Abstract
Intervertebral disc (IVD) disorders are believed to be related to aging-related cell loss and phenotypic changes, as well as biochemical and structural changes in the extracellular matrix of the nucleus pulposus (NP) region. Previously, we found that the laminin γ1 chain was more highly expressed in immature NP porcine tissues, in parallel with the expression pattern for a laminin receptor, integrin α6 subunit, as compared to adjacent anulus fibrosus region; suggesting that cell-matrix interactions may be unique to the immature NP. However, the identity of laminin isoforms specific to immature or mature NP tissues, their associated receptors and functional significance are still poorly understood. In this study, we evaluated the zonal-specific expression of the laminin chains, receptors (i.e. integrins) and other binding proteins in immature tissue and isolated cells of rat, porcine and human intervertebral disc, towards the goal of revealing features of cellular environment and cell-matrix interactions in the immature NP. Results from both immuno-histochemical staining and flow cytometry analysis found that NP cells expressed higher levels of the laminin α5 chain, laminin receptors (integrin α3, α6, β4 subunit and CD239) and related binding proteins (CD151), as compared to cells from adjacent anulus fibrosus. These differences suggest that laminin interactions with NP cells are distinct from that of the anulus fibrosus, and that laminins may be important contributors to region-specific IVD biology. The revealed laminin isoforms, their receptors and related binding proteins may be used as distinguishing features of these immature NP cells in the intervertebral disc.
Keywords: intervertebral disc, nucleus pulposus, laminin, integrin, Lutheran glycoprotein, tetraspanin
INTRODUCTION
Intervertebral disc (IVD) disorders and age-related disc degeneration are major contributors to low back pain and spine-related disability, with enormous socioeconomic consequence [1, 2]. Intervertebral disc disorders are believed to be related to aging-related cell loss and phenotypic changes, as well as biochemical and structural changes in the extracellular matrix of the nucleus pulposus (NP) region [3, 4]. The intervertebral disc contains multiple cell populations that are morphologically and biosynthetically distinct. Cells of the anulus fibrosus (AF) originate from mesenchyme and exhibit characteristics of both fibroblasts and chondrocytes [5-9]. In contrast, cells of the NP are believed to originate from notochord with many morphologic features that reflect these unique origins [8, 10, 11]. The majority of NP cells in the neonatal and immature tissue are large and highly vacuolated, appear in multi-cell clusters with tight cell-cell connections and intense cytoskeletal staining [11-17]. With maturation, there is a shift in the population of cells in the NP towards fibrochondrocyte-like cells [16], although it is unknown if this reflects cell differentiation or the presence of cells that have migrated from the adjacent cartilage endplate and inner anulus fibrosus regions [4, 18-20]. Cells of the NP are responsible for synthesis and maintenance of intervertebral disc tissues [6, 10, 21-23], but experience major challenges to cell survival that result in decreased cell density and matrix synthesis with age [3, 18, 24, 25]. With no blood or lymph supply in the intervertebral disc, these cells exhibit little or no capacity for regeneration in the face of aging or matrix changes. NP cells are surrounded by a pericellular matrix, and embedded in a dense extracellular matrix, that includes types II, VI, III and IX collagens [17, 22], as well as the cell adhesion proteins, fibronectin and laminin [23]. Previous work has reported the expression of α6 and β4 integrin subunits associated with cells of the immature porcine NP, identified via tissue immunostaining [26] and flow cytometry analysis [14]. The α6 integrin subunit (in combination with β1 or β4) is a primary receptor for the matrix protein laminin, and the presence of α6 in the NP suggests that this integrin is important in regulating cellular interactions with laminin in the NP. These studies also reported a higher expression pattern for these integrin subunits in the NP as compared to adjacent anulus fibrosus regions, suggesting that cell-matrix interactions may differ amongst regions or cell types. Significant changes occur in the composition and structure of the NP matrix with age, with the NP becoming firmer and more opaque due to increased type II collagen fibril accumulation and decreased proteoglycan content [27]. Little is known of compositional changes in the minor extracellular matrix components in the NP with age, however, such as laminin. An understanding of the functional roles for laminins and cell-associated laminin receptors in the immature NP would be important for revealing features of the NP cellular environment and cell-matrix interactions that may affect NP cell aging process including matrix metabolic remodeling and cell survival.
Laminin (LM) is a heterotrimeric protein composed of three polypeptide chains, termed α, β and γ chains. To date, five different α chains (α1-α5), three different β (β1-β3) and γ (γ1-γ3) chains have been described in vertebrates. Combinations of these three different chains form at least 15 laminin isoforms, with expression profiles differing significantly amongst tissues and developmental stages [28, 29]. Laminins regulate many biological functions, including cell adhesion, migration, proliferation, differentiation and survival [29]. Cell interactions with laminins are mediated by many different cell surface receptors, including the integrins α3β1, α6β1, α6β4, and α7β1 [30-33]. Non-integrin proteins, including Lutheran blood group glycoprotein (Lu, CD239), α-dystroglycan and tetraspanin (CD151), also contribute to cell interactions with laminins and may potentiate the strength of cell adhesion in conjunction with integrin [34-37]. In prior studies, the laminin γ1 chain was found to be more highly expressed in immature NP porcine tissues, in parallel with the expression pattern for integrin α6 subunit [14, 26, 38]. Furthermore, NP cells were found to adhere to laminin-1 (LM-111) through integrin-mediated processes that were unique to NP cells, as compared to adjacent anulus fibrosus cells [38]. However, the identity of the laminin isoforms specific to immature or mature NP tissues, their associated receptors, and functional significance, are still poorly understood. Our prior work revealing the presence of integrins α6β1/α6β4 in the IVD that are known to interact with laminin-1/3 (LM-111/121) or laminin-10/11(LM-511/521) motivates interests in the expression of laminin α1, α5 and γ1 chain that comprise these laminin isoforms in the IVD.
The objective of this study was to evaluate the zonal-specific expression of the laminin chains (α1, α5 and γ1) that comprise the isoforms, laminin-1/3 (LM-111/121) or laminin-10/11(LM-511/521), receptors and related binding proteins in immature tissues of the rat, porcine and human intervertebral disc, towards the goal of revealing features of cellular environment and cell-matrix interactions in the immature NP.
Immunohistochemical staining and flow cytometry analyses were compared between tissues and cells of AF and NP regions harvested from rat, porcine and human sources. Findings reveal expression of the laminin α5 chain, laminin receptors (integrin α3, α6, β4 subunit, and CD239), and related binding proteins (CD151) in a pattern that suggests laminin interactions with NP cells to be distinct from that of the AF. Also these interactions could be of potential use as distinguishing features of these immature NP cells in the intervertebral disc.
MATERIAL AND METHOD
Tissue Harvesting
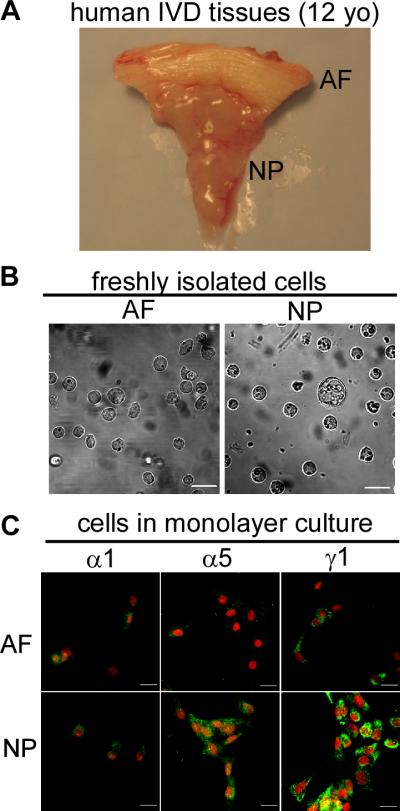
Human intervertebral disc samples were obtained as discarded surgical tissues from the lumbar spines of pediatric patients (2-14 year-old, n=5) undergoing procedures for treatment of scoliosis. The surgical tissues were kept at room temperature for no more than 2 hours until further processing. NP regions of these juvenile discs often exhibit a gelatinous-like appearance (Figure 3A), consistent with that reported for immature NP tissue [18]. It should be noted that a majority of NP cell sizes were similar to that of AF cells; however, approximately 1% of NP cells were observed to be of larger sizes as compared to AF cells (Figure 3B). Intervertebral discs were also obtained from the lumbar spine of an adult normal donor with no described spinal pathology (35 year-old, n=1). The gross appearance of all human IVD tissues were consistent with grades 0-1 of the Thompson grading scheme [39], and so were considered to be non-degenerate tissues. Porcine discs were obtained from the lumbar spines of immature (3 month-old, n=3) and skeletally mature pigs (24 month-old, n=3) within 8 hours of sacrifice (Duke University Vivarium or local abattoir). Rat intervertebral discs were similarly harvested from the lumbar and coccygeal spines of immature (Fisher 344, 1 month-old, n=19) and mature (12 month-old, n=19) rats within 1 hour of sacrifice (Duke University Vivarium). Tissues were procured and processed for immunostaining or histological evaluation, RNA isolation, or cell isolation as described below.
Figure 3.
Morphology of anulus fibrosus (AF) and nucleus pulposus (NP) tissues (A), freshly isolated cells of juvenile human (12 year-old) intervertebral disc (B) and expression of laminin subunits (α1, α5 and γ1) in juvenile human (12 year-old) intervertebral disc cells cultured in monolayer (3-7days) in vitro (C). Cell nuclei (red) counter-stained with propidium iodide. Scale bars: 20 μm.
Immunohistochemical Detection
Samples of NP and AF from human, pig and rat discs were embed in OCT medium then immediately flash-frozen in liquid nitrogen and stored at -80°C until cryosectioning. Frozen tissue sections (7 μm) were fixed in 4% formaldehyde (10 min at room temperature) for labeling with antibodies detecting specific laminin subunits, Lutheran glycoprotein (Lu, CD239) and tetraspanin (CD151). For labeling of integrin subunits, tissue sections were fixed in acetone (10 min at -20°C), incubated with a blocking solution (3.75% BSA/5% goat serum) for 30 min, Zymed, Carlsbad, CA), and then incubated for 2 hours with one of the following antibodies: polyclonal rabbit anti-human laminin α5, α1, γ1 chains, monoclonal anti-human-CD239, CD151, integrin subunits α3, α6, β4 and β1 (Table 1). The anti-human laminin α5, α1, γ1 chain antibodies are rabbit polyclonal antibodies against ~150-250 amino acids mapping within an specific internal region of laminin α5, α1, γ1 chains of human origin. They did not cross-react with other laminin chains, but cross-reacted with laminin α5, α1, γ1 chains of mouse and rat origin according to the manufacture's instruction. We also confirmed the specificity of these antibodies by western blot using positive control of laninin-1 protein (for α1, γ1 chains, Sigma, St. Louis, MO) and cell lysate from A549 cells (for α5, γ1 chains [30]).
Table 1.
Antibodies for laminin subunits, receptors and binding proteins used in immuno-hisotochemical staining (IH) and flow cytometry (FC) analysis (Hu: human, Rb: rabbit, Ms: mouse)
| Antibody | Vendor | Host/type | Cross-reactivity | Isotype control | Application |
|---|---|---|---|---|---|
| Laminin α1 | Santa Cruz (SC-5582) | Rb/polyclonal | Hu, pig, rat | none | IH |
| Laminin α5 | Santa Cruz (SC-20145) | Rb/polyclonal | Hu, pig, rat | none | IH |
| Laminin γ1 | Santa Cruz (SC-5584) | Rb/polyclonal | Hu, pig, rat | none | IH |
| Integrin α3 (CD49c) | Chemicon (AB1920) | Rb/polyclonal | Hu, pig, rat | none | IH |
| Chemicon (MAB2056) | Ms/monoclonal | Hu | Ms IgG1 | FC& IH | |
| Integrin α6 (CD49f) | BD Biosciences (GoH3, 555734) | Rat/monoclonal | Hu, pig | rat IgG2a | FC & IH |
| Serotec (MCA2034) | Ms/monoclonal | rat | Ms IgG1 | IH | |
| Integrin β1 (CD29) | Beckman Coulter (4B4, 6603113) | Ms/monoclonal | Hu, pig, rat | Ms IgG1 | FC & IH |
| Integrin β4 (CD104) | BD Biosciences (450-9D, 555721) | Ms/monoclonal | Hu | Ms IgG1 | FC & IH |
| Chemicon (AB1922) | Rb/polyclonal | Hu, pig, rat | none | IH | |
| Lu (CD239) | Serotec (MCA1982) | Ms/monoclonal | Hu, pig | Ms IgG2b | FC & IH |
| CD151 | BD Biosciences (PE-556057) | Ms/monoclonal | Hu, pig | Ms IgG1 | FC |
| Santa Cruz (SC-33123) | Rb/polyclonal | Hu, pig, rat | none | IH |
Sections were washed twice in PBS and incubated with appropriate secondary antibodies (AlexaFluor 488 or 633 secondary antibodies, Molecular Probes, Eugene, OR) for 30 min in blocking solution. Control sections were incubated with appropriate IgG controls (mouse IgG1 or rat IgG2a, Chemicon) instead of primary antibody, or with secondary antibody alone as a negative control for the polyclonal antibodies. All sections were counterstained with propidium iodide (1 mg/mL, Sigma) to label cell nuclei, and imaged using confocal laser scanning microscopy (Zeiss LSM 510; 20X NA 0.5 and 63X water immersion NA 1.2 objectives; Carl Zeiss, Thornwood, NY).
Flow Cytometry
Cells were isolated from the tissues of human intervertebral disc samples (9, 10, 14 year-old, n=3) for immunolabeling of specific proteins via flow cytometry. NP and AF cells were isolated with a sequential pronase–collagenase digestion [40], and were plated in subconfluent monolayers (50,000 cells/cm2) on 0.1% gelatin-coated (Sigma) tissue culture flasks for 3 to 7 days in culture media (F-12 media supplemented with 10% FBS, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C and 5% CO2. At confluence, cells were detached from the culture surface using 0.025% trypsin/EDTA (Cambrex, East Rutherford, NJ) and re-suspended in culture medium prior to labeling with antibodies. Cells (0.25~0.5×106) were incubated with monoclonal antibodies against human integrin subunits α3, α6, β1 and β4, CD239 and CD151 as described above (Table 1) using appropriate isotype controls. Cells were then labeled with AlexaFluor 633 conjugate secondary antibody. Cells were analyzed for fluorescence on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) to determine the percentage of cells with positive surface proteins (% cells) and mean fluorescence intensity (MFI).
In Vitro Expression of Laminins, Laminin Receptors, and Binding Proteins
Immunolabeling techniques were also used to confirm protein expression patterns for disc cells after short periods of culture in vitro. For these studies, cells were isolated from pig (3-month-old, n= 3) and human intervertebral discs (9-14 year-old, n=4) as described above, and cultured for 3-7 days on tissue culture flasks in culture media. For detection of laminin expression in cells under monolayer culture conditions, isolated human and pig cells were removed from the culture surface (0.025% trypsin/EDTA), re-suspended in culture media and plated on 8-well chamber slides (Nalge Nunc, Rochester, NY, 20,000 cells/well) coated with 0.1% gelatin. Cells were incubated overnight at 37°C to allow for attachment, followed by fixation in methanol (2 min at -20°C), incubation with a blocking solution (30 min), washing with PBS, and incubation with primary antibodies as described in Table 1 (2 hours). Chamber slides were imaged via confocal microscopy as described above.
CD151 AND CD239 mRNA Expression
Studies to quantify mRNA levels for the laminin-associated proteins CD151 and CD239 in rat tissues were undertaken as supplemental to the above-described studies of protein expression, as the specificity of available antibodies against these protein targets was not confirmed. Tissues from the rat (1 month-old, Fisher 344) coccygeal spines and rat-specific PCR primers were used for these studies. Intervertebral discs were dissected into zones corresponding to AF and NP and tissues were immediately flash-frozen in liquid nitrogen followed by pulverizing. Tissue powder was homogenized in TRIzol reagent and total RNA extracted with the RNeasy mini kit plus DNase I digestion (Qiagen, Valencia, CA). For one RNA sample, tissue was pooled across four animals to collect sufficient RNA. A total of four RNA samples (n=4 samples, 16 animals) for each tissue type (AF, NP) was analyzed to quantify the expression of CD239 and CD151 mRNA via RT-PCR as described previously (SmartCycler® system, Cepheid, Sunnyvale, CA) [40]. For each target gene, two rat-specific PCR primers and one fluorescently-labeled intron-spanning probe were used (CD239 # Rn 00582105-m1, CD151 # Rn 00574689-m1; Applied Biosystems, Foster City, CA). Relative mRNA for each target was quantified in NP and AF tissues using the comparative Ct method with 18S rRNA as an internal control [40]. Statistical analyses were performed to detect a difference in ΔCt (Ct of target- Ct of 18S rRNA) values between NP and AF samples using a one-factor ANOVA (StatView, SAS Institute, Cary, NC). Fold-differences of relative mRNA level (2-ΔΔCt) between NP and AF samples were reported if greater than or equal to 2, and where ANOVA detected a difference at p<0.05 [40].
RESULTS
Immunohistochemical Detection of Laminin Subunits
Intervertebral disc tissues of rat and pig spines exhibited region-specific expression of laminin subunits. In both immature rat (1 month-old) and immature pig (3 month-old) tissues, NP regions stained positively and often intensely for the laminin α5 chain, whereas little to no staining for the laminin α5 chain was observed in the AF regions (Figure 1). In contrast to the laminin α5 expression pattern, the AF stained positively and more intensely and more frequently than NP tissue for the laminin α1 chain (Figure 1). In the skeletally mature rat (12 month-old) and pig (24 month-old) tissues, the pattern of more intense staining for laminin α5 chain in the NP than AF regions was preserved, although with lower intensities than that of the immature NP tissues. Similar to the immature AF tissues, laminin α5 chain expression was not detected in the mature AF regions. For mature pig discs, laminin α1 staining was not observed in either NP or AF regions, while low intensity of staining for laminin α1 was observed in both AF and NP regions of the mature rat disc (Figure 1).
Figure 1.

Immunostaining for laminin α5 (left) and α1 subunit (right) in the intervertebral disc tissues of (A) rat (1 and 12 month-old) and (B) porcine (3 and 24 month-old). Cell nuclei (red) counter-stained with propidium iodide. Scale bars: 20 μm.
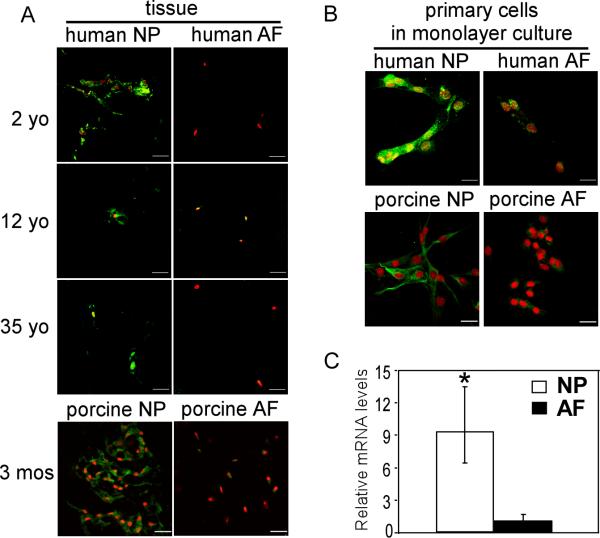
A similar region-specific laminin expression pattern was observed in tissues of the human intervertebral discs from ages 2 to 35 year-old. There was more intense staining for laminin α5 and γ1 chains in the NP tissues, as compared to AF, from the younger humans (2 and 12 year-old). The intensity and frequency of cell-associated staining for both laminin subunits were generally lower in the NP tissues from the older human (35 year-old) (Figure 2). Unlike rat and pig tissues, the laminin α1 chain was not detected in any immature or mature human NP or AF tissues (Figure 2). Laminin-10 (LM-511) and laminin-11 (LM-521) are both formed from the α5 and γ1 subunits, while laminin-1 (LM 111) and laminin-3 (LM-121) are formed from the α1 and γ1 subunits. In total, these findings suggest that laminin-10/11 may be uniquely present in the NP region of immature rat, pig and young human tissues, while laminin-1/3 may be the more abundant laminins present in the AF regions of immature rat and pig, but not human, intervertebral discs.
Figure 2.

Immunostaining for laminin α5 (left), α1 (middle) and γ1 subunit (right) in human intervertebral disc tissues (2, 12 and 35 year-old). Cell nuclei (red) counter-stained with propidium iodide. Scale bars: 20 μm.
Laminin subunit expression was also examined in cells isolated from human AF and NP tissues (9-14 year-old) following 3-7 days of monolayer culture. There was evidence of intense cell-associated expression of the laminin α5 subunit in the isolated human NP cells with much higher intensity as compared to AF cells (Figure 3C), consistent with a higher tissue-level expression for this subunit in the young human NP tissues. The laminin γ1 chain was also more highly expressed in isolated NP cells than AF cells, while both NP and AF cells expressed similar, modest levels of staining for the laminin α1 chain, in contrast to the expression pattern observed in the tissue (Figure 3C). As shown in Figure 3B, only a small population of cells isolated from NP regions of these juvenile disc samples were of sizes larger than 30μm and observed to contain vacuoles [18]. While a majority of NP cells were similar in size and morphology to that of the AF cells, it is noteworthy that a majority of NP cells from the juvenile NP retained expression of the distinct and specific laminin expression pattern observed at the tissue-level, suggesting that this molecular expression pattern may serve to distinguish juvenile NP cells from AF cells, in lieu of characteristic NP cell morphological features (i.e. larger size and vacuolated).
Region-Specific Expression of Laminin-Binding Integrins
Tissues isolated from NP regions of immature pig (3 month-old) and rat (1 month-old) demonstrated intensely positive staining for specific laminin-binding integrin subunits, including the α3, α6, β1 and β4 subunits (Figure 4). In contrast, adjacent AF regions did not stain at all for α3, α6, β1 or β4 integrin subunits in the rat and stained at a lower intensity in the pig (Figure 4). Similarly, tissues isolated from NP regions of young human (e.g., 12 year-old) also demonstrated positive and intense staining for α3, α6 and β1 integrin subunits but sparse and moderate staining for β4 subunits (Figure 4). The adjacent human AF regions of human did not stain for α3, α6 and β4 integrin subunits and only stained for β1 subunit with a modest intensity (Figure 4). These findings for higher intensity of α6 staining in the NP region, are consistent a previous report of both pig and human NP and AF tissues in situ [26].
Figure 4.
Immunostaining for laminin-binding integrin subunits in intervertevral disc tissues of immature human (12 year-old), porcine (3 month-old) and rat (1 month-old). Cell nuclei (red) counter-stained with propidium iodide. Scale bars: 20 μm.
When isolated from the tissue, human NP cells (e.g., 12 year-old) stained more intensely for integrin α3 than did isolated AF cells after short periods of culture; integrin α6 staining occurred with greater frequency for NP than AF cells, but was of low intensity when observed (Figure 5). A high and consistent expression of the β1 integrin subunit was observed for both NP and AF cells isolated from human tissues, when visualized via immunohistochemical staining (Figure 5) or quantified via flow cytometry (Table 2). The finding for higher expression of integrin α3 in isolated NP cells as compared to AF cells was corroborated by the results of flow cytometry (NP: 87% of all cells, MFI = 130; AF: 24%, MFI = 12, Table 2). The isolated human NP cells expressed only low levels of integrin α6 subunits via flow cytometry analyses; however, these levels were still higher than that in AF cells (NP: 41%, MFI=30; AF: 18%, MFI = 13, Table 2). These results were consistent with the low levels of immunohistochemical detection for integrin α6 subunit in the isolated NP cells (Figure 5). There was no detection of expression for the β4 integrin subunit in either isolated human NP or AF cells by both immunostaining (Figure 5) and flow cytometry (Table 2), a departure from that observed for staining of the human NP tissue regions in situ (Figure 4). In general, these results suggest that short periods of monolayer NP cell culture maintained higher expression for integrins α3 subunit as compared to AF cells, and uniform distribution of staining for the β1 subunit across AF and NP cell types, consistent with findings in disc tissues; however, the differential expression for integrin α6 and β4 subunit across NP and AF tissues was not apparently preserved well following culture of cells in vitro.
Figure 5.
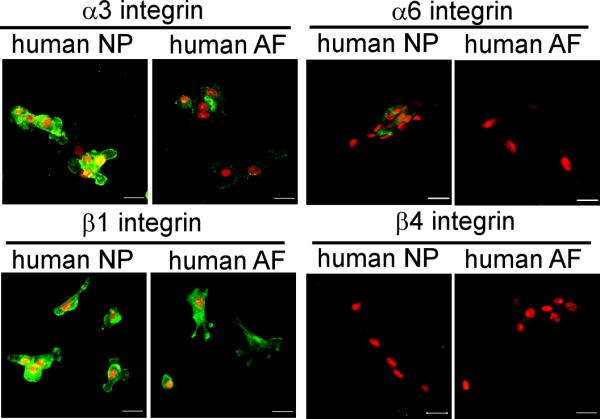
Expression of laminin-binding integrin subunits in immature human (12 year-old) intervertebral disc cells cultured in monolayer (3-7days) in vitro. Cell nuclei (red) counter-stained with propidium iodide. Scale bars: 20 μm.
Table 2.
Expression levels of laminin receptors and binding proteins in primary human intervertebral disc cells (9, 10, 14 year-old surgical samples) cultured in monolayer (3-7 days) in vitro via flow cytometry analysis.
| NP |
AF |
|||
|---|---|---|---|---|
| Binding protein | % (+) cellsa | MFIb | % (+) cellsa | MFIb |
| Integrin α3d | 87 | 130 | 24 | 12 |
| Integrin α6c | 41 ± 22 | 30 ± 9 | 18 ± 8 | 13 ± 4 |
| Integrin β1d | 91 | 404 | 98 | 1267 |
| Integrin β4d | 0 | 0 | 0 | 0 |
| Lu (CD239)d | 67 | 153 | 20 | 77 |
| CD151d | 95 | 181 | 90 | 244 |
%(+) cells: the percentage of cells with positive surface proteins
MFI: the mean fluorescence intensity of all positive cells
Values were the mean ± sd of three patient samples (9, 10 and 14 year-old)
Values were from one patient sample (9 or 10 year-old)
Region-Specific Expression of Laminin Receptors CD239 and Laminin-Binding Protein CD151
Tissues isolated from NP regions of young human (2 and 12 year-old) demonstrated more intense and positive staining for Lutheran glycoprotein (Lu, CD239), as compared to the adjacent AF regions (Figure 6A). The staining of CD239 was entirely lost in tissues of the older human NP (35 year-old, Figure 6A). A similar expression profile was noted for immature pig NP (3 month-old) which stained more intensely for CD239 than did adjacent AF (Figure 6A) or mature NP tissues (data not shown). When isolated from the tissue, both young human (9-14 year-old) and immature pig (3 month-old) NP cells exhibited a high intensity of staining for CD239 after only short periods of culture (Figure 6B). In contrast, a very slight staining for CD239 was found for human and porcine AF cells cultured under the same conditions (Figure 6B). The finding for higher expression of CD239 in isolated NP cells as compared to AF cells was corroborated by the results of flow cytometry (NP: 67%, MFI = 153; AF: 20%, MFI = 77, Table 2).
Figure 6.
Expression of Lutheran (Lu, CD239) in the intervertebral disc. (A) Immunostaining for CD239 in human (2, 12, 35 year-old) and porcine (3 month-old) tissue. (B) Immunostaining for CD239 in primary IVD cells (human 12 year-old, porcine 3 month-old) cultured in monolayer (3-7days). Scale bars: 20 μm. (C) RT-PCR for relative mRNA levels of CD239 in rat (1 month-old) tissues (relative fold changes normalized to AF=1.0, * p<0.05, ANOVA, n=4).
In a pattern similar to that observed for CD239 expression, tissues isolated from the NP of young human (2 and 12 year-old) and immature pig (3 month-old) stained positively and more intensely than AF tissues for the tetraspanin molecule, CD151 (Figure 7A). The higher staining for CD151 in the NP regions was maintained for the mature human tissue (35 year-old), however, in contrast to the pattern of decreased staining for CD239 in the older human NP (Figure 7A). When isolated from the tissue, the young human (9-14 year-old) and pig (3 month-old) NP cells stained with higher intensity for CD151 around the cell cytoplasm after short periods of culture as compared to AF cells (Figure 7B). The differential protein expression pattern for CD151 between NP and AF cells in the porcine and human IVD was not corroborated by the results of flow cytometry, however, as both AF and NP cells showed very high expression levels for this protein (90-95% of all cells; MFI = 181-244, Table 2).
Figure 7.
Expression of laminin-binding protein CD151 (tetraspanin) in the intervertebral disc. (A) Immunostaining for protein CD151 in human (2, 12 and 35 year-old) and porcine (3 month-old) tissues, (B) Immunostaining for CD151 in primary IVD cells (human 12 year-old, porcine 3 month-old) cultured in monolayer (3-7 days). Scale bars: 20 μm. (C) RT-PCR for relative mRNA levels of CD151 in the rat (1 month-old) tissues in vivo (relative fold changes normalized to AF=1.0, * p<0.05, ANOVA, n=4).
Region-Specific mRNA Levels for CD239 and CD151
Given evidence of higher protein expression for CD239 and CD151 in human cells of the immature NP regions, it was of interest to quantify constitutive mRNA levels for these proteins in an immature tissue. In immature rat NP tissue regions, mRNA levels for both CD239 and CD151 were significantly higher (8-30-fold higher) than that in AF tissue regions (1 month-old, Figures 6C and 7C) (p<0.05, ANOVA).
DISCUSSION
This study provides new findings for the expression of the laminin α5 chain, laminin receptors (integrin α3, α6, β4 subunit, CD239) and related binding proteins (CD151) in NP cells of immature human, porcine and rat discs. Distinct differences were noted in the expression of these laminins and laminin-binding proteins between NP and AF tissues, and for isolated cells after culture in vitro. NP cells expressed more intense and frequent staining for the laminin α5 chain, while AF cells expressed more staining for the laminin α1 chain in situ, supporting the conclusion that different isoforms of laminin are expressed in different intervertebral disc regions. Laminin-10 (α5β1γ1) or laminin-11 (α5β2γ1) may be those isoforms uniquely present in NP regions of immature and young rat, pig and human tissues, while laminin-1 (α1β1γ1) or laminin-3 (α1β2γ1) may be more abundant isoforms in the AF regions of immature rat and pig tissues. In general, staining for all laminin chains decreased in older specimens, possibly because of decreased cellularity in the older tissues. In human intervertebral discs of any age studied (2-35 year-old), we did not detect any expression of the laminin α1 subunit in either NP or AF regions, a finding that does not preclude the possibility for laminin α1 to be present at younger ages. The laminin γ1 subunit has been reported to be present in the developing rat intervertebral disc [23], largely in the notochord, and localized on cell surfaces and within the notochordal sheath. In intermediate developmental stages, weak pericellular staining of laminin γ1 has also been observed in the inner AF and some in the outer AF in the neonate. At all stages, however, laminin γ1 staining is closely associated with blood vessels at the periphery of the intervertebral disc and penetrating the cartilage endplates [41]. The presence of laminin γ1 in the NP is distinct from this description, as it is always pericellular and in close association with integrin localization [38], unlike the findings of no staining, or little vascular-associated staining in AF regions. These features are common across all three species, human (Figure 2), pig [38] and rat (data not shown).
As laminins have been found mostly in basement membranes and in association with type IV collagen, studies of laminin-cell interactions have focused largely on endothelial and epithelial cell types. The laminin α5 chain has been identified to be widely expressed in mouse and human tissues, including lung, heart, kidney and endothelia basement membranes [42], while laminin α1 chain shows a restricted distribution in epithelia basement membranes [43]. Laminins play key roles throughout development, in regulating cellular polarity and differentiation during tissue morphogenesis and organization [29, 44, 45]. Laminins and their receptors (α6β1 and α6β4 integrins) have also been shown to play important roles in the invasion, progression, and survival of cancers, including carcinomas [46, 47]. It is of interest that these laminin receptors are known to promote cell survival in response to environmental stress including serum-deprivation and hypoxia [46, 48], as the NP cells are known to experience low oxygen tension and limited nutrient supply [49]. Here, we further discovered that immature NP cells likely make use of the integrin receptor, α6β1, as well as integrin α3β1, Lutheran (Lu, CD239) and possibly α6β4, to bind to laminin, as well as laminin-related binding proteins such as the tetraspanin, CD151. A parallel functional role for these receptors and their interactions with laminin in protecting NP cells from environment stress is plausible, but currently unknown. While the potential roles for laminin-receptor interactions have rarely been investigated in the NP, we have previously observed that larger NP cells (i.e., likely of notochordal origins) not only express higher levels of integrin α6 than small NP cells and AF cells [14], but also rely upon an α6 integrin-mediated adhesion to laminin-1 substrates [38]. Thus, these laminin receptors do appear to be functional in the immature NP cells. As laminins are an essential component of the basement membrane surrounding the notochord during notochord formation [50] and differentiation [51] laminin receptors are also of chief importance during synthesis of the NP precursor tissues.
The parallel expression patterns for Lutheran (Lu, CD239) and the laminin α5 chain in NP tissues observed here are consistent with an understanding of the interactions between these two proteins in epithelia and other tissues. CD239 is a receptor which binds exclusively to the laminin α5 chain, and has been previously identified in human red blood cells and as co-receptors in epithelia, endothelia and smooth muscle cells [34]. Laminin α5 knockout mice show reduced basal concentration of CD239 on epithelial cells of embryos, while transgenic mice over-expressing the laminin α5 chain show increased amounts of CD239 in the heart [52]. Genetic inactivation of CD239 leads to developmental abnormalities in other laminin α5-expressing tissues (kidney and intestine) in the mouse [53]. That the tissue specific expression of both laminin α5 chain and CD239 are maintained in the immature NP but not AF regions points towards the unique and distinctly different developmental origins of the NP and AF regions [8]. It is indeed likely that this expression pattern could be linked to a notochordal origin of the NP tissue based on the known involvement of laminins in the basement membrane surrounding notochord during differentiation [51]. A functional role for these proteins and their interactions in postnatal growth, development and aging of the intervertebral disc is not known, although the findings here motivate studies of a role for CD239-mediated cell-laminin interactions in the NP with aging and degeneration. These findings also suggest that these unique laminin receptors, and related laminin isoforms and binding proteins may serve as phenotypic markers for immature NP cells, and markers that are distinct from unique morphological features.
Specific expression of the tetraspanin molecule, CD151, was another novel finding for immature NP tissues amongst all three species. CD151 is a cell surface glycoprotein belonging to the transmembrane 4 superfamily of proteins, tetraspanins. It is known that CD151 forms complexes with laminin binding integrins (α3β1, α6β1, and α6β4) and plays a key role in selectively strengthening α6β1 integrin-mediated adhesion to laminin [37]. Thus, CD151 may function as a transmembrane linker between extracellular integrin domains and intracellular cytoskeleton/signaling molecules in cellular processes including cell adhesion, spreading and integrin-dependent matrix remodeling [54]. Previously, immature NP tissues were reported to have higher expression levels of integrin α6 [26], while we additionally report here high expression of integrin α3 and CD151 in immature NP, in a pattern similar to that of integrin α6. Together, these findings suggest that CD151 may play a role in NP cell-laminin interactions through integrin α3 or α6 subunits.
While some tissue-specific expression of laminin chains and receptors were observed in situ, these patterns were not always preserved after short periods of monolayer culture in vitro. Whereas human NP cells expressed higher levels of integrin α6 and β4 in the tissue, high levels for these proteins were not observed after cells were isolated and cultured in monolayer culture conditions. The reasons for the discrepancy in staining patterns between tissue and in vitro cellular levels are unknown, but could be partly attributed to factors associated with cell isolation and trypsin digestion, as well as features of the tissue culture surfaces used. This may suggest that monolayer conditions used here may not be appropriate for maintaining NP-specific laminin and receptor expression in vitro. Studies are under way to find a 3D culture system which will maintain laminin and its receptor expression pattern in human intervertebral disc cells in vitro.
Numerous studies have focused on identification of unique features of NP cells in the intervertebral disc, towards the goal of maintaining cell phenotype of NP cells in vitro or promoting cell survival in vivo. In addition, there is an interest in of the use of progenitor cells (e.g., stem cells) to regenerate the immature NP in pathological situations [55-61], although no consensus has been achieved on appropriate outcome markers nor differentiation protocols appropriate for this cell type. The search for unique features of NP cells has also been confounded by the apparent heterogeneous size and morphology of the cell population, as well as biosynthesis that depends on age, species, and factors specific to culture conditions (e.g., mechanical loading, oxygen tension, pH, growth factors) [12, 24, 49, 62-66]. Studies of mRNA transcriptional profiling for cells of the immature NP [14, 67-69] reveal some patterns unique to immature NP cells, including lower mRNA expression of biglycan, decorin, lumican, some MMPs and TIMP1 in the large cells of the immature NP cells, compared to AF cells [14]. Quite a few protein “markers” of immature NP cells have been suggested, including high expression of vimentin [14], CD24 [70], GLUT-1, HIF-1α, MMP2 [67] and galectin-3 [71, 72]; however, few (if any) of these expression patterns were shown to persist for cells regenerating matrix in vitro or in vivo. The results presented here contribute a new set of laminin-associated proteins that may be relevant as NP-specific markers for distinguishing the primary cells as well as for evaluating NP cell phenotypes in situ and following in vitro culture.
A major limitation of the current study was the reliance on conclusions drawn from a small number of samples taken from juvenile, human intervertebral discs. Tissue procurement was limited to those tissue samples that were sufficiently large to support both cell isolation and tissue immunohistochemical staining portions of the study. For the low power afforded by these low sample numbers, it was determined that semi-quantitative grading of specimens would provide little additional information. For this reason, we sought to simultaneously obtain quantitative parameters of flow cytometry and mRNA levels for key proteins. Additional studies using a wider range of human ages, as well as a greater number of disc samples with the different severity of degeneration will be important for providing some quantitative evidence of the observations reported here. Data from these additional studies will also determine whether the expression these markers change in degenerating discs or if loss of function of any of these markers affect the disc.
In summary, our studies suggest that laminin interactions with NP cells are distinct from that of the anulus fibrosus, and that laminins may be important contributors to region-specific IVD biology. The revealed laminin isoforms, their receptors and related binding proteins appear to be distinguishing features of these immature NP cells in the intervertebral disc.
ACKNOWLEDGEMENTS
We gratefully acknowledge Steve Johnson and Tish Griffin for assistance with tissue harvesting, Tiffany Chang and Melissa Tsuboyama for assistance with immunostaining, Bob Nielsen for assistance with confocal microscopy, and Dr. Mike Cook for assistance with FACS. This study was supported with funds from the NIH (AR047442 & EB002263 & AR057410).
REFERENCES
- 1.Andersson GB, An HS, Oegema TR, Jr., Setton LA. Intervertebral disc degeneration. Summary of an AAOS/NIH/ORS workshop, September 2005. J Bone Joint Surg Am. 2006;88:895–9. doi: 10.2106/JBJS.F.00028. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Oegema TRJ. The role of disc cell heterogeneity in determining disc biochemistry: a speculation. Biochem Soc Trans. 2002;30:839–44. doi: 10.1042/bst0300839. [DOI] [PubMed] [Google Scholar]
- 6.Bayliss MT, Johnstone B. Biochemistry of the intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Back Pain. Churchill Livingstone; New York: 1992. pp. 111–31. [Google Scholar]
- 7.Postacchini F, Bellocci M, Massobrio M. Morphologic changes in anulus fibrosus during aging: An ultrastructural study in rats. Spine. 1984;9:596–603. doi: 10.1097/00007632-198409000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol. 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 9.Urban JP, Roberts S. Development and degeneration of the intervertebral discs. Mol Med Today. 1995;1:329–35. doi: 10.1016/s1357-4310(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 10.Oegema TRJ. Biochemistry of the intervertebral disc. Clin Sport Med. 1993;12:419–39. [PubMed] [Google Scholar]
- 11.Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307–14. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- 12.Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279–91. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–37. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–11. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilak F, Ting-Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells. Identification of two biomechanically distinct cell populations. Spine. 1999;24:2475–83. doi: 10.1097/00007632-199912010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JR, Twomey LT. The development of the human intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc. CRC Press; Boca Raton, FL: 1988. pp. 39–82. [Google Scholar]
- 17.Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211:444–52. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc: I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–69. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 19.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 20.Kim KW, Ha KY, Park JB, Woo YK, Chung HN, An HS. Expressions of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs - A cartilage endplate-fracture model using an intervertebral disc organ culture. Spine. 2005;30:1373–8. doi: 10.1097/01.brs.0000166155.48168.0e. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Winlove PC, Roberts S, Urban JP. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–75. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts S, Menage J, Duance V, Wotton S, Ayad S. 1991 Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16:1030–8. [PubMed] [Google Scholar]
- 23.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–21. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 24.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–30. doi: 10.1186/ar629. Epub 2003 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Nettles DL, Richardson WJ, Setton LA. Integrin expression in cells of the intervertebral disc. J Anat. 2004;204:515–20. doi: 10.1111/j.0021-8782.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 28.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 30.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113(Pt 5):869–76. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–23. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 32.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Nishiuchi R, Takagi J, Hayashi M, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Kikkawa Y, Miner JH. Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues. Connect Tissue Res. 2005;46:193–9. doi: 10.1080/03008200500344074. [DOI] [PubMed] [Google Scholar]
- 35.Nishiuchi R, Sanzen N, Nada S, et al. Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci U S A. 2005;102:1939–44. doi: 10.1073/pnas.0409493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ido H, Harada K, Futaki S, et al. Molecular dissection of the alpha-dystroglycanand integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–54. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 37.Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci U S A. 2003;100:7616–21. doi: 10.1073/pnas.1337546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilchrist CL, Chen J, Richardson WJ, Loeser RF, Setton LA. Functional integrin subunits regulating cell-matrix interactions in the intervertebral disc. J Orthop Res. 2007;25:829–40. doi: 10.1002/jor.20343. [DOI] [PubMed] [Google Scholar]
- 39.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Baer AE, Paik PY, Yan W, Setton LA. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun. 2002;293:932–8. doi: 10.1016/S0006-291X(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 41.Rudert M, Tillmann B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. Ann Anat. 1993;175:237–42. doi: 10.1016/s0940-9602(11)80009-9. [DOI] [PubMed] [Google Scholar]
- 42.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 43.Falk M, Ferletta M, Forsberg E, Ekblom P. Restricted distribution of laminin alpha1 chain in normal adult mouse tissues. Matrix Biol. 1999;18:557–68. doi: 10.1016/s0945-053x(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol. 2006;294:271–9. doi: 10.1016/j.ydbio.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Tzu J, Chen Y, et al. Laminin-10 is crucial for hair morphogenesis. Embo J. 2003;22:2400–10. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung J, Mercurio AM. Contributions of the alpha6 integrins to breast carcinoma survival and progression. Mol Cells. 2004;17:203–9. [PubMed] [Google Scholar]
- 47.Mercurio AM, Bachelder RE, Chung J, et al. Integrin laminin receptors and breast carcinoma progression. Journal of mammary gland biology and neoplasia. 2001;6:299–309. doi: 10.1023/a:1011323608064. [DOI] [PubMed] [Google Scholar]
- 48.Gu J, Fujibayashi A, Yamada KM, Sekiguchi K. Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J Biol Chem. 2002;277:19922–8. doi: 10.1074/jbc.M200383200. [DOI] [PubMed] [Google Scholar]
- 49.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 50.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 51.Parsons MJ, Pollard SM, Saude L, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–46. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- 52.Moulson CL, Li C, Miner JH. Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin alpha5 in vivo. Dev Dyn. 2001;222:101–14. doi: 10.1002/dvdy.1169. [DOI] [PubMed] [Google Scholar]
- 53.Rahuel C, Filipe A, Ritie L, et al. Genetic inactivation of the laminin {alpha}5 chain receptor Lu/BCAM leads to kidney and intestinal abnormalities in the mouse. American journal of physiology. 2008;294:F393–406. doi: 10.1152/ajprenal.00315.2007. [DOI] [PubMed] [Google Scholar]
- 54.Kazarov AR, Yang X, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 56.Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–41. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 57.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 58.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29:2627–32. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 59.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S306–13. doi: 10.1007/s00586-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson SM, Walker RV, Parker S, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–16. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 61.Crevensten G, Walsh AJL, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs. Annals of Biomedical Engineering. 2004;32:430–4. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–83. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Chiba K, Andersson GB, Masuda K, Thonar EJ. Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine. 1997;22:2885–93. doi: 10.1097/00007632-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 64.Thonar E, An H, Masuda K. Compartmentalization of the matrix formed by nucleus pulposus and annulus fibrosus cells in alginate gel. Biochem Soc Trans. 2002;30:874–8. doi: 10.1042/bst0300874. [DOI] [PubMed] [Google Scholar]
- 65.Wang JY, Baer AE, Kraus VB, Setton LA. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001;26:1747–51. doi: 10.1097/00007632-200108150-00003. discussion 52. [DOI] [PubMed] [Google Scholar]
- 66.Roberts S. Disc morphology in health and disease. Biochem Soc Trans. 2002;30:864–9. doi: 10.1042/bst0300864. [DOI] [PubMed] [Google Scholar]
- 67.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–7. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Jing L, Richardson WJ, Brown L, Setton LA. Gene expression profiling reveals age and zonal-specific differences in intervertebral disc tissue during aging. Trans Orthop Res Soc. 2007;32:1104. [Google Scholar]
- 69.Lee C, Grad S, Sakai D, Mochida J, Alini M. Comparison of gene expression profiles of nucleus pulposus, annulus fibrosus, and articular cartilage cells. Trans Orthop Res Soc. 2006;31:1199. [Google Scholar]
- 70.Fujita N, Miyamoto T, Imai J, et al. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–6. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- 71.Gotz W, Kasper M, Miosge N, Hughes RC. Detection and distribution of the carbohydrate binding protein galectin-3 in human notochord, intervertebral disc and chordoma. Differentiation. 1997;62:149–57. doi: 10.1046/j.1432-0436.1997.6230149.x. [DOI] [PubMed] [Google Scholar]
- 72.Oguz E, Tsai TT, Di Martino A, et al. Galectin-3 expression in the intervertebral disc: a useful marker of the notochord phenotype? Spine. 2007;32:9–16. doi: 10.1097/01.brs.0000250302.74574.98. [DOI] [PubMed] [Google Scholar]