Abstract
Objective
To examine the trends in incidence and prevalence of rheumatoid arthritis (RA) from 1995 to 2007.
Methods
To augment our preexisting inception cohort of RA patients (1955-1994), we assembled a population-based incidence cohort of individuals aged ≥18 years who first fulfilled ACR 1987 criteria for RA between 1/1/1995 and 12/31/2007 and a cohort of patients with prevalent RA on 1/1/2005. Incidence and prevalence rates were estimated and were age- and sex-adjusted to the 2000 US whites. Trends in incidence rates were examined using Poisson regression methods.
Results
The 1995-2007 incidence cohort comprised 466 patients (mean age 55.6 years; 66% female; 69% rheumatoid factor positive). The overall age- and sex-adjusted annual RA incidence was 40.9/100,000 population. The age-adjusted incidence in women was 53.1/100,000 population,(in men: 27.7/100,000 population). During 1995-2007 there was a moderate increase in RA incidence in women (p=0.02), but not in men (p=0.74). The increase was similar among all age groups. The overall age- and sex-adjusted prevalence on 1/1/2005 was 0.72% (95% CI 0.66–0.77) up from 0.62% (95% CI 0.55–0.69) in 1995 (p<0.001). Applying the 1/1/2005 prevalence to the US 2005 population, an estimated 1.5 million US adults are affected by RA. This is up from the 1.3 million reported previously.
Conclusion
The incidence of RA appears to be rising in women during 1995-2007 period. The reasons for this recent increase in incidence are unknown, but environmental factors may play a role. A corresponding increase in prevalence of RA was also found.
Keywords: rheumatoid arthritis, incidence, longitudinal trends
Introduction
Incidence and prevalence of rheumatoid arthritis (RA) in populations varies substantially between geographic areas and over time(1-12). This geographic and temporal variability cannot be explained by genetic factors alone. Rather, this implicates a combination of environmental exposures and gene-environmental interactions. Despite the variability of RA incidence rates in different populations at various time points, the trends of RA incidence in several countries during the past several decades appeared to follow a similar pattern. Indeed, a number of studies from different countries including studies from our group reported declines in RA incidence during the second half of the 20th century(2-8, 10). The declining incidence combined with the lack of improvement in survival of RA patients resulted in the reported decreases in prevalence of RA(3, 6, 11). However, the more recent trends in RA incidence are much less known. Thus, we sought to extend our studies of RA incidence and prevalence in Olmsted County, MN to include the 1995-2007 period, resulting in a total of more than 50 years of RA epidemiology.
Methods
The population of Olmsted County, MN, represents an optimal source for an investigation of RA epidemiology due to the availability of comprehensive medical records for all residents seeking medical care, and spanning for more than half a century. The unique population-based data resources of the Rochester Epidemiology Project (REP) medical record linkage system provide ready access to the medical records from all health care specialists for the local population. These health care providers include the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Group, the Olmsted Community Hospital, local nursing homes, and the few private practitioners. The unique features and potential of the REP medical record linkage system for population-based studies has been described previously(13-15). This system ensures virtually complete ascertainment of all clinically recognized cases of RA among the residents of Olmsted County, MN.
In order to identify all potential incident cases of RA in this population, the computerized diagnostic index was searched for any diagnosis of arthritis (excluding degenerative arthritis or osteoarthritis) made between 1/1/1995 and 12/31/2007 among Olmsted County, MN residents who were ≥18 years of age. Every person in the community ≥18 years of age who qualified over the defined period regardless of race, ethnicity, or socioeconomic status was included. The complete (inpatient and outpatient) medical record for each potential case was reviewed by a trained nurse abstractor using a pretested data collection form. All questionable cases were additionally reviewed by co-investigators supervised by the principal investigator, and confirmation or rejection of the diagnosis was accomplished based on the 1987 American College of Rheumatology criteria for RA(16). The incidence date was defined as the earliest date at which the subject fulfilled ≥4 1987 ACR criteria for RA. Subjects were allowed to accumulate the criteria over time until fulfillment of the 4th criteria (17). This inception cohort of RA patients diagnosed from 1995 to 2007 augmented the previously assembled cohort of Rochester, MN residents with incident RA from 1955 through 1994. The cohort was also augmented by all Olmsted County, MN residents (including those outside Rochester) with incident RA between 1985 and 1994 (5, 8). As the incidence rates based on this larger geographical area were essentially identical to those previously reported for the 1985 to 1994 time period, this report focuses on the 1995 to 2007 time period. Demographics and incidence rates based on the augmented cohort were used for comparison in the tables and figures.
The prevalence cohort on 1/1/2005 included persons in the incidence cohort who were still alive and living in Olmsted County on the prevalence date, as well as persons who moved into Olmsted County with previously diagnosed RA. The medical records were reviewed to confirm the diagnosis of RA.
Overall incidence rates were age- and/or sex-adjusted to the 2000 white population of the USA. Age- and sex-specific incidence rates were calculated by using the number of incident cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years(18). In order to compute 95% confidence intervals (95% CI) for incidence rates, it was assumed that the number of incident cases followed a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods with smoothing splines for age and calendar year(19). Tests for trends in incidence for women and men were performed using Poisson regression models adjusted for age by fitting a linear calendar year term to the incidence rates for the 1995-2007 time period. Annual incidence rates were illustrated using a 3-year–centered moving average. Trends in age over time were examined using linear regression models.
Prevalence was calculated on 1/1/1995 and 1/1/2005 as the number of Olmsted County, MN residents ≥18 years of age who fulfilled the 1987 ACR criteria for RA on these dates as the numerator, and the county population on the same date as the denominator. Prevalence estimates for 1/1/1995 and 1/1/2005 were compared using an F test (20). The number of affected US adults was estimated by multiplying the prevalence rate for each gender and 5 year age group to the US population in 2005 obtained from the US census (21).
Results
We screened the medical records of 1,761 Olmsted County, MN residents aged ≥18 years who had received one or more diagnoses of arthritis (excluding degenerative arthritis or osteoarthritis) between 1/1/1995 and 12/31/2007. After comprehensive review of the medical record for each potential case, the diagnosis of RA was confirmed for 466 patients. These RA patients comprised the final incidence cohort from 1/1/1995 to 12/31/2007. The mean age at RA incidence was 55.6 years and 321 (69%) were female. Median follow-up was 5.7 years (Min 0.1 years, Max 14.0 years). Rheumatoid factor was positive in 307 (66%) of patients (i.e. at least one positive test for RF during the follow-up period). Definite radiographic changes (erosions) during the first year after RA incidence were found in 92 (20%) patients slightly up from 33 (15%) patients in the previous decade (p=0.08). The proportion of subjects radiographed also increased (from 92% in 1985-1994 to 97% in 1995-2007) which may explain this change in the proportion of patients with erosions. Other baseline demographics and disease characteristics in the incidence cohort from 1995-2007 period were not different from those in the 1985-1994 period, except that a lower percentage of males with RA were smokers in the 1995-2007 period (59%) compared to the 1985-1994 period (78%; p=0.006) (Table 1). During the same period smoking rates in women remained essentially unchanged from the previous decade (51% vs 46%, respectively, p=0.38). For each gender, the changes in smoking status between the decades when current and former smoking was examined separately were similar to those reported for current and former smokers combined (data not shown).
Table 1.
Characteristics of Olmsted County, MN residents ≥18 years of age with incident RA between 1/1/1985 and 12/31/2007
| Variable | Time period |
||
|---|---|---|---|
| 1985-1994 | 1995-2007 | p-value# | |
| Total no. of patients | 240 | 466 | |
| No. (%) female | 160 (67%) | 321 (69%) | 0.55 |
| Age at incidence, years | |||
| Mean, median | 56.6, 55.9 | 55.6, 54.8 | 0.42 |
| Minimum, maximum | 18 - 94 | 19 – 89 | |
| Rheumatoid factor (RF) | |||
| No. (%) of RF positive of those tested* | 165 (69%) | 307 (66%) | 0.44 |
| % tested | 100% | 99.7% | |
| No. (%) with erosions on radiographs in | |||
| 1st year after RA incidence | 33 (15%) | 92 (20%) | 0.08 |
| % radiographed | 92% | 97% | |
| Cigarette smoking (former or current) | 143 (60%) | 233 (50%) | 0.02 |
| Females | 81 (51%) | 148 (46%) | 0.38 |
| Males | 62 (78%) | 85 (59%) | 0.006 |
No. (%) of RA patients who had positive test for RF at least once during the follow-up period
indicates the differences between the two time periods.
The overall age- and sex-adjusted annual RA incidence among Olmsted County, MN residents ≥18 years for the 1995-2007 period was 40.9/100,000 population (95% CI 37.2-44.7) (Table 2). The age-adjusted incidence in women was 53.1/100,000 population (95% CI 47.3–58.9), in men: 27.7/100,000 population (95% CI 23.1–32.2). The incidence of RA overall and in both genders was low in the 18-34 age group, after which it progressively increased with age resulting in the maximal incidence in the 65-74 age group. There was a decline in RA incidence in older ages (≥75 years) (Table 2).
Table 2.
Annual incidence of RA (per 100,000), in Olmsted County, Minnesota between 1995 and 2007, by sex and age group
| Age group | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| No. of pts |
Rate | No. of pts |
Rate | No. of pts |
Rate | |
| 18-34 years | 7 | 3.6 | 27 | 13.8 | 34 | 8.7 |
| 35-44 years | 26 | 19.1 | 73 | 55.0 | 99 | 36.2 |
| 45-54 years | 30 | 26.9 | 72 | 62.4 | 102 | 44.9 |
| 55-64 years | 36 | 51.7 | 55 | 74.2 | 91 | 63.3 |
| 65-74 years | 31 | 72.4 | 51 | 104.4 | 82 | 89.4 |
| 75-84 years | 13 | 52.3 | 30 | 81.1 | 43 | 69.5 |
| > 85 years | 2 | 26.1 | 13 | 63.7 | 15 | 53.5 |
| Overall (95% CI) x | 145 | 27.7 (23.1-32.2) # | 321 | 53.1 (47.3-58.9) # | 466 | 40.9 (37.2-44.7) * |
RA=rheumatoid arthritis
age-adjusted to the 2000 US white population
age- and sex-adjusted to the 2000 US white population
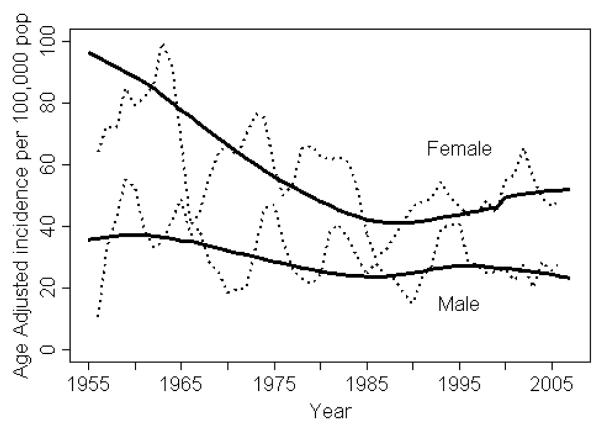
Figure 1 illustrates the trends in the annual incidence rates of RA by gender per 100,000 population from 1995-2007, along with the previously reported data from the 1955-1994 period. RA incidence appeared to increase by 2.5% per year from 1995 to 2007 in women (95% CI: 0.3%, 4.7% per year, p=0.02), but not in men (0.5% decrease per year; 95% CI: −3.6%, 2.7%; p=0.74). There was no apparent difference in the trend for RF positive patients compared to RF negative patients (p=0.26 for women and p=0.70 for men).
Figure 1.
Annual incidence of rheumatoid arthritis per 100,000 population, 1955-2007, in Olmsted County, MN residents, by gender The dashed lines are calculated as a 3-year–centered moving average; the solid lines are trends in incidence rates after adjustment for age.
We then examined whether age at RA incidence has changed over time. The distribution of age at RA incidence was stable from 1980 to 2007 (p=0.32). Furthermore, the increases in incidence rates from the 1985-1994 period to the 1995-2007 period for each gender were proportional to the incidence rates in each age category. In other words, there were no disproportionate increases in RA incidence in particular age groups over time.
The overall age- and sex-adjusted prevalence of RA among those aged ≥18 on 1/1/2005 was 0.72%; up from the estimate of 0.62% on 1/1/1995 (p<0.001) (Table 3). Compared to 1/1/1995 the prevalence of RA in women increased significantly from 0.77% to 0.98% on 1/1/2005 (p<0.001); the prevalence of RA in men on 1/1/2005 was unchanged (p=0.18). Applying the prevalence of RA on 1/1/2005 to the US 2005 population, an estimated 1.5 million US adults are affected by RA. This is up from the 1.3 million reported previously (11).
Table 3.
Prevalence of RA per 100,000 population (age ≥18), overall and by gender on 1/1/1995 and 1/1/2005
| Time point | Male# | Female# | Total* |
|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | |
| 1/1/1995 | 0.44 (0.34-0.53) | 0.77 (0.66-0.87) | 0.62 (0.55-0.69) |
| 1/1/2005 | 0.41 (0.35-0.48) | 0.98 x x (0.90-1.07) | 0.72 x x (0.66-0.77) |
RA=rheumatoid arthritis
age-adjusted to the 2000 US white population
age- and sex-adjusted to the 2000 US white population
indicates significant differences in the prevalence as compared to the previous decade (p<0.001)
Discussion
This study reports the most recent trends in RA incidence in a population-based US cohort. We demonstrated a modest increase in RA incidence among women during the period from 1995 to 2007. This increase followed a sharp decline in RA incidence observed over the previous 4 decades. As expected, the increased incidence was accompanied with an increase in the prevalence of RA.
From the middle of the 20th century, RA occurrence has been widely studied in multiple centers, predominantly in the US and Western Europe (1-8, 10-12, 22). Despite the variable disease frequency reported for different populations, all these studies (including our own) showed a compelling decline in RA incidence over the period 1955-1994. Very few epidemiological studies have reported secular trends in RA incidence after 1995. Pedersen et al. demonstrated an increasing incidence of RA in from 1995-2001, particularly in women(23). These findings are consistent with our own. Kaipiainen-Seppänen et al. compared RA incidence in 1980, 1985, 1990, 1995 and 2000, verifying the previously observed declining trend in our cohort(5, 8). The observed declines in that study were less apparent among cases >64 years of age where a modest increase in incidence was shown from 1980 to 2000(10). This increasing trend in RA incidence is concordant with our data. However, unlike these other reports, we did not observe a difference in increase of RA incidence among different age-groups.
Below we speculate on the possible explanations for the modest increase in RA incidence observed in our study. Oral contraceptives (OC) have been clearly shown to be associated with a dose dependent protective effect against the development of RA in women(1, 24-29). Modern day OC contain significantly lower doses of synthetic estrogens as compared to OC used decades ago(30, 31). Because these lower doses of synthetic estrogens confer less protection against the development of RA than higher doses, it is almost certain that the protective effect of OC has diminished over time. Thus, this exposure may contribute to the observed increase in RA incidence in women.
Breastfeeding is another gender-specific factor reported to have a dose-dependent protective effect against the development of RA(32, 33). Moreover, rates of breastfeeding in the US have been increasing in recent years(34). However, this protective effect is limited to the population of parous women who chose breastfeeding, and the effects are most significant among those who choose long-time (>24 months) breastfeeding, an even smaller number. Therefore, this factor is unlikely to have a major impact on RA incidence.
Cigarette smoking is perhaps the strongest environmental risk factor clearly associated with the development of RA in both genders(35-42). While smoking is less prevalent in the US in general, smoking rates are declining at a significantly slower pace among women compared to men. Thus, the gap in the proportion of male and female smokers has significantly and progressively narrowed since 1965(43). The slower decline in smoking rate among women can, in part, explain the lack of decline in RA incidence rate among women.
Several lines of evidence suggest that vitamin D deficiency may be associated with the development of RA(44-50). In addition, vitamin D deficiency has been demonstrated to be increasing over the previous decades, particularly in women(44). Thus, vitamin D deficiency may also contribute to the observed RA incidence trends.
A number of other environmental factors postulated to play a role in RA incidence, especially in women, may also be implicated in the observed trends. These include infections, immunizations, obesity, socioeconomic status, etc.(51-56).
While speculative, the above findings, when taken together, suggest that the cumulative effect of multiple environmental factors (each associated with either an increased in risk or a loss in protection, particularly in women) could explain the observed rise in RA incidence over the study period. Besides those mentioned, the effect of other, unknown risk factors on the changes in RA incidence over time cannot be excluded.
Our study has several important strengths including its longitudinal population-based design and the use of a systematic and standardized approach to case identification. Study limitations include the possibility of an increased awareness of RA in recent years, potentially including the awareness of the 1987 ACR criteria for RA. The increase in female RA incidence without changes in male incidence and lack of differences in incidence of RF positive and RF negative RA argues against ascertainment bias. Small sample size and resulting lack of statistical power could be responsible for our inability to detect a change in incidence in males. While it is possible that the observed increase in incidence in RA females merely represents a lack of decline, we believe this is unlikely because the increase was observed across a number of years. Furthermore, there is a possibility of underascertainment of cases in studies involving medical record review. However, the comprehensive and standardized approach to case ascertainment described in methods makes selection bias unlikely. Finally, the population of Olmsted County, MN is 90% white suggesting that the results of our study may not be generalizable to other, more racially diverse populations. Furthermore, the demographic characteristics in Olmsted County, MN (including survival rates, aging, race, % of emigrants) have not changed substantially over the recent years and were thus unlikely to influence the observed epidemiological trends.
In conclusion, we observed that the incidence of RA appears to be rising during the period from 1995 to 2007. This rise in incidence followed a period of 4 decades of declining incidence and appeared to be limited to women. The reasons for this increase in incidence are unknown, but environmental factors likely play a role.
Acknowledgments
Funding Source: This work was funded by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and the National Institutes of Health (AR-30582) US Public Health Service
Footnotes
Financial Disclosures: None
References
- 1.Hochberg MC. Changes in the incidence and prevalence of rheumatoid arthritis in England and Wales, 1970-1982. Sem Arthritis Rheum. 1990;19(5):294–302. doi: 10.1016/0049-0172(90)90052-h. [DOI] [PubMed] [Google Scholar]
- 2.Dugowson CE, Koepsell TD, Voigt LF, Bley L, Nelson JL, Daling JR. Rheumatoid arthritis in women. Incidence rates in group health cooperative, Seattle, Washington, 1987-1989. Arthritis Rheum. 1991;34(12):1502–7. doi: 10.1002/art.1780341205. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsson LTH, Hanson RL, Knowler WC, Pillemer S, Pettitt DJ, McCance DR, et al. Decreasing incidence and prevalence of rheumatoid arthritis in Pima Indians over a twenty-five-year period. Arthritis Rheum. 1994;37:1158–1165. doi: 10.1002/art.1780370808. [DOI] [PubMed] [Google Scholar]
- 4.Kaipiainen-Seppanen O, Aho K, Isomaki H, Laakso M. Incidence of rheumatoid arthritis in Finland during 1980-1990. Ann Rheum Dis. 1996;55:608–611. doi: 10.1136/ard.55.9.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, MN, 1955-1985. Arthritis Rheum. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Shichikawa K, Inoue K, Hirota S, Maeda A, Ota H, Kimura M, et al. Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda, Wakayama, Japan, 1965-1996. Ann Rheum Dis. 1999;58(12):751–6. doi: 10.1136/ard.58.12.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silman AJ. The changing face of rheumatoid arthritis: why the decline in incidence? Arthritis Rheum. 2002;46(3):579–81. doi: 10.1002/art.508. [DOI] [PubMed] [Google Scholar]
- 8.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 9.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–8. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Kaipiainen-Seppanen O, Kautiainen H. Declining trend in the incidence of rheumatoid factor-positive rheumatoid arthritis in Finland 1980-2000. J Rheumatol. 2006;33(11):2132–8. [PubMed] [Google Scholar]
- 11.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 12.Silman AJ, Hochberg MC. Descriptive epidemiology of rheumatoid arthritis. In: Hochberg MC, editor. Rheumatoid Arthritis. Elsevier Inc.; 2009. pp. 15–22. [Google Scholar]
- 13.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 14.Melton L. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Maradit Kremers H, Crowson CS, Gabriel SE. Rheum Dis Clin North Am. 2004. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases; pp. 819–34. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor AJ, Bamber S, Silman AJ. A comparison of the performance of different methods of disease classification for rheumatoid arthritis. Results of an analysis from a nationwide twin study reference. J Rheumatol. 1994 Aug;21(8):1420–6. [PubMed] [Google Scholar]
- 18.Bergstralh EJ, Offord KP, Chu CP, Beard CM, O’Fallon WM, Melton LJ., III . Calculating incidence, prevalence and mortality rates for Olmsted County, Minnesota: An update. 1992. (Technical Report Series). No. 49. [Google Scholar]
- 19.McCullagh P, Nelder JA. Generalized Linear Models. Chapman and Hall Lt.; New York: 1983. [Google Scholar]
- 20.Cox DR. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354–60. [Google Scholar]
- 21.U.S. Census Bureau; Annual Estimates of the Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2005 (NC-EST2005-01) Source: Population Division. Release Date: May 10, 2006.
- 22.Linos A, Worthington JW, O’Fallon WM, Kurland LT. The epidemiology of rheumatoid arthritis in Rochester, Minnesota: A study of incidence, prevalence, and mortality. Am J Epidemiol. 1980;111(1):87–98. doi: 10.1093/oxfordjournals.aje.a112878. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen JK, Svendsen AJ, Horslev-Petersen K. Incidence of Rheumatoid Arthritis in the Southern part of Denmark from 1995 to 2001. Open Rheumatol J. 2007;1:18–23. doi: 10.2174/1874312900701010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doran MF, Crowson CS, O’Fallon WM, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol. 2004;31(2):207–13. [PubMed] [Google Scholar]
- 25.Pladevall-Vila M, Delclos GL, Varas C, Guyer H, Brugues-Tarradellas J, Anglada-Arisa A. Controversy of oral contraceptives and risk of rheumatoid arthritis: meta-analysis of conflicting studies and review of conflicting meta-analyses with special emphasis on analysis of heterogeneity. Am J Epidemiol. 1996;144(1):1–14. doi: 10.1093/oxfordjournals.aje.a008846. [DOI] [PubMed] [Google Scholar]
- 26.Spector TD, Hochberg MC. The protective effect of the oral contraceptive pill on rheumatoid arthritis: an overview of the analytic epidemiological studies using meta-analysis. J Clin Epidemiol. 1990;43(11):1221–30. doi: 10.1016/0895-4356(90)90023-i. [DOI] [PubMed] [Google Scholar]
- 27.Brennan P, Bankhead C, Silman A, Symmons D. Oral contraceptives and rheumatoid arthritis: results from a primary care-based incident case-control study. Semin Arthritis Rheum. 1997;26:817–823. doi: 10.1016/s0049-0172(97)80025-x. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen C, Picot MC, Bologna C, Sany J. Oral contraception, parity, breast feeding, and severity of rheumatoid arthritis. Ann Rheum Dis. 1996;55(2):94–8. doi: 10.1136/ard.55.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia SS, Majka DS, Kittelson JM, Parrish LA, Ferucci ED, Deane KD, et al. Rheumatoid factor seropositivity is inversely associated with oral contraceptive use in women without rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):267–9. doi: 10.1136/ard.2006.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot HB, Harris AL. The latest advances in hormonal contraception. J Obstet Gynecol Neonatal Nurs. 2008;37(3):369–74. doi: 10.1111/j.1552-6909.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- 31.Sulak PJ. Continuous oral contraception: changing times. Best Pract Res Clin Obstet Gynaecol. 2008;22(2):355–74. doi: 10.1016/j.bpobgyn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50(11):3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 33.Pikwer M, Bergstrom U, Nilsson JA, Jacobsson L, Berglund G, Turesson C. Breast feeding, but not use of oral contraceptives, is associated with a reduced risk of rheumatoid arthritis. Ann Rheum Dis. 2009;68(4):526–30. doi: 10.1136/ard.2007.084707. [DOI] [PubMed] [Google Scholar]
- 34.Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics. 2002;110(6):1103–9. doi: 10.1542/peds.110.6.1103. [DOI] [PubMed] [Google Scholar]
- 35.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol. 1999;26(1):47–54. [PubMed] [Google Scholar]
- 36.Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–5. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. J Rheumatol. 2000;27(3):630–637. [PubMed] [Google Scholar]
- 38.Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62(9):835–41. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465–71. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 40.Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35(3):169–74. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- 41.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988-2006. Am J Med. 2009;122(6):528–34. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 43.Tomar SL. Trends and patterns of tobacco use in the United States. Am J Med Sci. 2003;326(4):248–54. doi: 10.1097/00000441-200310000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 45.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 47.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 48.Leventis P, Patel S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology (Oxford) 2008;47(11):1617–21. doi: 10.1093/rheumatology/ken296. [DOI] [PubMed] [Google Scholar]
- 49.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143–9. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 50.Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, et al. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol. 1999;17(4):453–6. [PubMed] [Google Scholar]
- 51.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 52.Dayer JM, Chicheportiche R, Juge-Aubry C, Meier C. Adipose tissue has anti-inflammatory properties: focus on IL-1 receptor antagonist (IL-1Ra) Ann N Y Acad Sci. 2006;1069:444–53. doi: 10.1196/annals.1351.043. [DOI] [PubMed] [Google Scholar]
- 53.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40(11):1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 54.Carty SM, Snowden N, Silman AJ. Should infection still be considered as the most likely triggering factor for rheumatoid arthritis? J Rheumatol. 2003;30(3):425–9. [PubMed] [Google Scholar]
- 55.Molina V, Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38(3):235–45. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 56.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(11):1588–94. doi: 10.1136/ard.2004.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]