Abstract
PURPOSE
The purpose of this study was to estimate the 10-year absolute risk of CV events in newly diagnosed RA subjects and the potential contribution of CV risk factors to absolute risk assessment.
METHODS
A population-based incidence cohort of RA subjects (ascertained according to ACR criteria) was assembled and compared to an age and sex-matched cohort of non-RA subjects. Data were collected on CV risk factors and CV events. Cox regression models were used to estimate the 10-year risk of a combined CV endpoint, adjusting for CV risk factors. Subjects were classified into 5 risk categories based on their 10-year absolute risk.
RESULTS
The absolute CV risk in RA subjects was similar to that of non-RA subjects who were 5-10 years older. The absolute risk varied substantially according to presence of CV risk factors. The 10-year absolute CV risk among 60-69 year old RA subjects with no risk factors was 16.8% but rose to 60.4% in the presence of smoking, hypertension, dyslipidemia, diabetes and obesity. Among RA subjects with low, as compared to normal or high BMI, in addition to the above risk factors, the 10-year absolute CV risk rose to 86.2%.
CONCLUSION
More than half of 50-59 year old newly diagnosed RA subjects and all of those >60 years of age had a 10% or greater risk of CV disease within the next 10 years and should be targeted for specific CV risk reduction strategies tailored to their personal risk profiles.
Keywords: Mortality, Rheumatoid Arthritis, Cardiovascular disease, absolute risk
INTRODUCTION
Patients with rheumatoid arthritis (RA) are at increased risk of cardiovascular (CV) disease. The risk of myocardial infarction, heart failure and CV death among RA subjects has been reported to be approximately 2-3 fold greater than in the general population1-3. Even though the CV risk in RA is well-recognized, a major challenge is detection, treatment, and prevention of CV disease in RA subjects who have no symptoms of heart disease and yet are at increased risk. We have previously shown that RA subjects are less likely to report symptoms of angina and more likely to experience unrecognized MI1. Even more importantly, RA subjects are twice as likely to experience sudden deaths, indicating that the first presentation of CV disease in RA subjects may be a sudden cardiac death1. Therefore, identifying high risk asymptomatic RA patients who may potentially benefit from primary prevention is an important challenge for the treating physicians.
High risk asymptomatic individuals in the general population are typically identified using CV risk scores which rely on assessment of age, gender, total cholesterol, HDL cholesterol levels, smoking status and blood pressure and then calculating the 10-year absolute CV risk4. We believe that CV risk factor assessment and risk reduction tailored to specific risk profiles of asymptomatic RA subjects have the potential to prevent a substantial number of future CV events in RA subjects. Thus, the purpose of our study was to estimate the age-specific 10-year absolute risk of CV events and to describe the potential contribution of CV risk factors to absolute risk assessment in newly diagnosed RA subjects.
METHODS
This is a retrospective population based cohort study performed using the resources of the Rochester Epidemiology Project medical records linkage system5 in Olmsted County, Minnesota. Population-based epidemiologic research can be conducted in Olmsted County because medical care is virtually self-contained within the community, and complete (inpatient and outpatient) medical records for county residents are available for review. After approval by the Mayo Clinic’s Institutional Review Board, we used the unique resources of the Rochester Epidemiology Project to identify all subjects with RA, first diagnosed between January 1, 1955 and January 1, 1995, among Rochester, MN residents ≥ 18 years of age1. All RA subjects fulfilled the 1987 American College of Rheumatology (ACR) criteria for RA6 and the incidence date was defined as the first date of fulfillment of (four out of the seven) classification criteria. Each RA subject was then individually matched by sex, birth year (± 3 years) and length of prior medical history to a randomly selected subject without RA living in Rochester, MN. Matching on length of prior medical history ensured that RA and corresponding non-RA subjects had the same opportunity for clinical detection of study outcomes. Each subject in the matched non-RA comparison cohort was assigned an index date corresponding to the RA incidence date.
Subjects in the RA cohort and the non-RA comparison cohort were then followed forward in time through their linked medical records in the community. Data were collected on CV risk factors and CV events using established epidemiological criteria. Cigarette smoking status was categorized as current, former or never. Dyslipidemia was defined as LDL-cholesterol level ≥160 mg/dl, total cholesterol level ≥240 mg/dl, high-density lipoprotein cholesterol level <40 mg/dl or triglycerides >150 mg/dl, or a clearly documented history of dyslipidemia or treatment with specific lipid-lowering therapy7. Diabetes mellitus was defined as at least 2 measurements of fasting plasma glucose ≥126 mg/dl or 2-hour plasma glucose level ≥200 mg/dl, or a clearly documented history of diabetes or treatment with hypoglycemic agents8. Hypertension was defined as 2 or more ambulatory blood pressure readings of ≥140 mm Hg systolic and/or 90 mm Hg diastolic, or a physician’s diagnosis of hypertension or treatment with antihypertensive agents 9. Height and weight were recorded at baseline and body mass index (BMI) was computed. Obesity was defined as BMI ≥30 kg/m2 and low BMI defined as <20 kg/m2 210. Personal cardiac history was ascertained at baseline and included presence of angina pectoris, coronary artery disease and ischemic heart disease.
Combined CV outcome comprised coronary revascularization procedures, silent or non-fatal myocardial infarctions (MI), heart failure (HF) and CV deaths. Coronary revascularization procedures included percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass grafting (CABG). Non-fatal hospitalized MI were classified as definite or probable, based on the presence of cardiac pain, biomarker values and the Minnesota ECG coding system11. Silent MI was defined as the presence of a characteristic ECG in a non-acute setting, or recorded physician diagnosis of a characteristic ECG in patients without any documentation of previous MI. HF was defined according to Framingham criteria12.
All subjects (irrespective of residency status) were tracked nationally (using the National Death Index and other sources) to ascertain vital status. Death certificates were obtained from the respective states for subjects who were deceased out of state. CV deaths were classified based on the underlying cause of death and included ICD-9 codes 390–459 and ICD 10 codes I00–I99. Follow-up continued until death, migration or January 1st, 2001.
Statistical Methods
Subjects with a history of CV disease at baseline (i.e. RA incidence date for RA subjects and index date for non-RA subjects) were excluded. Kaplan-Meier methods were used to estimate the cumulative incidence of CV events by decade of age13. Cox regression models were used to estimate the 10-year risk of the combined CV endpoint, adjusting for CV risk factors. These 10 year risk estimates represent the overall RA (or non-RA) cohort because they correspond to obtaining a prediction for the risk profile of each patient and averaging these predicted 10 year risks. Subjects were classified into 5 risk categories based on their 10-year absolute risk of combined CV endpoint: low-risk (<6% CV risk over the next 10 years), low- moderate (6%-10% risk), intermediate (>10%-20 % risk), high (>20-50% risk) and very high risk (>50%). Absolute risk estimates corresponding to specific risk factor profiles were obtained as predictions from the Cox models.
RESULTS
The study population comprised 553 RA subjects and 574 matched non-RA subjects free of CV disease at baseline (i.e. RA incidence date for RA subjects and index date for matched non-RA subjects). The baseline characteristics of the RA and the non-RA cohorts are shown in Table 1. In both cohorts, 73% were women and the mean age at baseline was 57 years. Median follow-up time was 14.7 years for RA subjects and 16.1 years for non-RA subjects, corresponding to 9484 and 10244 person-years of follow-up, respectively. RA subjects were significantly more likely to have been former or current smokers when compared to non-RA subjects. The baseline prevalence of family cardiac history, hypertension, dyslipidemia, obesity and diabetes mellitus were similar in both groups (Table 1).
Table 1.
Baseline Characteristics
| RA cohort N=553 | Non-RA cohort N=574 | p-value | |
|---|---|---|---|
| Age at RA incidence, mean (years) | 56.8 (±15.0) | 57.3 (±15.0) | 0.60 |
| Female, n (%) | 406 (73) | 425 (74) | 0.81 |
| Follow-up (years), median | 14.7 | 16.1 | -- |
| CV risk factors | N (%) | N (%) | |
| Current/former cigarette smoking status | 285 (52) | 247 (43) | 0.004 |
| Hypertension | 272 (49) | 277 (48) | 0.76 |
| Dyslipidemia * | 137 (48) | 155 (51) | 0.43 |
| High BMI (≥30 kg/m2) | 66 (12) | 66 (12) | 0.82 |
| Low BMI (<20 kg/m2) | 64 (12) | 58 (10) | 0.43 |
| Diabetes mellitus | 36 (7) | 36 (6) | 0.87 |
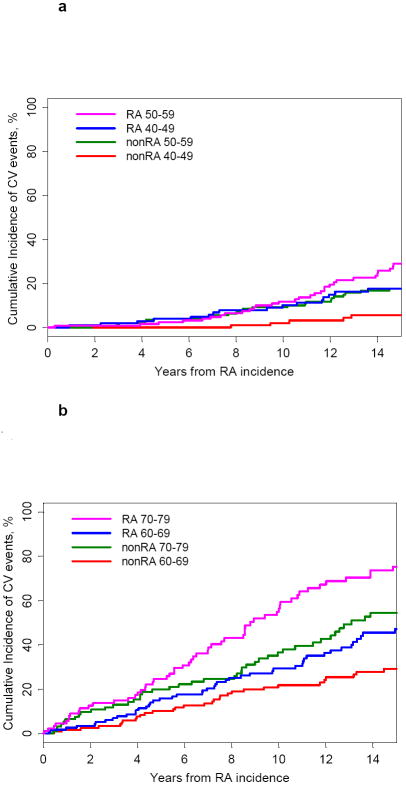
During the entire follow-up period, we ascertained a total of 254 first CV events in 553 RA subjects (absolute risk of 49.5 per 1000 person-years) and 209 first CV events in 574 age- and sex-matched non-RA subjects (absolute risk of 31.7 per 1000 person-years). Figure 1 shows the cumulative incidence of combined CV outcome by age groups starting at RA incidence for RA subjects and index date for non-RA subjects. The absolute risk of CV events at 10 years was 2.1% in 40-49 years old non-RA subjects and 10.2% in 40–49 years old RA subjects (figure 1a). In the 50-59 years age group, the absolute risk at 10 years was 9.2% in 50-59 years old non-RA subjects and 11.9% in 50–59 years old RA subjects. Therefore, the absolute risk in 40-49 years old RA subjects (10.2%) was similar to non-RA subjects who were 5-10 years older (9.2%). Similarly, the absolute risk of CV events in 60–69 years old RA subjects (29.4%) was similar to 65-74 years non-RA subjects (32.5%) (figure 1b). As illustrated in figures 1a and 1b, the absolute CV risk difference between RA and non-RA subjects increased over time since RA incidence date for RA subjects and index date for non-RA control subjects.
Figure 1. Cumulative incidence of combined CV endpoint by age groups in RA and non-RA subjects.
a. 40-49 and 50-59 years old RA (solid lines) and non-RA (dotted lines) subjects
b. 60-69 and 70-79 year old RA (solid lines) and non-RA (dotted lines) subjects
We then estimated the 10-year absolute risk categories for the combined CV endpoint, accounting for CV risk factors, separately for the RA and non-RA subjects. Figure 2 and Table 2 show the 10-year absolute risk of a CV event in RA and non-RA subjects according to age groups. Within each age group, the first column in Figure 2 corresponds to the non-RA cohort and the second column corresponds to the RA cohort. Subjects within each age group were categorized into 5 CV risk groups (low, low- moderate, intermediate, high and very high) according to their 10-year risk of a CV outcome. Irrespective of RA disease status, the proportion of subjects with intermediate or high risk of CV events increased sharply with age, reinforcing the fact that the key determinant of CV disease in both RA non-RA subjects is age at baseline. At their RA incidence date, almost 20% of 40-49 year old RA subjects had an intermediate – high risk (10% or more) of CV event as compared to only 5% among 40-49 year old non-RA subjects. In other words, 20% of 40-49 years old RA subjects can be expected to experience a CV event within 10 years of their RA incidence date as compared to only 5% of 40-49 year old non-RA subjects. The CV risk was substantially higher in the 50-59 year age group. The proportion of newly diagnosed 50-59 year old RA subjects with intermediate or higher 10-year risk of CV events was 85% as compared to 27% among non-RA subjects. The proportion of 60-69 year old RA subjects with intermediate or higher risk of CV events was 100% as compared to 79% among non-RA subjects. Similarly, the proportion of 60-69 year old RA subjects with a high or very high risk (higher than 20%) of CV events was 85% as compared to only 40% among non-RA subjects. In other words, these data indicate that all the 60-69 years old RA subjects have greater than 10% risk of experiencing a CV event within 10 years of their RA incidence date and, depending on their CV risk factor profiles, the risk may be as high as 20%.
Figure 2. Estimated 10-year cardiovascular risk by age groups among non-RA (first column) and RA (second column) subjects, Rochester, MN.
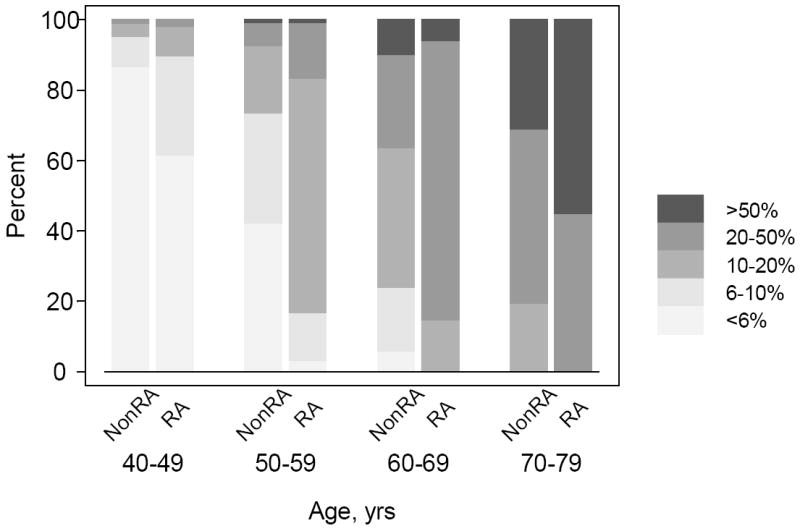
Table 2.
Distribution of non-RA and RA subjects according to estimated 10-year cardiovascular risk
| Cardiovascular risk over the next 10 years | 40-49 years | 50-59 years | 60-69 years | 70-79 years | ||||
|---|---|---|---|---|---|---|---|---|
| non-RA | RA | non-RA | RA | non-RA | RA | non-RA | RA | |
| < 6% | 86.7 | 61.7 | 42.2 | 3 | 5.8 | 0 | 0 | 0 |
| 6-10% | 8.6 | 28 | 31.3 | 13.6 | 18.2 | 0 | 0 | 0 |
| >10-20% | 3.8 | 8.4 | 19 | 66.7 | 39.7 | 14.7 | 19.4 | 0 |
| >20-50% | 1 | 1.9 | 6.8 | 15.9 | 26.4 | 79.3 | 49.5 | 44.9 |
| >50% | 0 | 0 | 0.7 | 0.8 | 9.9 | 6 | 44.9 | 55.1 |
We then explored the role of the CV risk factors in absolute risk prediction. Table 2 shows the age-specific absolute risk of CV events within the next 10 years, according to presence of various CV risk factors in RA and non-RA subjects. The shading in the table represents the five CV risk groups (low, low-moderate, intermediate, high and very high). The last two columns in the table illustrate CV risk in subjects with either high or low BMI. The increased absolute risk for CV events in RA subjects compared with non-RA subjects was apparent across all age groups and risk factor categories. For example, among 40-49 year old non-RA subjects with no CV risk factors, the absolute risk of a CV event was 1.4% whereas, the absolute risk among 40-49 year old RA subjects with no risk factors was 3.0%. If the 40-49 year old person was a smoker, the absolute risk was 2.9% among non-RA subjects and 5.1% among RA subjects. The absolute risk of a CV event was as high as 14.4% among 40-49 year old RA patients with several risk factors, and 28.3% among 40-49 year old RA subjects with low BMI in addition to other CV risk factors. The risk was even higher (32.6%) among RA subjects with a BMI of <18. Similarly, a 50-59 year old RA subject with no risk factors had an absolute risk of 7.3% but the absolute risk was as high as 31.6% if the person was a smoker, obese and had hypertension, dyslipidemia and diabetes. If the 50-59 year old RA subject had low BMI at RA incidence, the absolute risk was 55.6%. The risk was 61.3% among 50-59 year RA subjects with a BMI of <18. The pattern was essentially similar in older RA subjects. The absolute risk among 60-69 year old RA subjects with no risk factors was 16.8% but could be as high as 60.4% if several risk factors were present, and 86.2% if 60-69 year old RA subjects had low BMI in addition to the CV risk factors. Hence, Table 2 illustrates how absolute CV risk in RA subjects varies depending on the CV risk factor profile of individual subjects.
DISCUSSION
In this retrospective population-based cohort study, we estimated the age-specific 10-year absolute risk of CV events in newly diagnosed RA subjects in comparison to subjects without RA. Our findings confirm that age is the key determinant of CV disease in both RA non-RA subjects. In general, absolute CV risk in RA subjects is similar to that of non-RA subjects 5-10 years older. More than half of the 50-59 year old newly diagnosed RA subjects and all of those >60 years of age have 10% or greater risk of CV disease within 10 years of their RA incidence. Our analyses underscore the importance of CV risk factor assessment for accurate prediction of CV risk in individual RA subjects. These subjects could benefit from early CV risk detection and risk reduction strategies tailored to their specific CV risk profiles. Importantly, consideration of absolute CV risk categories and CV risk factors in RA can facilitate clinical decisions concerning CV disease prevention in asymptomatic RA subjects.
Most studies of CV risk in RA have reported that the excess risk of myocardial infarction and heart failure in RA subjects cannot be explained by an increased frequency or an increased effect of the traditional CV risk factors1, 2, 14, 15. The findings of these etiological studies have been misinterpreted by some to imply that traditional CV risk factors are less important for CV risk assessment in the RA compared to the non-RA population. This is because the relative risks associated with traditional CV risk factors disease have been reported to be lower in RA compared to non-RA subjects15. However, this does not imply that traditional risk factors are unimportant in the etiology of CV disease in RA. Instead, we believe that these relatively smaller relative risks indicate that RA specific disease mechanisms, (eg. systemic inflammation) are competing with the more traditional CV risk factors thereby lessening their magnitude. By focusing on the absolute CV risk associated with RA we showed that assessment and management of traditional CV risk factors remain of the utmost importance to identify high risk patients and potentially reduce CV risk in RA patients.
Excess risk of CV disease in RA is well recognized but much less is known as to when the CV risk starts and the potential predictors associated with this excess risk. Our findings indicate that CV risk is high even in newly diagnosed RA subjects, and emphasize the need for early efforts to undertake CV risk detection and prevention in RA subjects. Ironically, the need for early intervention is analogous to the concept of a therapeutic “window of opportunity” to reduce joint damage in RA16, 17. Likewise, there is possibly a “window of opportunity” to detect and prevent CV disease in RA. Although evidence is currently lacking, early detection and early CV interventions may modify the underlying CV risk and improve survival in RA. Additionally, our findings have implications regarding the clinical diagnosis and classification of RA based on ACR criteria. Increasing evidence suggests that there is a preclinical or asymptomatic phase of the disease where autoantibodies are present several years before RA manifests clinically18. The increased CV risk is almost certainly present during this preclinical phase of the disease and by the time a patient fulfills ACR criteria, the window of opportunity for CV prevention may already be closing. Hence, CV risk is one additional motivation to intensify efforts to identify RA as early as possible rather than waiting for the emergence of its cardinal clinical manifestations19.
Assessing a person’s CV risk has become the accepted way of targeting CV preventions toward asymptomatic individuals. In fact, this approach has been credited, in part, with the significant improvements in cardiovascular survival in the general population20. This is typically achieved using CV risk scores, such as the Framingham risk score4. Asymptomatic individuals are then treated with cholesterol and blood pressure-lowering medications and aspirin, as well as advice about relevant health behaviors, depending on their personal risk profiles. However, the predictive performance of risk scores is highly dependent upon the absolute CV risk, and risk scores may under-estimate true CV risk in subjects with a high absolute risk of CV disease, such as RA21, 22. Obtaining accurate estimates of absolute risk for CV disease in RA will require RA-specific risk equations. Thus, further research is needed in this area.
Finally, it can be challenging for treating physicians to communicate excess CV risk to newly diagnosed RA patients whose primary concern at the time is RA and the challenging treatment course ahead. The ability to communicate CV risk using 10-year absolute risks may increase patients’ understanding of the risk and their willingness to accept CV preventive measures.
Potential limitations of this study originate mainly from the retrospective study design. We relied on information recorded in the medical records accrued over several decades. For a CV condition to be ascertained, it had to come to medical attention, be diagnosed by the physician and documented in the records. CV events which were unrecognized by patients and/or health professionals would not have been ascertained and this may result in underestimation of the number or timing of CV events, particularly of silent MI. We cannot rule out the possibility of differential ascertainment of CV events in the RA and non-RA cohorts. However, the comprehensive medical records linkage system of the Rochester Epidemiology Project (covering inpatient and outpatient care from all local providers) allowed us to access complete healthcare information for the entire study population over approximately 15 years of follow-up and enabled us to validate CV events. In this geographically well-defined population, almost all residents come to medical attention in any three-year period5, and so differential ascertainment is unlikely to affect our results. Absolute risk for individual CV events (e.g. MI or HF) could also be as informative as all CV events combined. However, this study had inadequate power to examine absolute risk for each CV event individually. Although the impact of risk factors may vary from one specific CV event to another, there is sufficient commonality of risk factors to warrant examining overall CVD risk. Furthermore, preventive measures taken to prevent any one CV event would be expected to also prevent risk of the other CV events. Our study population was largely composed of white individuals, which may limit the generalizability of our findings to more diverse populations. The absolute cardiovascular event rates may have changed in the general population in recent years and therefore, the event rates reported in this manuscript may not reflect the current rates. Finally, the absolute risk estimates for CV events reported in this manuscript reflect the overall risk for RA subjects at various levels of risk, from very high to low risk. Extending this research to develop a RA-specific risk prediction score which can differentiate RA subjects according to level of risk is the subject of our ongoing research.
This study has a number of strengths, including the inclusion of an unselected population-based incidence cohort of RA patients ascertained at their RA incidence, the longitudinal design with approximately 15 years of follow-up and complete and accurate ascertainment of CV events and risk factors using established criteria. Furthermore, inclusion of a matched non-RA comparison cohort ascertained from the same underlying population and with identical data collection methods allowed us to make absolute risk comparisons which would not be feasible otherwise.
In conclusion, the 10-year absolute CV risk in RA subjects is high, even at incidence and is similar to that of non-RA subjects 5-10 years older. This difference is even more profound among women. The absolute CV risk varies substantially according to presence of CV risk factors. These findings underscore the importance of careful CV risk assessment for each newly diagnosed RA subject, followed by individualized preventive care. Future research should focus on the development of RA specific CV risk scores designed to more accurately identify RA subjects’ unique risk profiles and guide individualized therapy.
Table 3.
Age-specific 10-year cardiovascular risk according to presence of various cardiovascular risk factors among non-RA and RA subjects
| Age groups | No risk factors | Smoker | Smoker Hypertension | Smoker Hypertension Dyslipidemia | Smoker Hypertension Dyslipidemia Diabetes | Smoker Hypertension Dyslipidemia Diabetes Obesity | Smoker Hypertension Dyslipidemia Diabetes Low BMI | |
|---|---|---|---|---|---|---|---|---|
| 40-49 | non-RA | 1.4 | 2.9 | 5.2 | 5.1 | 7.0 | 8.4 | 7.8 |
| RA | 3.0 | 5.1 | 6.4 | 8.2 | 14.4 | 14.4 | 28.3 | |
| 50-59 | non-RA | 3.2 | 6.6 | 11.5 | 11.3 | 15.4 | 18.1 | 17.1 |
| RA | 7.3 | 12.1 | 14.9 | 18.8 | 31.6 | 31.6 | 55.6 | |
| 60-69 | non-RA | 7.2 | 14.5 | 24.4 | 24.0 | 31.8 | 36.7 | 34.8 |
| RA | 16.8 | 26.9 | 32.5 | 39.7 | 60.3 | 60.4 | 86.2 | |
| 70-79 | non-RA | 15.7 | 30.2 | 47.4 | 46.6 | 58.4 | 65.0 | 62.5 |
| RA | 36.1 | 53.4 | 61.6 | 70.9 | 89.5 | 89.5 | 99.2 |
The shading in the table represents the five CV risk groups: : low-risk (<6% CV risk over the next 10 years), low- moderate (6%-10% risk), intermediate (>10%-20 % risk), high (>20-50% risk) and very high risk (>50%)
Acknowledgments
Funding Source: This work was supported in part by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and the National Institutes of Health (AR-30582) US Public Health Service.
Footnotes
Financial Disclosures: None.
References
- 1.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005 Feb 3;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005 Feb 3;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005 Mar;52(3):722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991 Jan;83(1):356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 6.Arnett FC, E S, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 7.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003 May 21;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Archives of Internal Medicine. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 11.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clini Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Statistical Assoc. 1958;53:457–481. [Google Scholar]
- 14.Crowson CS, Nicola PJ, Maradit Kremers H, et al. How much of the Increased Incidence Of Heart Failure In Rheumatoid Arthritis Is Attributable To Traditional Cardiovascular Risk Factors And Ischemic Heart Disease? Arthritis & Rheumatism. 2005;52(10):3039–3044. doi: 10.1002/art.21349. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez A, Kremers HM, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008 January 1;67(1):64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 16.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003 Jul;48(7):1771–1774. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 17.O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002 Feb;46(2):283–285. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 18.Aho K, Heliovaara M, Maatela J, Tuomi T, Palosuo T. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991 Sep;18(9):1282–1284. [PubMed] [Google Scholar]
- 19.Kim JM, Weisman MH. When does rheumatoid arthritis begin and why do we need to know?[comment] Arthritis & Rheumatism. 2000;43(3):473–484. doi: 10.1002/1529-0131(200003)43:3<473::AID-ANR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007 Jun 7;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen TF, McGee D, Davidsen M, Jorgensen T. A cross-validation of risk-scores for coronary heart disease mortality based on data from the Glostrup Population Studies and Framingham Heart Study. Int J Epidemiol. 2002 Aug;31(4):817–822. doi: 10.1093/ije/31.4.817. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RL, Stevens RJ, Retnakaran R, Holman RR. Framingham, SCORE, and DECODE Risk Equations Do Not Provide Reliable Cardiovascular Risk Estimates in Type 2 Diabetes. Diabetes Care %R. 2007 May 1;30(5):1292–1293. doi: 10.2337/dc06-1358. [DOI] [PubMed] [Google Scholar]