Abstract
The ubiquitously expressed G protein α-subunit Gsα mediates the intracellular cAMP response to glucagon-like peptide 1 (GLP1) and other incretin hormones in pancreatic islet cells. We have shown previously that mice with β cell-specific Gsα deficiency (βGsKO) develop severe early-onset insulin-deficient diabetes with a severe defect in β cell proliferation. We have now generated mice with Gsα deficiency throughout the whole pancreas by mating Gsα-floxed mice with Pdx1-cre transgenic mice (PGsKO). PGsKO mice also developed severe insulin-deficient diabetes at a young age, confirming the important role of Gsα signaling in β cell growth and function. Unlike in βGsKO mice, islets in PGsKO mice had a relatively greater proportion of α cells which were spread throughout the interior of the islet. Similar findings were observed in mice with pancreatic islet cell-specific Gsα deficiency using a neurogenin 3 promoter-cre recombinase transgenic mouse line. Studies in the a cell line αTC1 confirmed that reduced cAMP signaling increased cell proliferation while increasing cAMP produced the opposite effect. Therefore it appears that Gsα/cAMP signaling has opposite effects on pancreatic α and β cell proliferation, and that impaired GLP1 action in α and β cells via Gsα signaling may be an important contributor to the reciprocal effects on insulin and glucagon observed in type 2 diabetics. In addition, PGsKO show morphological changes in exocrine pancreas and evidence for malnutrition and dehydration, indicating an important role for Gsα in the exocrine pancreas as well.
Keywords: G protein, insulin, glucagon, islet cells
Introduction
Both type 1 and type 2 diabetes have been associated with pancreatic β cell dysfunction, with reduced glucose-stimulated insulin secretion and decreased β cell mass due to impaired β cell proliferation and survival (Butler et al. 2003). In addition, type 2 diabetics also have increased glucagon secretion from pancreatic α cells and inappropriately increased serum levels of glucagon (Goke 2008). Glucagon-like peptide 1 (GLP1) and other incretin hormones promote glucose-stimulated insulin secretion and β cell growth and inhibit glucagon secretion from α cells by activating the G protein α-subunit Gsα which stimulates intracellular cAMP production (Ahren 2009; Brubaker and Drucker 2004; Preitner et al. 2004; Weinstein et al. 2001). Because of these beneficial effects, the GLP1 receptor agonist exendin 4 has recently been approved as a therapeutic agent for diabetes.
We previously showed in mice with β cell-specific Gsα deficiency (βGsKO mice) that in fact Gsα/cAMP signaling is critical for normal β cell function and growth (Xie et al. 2007). Although GLP-1 tends to inhibit glucagon secretion in vivo, these effects may not be due to direct effects of GLP1 on glucagon secretion from α cells as several studies have shown that Gsα/cAMP signaling stimulates both proglucagon gene expression (Drucker et al. 1991; Islam et al. 2009) and glucagon release (Ding et al. 1997; Gromada et al. 1997; Hermansen 1985; Ma et al. 2005) in isolated α cells. Little is known about the role of GLP1 or Gsα/cAMP signaling on the regulation of pancreatic α cell proliferation.
In this present study we generated mice with Gsα deficiency throughout the endocrine and exocrine pancreas (PGsKO mice) by mating Gsα-floxed mice with Pdx1-cre mice in order to directly examine the in vivo role of Gsα/cAMP signaling in both pancreatic α and β cells. These mice had the same β cell defect with early-onset insulin-deficient diabetes as that seen in βGsKO mice. In addition, PGsKO had relatively increased numbers of α cells and a similar finding was observed in a similar mouse model that was generated using neurogenin 3 promoter-cre mice. Studies in the α cell line αTC1 showed that Gsα/cAMP signaling inhibits α cell proliferation, an effect opposite to that known to occur in β cells. Therefore reduced GLP1 actions on both α and β cells via Gsα signaling may be an important contributor to the reciprocal effects on insulin and glucagon observed in type 2 diabetics. In addition we show evidence that Gsα is also important in pancreatic exocrine function.
Materials and Methods
Mice
Mice with loxP sites surrounding Gsα exon 1 (E1fl/fl) (Chen et al. 2005) were maintained on Black Swiss genetic background. Pdx1-cre+ mice (Lammert et al. 2003) and neurogenin 3 (Ngn3) promoter-cre+ mice (Schonhoff et al. 2004) were crossed with Black Swiss WT mice for three or more generations before the studies in order to obtain a purer genetic background. Female E1fl/fl mice were mated to male E1fl/+:Pdx1-cre+/− mice to generate pancreatic Gsα knockout mice (PGsKO; E1fl/fl:cre+), mice with heterozygous pancreatic Gsα deletion (E1fl/+:cre+ ), and control cre− littermates, or were mated with Elfl/+:Ngn3-cre+/− mice to generate mice with pancreatic islet-specific Gsα deficiency. Mice were maintained on standard pellet diet (NIH-07, 5% fat by weight) and 12:12-h light/dark cycle. Except where noted, studies were performed in 3- to 4-month-old mice and were approved by the NIDDK Animal Care and Use Committee. Mice were genotyped by PCR as previously described (Sakamoto et al. 2005).
Body composition and food intake
Body composition was measured by using the Minispec mq10 NMR analyzer (Bruker Optics Inc., Woodlands, TX). Food intake was measured over 21 days in individually caged mice.
Serum chemistry
Blood was obtained by retroorbital bleed. Serum insulin (Crystal Chem, Downers Grove, IL), glucagon (R&D Systems, Minneapolis, MN), insulin-like growth factor 1 (IGF1) (Alpco Diagnostics, Salem, NH) and the circulating active forms of GLP-1 (Millipore, Billerica, MA) were measured by ELISA or RIA. The remainder of the chemistries was measured in the NIH Clinical Chemistry Laboratory.
Glucose and insulin tolerance tests and insulin secretion
Glucose and insulin-tolerance tests were performed in overnight-fasted mice after i.p. injection of glucose (2 mg/g) or insulin (Humulin; 0.75 mIU/g), respectively. Plasma glucose was measured by glucometer.
Immunostaining and islet morphometry
Pancreata were fixed in 4% paraformadehyde and paraffin-embedded, and sections were H&E-stained. For immunostaining, 5-μm sections of paraffin blocks were deparaffinized and rehydrated with xylene followed by decreasing concentrations of ethanol; microwaved in 0.01 M sodium citrate, pH 6.0, for 20 min; and permeabilized with 1% Triton X-100 in PBS. Sections were then incubated with mouse anti-insulin (Invitrogen, Carlsbad, CA), rabbit anti-glucagon, rat anti-somastatin (Lab Vision, Fremont, CA), rabbit anti-PC1/3, rabbit anti-PC2 (Millipore) or rabbit anti-Gsα (Simonds et al. 1989) antibodies, followed by secondary antibodies that were labeled with rhodamine or Cy2 (Jackson ImmunoResearch, West Grove, PA). Sections were counterstained with DAPI (Invitrogen). For α cell proliferation studies, pancreatic sections of 7-day-old mice (n = 3 per group) were stained with anti-glucagon and rabbit anti-Ki67 (Vector, Burlingame, CA) antibodies.
Cell proliferation and glucagon secretion in culture
A pancreatic α cell line (αTC1.9) (Powers et al. 1990) was purchased from ATCC (catalog # CRL-2350) and grown in 37°C under 10% CO2 in DMEM modified to contain 0.3% glucose, 15 mM HEPES, 0.1 mM non-essential amino acids, 0.02% bovine serum albumin and 10% fetal bovine serum. For RNAi Gsα knockdown assay, Gsα-specific siRNA and negative control siRNA were purchased from Ambion (Austin, TX; catalog #16706, Gene ID46627) and lipofectamine 2000 (Invitrogen) was used for transfection. To test the effect of increased intracellular cAMP on αTC1 growth, cells were seeded into 24 well plates at 2 × 105 cell/well and incubated overnight before medium containing either forskolin (20 mM) and isobutylmethylxanthine (IBMX) (100 mM) or vehicle alone were added and then refreshed every 24 hours. Treated and control cells were stained with 0.1% thiazolyl blue tetrazolium bromide (MTT; Sigma, St. Louis, MO) in PBS at 37°C for 30 minutes. Stained cells were incubated with isopropanol to elute the stain and absorbances were read at OD560. To test the effects on glucagon secretion media was changed at 24 hours after transfection or treatment and replaced with new media containing 16.7mM or 5.6 mM glucose. The cells were then incubated for 4 hours and the glucagon concentrations in the culture media were measured by ELISA (R&D systems)
Quantitative RT-PCR
Islet were isolated and RNA samples were prepared as previously described (Xie et al. 2007) and quantitative real-time PCR was performed using SYBR-Green based detection in an Mx3000P (Stratagene, La Jolla, CA). Gene expressions were normalized by geometric averaging of the levels of β-actin, Hprt and Gapdh mRNAs. Primer sequences are available upon request.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance was determined by using unpaired Student’s t test (two-tailed) or one-way ANOVA with Tukey’s post hoc test with differences considered significant at p < 0.05.
Results
PGsKO mice have reduced body mass and adiposity
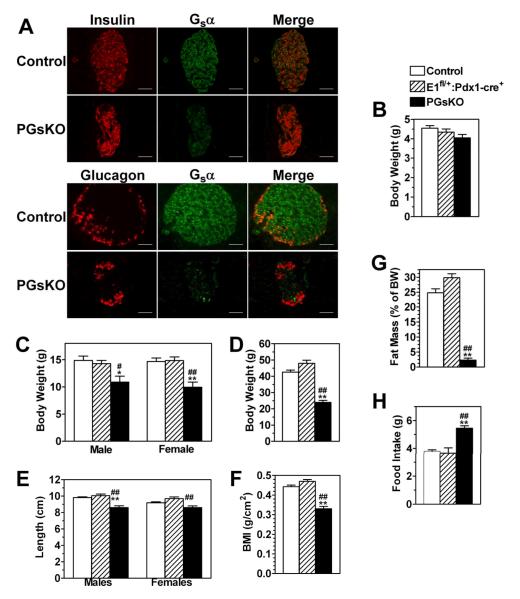
PGsKO (E1fl/fl:Pdx1-cre+) mice with pancreatic Gsα deficiency were generated by mating of E1fl/fl females to E1fl/+:Pdx1-cre+/− males, and their phenotype were compared to cre− control and E1fl/+:Pdx1-cre+ littermates. Coimmunostaining of pancreatic sections with a Gsα antibody and either an insulin or glucagon antibody showed robust Gsα expression in both α- and β cells of control islets which was markedly reduced in PGsKO islets (Fig. 1, panel A). This is in contrast to βGsKO mice, in which Gsα deficiency was confined to β cells (Xie et al. 2007). Compared to islets, Gsα expression in the surrounding exocrine pancreas was much lower even in control mice (Fig. 1, panel A). PGsKO mice showed no change in Gsα expression in the pituitary or hypothalamus (data not shown). Unlike βGsKO mice, which had significant lethality throughout the first few months of postnatal life (Xie et al. 2007), PGsKO mice showed minimal effects on postnatal survival (102% and 89% of expected survival at 7 days and 4 weeks of age, respectively; n = 82 and 282 total offspring, respectively).
Figure 1.
PGsKO mice have lower body weight in spite of being hyperphagic. (A) Immunostaining of a control and PGsKO islet for either insulin (red) or Gsα (green) (top panels) or for either glucagon (red) or Gsα (green) (bottom panels). In both cases the merged images are shown on the right. Body weight of control, E1fl/+:Pdx1-cre+, and PGsKO mice at (B) 7 days (males only, n = 21-36/group) (C) 3.5 weeks (males and females, n = 3-8/group), and (D) 3 months (males only, n = 6-10/group) after birth. (E) Body length (males and females, n = 3-10/group), (F) body mass index (males, n = 4-10/group), (G) % fat mass (males, n = 5-13/group), and (H) food intake (males, n = 4-8/group) in 3 month old mice. Data are mean ± S.E.M. (*p<0.05 or **p<0.01 vs. controls. #p<0.05 or ##p<0.01 vs. E1fl/+:Pdx1-cre+). Bars equal 50 μm.
PGsKO mice had a severe postnatal growth defect. Body weights of control, E1fl/+:Pdx1-cre+, and PGsKO mice were similar on postnatal day 7 (Fig. 1, panel B) but by 3.5 weeks both male and female PGsKO had markedly reduced body weight compared to either Elfl/+:Pdx-cre+ mice or controls (Fig. 1, panel C) and this difference became even greater by 3 months of age (Fig. 1, panel D). Although adult PGsKO mice had slightly reduced body length compared to E1fl/+:Pdx1-cre+ mice and controls (Fig. 1, panel E), their overall body mass index (body weight/body length2) was significantly reduced (Fig. 1, panel F). Body composition analysis showed PGsKO mice to have markedly reduced fat mass (Fig. 1, panel G). While impaired growth in βGsKO mice was associated with reduced circulating levels of insulin-like growth factor 1 (IGF1) (Xie et al. 2007), IGF1 levels were similar in PGsKO, E1fl/+:Pdx1-cre+, and control mice (Table 1). In spite of their impaired growth, these mice had significantly increased food intake (Fig. 1, panel H). One possible explanation for the poor growth in PGsKO mice may be malabsorption due to pancreatic exocrine dysfunction, as PGsKO mice showed markedly abnormal histology of the exocrine pancreas (Fig. 4, panel B) presumably as a result of Gsα deficiency in these regions as well as the endocrine pancreas. Consistent with severe malnutrition, serum albumin levels were significantly decreased in PGsKO mice (Table 1).
Table 1.
Serum chemistries in adult micea
| Control |
E1fl/+:Pdx-cre+ |
PGsKO |
|
|---|---|---|---|
| Glucose (mg/dl) | 184 ± 8 | 238 ± 11** | 602 ± 2**,## |
| Cholesterol (mg/dl) | 129 ± 6 | 160 ± 14 | 206 ± 15**,# |
| Triglycerides (mg/dl) | 277 ± 60 | 320 ± 49 | 523 ± 73* |
| Sodium (mEq/l) | 153 ± 1 | 152 ± 3 | 147 ± 4 |
| Potassium (mEq/l) | 5.9 ± 0.2 | 5.8 ± 0.3 | 6.6 ± 0.3 |
| Chloride (mEq/l) | 117 ± 1 | 113 ± 2 | 109 ± 4 |
| Creatinine (mg/dl) | 0.40 ± 0.03 | 0.56 ± 0.09 | 0.90 ± 0.13** |
| BUN (mg/dl) | 24 ± 2 | 22 ± 1 | 49 ± 16 |
| Albumin (g/dl) | 1.52 ± 0.04 | 1.48 ± 0.02 | 1.22 ± 0.07**,## |
| IGF1 (ng/ml) | 641 ± 39 | 682 ± 25 | 673 ± 19 |
| Glucagon (pg/ml) | 155 ± 30 | 160 ± 46 | 185 ± 61 |
| Insulin (ng/ml) | 1.23 ± 0.16 | 1.02 ± 0.14 | 0.35 ± 0.08**,# |
| GLP-1 (active) pM | 6.63 ± 0.09 | 6.42 ± 0.23 | 6.40 ± 0.14 |
measured in 3-4 month-old males, n = 3-12/group.
p<0.01 vs. control;
p<0.05 or
p<0.01 vs E1fl/+:pdx1-cre+ by ANOVA.
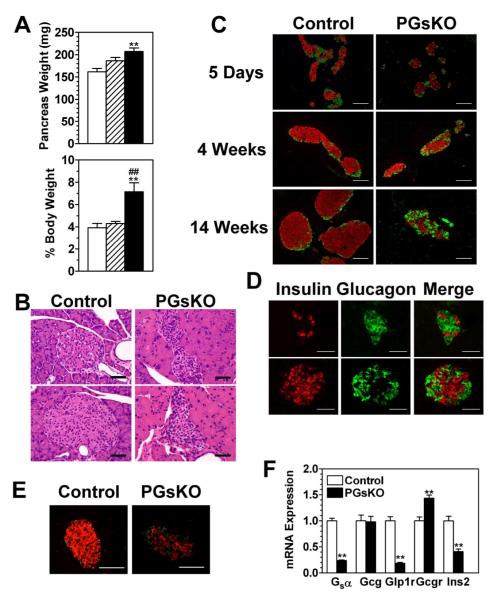
Figure 4.
Pancreatic exocrine and islet pathology in PGsKO mice. (A) Pancreatic weights in mg. (top panel; n = 7-15/group) and as % body weight (lower panel; n = 4-7/group) in adult male control (open bars), E1fl/+:Pdx1-cre+ (striped bars), and PGsKO mice (solid bars). (B) H&E-stained sections (2 each) of an islet and surrounding exocrine pancreas from a control and a PGsKO mouse. (C) Immunostaining of pancreatic sections from 5 day-, 4 week-, and 14 week-old control and PGsKO mice for insulin (red) and glucagon (green). (D) Two sections showing islets from E1fl/fl:Ngn3-cre+ mice that were immunostained for insulin (red) or glucagon (green) with the merged images on the right. (E) Sections of a control and a PGsKO islet immunostained for insulin (red) and somatostatin (green). (E) mRNA expression levels of the Gsα, glucagon (Gcg), GLP-1 receptor (Glp1r), glucagon receptor (Gcgr), and insulin (Ins2) genes in islets isolated from control and PGsKO mice (n = 7/group). Data are mean ± S.E.M. (**p<0.01 vs. controls; ##p<0.01 vs. E1fl/+:Pdx1-cre+). Bars equal 50 μm.
PGsKO mice develop early-onset insulin-deficient diabetes
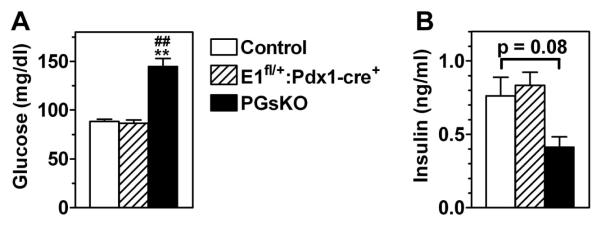
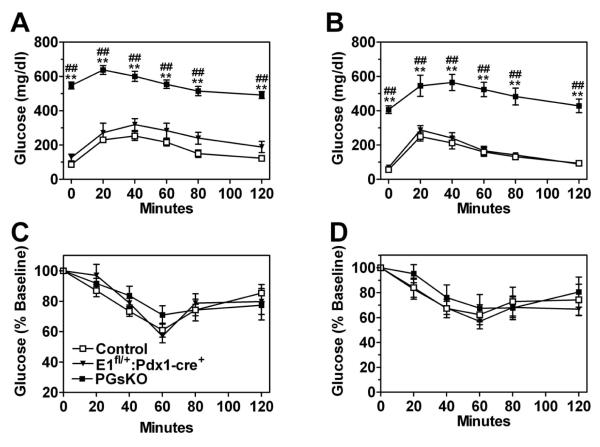
Even by day 7 postpartum PGsKO mice were very hyperglycemic as compared to E1fl/+:Pdx1-cre+ and control mice (Fig. 2, panel A). Serum insulin levels on day 5 were ~50% lower in PGsKO as compared to the other two groups (p = 0.08 vs. controls) (Fig. 2, panel B). In adult mice (3-4 months of age) glucose levels were slightly higher in E1fl/+:Pdx1-cre+ mice as compared to controls while they were > 3-fold higher in PGsKO mice (Table 1). Insulin levels were markedly reduced in adult PGsKO mice as compared with controls and E1fl/+:Pdx1-cre+ mice (Table 1). Adult male and female PGsKO mice had markedly impaired glucose tolerance compared to E1fl/+:Pdx1-cre+ and control mice (Fig. 3, panels A & B), while insulin tolerance tests showed similar levels of insulin sensitivity between the three groups (Fig. 3, panels C & D). In addition adult PGsKO mice had increased serum triglyceride and cholesterol levels as compared to the other two groups of mice (Table 1). Serum IGF-1, GLP-1 (active forms) and glucagon levels were similar between the three groups (Table 1). While electrolytes were normal, serum creatinine was significantly increased and blood urea nitrogen (BUN) levels were more than double in PGsKO mice, although this was not statistically significant (Table 1), indicating that PGsKO mice were dehydrated, most likely secondary to severe hyperglycemia. Therefore PGsKO mice mimic βGsKO mice in terms of developing very early-onset insulin-deficient diabetes, although βGsKO mice had low IGF1 levels while IGF1 remained unaffected in PGsKO mice (Xie et al. 2007).
Figure 2.
Mice develop insulin deficiency and hyperglycemia at a young age. (A) Serum glucose (n = 21-38/group) and (B) insulin (n = 3-7/group) in control, E1fl/+:Pdx1-cre+, and PGsKO mice at day 7 and 5 postpartum, respectively. Data are mean ± S.E.M. (**p<0.01 vs. controls; ##p<0.01 vs. E1fl/+:Pdx1-cre+).
Figure 3.
PGsKO mice are glucose intolerant but have normal insulin sensitivity. Glucose tolerance tests performed in (A) male (n = 4-5/group) and (B) female 3 month old control (□), E1fl/+:Pdx1-cre+ (▼), and PGsKO mice (⑤) (n = 4-7/group). Insulin tolerance tests performed in (C) male (n = 6-8/group) and (D) female mice (n = 4-6/group). Data are mean ± S.E.M. (**p<0.01 vs. controls; ##p<0.01 vs. E1fl/+:Pdx1-cre+).
PGsKO mice have reduced islet size and β cell mass with increased numbers of α cells
Total pancreas weights of PGsKO mice were greater than controls (Fig. 4, panel A). When pancreas weights were normalized to total body weights, they were ~75% greater in PGsKO mice as compared to E1fl/+:Pdx1-cre+ or control mice (Fig. 4, panel A). This was primarily due to markedly enlargement of the pancreatic exocrine ducts in PGsKO mice, which were also more eosinophilic as compared to those in controls on H & E staining (Fig. 4, panel B). These changes are probably the result of Gsα deficiency in exocrine pancreas and suggest that PGsKO mice may have pancreatic exocrine insufficiency and malabsorption.
H & E staining of adult pancreas showed that PGsKO mice, like βGsKO mice (Xie et al. 2007), had markedly smaller islets than controls with irregular shapes and increased cellularity (Fig. 4, panel B). Co-immunostaining of islets with antibodies for insulin and glucagon showed that at 5 days of age PGsKO islets were only slightly smaller than controls with a similar distribution of α- and β cells (Fig. 4, panel C). By 4 weeks PGsKO islets had a greater ratio of α to β cells than controls, although the α cells remained on the periphery. At 14 weeks PGsKO islets were much smaller than in controls and had less β cells with reduced intensity of insulin staining, similar to what was observed in βGsKO mice (Xie et al. 2007). However, unlike what was observed in βGsKO mice (Xie et al. 2007), PGsKO mice had increased numbers of α cells with a very significant number of α cells mixed with β cells throughout the islet (Fig. 4, panel C). A similar pattern was also observed in E1fl/fl mice:Ngn3-cre+ (Schonhoff et al. 2004), another model with Gsα deficiency throughout the pancreatic islet (Fig. 4, panel D). Coimmunostaining of PGsKO islets with insulin and somatostatin antibodies showed reduced numbers of β cells and a slight increase in somatostatin-staining δ cells with some intermingling of δ cells with β cells within the interior of the islet (Fig. 4, panel E). Immunostaining of duodenal sections for serotonin, GLP-1, cholecystokinin, or ghrelin showed no obvious change in the number or distribution of endocrine cells in the duodena of PGsKO mice (data not shown).
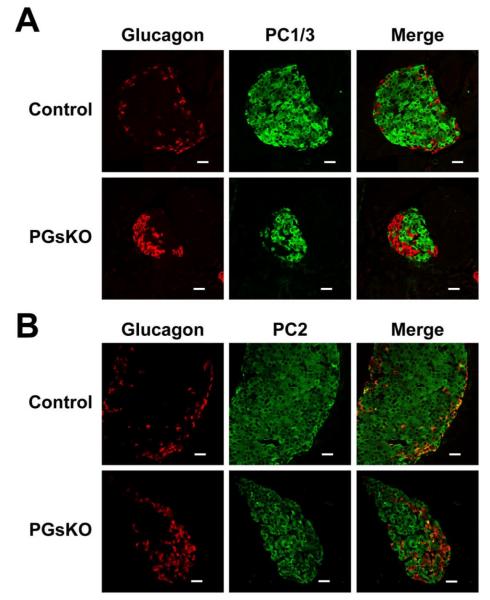
The ductal enlargement observed in PGsKO mice suggests that the relative greater proportion of islet α cells may be due to increased α cell neogenesis. During embryonic development fetal α cells transiently express the prohormone convertase PC1/3 which converts proglucagon to GLP-1 (Wilson et al. 2002) whereas adult α cells express PC2 which converts proglucagon to glucagon. Both PC1/3 and PC2 are expressed in islet β cells and are required for proinsulin processing. To determine if α cell neogenesis is increased in PGsKO mice we looked for the presence of α cells expressing PC1/3 by coimmunostaining islets for glucagon and either PC1/3 or PC2 (Fig. 5, panels A and B, respectively). We found that control and PGSKO islets had similar distributions of PC1/3 and PC2, with the absence of PC1/3 and presence of PC2 within glucagon-producing α cells, and the presence of both PC1/3 and PC2 in the non-glucagon staining cells, which are primarily β cells. Therefore we found no evidence for increased α cell neogenesis in PGsKO mice.
Figure 5.
Prohormone convertase 1/3 (PC1/3) is absent in α cells from control and PGsKO mice. Immunostaining of a control and PGsKO islet for (A) either glucagon (red) or PC1/3 (green) or for (B) either glucagon (red) or PC2 (green). In both cases the merged images are shown on the right. Bars equal 20 μm.
Gene expression studies in control and PGsKO islets by quantitative RT-PCR (Fig. 4, panel F) showed a significant reduction in Gsα mRNA in PGsKO islets, as expected. Insulin (Ins2) mRNA was also markedly reduced, consistent with the marked reduction in β cells. Expression of the proglucagon gene (Gcg) which generates glucagon was similar in control and PGsKO islets. Given that PGsKO islets have a greater proportion of α cells, this suggests that expression levels per α cell may be decreased, although we were unable to detect a change in Gcg mRNA levels in cultured α (αTC1) cells transfected with Gsα RNAi (data not shown). GLP-1 receptor (Glp1r) receptor gene expression was also markedly reduced in PGsKO islets. This is probably due mostly to the reduced proportion of β cells, as these receptors were recently shown to be exclusively expressed within β cells in mouse islets (Tornehave et al. 2008). In addition, Glp1r expression in islets has been shown to be inhibited by hyperglycemia, but to be unaffected by Gsα/cAMP signaling (Abrahamsen and Nishimura 1995). Finally, glucagon receptor (Gcgr) expression was upregulated in PGsKO islets. This is consistent with prior studies showing that Gsα/cAMP signaling downregulates while high extracellular glucose levels upregulate this gene in pancreatic islets (Abrahamsen and Nishimura 1995) and cultured hepatocytes (Abrahamsen et al. 1995).
Gsα deficiency leads to increased proliferation of pancreatic α cells
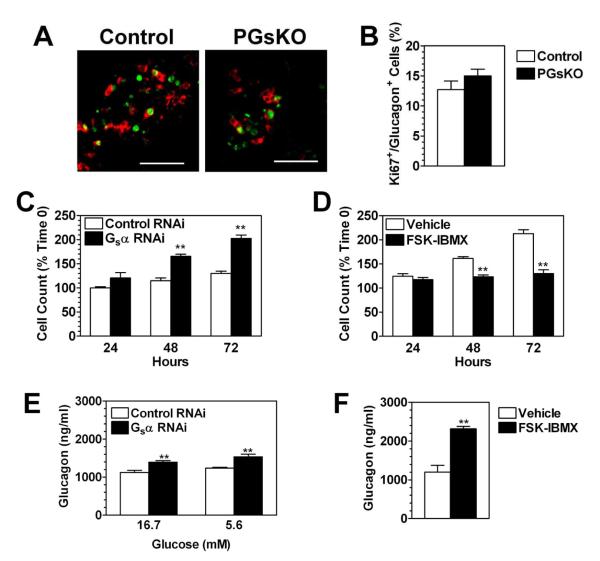
We have previously shown that Gsα deficiency in β cells leads to marked reduced β cell proliferation and reduced β cell mass (Xie et al. 2007), and our findings in PGsKO mice are consistent with these prior observations. However in PGsKO mice, which also had Gsα deficiency in α cells, costaining of islets from 7 day old mice with antibodies to glucagon and the proliferative marker Ki67 showed no evidence for a proliferative defect in α cells and in fact the proportion of proliferating α cells in PGsKO mice tended to be slightly higher than in controls, although the differences were not statistically significant (Fig. 6, panels A & B).
Figure 6.
Gsα deficiency leads to increased pancreatic α cell proliferation. (A) Staining of a control and PGsKO islet for glucagon (red) and Ki-67 (green). (B) % of glucagon-staining cells that are also Ki-67+ in islets examined from 3 control and 3 PGsKO mice. (C) Proliferation of αTC1 cells after transfection with either control (scrambled) or Gsα RNAi (n = 6/group). (D) Proliferation of αTC1 cells after treatment with either forskolin plus IBMX (FSK-IBMX) or with vehicle alone (n = 12/group). For panels C and D are expressed as % of cell number at time 0. (E) Glucagon secreted into media of αTC1 cells from hours 24-28 after transfection with either control (scrambled) or Gsα RNAi in the presence of 16.7 or 5.6 mM glucose (n = 6/group). (F) Glucagon secreted into media of αTC1 cells from hours 24-28 after treatment with either forskolin plus IBMX (FSK-IBMX) or with vehicle alone in the presence of 5.6 mM glucose (n = 6/group). Data are mean ± S.E.M. (**p<0.01 vs. control or vehicle). Bars equal 50 μm.
We further examined the effects of Gsα signaling on proliferation in the α cell line αTC1. αTC1 cells were treated with either a control (scrambled) RNAi or a Gsα RNAi which reduced Gsα expression by ~70% as determined by quantitative RT-PCR (data not shown). Cells treated with the Gsα RNAi had a markedly higher proliferative rate than the control cells with the Gsα RNAi-treated cells doubling at 72 hours while the control cell number only increased by ~30% during the same time period (Fig. 6, panel C). We also performed the opposite experiment in which αTC1 cells were treated with the adenylyl cyclase stimulator forskolin and the cAMP phosphodiesterase inhibitor isobutylmethylxanthine, which lead to increased intracellular cAMP levels (FSK-IBMX). In contrast to the cells treated with Gsα RNAi, proliferation of FSK-IBMX-treated cells was significantly lower than controls (Fig. 6, panel D). These in vitro results confirm that Gsα signaling pathways have an antiproliferative effect in α cells, which is opposite to their known proproliferative effect in β cells.
We next examined the effect of perturbing Gsα/cAMP signaling on glucagon secretion in αTC1 cells. Cells were transfected with either control or Gsα RNAi and glucagon secretion into the media was measured from 24 to 28 hours later at both 5.6 and 16.7 mM glucose to avoid the potential confounding effects of differing glucose concentrations on glucagon secretion. While glucagon secretion was increased after Gsα RNAi transfections at both glucose concentrations (Fig. 6, panel E), the percent increase was similar to the percent increase in αTC1 cell numbers at 24 hours after Gsα RNAi treatment (Fig. 6, panel C), and therefore this does not represent an increase in the amount of glucagon secreted per cell. Interestingly, αTC1 cells treated with FSK-IBMX showed a more marked two-fold increase in glucagon secretion at 24-28 hours in the presence of 5.6 mM glucose (Fig. 6, panel F) despite the fact that there was no increase in cell numbers (Fig. 6, panel D), indicating that excess Gsα/cAMP signaling directly promotes glucagon secretion in α cells.
Discussion
Previously we generated mice with β cell-specific Gsα deficiency (βGsKO mice) using Rip2-cre, and demonstrated that Gsα/cAMP pathways are critical regulators of β cell function and promote β cell proliferation (Xie et al. 2007). However the βGsKO model is limited by the fact that Gsα deficiency occurs in other tissues such as the hypothalamus and pituitary due to expression of the Rip2 gene in these tissues. In this work we extended the study of Gsα function in pancreatic cells by deleting Gsα from all major types of pancreatic endocrine and exocrine cells using Pdx1-cre transgenic mice (Gu et al. 2002; Jorgensen et al. 2007). The utilization of Pdx1-cre mice avoids the confounding effects of Gsα deficiency in the central nervous system present in βGsKO mice as Pdx1 is not expressed in the central nervous system. Similar to βGsKO mice, PGsKO mice developed early-onset hypoinsulinemia and hyperglycemia with reduced β cell mass. However, glucagon levels in PGsKO mice remained similar to those in control mice, indicating that Gsα deficiency likely does not lead to a similar effect on α cell function and hormone production as it does in β cells.
The production and secretion of glucagon can be regulated by many factors including nutrients, hormones and neurotransmitters. Although the net effect of GLP1 on glucagon secretion is inhibitory (Ahren 2009), several studies have shown that Gsα/cAMP stimulating agents increase both proglucagon gene expression (Drucker et al. 1991; Islam et al. 2009) and glucagon release (Ding et al. 1997; Gromada et al. 1997; Hermansen 1985; Ma et al. 2005) in pancreatic α cells. We also found a stimulatory effect of increased cAMP on glucagon release in αTC1 cells, although the effect of lowering cAMP on glucagon release was not significant. Overall, these observations would predict that loss of Gsα expression in α cells of PGsKO mice should lead to a reduction in serum glucagon levels. Another factor that might be expected to reduce glucagon levels in PGsKO mice is hyperglycemia (Dumonteil et al. 2000), as elevated glucose levels do not appear to affect glucagon expression levels but do inhibit glucagon secretion from α cells (Gromada et al. 2007). In fact this may help to explain the moderate reduction in glucagon levels in βGsKO mice (Xie et al. 2007). However glucagon levels and islet glucagon mRNA levels were maintained in PGsKO mice despite the fact that they have more severe hyperglycemia than βGsKO mice. Low insulin levels would be expected to increase glucagon levels in PGsKO mice as insulin has an inhibitory effect on α cell activity (Kawamori et al. 2009). However insulin levels were similarly reduced in βGsKO mice as in PGsKO mice (Xie et al. 2007).
Maintenance of normal glucagon levels and islet glucagon mRNA levels in PGsKO mice in spite of the presence of hyperglycemia and loss of Gsα expression in α cells may be partially explained by an increment of the α cell population. While it is difficult to accurately measure the extent of total α cell mass alteration in PGsKO mice as all pancreatic endocrine and exocrine cells were morphologically abnormal and therefore could be used as reference controls for each other, there are morphological characteristics to suggest that α cells are overrepresented in PGsKO mice compared to other pancreatic cell types. In normal mouse islets β cells are the most prominent cell population and are located in the interior of the islet while α cells are less numerous and located at the periphery. In comparison to islets from βGsKO mice which maintained the normal architecture (Xie et al. 2007), PGsKO islets were small and deformed with a significantly greater number of α cells within the interior of the islet and a greater α to β cell ratio. The increment in α to β cell ratio happened relatively early in postnatal life, occurring within the first 4 weeks after birth. These findings are consistent with our results in the αTC1 cell line showing that Gsα signaling pathways have an antiproliferative effect in α cells, which would suggest that α cells in PGsKO mice may have growth advantage due to loss of Gsα expression. However, using Ki-67/glucagon costaining we were unable to document a clear increase in α cell proliferation in PGsKO mice, at least at day 7 postpartum. We also found no evidence for the presence of fetal α cells in PGsKO islets, suggesting that increased neogenesis was not an important factor. Hyperglycemia and hyperlipidemia also likely contributes to an increased α/β cell ratio through β cell toxicity (Poitout and Robertson 2008), although these factors were also present in βGsKO mice (Xie et al. 2007).
The mechanisms underlying the antiproliferative and proproliferative effects of Gsα/cAMP signaling in α and β cells, respectively, are unknown. One possible mechanism may be the stimulatory effect of Gsα/cAMP signaling on Pdx1 expression (Jhala et al. 2003), as both in vivo and in vitro studies in β cells have shown that loss of Pdx1 expression leads to conversion of insulin-secreting cells to glucagon-secreting cells (Ahlgren et al. 1998; Lottmann et al. 2001; Wang et al. 2001). Conversely, ectopic expression of Pdx1 in cultured α cells inhibits glucagon gene expression (Ritz-Laser et al. 2003). However Pdx1 is not expressed in mature α cells, so Pdx1-independent mechanisms may be responsible for the antiproliferative effect of Gsα on α cells that we observed in vitro. Moreover, β cell-specific Gsα deficiency did not lead to reduced Pdx1 expression in βGsKO mice (Xie et al. 2007). Further studies will be required to determine whether Pdx1 plays a role in postnatal α-cell neogenesis and contributes to enlarged α cell mass. Recent studies have also shown islet-specific microRNA miR-375 to be a powerful regulator of insulin secretion and pancreatic α and β cell mass (Poy et al. 2004; Poy et al. 2009). miR-375 null mice develop hyperglycemia with decreased β cell mass and increased α cell mass, similar to PGsKO mice, while overexpression of miR-375 in β cells leads to a β cell defect with diabetes (Poy et al. 2009).
In conclusion, our studies in βGsKO and PGsKO mice as well as cultured α cells show that Gsα/cAMP pathways play important and distinct roles in pancreatic α and β cell growth and function. While many studies, including those in βGsKO mice, suggest that these pathways stimulate hormone production in both types of cells, our results show that there are opposite effects of Gsα/cAMP on cell proliferation (proproliferative in β cells, antiproliferative in α cells). In both βGsKO and PGsKO mice the primary defect leading to hyperglycemia is hypoinsulinemia due to a primary β cell defect. However, compared to βGsKO mice, PGsKO mice had higher glucagon levels and α cell mass relative to β cell mass, leading to a higher serum glucagon/insulin ratio which may contribute to the more severe hyperglycemia in PGsKO mice. This is similar to what is typically observed in type 2 diabetes mellitus and opposite to the effects of GLP1 administration (Ahren 2009). As Gsα mediates the signals of GLP1, impaired GLP1 action in α and β cells may be an important factor in the observed changes in the islets of type 2 diabetics (Goke 2008). In addition to its role in the endocrine pancreas, morphology in PGsKO mice also indicates an important role in normal maturation of pancreatic exocrine cells.
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the National Institute of Diabetes, Digestive, and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services.
Footnotes
Declaration of Interest: The authors have no conflict of interest that could be perceived as prejudicing the impartialility of the research reported.
References
- Abrahamsen N, Lundgren K, Nishimura E. Regulation of glucagon receptor mRNA in cultured primary rat hepatocytes by glucose and cAMP. Journal of Biological Chemistry. 1995;270:15853–15857. doi: 10.1074/jbc.270.26.15853. [DOI] [PubMed] [Google Scholar]
- Abrahamsen N, Nishimura E. Regulation of glucagon and glucagon-like peptide-1 receptor messenger ribonucleic acid expression in cultured rat pancreatic islets by glucose, cyclic adenosine 3′,5′-monophosphate, and glucocorticoids. Endocrinology. 1995;136:1572–1578. doi: 10.1210/endo.136.4.7534705. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. β-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes and Development. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Reviews Drug Discovery. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β cell deficit and increased β cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Chen M, Gavrilova O, Zhao W-Q, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gsα deficiency. Journal of Clinical Investigation. 2005;115:3217–3227. doi: 10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WG, Renstrom E, Rorsman P, Buschard K, Gromada J. Glucagon-like peptide I and glucose-dependent insulinotropic polypeptide stimulate Ca2+-induced secretion in rat α cells by a protein kinase A-mediated mechanism. Diabetes. 1997;46:792–800. doi: 10.2337/diab.46.5.792. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Campos R, Reynolds R, Stobie K, Brubaker PL. The rat glucagon gene is regulated by a protein kinase A-dependent pathway in pancreatic islet cells. Endocrinology. 1991;128:394–400. doi: 10.1210/endo-128-1-394. [DOI] [PubMed] [Google Scholar]
- Dumonteil E, Magnan C, Ritz-Laser B, Ktorza A, Meda P, Philippe J. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology. 2000;141:174–180. doi: 10.1210/endo.141.1.7230. [DOI] [PubMed] [Google Scholar]
- Goke B. Islet cell function: α and β cells – partners towards normoglycaemia. International Journal of Clinical Practice. 2008;62(Supp 159):2–7. doi: 10.1111/j.1742-1241.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renstrom E, Rorsman P. Adrenaline stimulates glucagon secretion in pancreatic α cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. Journal of General Physiology. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. α cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine Reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hermansen K. Forskolin, an activator of adenylate cyclase, stimulates pancreatic insulin, glucagon, and somatostatin release in the dog: studies in vitro. Endocrinology. 1985;116:2251–2258. doi: 10.1210/endo-116-6-2251. [DOI] [PubMed] [Google Scholar]
- Islam D, Zhang N, Wang P, Li H, Brubaker PL, Gaisano HY, Wang Q, Jin T. Epac is involved in cAMP-stimulated proglucagon expression and hormone production but not hormone secretion in pancreatic alpha- and intestinal L-cell lines. American Journal of Physiology Endocrinology and Metabolism. 2009;296:E174–181. doi: 10.1152/ajpendo.90419.2008. [DOI] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic β cell survival via CREB-mediated induction of IRS2. Genes and Development. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocrine Reviews. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metabolism. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Current Biology. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lottmann H, Vanselow J, Hessabi B, Walther R. The Tet-On system in transgenic mice: inhibition of the mouse Pdx1 gene activity by antisense RNA expression in pancreatic β cells. Journal of Molecular Medicine. 2001;79:321–328. doi: 10.1007/s001090100229. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, Salehi A, Vikman J, Rorsman P, Eliasson L. Glucagon stimulates exocytosis in mouse and rat pancreatic α cells by binding to glucagon receptors. Molecular Endocrinology. 2005;19:198–212. doi: 10.1210/me.2004-0059. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β cell dysfunction. Endocrine Reviews. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AC, Efrat S, Mojsov S, Spector D, Habener JF, Hanahan D. Proglucagon processing similar to normal islets in pancreatic α-like cell line derived from transgenic mouse tumor. Diabetes. 1990;39:406–414. doi: 10.2337/diab.39.4.406. [DOI] [PubMed] [Google Scholar]
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic α and β cell mass. Proceedings of the National Academy of Science of the United States of America. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. Journal of Clinical Investigation. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz-Laser B, Gauthier BR, Estreicher A, Mamin A, Brun T, Ris F, Salmon P, Halban PA, Trono D, Philippe J. Ectopic expression of the β cell specific transcription factor Pdx1 inhibits glucagon gene transcription. Diabetologia. 2003;46:810–821. doi: 10.1007/s00125-003-1115-7. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS. Chondrocyte-specific knockout of the G protein Gsα leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. Journal of Bone Mineral Research. 2005;20:663–671. doi: 10.1359/JBMR.041210. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Developmental Biology. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Simonds WF, Goldsmith PK, Woodard CJ, Unson CG, Spiegel AM. Receptor and effector interactions of Gs. Functional studies with antibodies to the αs carboxyl-terminal decapeptide. FEBS Letters. 1989;249:189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. Journal of Histochemistry and Cytochemistry. 2008;56:841–851. doi: 10.1369/jhc.2008.951319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. Journal of Biological Chemistry. 2001;276:25279–25286. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocrine Reviews. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Kalamaras JA, German MS. Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mechanisms of Development. 2002;115:171–176. doi: 10.1016/s0925-4773(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Xie T, Chen M, Zhang QH, Ma Z, Weinstein LS. β cell-specific deficiency of the stimulatory G protein α-subunit Gsα leads to reduced β cell mass and insulin-deficient diabetes. Proceedings of the National Academy of Science of the United States of America. 2007;104:19601–19606. doi: 10.1073/pnas.0704796104. [DOI] [PMC free article] [PubMed] [Google Scholar]