5–300 mg). |
8 weeks | HAM-D, KSQ, CGI |
Response and remission rates by week were 75% and 37.5%, respectively. None of the 3 patients with PPD and comorbid anxiety disorder achieved remission. Response criteria: 50% reduction of HAM-D and KSQ scores. Remission criteria: HAM-D ≤7 |
Abbreviations: VENLA: venlafaxine; BUPRO: bupropion; FLUVO: fluvoxamine, FLUO: fluoxetine; SER: sertraline; SD:standard deviation; NS: not specified in the published article: BDI:Beck Depression Inventory; HAM-D:Hamilton Depression Rating Scale;HAM-A: Hamilton Anxiety Rating Scale;CGI: Clinical Global Impression Scale; MADRS:Montgomery-Åsberg Depression Rating Scale; KSQ: Kellner Symptom Questionnaire; SIGH-D: Structured Interview Guide for the Hamilton Rating Scale for Depression
Roy et al.[30] reported 4 cases of moderate to severe PPD with onset within 12 weeks after birth. The women were treated with fluoxetine (20 mg daily) for 3 to 6 weeks. All subjects achieved recovery, defined as Hamilton Depression Scale (HAM-D) ≤7 or Clinical Global Impression Scale (CGI) = 1. The description of 4 single cases limits the generalizability of the results to the general population. Stowe et al.[31] reported the efficacy of sertraline in a prospective open-label study of 26 women with moderate PPD onset within 24 weeks after birth. The dose was 50 mg/day for the first two weeks and increased to a maximum of 200 mg/day depending on depressive symptoms and tolerance. All women in the study also received supportive psychotherapy. By week 8, 14 subjects experienced recovery defined as HAM-D ≤7 or CGI = 1), and 20 subjects achieved response (defined as >50% reduction from baseline HAM-D scores). Definitive conclusions from this study are limited secondary to the open design, small sample size, and the use of concomitant psychotherapeutic interventions.
Cohen et al [32] reported data on an 8-week open label trial of venlafaxine in 15 women with severe PPD with comorbid anxiety (mean HAM-D at baseline = 26.13 ± 5.15). The mean dose of venlafaxine across the study was 162.5 mg/day (range 75–225). After 2 weeks of treatment, the subjects had a significant decrease in depression and anxiety symptoms, and the rate of remission at week 8 was 80% (defined as HAM-D ≤7 or CGI ≤2). The major limitations of this study were the small sample size and the lack of double-blind randomized design.
Suri et al [33] conducted an 8-week open label trial of the use of fluvoxamine for the treatment of PPD in 6 subjects with scores of ≥17 on the 21-item HAM-D and ≥12 on the Edinburgh Postnatal Depression Scale (EPDS). The fluvoxamine dose was initiated at 50 mg/day and titrated to 150 mg/day over the first 2 weeks. The mean final daily dose of fluvoxamine for all subjects was 142 mg/day ±20 (150 mg/day for the three responders and both nonresponders and 100 mg/day for one responder). The rate of remission was 67% (defined as HAM-D ≤7) and the greatest degree of improvement occurred between weeks 2 and 3. The major limitations of this study were the small sample size, lack of randomized double-blind design. Moreover, the baseline HAM-D scores were not reported.
Nonacs et al [34] conducted a pilot study on the use of bupropion for the treatment of PPD. Eight women with moderate PPD were enrolled in an 8-week open label trial using a flexible dosing schedule (median HAM-D at baseline=20.5, range15–38). Three women also had comorbid anxiety disorders. Dosage started at 150 mg/day and was adjusted at each visit to a maximum of 400 mg/day depending on side effects and depressive symptoms. The median effective dosage across the study was 262.5 mg (range 37.5–300 mg). Concomitant zolpidem (up to 10 mg) and lorazepam (up to 2 mg/day) were permitted. At the study end-point, 6 of the 8 patients (75%) achieved response and 3 achieved remission (37.5%). Response rates were similar of those found by Cohen et al.[32] and Stowe et al. [31] with venlafaxine and sertraline, respectively. However, the rates of remission were lower. Only a trend toward significant improvement in anxiety symptoms was observed and none of the 3 patients with PPD and comorbid anxiety disorder achieved remission. The small sample size and the open-label design limit the generalizability of the results.
All studies cited above were conducted among clinically significant depressed subjects and included antidepressants with different mechanisms of action (venlafaxine, bupropion). Only venlafaxine adequately treated both depressive and anxiety symptoms, which are common in women with PPD.
Estradiol has been evaluated for the treatment of PPD. Ahokas et al.[35] described the treatment of 2 women with severe PPD according to ICD-10 criteria. The women had low pretreatment estradiol levels (140 pmol/L and 23 pmol/). They received 2 weeks of sublingual estradiol monotherapy (4 mg daily for the first week and 3 mg daily for the second week for one subject; the other subject received 4 mg both weeks). By the end of the second week, depressive symptoms significantly decreased as estradiol serum levels increased. The same author [36] also conducted a 8-week open study of sublingual physiologic micronized beta-estradiol (1 mg 3–8 times daily depending on estradiol serum levels) in 23 women with severe PPD with onset within 6 months postpartum. The women had baseline serum estradiol concentrations equal to or less than 200 pmol/L. During the first 2 weeks the depressive symptoms decreased and the serum estradiol levels increased significantly. At week 8, all subjects achieved recovery, as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤7. Of note, 14 of the 23 subjects were also taking antidepressants, which creates uncertainty about the unique contribution of estradiol therapy for the treatment of PPD. The open design, small sample size, and the use of concomitant pharmacological psychotherapeutic interventions are the major limitations of this study.
3.4 Treatment of PPD – Randomized Clinical Trials (Table 4)
TABLE 4.
PPD TREATMENT - RANDOMIZED CLINICAL TRIALS
| Article | Sample Size | Depression Baseline |
Pharmacological Intervention |
Duration Treatment |
Measures | Main Outcome/Results |
|---|---|---|---|---|---|---|
| Gregoire et al.,1996(41) |
61 (34 in E2 group, 27 in the placebo group). Breastfeeding women: Excluded |
Mean (SD) EPDS tot. score in the E2 group and the placebo group were 21.8 (3) and 21.3 (2.9), respectively |
Transdermal 17β- estradiol: dose begun at 200 microgram/day for 6 months. After 3 months dydrogesterone was added (10 mg/day for 12 days monthly) |
6 months | EPDS, SAD | Both groups improved over time; however, the E2 treated group improved rapidly during the first month. Remission rate by month 3 in E2 group and placebo group were 80% and 31%, respectively Remission criteria: EPSD <14 |
| Appleby et al.,1997 (38) |
87: FLUO + 1 session of counseling (22), FLUO + 6 sessions of counseling (2), placebo + 1 session (23), placebo + 6 sessions (2) Breastfeeding women: Excluded |
Mean HAM-D total score in the FLUO group = 13.3 (range 12.2– 14.5). Mean HAM-D score in the placebo group=14 (range 12.5– 15.7) |
Combination of FLUO or placebo plus either 1 or 6 sessions of counseling |
12 weeks | EPDS, HAM-D, Revised clinical Interview Schedule |
After 1 week of treatment improvement in the FLUO group was > placebo group. Patients receiving 6 sessions of counseling improved more than patients receiving 1 session. |
| Misri et al., 2004(39) |
35 (16 in the PARO group, 19 in the CBT+PARO group) Breastfeeding :NS |
Mean (SD) HAM-D in the PARO group and CBT+PARO group were 22.06 (3.38)and 21.16 (2.03), respectively. Mean (SD) HAM-A were 20.31 (6.58)and 21.32 (8.22), respectively |
PARO: dose begun at 10 mg/day, tailored up to a maximum of 50 mg/day. CBT+PARO: Paroxetine plus 12 sessions of CBT |
12 weeks | HAM-D, HAM-A, EPSD, CGI, YBOCS |
Response rates at final visit in the PARO group and in the CBT+PARO group were 87.5% and 78.9 (p=0.50), respectively. Anxiety response rates at final visit in the PARO group and in the CBT+PARO group were 75% and 84.2 % (p= 0.50), respectively. Response criteria: ≥50%baseline HAM scores |
| Wisner et al.,2006(37) |
109 (55 in the SER group, 54 in the NTP group). Breastfeeding: Included |
Mean (SD) HAM-D in the NTP group and in the SER group were 24.7 (7.2) and 25.8 (SD 5.9), respectively |
NTP: starting dose 10 mg/d up to a maximum of 150 mg/day. SER: starting dose 25 mg/d up to a maximum of 200 mg/day. |
8 weeks | HAM-D, CGI-I | Response rates and median time to remit did not differ between NTP and SER group at week 8 and 24 : 55% and 67% at week 8; 79% and 73% at week 24, respectively (p=1). Remission criteria: HAM-D <7 |
| Yonkers et al.,2008(40) |
70 (35 in the PARO group, 35 in the placebo group). Breastfeeding: Included |
Mean (SD) HAM-D score in the PARO group and placebo group were 23.6 (4.7) and 24(5), respectively |
PARO: dose begun at 10 mg/day for the first 2 weeks, increased to 20 mg/day for the third and forth week and further increased to 30 or 40 mg/day depending on clinical status. Mean (SD) dose at study end point = 21.1 (10.7) mg/day |
8 weeks | HAM-D, CGI Inventory of Depressive Symptomatology- Self Report |
Paroxetine was associated with higher rates of remission at week 8 compared to placebo (37% and 14%, respectively). OR (95% interval confidence) =3.5 (1.1–11.5) (p=.04). Remission criteria: HAM-D ≤8 |
Abbreviations: FLUO: fluoxetine;E2: estradiol treated group; PARO:paroxetine monotherapy; SER: sertraline; NTP: nortriptyline; NS:not specified in the published article; PDS : Edinburgh Postnatal Depression Scale; HAM-D:Hamilton Depression Scale ; CGI:Clinical Global Impression Scale; YBOCS: Yale-Brown Obsessive Compulsive Scale; MADRS:Montgomery-Åsberg Depression Rating Scale; SAD:Schedule for Affective Disorders and Schizophrenia; CBT: cognitive behavioural therapy
In a double-blind randomized controlled trial, 54 subjects taking nortriptyline were compared with 55 subjects taking sertraline for PPD [37]. The study included an acute phase 8 week randomized clinical trial, followed by a 16-week continuation phase to test durability of response. Women were treated with a fixed dosage strategy that required dose escalation unless the woman had prohibitive side effects or remission (starting dose of nortriptyline and sertraline 10 mg/day and 25 mg/day and maximum dose 150 mg/day and 200 mg/day, respectively). The proportion of women responding and remitting did not differ between drugs at 4, 8 or 24 weeks, and time to response and remission also did not differ between the two drug groups. For both drugs, over 75% of women who remitted by week 8 also met response criteria by week 4. Psychosocial functioning improved also similarly in both drug-treated groups of mothers. The total side effect burden of each drug was similar, although side effect profiles differed between agents. The dosages required to achieve remission after 8 weeks of treatment were: for the 25 sertraline-treated remitters: only 1 = less than 100 mg/d, 12 = 100 mg/d, 5 = 125 or 150 mg/d, and 7 = 200 mg/d; for 26 nortriptyline remitters: 15 = less than 100 mg/d, 7 = 100 mg/d, and 4 = 125 or 150 mg/d.
Appleby et al [38] reported a very large effect size (2.4) for fluoxetine versus placebo after 4 weeks of treatment. Eighty seven women with major or minor depressive disorder at 6–8 weeks after birth participated in a 12-week double-blind (for pharmacotherapy), randomized clinical trial with 4 treatment cells: fluoxetine (20 mg/day) or placebo plus one or six sessions of counseling derived from cognitive-behavioral therapy. The mean HAM-D total scores in the fluoxetine group and in the placebo group were 13.3 (12.2–14.5) and 14 (12.5–15.7) respectively. Both fluoxetine and counseling were more effective than placebo in improving depressive symptoms. No advantage in treatment outcomes was observed with the combination of counseling and fluoxetine. Since the baseline depression severity was mild (according to HAM-D score) the results of this study cannot be generalized to women with moderate to severe depression. Moreover, the outcome was limited to improvement in depressive symptoms.
Similarly, Misri et al.[39] did not observe additional advantage in combining paroxetine with 12 sessions of cognitive behavior therapy in the treatment of PPD with comorbid anxiety disorders. In this study, 16 subjects receiving paroxetine in monotherapy (dose begun at 10 mg daily, individually tailored up to a maximum dose of 50 mg daily) were compared to 19 subjects receiving paroxetine in combination with cognitive behavioral therapy (1 hour session weekly for 12 weeks) in a 12-week randomized controlled trial. The mean HAM-D score at baseline in the paroxetine group was 22.06±3.38, and in the combined group was 21.16±2.03). Both groups had a high response rate with no significant difference (87.5% in the paroxetine group versus 78.9% in the combined therapy group). Also, the mean time (weeks) to achieve remission and the mean dose of paroxetine used to achieve remission were not significantly different between groups. Both treatments were equally efficacious in reducing anxiety symptoms. The major limitation of this study is the small sample size.
Yonkers et al.[40] conducted an 8-week double-blind placebo-controlled randomized clinical trial in 70 women with onset of PPD within 3 months after birth. Thirty-five women received immediate-release paroxetine (begun at 10 mg/day for the first 2 weeks, increased to 20 mg/day for the third and forth week and further increased to 30 or 40 mg/day if needed). Thirty-five women received placebo. The rate of remission at week 8 (defined as HAM-D ≤ 8) was significantly higher in the paroxetine group compared to placebo (37% compared to 14%; p= .04). On the contrary, response rate at week 8 (defined as CGI= 1 or 2) was higher in the paroxetine group but did not achieve statistical significance (43% compared to 32% in the placebo group; p= .94), and the dose of paroxetine did not differ in the responders compared to non responders (22.9 mg/day +/− 12.1 and 19.4 mg/day +/−9, respectively; p=.34). The major limitation of this study is the small sample size.
Gregoire et al [41] randomized 61 women with severe PPD to placebo (N=27) or 17β-estradiol (17β-E2) (N=34) (200 mcg/day) delivered by transdermal patch for 6 months. The mean EPDS total score in the E2 group was 21.8 ± 3.0, and in the placebo group was 21.3±2.9. The mean E2 concentration of actively treated women was 680 pmol/L (as a comparison, the mean E2 concentration across the menstrual cycle is 370 pmol/L). Both groups improved over time; however, the E2 treated group improved rapidly. The mean EPDS score for E2-treated subjects was <14) and was 4 points lower than that of the placebo group at study completion. Symptomatic improvement in the E2 group was 2-fold higher than that of the placebo group. By 3 months of treatment, 80% of the E2 group but only 31% of placebo group had EPDS scores <14. Because assessments were done once a month, the time course of response in the early weeks of treatment is unknown. E2 treatment was well-tolerated as judged by low attrition. Endometrial changes were found in 3 participants at the study conclusion (6 months), despite co-administration of dydrogesterone (10mg/day, 12 days per month in the final 3 months of the E2 trial). The endometrial changes resolved at followup. The inclusion of women who took concurrent antidepressant medications (47% and 37% in the E2 and placebo arms, respectively) limits the ability to discern an E2-specific treatment effect. Moreover, the validity of the findings would be increased if they were confirmed with a clinician interview-based measure.
The studies of Gregoire et al [41] and Ahokas et al [36] suggest that E2 treatment is associated with a rapid and robust response in women with PPD. The recovery rate reported in both E2 trials was higher than that of standard antidepressants [42, 43]. Although symptom reduction was considered rapid in the E2 trials compared to that of antidepressants, the dictum that the response to an antidepressant response is delayed by many weeks was challenged by the findings of a meta-analysis [44]. The authors selected 47 studies that included antidepressant medications with established efficacy, obtained weekly or biweekly assessments, and presented the time course of improvement as measured by the HAM-D. The greatest amount of improvement occurred during the first 2 weeks. Drug-placebo differences were not only present but were most pronounced during the first 2 weeks of treatment and diminished thereafter.
3.5 Prevention and Treatment of PPD: The Impact of Maternal Breastfeeding
The many short and long-term benefits of breastfeeding for maternal and infant health are well-documented [45]. Although beyond the scope of this article, we acknowledge that breastfeeding has an impact on both a woman’s acceptance of pharmacotherapy and the agent selected. Women avoid pharmacotherapy because of concerns of effects on the nursing infants [46]. [47]. Physicians frequently encounter challenging questions such as whether breastfeeding and antidepressants are mutually exclusive or whether there is evidence to support that specific antidepressants are more favorable for use during breastfeeding. Results of a detailed review and pooled analysis of antidepressant level in lactating mothers, breast milk and nursing infants published by Weissman et al. [48], indicated that antidepressants differ with respect to infant exposure. Results of the pooled analysis suggested that infants exposed to paroxetine and sertraline through breast milk are unlikely to develop detectable serum drug levels, whereas infants exposed to fluoxetine are more likely to develop elevated levels of the drug especially if the mothers started the treatment during pregnancy (due to prenatal loading). The data on citalopram, although limited compared the other SSRIs, suggested that some infants rarely develop quantifiable serum levels of the drug which may be associated with adverse effects. With respect to significant clinical impact in the nursing infant short-term (within 6 months of age) adverse effects have been identified for the tricyclic doxepin (i.e. respiratory depression, hypotonia, vomiting), citalopram (uneasy sleep), paroxetine (lethargy, poor weight gain, hypotonia) and fluoxetine (incontrollable crying, irritability, poor feeding), whereas data on the long-term effects (beyond 6 months of age) were limited.
We recently updated the review by Weissman et al. and presented evidence on the more recently released antidepressants [49]. The recent studies evaluating the long-term effects of antidepressants delivered through breast milk are limited to infants one year of age and are often confounded by prenatal exposure to either drug or major depression. The only indirect biological evidence that serotonin transmitters are not affected are studies [50, 51] with no observed platelet serotonin effects in breastfed infants with mothers treated with sertraline or fluoxetine. Empirical studies on escitalopram, buproprion and duloxetine are represented by uncontrolled studies and case reports and are limited to short-term evaluation. The pharmacokinetics of duloxetine (but no infant adverse events) was evaluated in one uncontrolled study of 6 women taking 40 mg of the drug every 12 hours for 3.5 days [52]. After the seventh dose plasma and milk samples were obtained over 12 hours. The milk to M/P was 0.25, and the relative dose of duloxetine was 0.14%. Data on the recently released venlafaxine metabolite (desmethylvenlafaxine) are not yet available
4 CONCLUSIONS
Studies of the efficacy of antidepressants and hormonal supplements for the prevention of PPD are limited to 3 randomized controlled trials and 4 open label studies. The only agent shown to be efficacious in a randomized controlled trial is the SSRI sertraline [25]. In open label studies progesterone has been shown to prevent PPD recurrence [22, 23] but a placebo-controlled randomized trial of norethisterone enanthate (synthetic progesterone) has been shown to increase the risk depressive symptoms at 6 weeks postpartum [29]. Estrogen was efficacious in preventing prevent PPD recurrence in one open label study [21], but its preventive efficacy has not been evaluated in randomized clinical trials.
The efficacy of antidepressants and hormonal supplements in the treatment of PPD is limited to 8 open label studies and 5 randomized controlled trials. Venlafaxine has been shown to adequately treat depressive and anxiety symptoms in an open label study of women with PPD with comorbid anxiety [32]. This result is important, since PPD is characterized by prominent anxiety symptoms [9]. Sertraline has been shown to be as effective for PPD as nortriptyline with a similar side effect burden. [37]. Transdermal estradiol and paroxetine were efficacious in the treatment of PPD in placebo-controlled randomized clinical trials [40, 41]. In a randomized clinical trial comparing paroxetine monotherapy and paroxetine in combination with cognitive-behavioral therapy in women with PPD and comorbid anxiety symptoms, both treatments were associated with a high response rate for depression and anxiety. Fluoxetine and paroxetine in monotherapy have been found to be as efficacious as CBT and no additional advantage was found in combining pharmacotherapy to CBT in two randomized clinical trials. [38, 39]
Sertraline and paroxetine (among SSRIs) and nortriptyline and imipramine (among TCAs) are the most evidence-based medications for use during breastfeeding because of similar findings across multiple laboratories, usually nonquantifiable infant serum levels and no reports of short term adverse events. [48]. However, evidence on the more recently released antidepressants (buproprion, duloxetine, escitalopram) are represented by uncontrolled studies and case reports and are limited to short-term evaluation. For the recently released venlafaxine metabolite (desmethylvenlafaxine) no data are available [34, 52].
5 EXPERT OPINION
5.1 General consideration
PPD is a major public health problem that compels clinical research prioritization [53]. An issue that deserves clarification is the definition of PPD, which differs across the DSM-IV and ICD-10 with respect to time of onset and illnesses that can be assigned the postpartum onset specifier. An international expert panel convened at Satra Bruk, Sweden, recommended 3 months as the time frame for specifying postpartum onset [54]. This decision was influenced by epidemiologic studies conducted by Kendell and colleagues [24] and confirmed by Munk-Olsen et al [5]. The lack of a standard definition based upon evidence results in difficulty comparing treatments for the disorder.
Given the point prevalence of PDD [3] women should be screened during the first postpartum year. Health care professionals specialized in postpartum care (e.g. obstetrician-gynecologists, pediatricians, health visitors and community midwives) are potential resources for screening. The Edinburgh Postpartum Depression Scale (EPDS) [55] [56] and the Postpartum Depression Screening Scale (PDSS) [57] are screening tools with high degree of specificity and sensitivity to identify women at risk or having PPD in the first year after birth. Any women scoring ≥ 10 on the EPDS or ≥ 80 on the PDSS should be referred for psychiatric evaluation to confirm diagnosis of major or minor PPD. To date, the hypothesis that postpartum screening leads to improved maternal and infant health outcomes has not been confirmed, largely due to multiple barriers to mental health care for patients identified in primary care settings [58] with additional factors related to childbearing [59].
5.2 Preventive treatment
The lasting impact of depression on the mother-child dyad’s health [18] [19]supports preventive treatment for women with history of PPD. Physicians and health care providers should guide the patient through the decision making process identifying risks of untreated illness, infant medication exposure, treatment options, and alternative therapeutic choices. If preventive pharmacotherapy is not acceptable, closely monitoring for recurrence and rapid implementation of treatment is prudent.
The one positive placebo controlled pharmacologic prevention study demonstrated the efficacy of sertraline [25]. Women who have a history of PPD are candidates for the initiation of sertraline immediately following birth to prevent the emergence of episodes during this high-risk period. The physician has the option of following the dosing protocol utilized in the investigation of Wisner et al [25] as well as more flexible dosing for individual patients. For example, doses higher than the maximum used in that protocol (75 mg/day) could be prescribed for women who had previously responded to and tolerated higher doses. The duration of the treatment should be a minimum of 6 months. Although other serotonergic antidepressants have not been evaluated for preventive efficacy, the explanation for the efficacy of sertraline is based upon its serotonergic impact. Therefore, use of another serotonergic agent, particularly one to which the woman has responded in the past, is a reasonable preventive option if sertraline is not suitable.
5.3 Active treatment
As discussed above, antidepressants appear to be similarly effective for PPD as for major depressive disorder at other times in the life cycle. Consistent with the clinical management of PPD developed by three scientific commissions (National Institute for Clinical Excellence, American College of Obstetrics and Gynecologists, Academy of Breastfeeding Medicine) [60–62],we conclude:
Antidepressants are efficacious for women with PPD. They should be considered for women with moderate to severe depression or for women not responding to (or declining) psychological treatments.
The selection of the specific antidepressant for a woman with PPD must derive from a personalized risk-benefit analysis considering the risk of untreated maternal illness, the risk/benefit of the specific treatment, and (if appropriate) the risk/benefit of breastfeeding for the mother/infant pair [63].
The choice of the drug is based on clinical factors, particularly previous efficacious treatments and patient preference [53].
If the woman is suffering from the first lifetime episode, physicians should consider that fluoxetine and paroxetine were shown to be more efficacious than placebo in randomized controlled trials [42] [44], and nortriptyline and sertraline had similar efficacy in a randomized comparison trial [41].
Sertraline or paroxetine (among SSRIs) or nortriptyline or imipramine (among TCAs) should be the agents of first choice during breastfeeding because they are the most evidence-based medications (similar findings across multiple laboratories, usually undetectable infant serum levels and no reports of short term adverse events). Although TCAs are reasonable treatment options for PPD, they remain second line drugs due to their toxicity in overdose and suicidal risk. The more recently released antidepressants (escitalopram, duloxetine, bupropion) should not be first choices in postpartum women who continue breastfeeding unless demonstrated efficacy in past episodes is clear and no new contraindications exist for their use in the individual woman [48, 49]
Nursing infants’ clinical conditions, particularly for sick or low birth weight infants, should be monitored to detect initial adverse effects that may be attributed to the maternal drug (behavioral activation or sedation; new onset feeding or sleeping problems). Routine laboratory measurement of infant serum drug levels for clinical purpose was not considered warranted in a major review [48].
Much potential for pharmacologic intervention development exists for PPD over the next 5 – 10 years. With respect to preventive treatment, an injectable progestogen did not demonstrate preventive efficacy compared to placebo; however, in a small open trial, estrogen yielded promising results that compel further study in a preventive model.
A convergence of research compels further evaluation of estradiol as a treatment for PPD because both open and a randomized double blind controlled trials have shown robust and rapid response in women with PPD. Many women welcome a natural alternative to pharmacotherapeutic agents, and major side effects of estradiol were rare within the dose ranges investigated. Estradiol is a mechanistically appropriate treatment since women with PPD develop depressive symptoms in conjunction with steroid hormone withdrawal. The treatment is convenient in once or twice weekly skin patch form, and it is inexpensive.
Little is known about estradiol secretion into breast milk; however, Perheentupa et al [64] did not detect estradiol (limit of detection=25 pmol/L) in the breast milk of 18 lactating women who used transdermal estradiol at a dose of 50–100 µg/day. While high doses of E2 can reduce lactation [65], breastfeeding frequency was no different between women randomized to treatment with transdermal E2 (50–100 µg/d) or placebo for 12 weeks [64].
An entirely unstudied area in the pharmacotherapy of PPD is management of women who do not achieve remission to the initial pharmacologic agent. The applicability of strategies for subsequent pharmacotherapy for major depressive disorder specifically for PPD has not been studied. The large-scale NIMH–funded Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study did not include pregnant or breastfeeding women. This study included four successive steps in an adult outpatient group with nonpsychotic major depression, and the overall cumulative remission rate was only 67% [66].
Finally, the expansion of information on the efficacy of complementary and alternative or integrative pharmacologic therapies (reviewed by Freeman [67]) would allow assessment of their appropriate role in the armamentarium of therapies for PPD.
Acknowledgments
Dr Wisner is sponsored by NIMH: R01, Identification and Therapy for Postpartum Depression. NIMHR01, Antidepressant Use during Pregnancy, NIMH R01, Antimanic Use during Pregnancy. By the State of Pennsylvania for Increasing Maternal Infant Mental Health Services; feasibility study for depression education for Nurse Family Partnerships (PA). Conference support was provided by the Fine Foundation and Staunton Farms Foundation. Support was also provided by the Heinz Foundation: Teens—Building Options and Opportunities for Mothers grant. Finally, support was provided by Nova-Gyne for the donation of transdermal placebo patches for an NIMH funded study of estradiol patch for postpartum depression treatment.
Footnotes
Declaration of interest
Contributor Information
Teresa Lanza di Scalea, Email: lanzadiscaleat@upmc.edu, Operative Unity of Psychiatry, University of Rome Tor Vergata - Neuroscience Via Nomentana 1362, Rome, Italy.
KL Wisner, University of Pittsburgh - Epidemiology and Women's Studies USA.
REFERENCES
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatry Association; 1994. [Google Scholar]
- 2.WHO. Geneva: World Health Organization; Classification of Mental and Behavioral Disorders - Clinical Descriptions and Diagnostic Guidelines ICD-10. (10 ed.) 1992
- 3.Gaynes B, et al. [Last accessed 2009 April, 21];Perinatal Depression: Prevalence,Screening Accuracy, and Screening Outcomes. 2005 doi: 10.1037/e439372005-001. Available from: http://www.ahrq.gov/downloads/pub/evidence/pdf/peridepr/peridep.pdf. [DOI] [PMC free article] [PubMed]
- 4.O'Hara M, Swain A. Rates and risk of postpartum depression: a meta analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- 5.Munk-Olsen T, et al. New parents and mental disorders: a population-based register study. JAMA. 2006;296(21):2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 6.Nonacs R, Cohen LS. Postpartum psychiatric syndromes. In: Sadock BJ, Sadock VA, editors. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. Philadelphia, PA: 2000. pp. 1276–1283. [Google Scholar]
- 7.Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77–87. doi: 10.1016/0165-0327(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, et al. Non-psychotic psychiatric disorder after childbirth A prospective study of prevalence, incidence, course and nature. Br J Psychiatry. 1988;152:799–806. doi: 10.1192/bjp.152.6.799. [DOI] [PubMed] [Google Scholar]
- 9.Wisner KL, et al. Obsessions and compulsions in women with postpartum depression. J Clin Psychiatry. 1999;60(3):176–180. doi: 10.4088/jcp.v60n0305. [DOI] [PubMed] [Google Scholar]
- 10.Angold A, Worthman C. Puberty onsent of gender differences in rates of depression: a developmental, epidemiological, and neuroendocrine perspective. Journal of Affective Disorders. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 11.Bebbington P, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Psychol Med. 1998;28:9–19. doi: 10.1017/s0033291797006077. [DOI] [PubMed] [Google Scholar]
- 12.Parry BL. Postpartum psychiatric syndromes. In: Kaplan H SB, editor. Comprehensive Textbook of Psychiatry. IV edition. Volume 1. Philadelphia: Williams & Wilkins; 1995. [Google Scholar]
- 13.Parry BL. Mood Disorders Linked to the Reproductive Cycle in Women. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd.; 1995. pp. 1029–1042. [Google Scholar]
- 14.Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity--adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation An overview. Prog Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- 15.O'Hara MW, et al. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- 16.Bloch M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 17.Wisner KL, et al. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62(2):82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- 18.Hay DF, et al. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J Child Psychol Psychiatry. 2008;49(10):1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 19.Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33(3):543–561. doi: 10.1111/j.1469-7610.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 20.Wisner KL, Wheeler SB. Prevention of recurrent postpartum major depression. Hosp Community Psychiatry. 1994;45(12):1191–1196. doi: 10.1176/ps.45.12.1191. [DOI] [PubMed] [Google Scholar]
- 21.Sichel DA, et al. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38(12):814–818. doi: 10.1016/0006-3223(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 22.Dalton K. Progesterone prophylaxis used succesfully in postnatal depression. Practitioner. 1985;229:507–508. [Google Scholar]
- 23.Dalton K. Succesful prophylactic progesterone for idiopathic postnatal depression. Int. J. Prenatal and Perinatal Studies. 1989:323–327. [Google Scholar]
- 24.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 25.Wisner KL, et al. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161(7):1290–1292. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- 26.APA. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders. American Psychiatric Publishing, Inc; 2006. [Google Scholar]
- 27.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britton KT, Koob GF. Neuropharmacology. Premenstrual steroids? Nature. 1998;392(6679):869–870. doi: 10.1038/31816. [DOI] [PubMed] [Google Scholar]
- 29.Lawrie TA, et al. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 1998;105(10):1082–1090. doi: 10.1111/j.1471-0528.1998.tb09940.x. [DOI] [PubMed] [Google Scholar]
- 30.Roy A, et al. Fluoxetine treatment of postpartum depression. Am J Psychiatry. 1993;150(8):1273. doi: 10.1176/ajp.150.8.1273a. [DOI] [PubMed] [Google Scholar]
- 31.Stowe Z, Casarella J, Landry J, Nemeroff CB. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3:49–55. [Google Scholar]
- 32.Cohen LS, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592–596. doi: 10.4088/jcp.v62n0803. [DOI] [PubMed] [Google Scholar]
- 33.Suri R, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739–1740. doi: 10.1176/appi.ajp.158.10.1739. [DOI] [PubMed] [Google Scholar]
- 34.Nonacs RM, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445–449. doi: 10.1017/S1461145705005079. [DOI] [PubMed] [Google Scholar]
- 35.Ahokas AJ, Turtiainen S, Aito M. Sublingual oestrogen treatment of postnatal depression. Lancet. 1998;351(9096):109. doi: 10.1016/S0140-6736(05)78152-6. [DOI] [PubMed] [Google Scholar]
- 36.Ahokas A, et al. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):332–336. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- 37.Wisner KL, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 38.Appleby L, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932–936. doi: 10.1136/bmj.314.7085.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misri S, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236–1241. doi: 10.4088/jcp.v65n0913. [DOI] [PubMed] [Google Scholar]
- 40.Yonkers KA, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659–665. doi: 10.4088/jcp.v69n0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregoire AJ, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006):930–933. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- 42.Walsh BT, et al. Placebo response in studies of major depression: variable, substantial, and growing.[see comment] JAMA. 2002;287(14):1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 43.Walsh BT, et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 44.Posternak MA, Zimmerman M. Is there a delay in the antidepressant effect? A meta-analysis. J Clin Psychiatry. 2005;66(2):148–158. doi: 10.4088/jcp.v66n0201. [DOI] [PubMed] [Google Scholar]
- 45.Goldman AS, Hopkinson JM, Rassin DK. Benefits and risks of breastfeeding. Adv Pediatr. 2007;54:275–304. doi: 10.1016/j.yapd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Pearlstein TB, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303–308. doi: 10.1007/s00737-006-0145-9. [DOI] [PubMed] [Google Scholar]
- 47.Battle CL, et al. Depression and breastfeeding: which postpartum patients take antidepressant medications? Depress Anxiety. 2008;25(10):888–891. doi: 10.1002/da.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman AM, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066–1078. doi: 10.1176/appi.ajp.161.6.1066. [DOI] [PubMed] [Google Scholar]
- 49.Lanza di Scalea T, Wisner KL. Antidepressant Medication Use during Breastfeeding. Clinical Obstetrics and Gynecology. 2009 doi: 10.1097/GRF.0b013e3181b52bd6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epperson CN, et al. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003;112(5):e425. doi: 10.1542/peds.112.5.e425. [DOI] [PubMed] [Google Scholar]
- 51.Epperson N, et al. Maternal sertraline treatment and serotonin transport in breast-feeding mother-infant pairs. Am J Psychiatry. 2001;158(10):1631–1637. doi: 10.1176/appi.ajp.158.10.1631. [DOI] [PubMed] [Google Scholar]
- 52.Lobo ED, et al. Pharmacokinetics of duloxetine in breast milk and plasma of healthy postpartum women. Clin Pharmacokinet. 2008;47(2):103–109. doi: 10.2165/00003088-200847020-00003. [DOI] [PubMed] [Google Scholar]
- 53.Wisner KL, Chambers C, Sit DK. Postpartum depression: a major public health problem. JAMA. 2006;296(21):2616–2618. doi: 10.1001/jama.296.21.2616. [DOI] [PubMed] [Google Scholar]
- 54.Elliott S. Report on the satra bruk workshop on classification of postnatal mental disorders on november 7–10, 1999, convened by Birgitta Wickberg, Philip Hwang and John Cox with the support of Allmanna Barhuset represented by Marina Gronros. Archives of Women's Mental Health. 2000;3:27–33.
- 55.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 56.O'Hara M. Postpartum depression: identification and measurement in a cross-cultural context. In: Cox J, Holden J, editors. Perinatal Psychiatry. London: Gaskell; 1994. pp. 161–162. [Google Scholar]
- 57.Beck CT, Gable RK. Comparative analysis of the performance of the Postpartum Depression Screening Scale with two other depression instruments. Nurs Res. 2001;50(4):242–250. doi: 10.1097/00006199-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Gilbody S, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 59.Sit D, et al. An Emerging Best Practice Model for Perinatal Depression Care. Psychiatric Services. doi: 10.1176/appi.ps.60.11.1429. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NICE. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance, Clinical Guideline No. 45. [Last accessed 2009 July 10];2007 Available from: http://www.nice.org.uk/nicemedia/pdf/CG045NICEGuidelineCorrected.pdf.
- 61.ABM. The Academy of Breastfeeding Medicine Protocol Committee clinical protocol #18: use of antidepressants in nursing mothers. Breastfeed Med. 2008;3(1):44–52. doi: 10.1089/bfm.2007.9978. [DOI] [PubMed] [Google Scholar]
- 62.ACOG. Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001–1020. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 63.Sit DKY, Wisner KL. Decision Making for Postpartum Depression treatment. Psychiatric Annals. 2005;35(7):577–584. doi: 10.3928/0048-5713-20050701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perheentupa A, Ruokonen A, Tapanainen JS. Transdermal estradiol treatment suppresses serum gonadotropins during lactation without transfer into breast milk. Fertility & Sterility. 2004;82(4):903–907. doi: 10.1016/j.fertnstert.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 65.Booker DE, Pahyl IR. Control of postpartum breast engorgement with oral contraceptives. Am J Obst Gyn. 1967;98:1099–1101. doi: 10.1016/0002-9378(67)90034-8. [DOI] [PubMed] [Google Scholar]
- 66.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 67.Freeman MP. Complementary and alternative medicine for perinatal depression. J Affect Disord. 2009;112(1–3):1–10. doi: 10.1016/j.jad.2008.06.017. [DOI] [PubMed] [Google Scholar]