Abstract
Dynamic Magnetic Resonance Elastography (MRE) quantitatively maps the stiffness of tissues by imaging propagating shear waves in the tissue. These waves can be produced from intrinsic motion sources (e.g., due to cardiac motion), from external motion sources that produce motion directly at depth in tissue (e.g., amplitude-modulated focused ultrasound), and from external actuators that produce motion at the tissue surface that propagates into the tissue. With external actuator setups, typically only a single transducer is used to create the shear waves, which in some applications might have limitations due to shadowing and attenuation of the waves. To address these limitations, a phased-array acoustic driver system capable of applying independently controlled waveforms to each channel was developed and tested. It was found that the system produced much more uniform illumination of the object, improving the quality of the elastogram. It was also found that the accuracy of the stiffness value of any arbitrary region of interest could be improved by obtaining maximal shear wave illumination with the phased array capability of the system.
Keywords: Magnetic resonance elastography, multiple transducers, shear wave illumination, phased array transducers, tissue stiffness
Introduction
Magnetic Resonance Elastography (MRE, (1)) is a dynamic phase-contrast MRI technique that can assess the mechanical properties of tissues and is emerging as a diagnostic tool for assessing pathological conditions in organs such as the liver, breast, and brain (2-4). In conventional MRE, repetitive time-harmonic shear motions are applied to the tissue of interest using either external or internal sources of motion and the resultant cyclic displacements are mapped into the phase of the reconstructed MR images with the use of motion-sensitive imaging gradients inserted into conventional pulse sequences. With appropriate manipulation of the motion-encoding gradients, a set of images sampled at several time points, called wave images, are collected. From these wave images, an elastogram that reflects the stiffness of the tissue is produced using a mathematical inversion algorithm (5). Since the mechanical properties of tissues vary over many orders of magnitude (6) and are sensitive indicators of underlying pathological conditions (7), these elastograms provide clinically useful diagnostic information.
Typically with external actuator systems, only a single mechanical driver is used to introduce shear waves into tissues. As a result, several types of image artifacts may occur due to attenuation and shadowing effects. The physical attenuation of the propagating shear waves due to viscous losses of wave energy and geometric dispersion of wave fronts can cause the tissues distant from the wave source to have very low amplitude wave motion. This decreases the accuracy of the inversion results due to the increased impact of noise in the data. Also, waves propagating through stiff tissue regions can be significantly attenuated, resulting in low-amplitude wave motion behind the stiff regions. This results in a shadowing artifact in which stiffness estimates can be less reliable behind stiff regions. Another concern regarding attenuation deals with the penetration-resolution trade-off. At higher frequencies of motion, shear wavelengths are smaller (λ = c/f, λ = wavelength, c = wave speed, f = vibration frequency) and are more desirable for characterizing tissue mechanical properties. However, the higher attenuation of shear waves at these frequencies limits the upper frequency that can be used. Initial feasibility studies with multiple drivers have been done and are reported in (8). Work by Zheng et al. (9) has demonstrated that the use of two, coupled drivers can improve MRE stiffness estimation, but the advantages and opportunities offered by the use of multiple, independent drivers have not been thoroughly explored.
To address these limitations, a phased-array acoustic driver system with eight mechanical drivers capable of applying independently controlled waveforms to each channel was developed and investigated. The first hypothesis of this work is that the attenuation of shear waves in MRE can be compensated by using an increased number of wave sources, and thus nearly uniform illumination can be achieved. The second hypothesis is that by suitably adjusting the waveform applied to each driver in the phased array, it is possible to increase the shear wave amplitude of any arbitrary region of interest (ROI), which improves the accuracy and robustness of the stiffness estimates in the ROI.
Methods
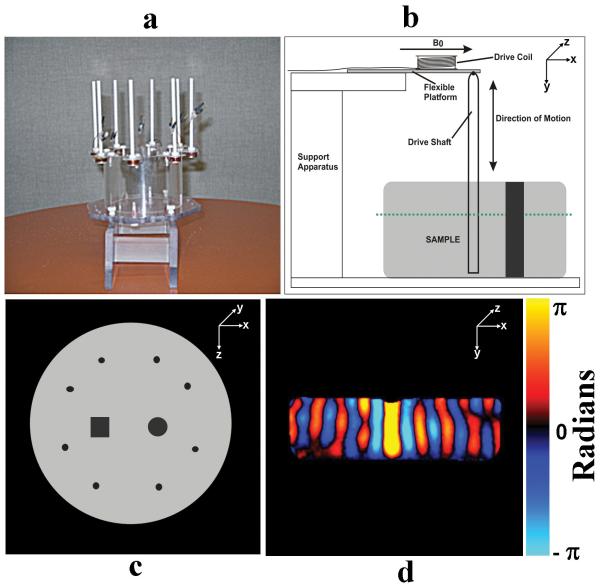
A series of phantom studies were designed to demonstrate the improved wave illumination and elastograms resulting from MRE with a phased-array driver. A 1.5-Tesla whole-body scanner (General Electric, Milwaukee, WI), a custom-made Helmholtz surface coil, and a gradient echo based MRE pulse sequence were used for the following experiments. Typical MRE imaging parameters were a coronal imaging plane (cross section of the phantom), 24-cm FOV, 256×64 acquisition matrix, 100-ms TR, 18-ms TE, 30° flip angle, 16-kHz receiver bandwidth, 10-mm slice thickness, 200-Hz vibrations, 4 time offsets, and through-plane motion-encoding with a 10-ms motion-encoding gradient with an amplitude of 1.84 G/cm. A prototype phased-array driver system was constructed with a set of eight electromechanical drivers, with 2-Ω resistance in each coil (fig. 1a). The eight drivers could be driven simultaneously and independently using a commercially available 8-channel 16-bit analog-output board (PD2-AO-8/16, United Electronics Industries) controlled by a computer. A schematic setup of one of the electromechanical drivers is shown in fig. 1b. When an alternating electric current is sent through the drive coil, the local magnetic moment created tries to align with the main field B0, moving the flexible platform in one direction. When the alternating current reverses, the local magnetic moment switches to the opposite direction, and the platform deflects in the other direction. The flexible platform makes the drive shaft embedded in the sample move up and down inducing shear waves inside it. Cylindrical gel phantoms 20 cm in diameter and 7 cm in height were made with 1.5% agar. As indicated, some experiments were performed with a phantom which also included two stiff 3.0% agar gel inclusions (one with a square cross section and another with a circular cross section) to simulate the presence of tumors in normal soft tissues (shown schematically in fig. 1c). The experimental setup was designed with the phantom and the actuator rods uniform along the Y direction (the axis of the phantom). In a coronal imaging plane, all the actuators are co-planar and the shear waves created are vertically polarized, cylindrically symmetric shear waves. In an axial or sagittal plane, the wave pattern appears planar and uniform along Y, but only 1 or 2 drivers can be studied at once (fig. 1d). Stiffness estimates were derived from the wave images using the local frequency estimation (LFE) algorithm (5). Since the wave fields in the multiple driver experiments are inherently multidirectional with many interfering wave fronts, directional filtering (10, 11) was performed before the calculation of the elastograms from the wave data.
Figure 1. Experimental Setup.
a. An image of the prototype eight driver phased array. b. A schematic representation of an axial or sagittal cross section of the experiment setup showing a single electromechanical driver, the phantom, and the level of the coronal images shown in this work (dotted line). c. A schematic of the coronal cross section of the inclusion phantom used in some of the experiments. d. A single wave image from an axial plane of the phantom through a single active driver indicating the uniform, planar nature of the motion along the length of the driver (the vertical component of the motion is shown).
Uniform Illumination
To demonstrate the improved overall wave field illumination provided by phased-array MRE, wave images were collected in the inclusion phantom with a single driver active and with all 8 elements active. The overall wave field intensity and the quality of the elastograms in and around the inclusions were examined.
Wave Optimization: Harmonic wave MRE
Several phantom experiments were performed to test the improved capabilities for localized wave field illumination provided by the phased-array MRE system. Wave images were first collected in a uniform phantom (no inclusions) with each element active individually (“calibration data”). The phase shift between each active driver and any location in the phantom can be determined by calculating the phase of the first temporal harmonic of the calibration wave data at that location. If this phase is introduced as a phase delay for that driver, the adjusted wave field from that driver will have 0° of phase at that location. This can be done during the MRE acquisition by introducing a delay to the appropriate channel of the analog-output board. This can also be done synthetically by subtracting the extra phase as a constant value from the phase of the first harmonic of the original MRE data, and then generating a new time series of wave images from that modified first harmonic data.
The displacements used throughout in MRE experiments are small enough (in the range of tens of microns) that linear behavior of the materials should be a good assumption. To validate the linearity of the superposition of the shear waves from different actuators and the process of wave optimization, wave data were acquired with each of the driver elements inducing shear waves with the necessary phase delays individually. These data were added together to simulate a composite wave field acquired with all the elements inducing shear waves simultaneously. An additional acquisition was performed with all 8 drivers on simultaneously with the calculated phase shifts introduced into the appropriate channels of the analog-output board. The simulated and measured wave fields were then compared to verify the equivalence of the two techniques.
Wave data with 2, 4, and 8 drivers on simultaneously with the waves from each driver adjusted to have zero relative phase (i.e., constructively interfere) at the center of the phantom were acquired. The wave amplitude was inspected at this location to verify the improved wave amplitude at this location. To demonstrate that phase shifting the 8 drivers in this fashion yields a maximum, optimal wave amplitude, 100 simulated wave fields were produced in which the phase shift introduced into the synthetic wave data from each driver was randomly chosen. The mean and maximum wave amplitude in several regions in the phantom were calculated over the 100 trials and compared to the wave amplitudes when the phase delays introduced were determined from the calibration data to produce zero phase in each of these regions.
To evaluate the potential of wave optimization to improve MRE stiffness estimates, calibration data were collected from the 8 individual drivers in the inclusion phantom. Appropriate phase delays were calculated to optimize the wave field in each of the two inclusions. Two wave datasets where all the driver elements were inducing shear waves in the sample were acquired, one without any phase shifts applied and the other with the calculated phase shifts applied to all the drivers. Elastograms were then calculated from the single driver calibration data, the multiple driver wave data without wave optimization and the multiple driver wave data with wave optimization. Average stiffness estimates were obtained for the stiff inclusions and compared to stiffness values obtained with MRE on a large reference batch of the same 3.0% agar gel used to make the inclusions. Additional random noise was then added to the optimized and non-optimized data and new elastograms were calculated and studied to determine differences in the depiction of the stiff inclusions.
Wave Optimization: Transient wave MRE
Mechanical transient waves (12) are being investigated as a tool to overcome some of the problems that arise due to the use of harmonic waves, such as wave reflection and interference. To demonstrate how wave optimization using phased-array MRE can be used for targeted transient MRE analysis, calibration data were again collected from the 8 individual drivers. Rather than vibrating each driver using continuous vibrations at 200 Hz as in the previous studies, a single period of a sinusoid with duration of 5 ms was delivered to each driver. A series of 32 images of various delay values were acquired for each driver to adequately sample the transient wave front as it propagated through the phantom. To force the transients from each driver to converge at a particular location, relative time delays between the drivers and the location of interest were calculated from the calibration data. These delays were introduced into the appropriate channels of the analog-output board. Wave data were again collected, now with all 8 drivers active and each with its own optimized delay. The wave amplitude at the desired point of convergence was then inspected.
Results
Uniform Illumination
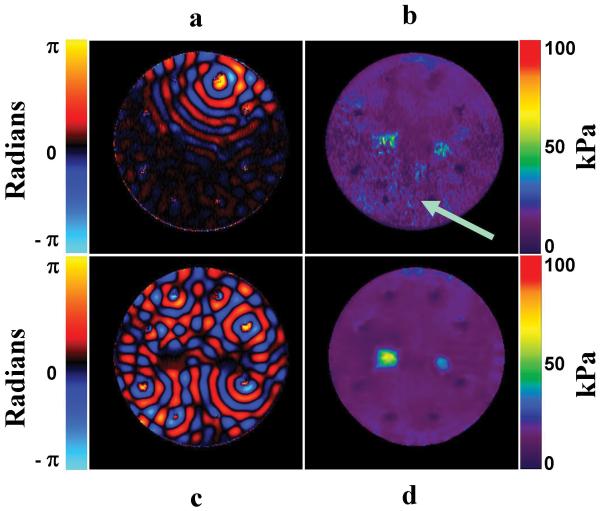
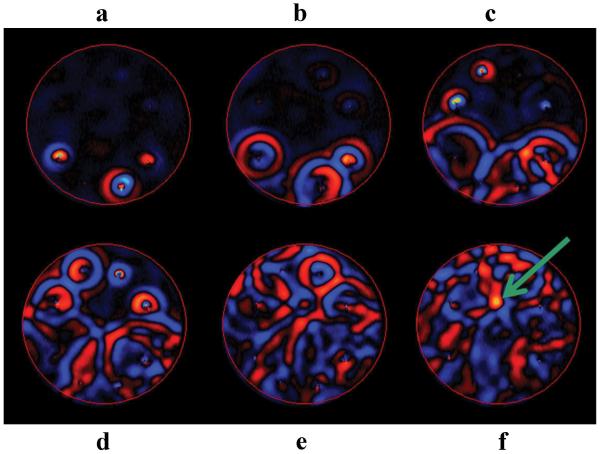
A single wave image of the inclusion phantom with only one of the eight drivers actively producing shear waves is shown in fig. 2a. The wave image shows the shear wave motion as phase of the image (with units in radians), which is directly proportional to the displacement according to the equation in (1). The attenuation of the shear waves is apparent in the regions distant from the wave source, which is indicated by the arrow. It can also be seen that the regions hidden from the wave source by the stiff inclusions experience low-amplitude shear wave motion. The elastogram obtained from the single-driver data is shown in fig. 2b and it is apparent that the regions with low-amplitude shear waves have noisier, less accurate stiffness estimates. A single wave image of the same phantom with multiple drivers inducing shear waves in the sample is shown in fig. 2c. Due to the presence of multiple wave sources, the whole sample is well illuminated with similar wave amplitude. Further, the stiff regions are illuminated from different directions, thus significantly reducing the shadowing around the stiff inclusions present in the single-driver data. Due to uniform illumination of the object, the corresponding elastogram (shown in fig. 2d) is free from the artifacts that were present in the single-driver case resulting in more uniform assessments of the stiffness of the background gel and the stiff inclusions and better boundary depiction of the stiff regions.
Figure 2. Uniform Illumination Study.
a. A single wave image of a phantom with only one driver active. b. The elastogram obtained from the single driver data. The wave attenuation dependent artifacts are evident in the low amplitude regions. c. A single wave image of a phantom with multiple active drivers. d. The elastogram obtained from the multi aspect driver data, showing that the use of multiple drivers makes the elastogram more uniform and artifact free.
Wave Optimization: Harmonic wave MRE
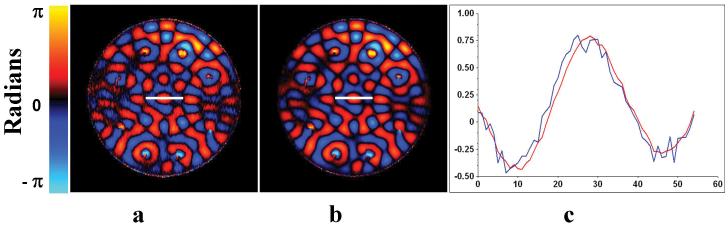
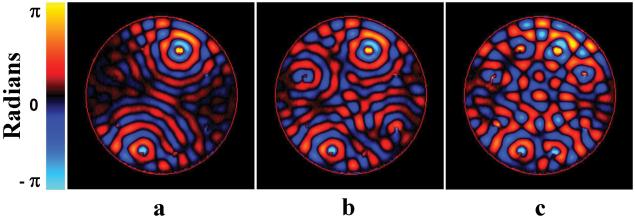
Figure 3 shows the comparison between the simulated wave field constructed by adding the acquired phase-adjusted individual driver wave images of the uniform phantom together (fig. 3a) and the measured wave image when all the driver elements were creating shear waves with the calculated phase shifts simultaneously (fig. 3b). The phases were adjusted to produce constructive interference, and thus increased displacement amplitude, at the center of the phantom. Figure 3c shows a line profile through the middle of each image showing that the two wave fields are similar, indicating that the system is linear and that the process of wave optimization is valid. Wave images of the same homogenous phantom shown in fig. 3 with 2, 4, and 8 active drivers that were phase adjusted to create an increased displacement in the center of the phantom are shown in figs. 4a-c, respectively. The constructive interference of the shear waves at the center can be seen and the displacement amplitude increases with the number of active drivers, as expected.
Figure 3. Validation of Superposition of Shear Waves.
a. A single wave image from the offline addition of eight individual driver datasets with phase adjustments b. A wave image acquired with all the drivers “ON” simultaneously c. A line profile comparison (as indicated) of the two data: The red line indicates the acquired data and the blue line indicates the offline added individual driver data.
Figure 4. Phased Array Wave Optimization.
Wave images of the phantom with two (a), four (b) and eight (c) drivers inducing shear waves with the phases adjusted to give a higher displacement in the center of the phantom
Figure 5 shows the results from the randomized phase-adjustment study. The chart shows the mean and maximum displacement amplitudes from 100 randomized phase adjustments for three different locations (of the several locations) in the uniform phantom. The displacement amplitudes obtained using the optimized phase adjustments to produce constructive interference of the waves at those locations are also shown. It can be seen that the displacement amplitude at the regions of interest with optimized phases is two to three times higher than the mean displacement amplitude obtained with no phase adjustments. Also, the optimal displacement is always higher than the maximum displacement obtained with the drivers at random phases, showing that the probability of getting the maximum wave amplitude at a particular location purely by chance is low.
Figure 5. Wave Optimization Amplitude Analysis.
The mean and maximum displacement for 100 random phase adjustments of the drivers and the displacement for the optimized phase adjustment at three different regions of interest is shown. The displacement amplitude at each region with the drivers at optimized phases is always higher than the mean or maximum displacement likely to occur with a random driver phase relationship.
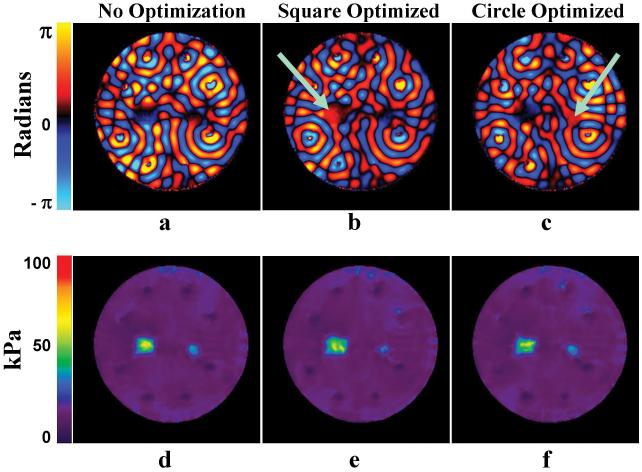
Figure 6 shows the results of optimizing the wave amplitude in the stiff inclusions of the inclusion phantom. Figure 6a shows a wave image using individual driver data with no phase adjustments. Wave images with the phases of the drivers adjusted to give a higher displacement in the square inclusion and circular inclusion (shown by the arrows) are shown in figs. 6b-c, respectively, and indicate a noticeable increase in the displacement in the wave optimized regions. These images also indicate that the presence of stiff regions in the path of the waves does not affect the wave optimization process. The elastograms obtained from these wave data are shown in figs. 6d-f. The mean stiffness values in a ROI drawn inside the stiff regions in the single-driver case (fig. 2a), non-optimized multiple-driver case (fig. 6d) and the wave-optimized multiple-driver case were found to be 43 kPa, 62 kPa, and 65 kPa, respectively. In a separate experiment on a larger 3.0% agar gel phantom of the same batch of the gel that the inclusions were made of, the stiffness value was found to be 80 kPa. Comparing this reference stiffness value to the values obtained in our experiments, the multiple-driver data provide improved estimates of the stiffness of these small, stiff inclusions.
Figure 6. Wave-Optimized Elastograms.
Single wave images of the phantom with a. no phase optimization b. phases adjusted for square inclusion c. phases adjusted for circular inclusion. The corresponding elastograms obtained from data with d. no phase optimization e. phases adjusted for square inclusion f. phases adjusted for circular inclusion. The increase in the displacement in the regions of wave optimization is easily noticeable, although it does not translate to improvement in the accuracy of stiffness estimation in this case.
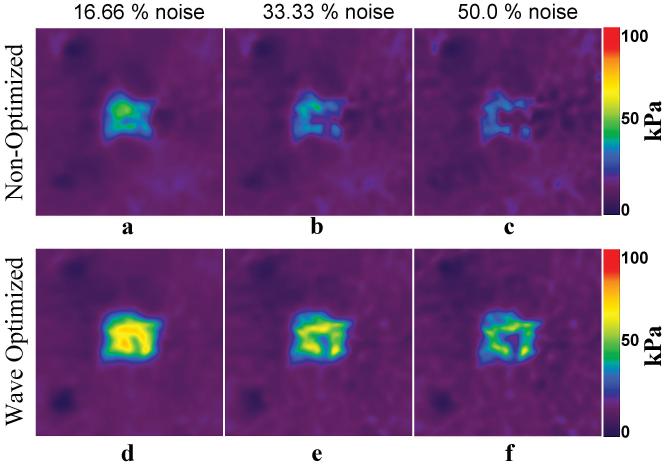
Figure 7 shows the elastograms of the region surrounding the square inclusion from non-optimized and optimized (for the square inclusion region) wave data with different amounts of additional random noise added to the data. Elastograms obtained from data with ±30°, ±60°, and ±90° Gaussian noise (corresponding to 16.66%, 33.33% and 50 % of the original signal) are shown for both cases and it can be seen that wave optimization increases the stability and robustness of the stiffness estimation .
Figure 7. Wave Optimization and Noise Analysis.
Elastograms of the region surrounding the square inclusion obtained from the wave data without wave optimization (a-c) and with wave optimization for the square inclusion (d-f) with the indicated Gaussian noise added. These results indicate that wave optimization increases the stability of MRE in the presence of noise.
Wave Optimization: Transient wave MRE
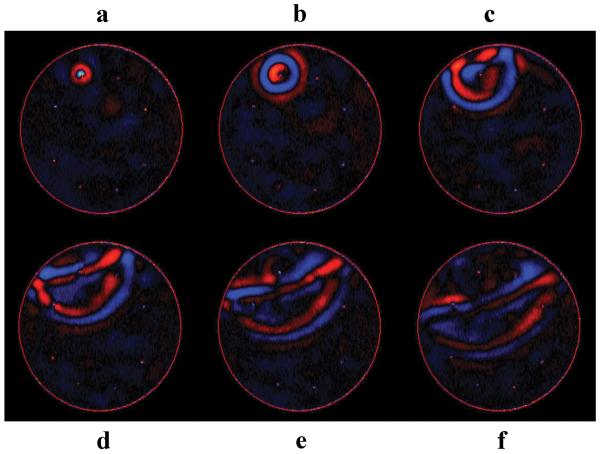
Figures 8 and 9 show the phased-array driver optimization technique applied to transient MRE. In these experiments, a single shear wave impulse is produced from each driver in the uniform phantom, which is imaged as it propagates through the phantom. As an example, 6 time points of the transient wave MRE data with only one driver active are shown in fig. 8. It should be noted that due to mechanical limitations, a true single impulse could not be created. From these images, it is evident that both source and reflected waves are readily distinguished in transient wave MRE. Wave optimization was performed at selected regions of interest inside the sample by adjusting the delay of the transient impulses produced by each driver, based on the previously acquired transient calibration wave data (e.g., fig. 8). Selected images from one such wave-optimized dataset are shown in fig. 9. It can be seen that the transient waves created from all the drivers are in phase in the region of interest (arrow) and hence constructively interfere to create a high displacement. It can also be seen that the drivers are activated sequentially, with the driver furthest from the point of interest starting first and the closest driver starting last, as expected.
Figure 8. Transient Wave Example.
6 sequential time points (a - f) from a single-driver transient data acquisition. Reflection of the transient wave can be seen in 8c, and successive frames have easily distinguishable incident and reflected waves.
Figure 9. Wave-Optimized Transient Data.
6 sequential time points (a - f) from a time-delay-adjusted transient wave experiment with eight active drivers optimized to give higher displacement in the region showed by the arrow mark. The waves from all the drivers can be seen to be in-phase and to be interfering constructively at that location.
Discussion
The results demonstrate that the use of phased-array driver techniques for MRE have potential to improve MRE stiffness estimates by reducing artifacts due to wave attenuation and shadowing. While the stiffness estimates for the inclusions are closer to the reference value with the wave-optimized data, there is still an underestimation of the stiffness which is likely an artifact due to the inclusions being smaller than the shear wavelength of the waves inside them. It is known that as the frequency of a wave increases, the wavelength decreases and the MRE resolution increases, while the depth of penetration of the wave decreases due to the increase in attenuation. This places an upper limit on the frequency that can be used, which in turn affects the resolution that can be obtained with MRE. However, with the use of multiple shear wave sources, an entire object can be more uniformly illuminated and hence a higher frequency wave could be used. However it has been noted that in a few cases, though the stiffness estimation for the whole object becomes smooth and uniform due to the use of multiple drivers(without wave optimization), the wave amplitude within small stiff regions could be low due to unintentional destructive interference of the waves. This further emphasizes the advantage obtained with the wave optimization process.
The results obtained from the wave-optimization experiments indicate that the technique can be used to increase the quality of elastograms and thus could prove very useful when applied to in vivo acquisitions. Though the experimental setup used in this study (with embedded rod type actuators) was designed to produce simple, symmetric, vertically polarized shear waves, the wave optimization process described here makes no assumptions dependent upon the driver system configuration or orientation. Hence, the results obtained from this study can be readily extended to clinically usable phased array driver designs. In clinical applications, the SNR may be low enough that wave optimization would significantly improve the reliability of the stiffness estimates. Similarly, the improved wave amplitude obtained using wave optimization could be used to compensate for SNR losses associated with faster MR imaging protocols being investigated for MRE, like EPI based MRE (4), localized MRE (13) etc. One of the potential applications of wave optimization and phased-array techniques in MRE is breast imaging(14) where the locations of suspicious lesions are known after contrast enhanced-MRI. Using a phased array actuator system capable of applying shear waves to the breast, wave-optimized MRE could be used to sensitively characterize the mechanical properties of these lesions, potentially contributing to the specificity of the combined imaging procedure and thus reduce the number of unnecessary biopsies.
Additional future work involving this methodology includes developing techniques to reduce the acquisition time for the calibration data. Since a set of calibration data has to be obtained for wave optimization in each slice, the total scan time might become substantially long. Hence, rather than collecting 2D datasets for each driver individually, a more efficient method could involve a single acquisition with all the drivers active and the data processed using directional filters to isolate the waves from individual drivers. Another approach would be to use 1D or reduced FOV imaging techniques (13) to collect just the image data between the point of interest and each driver individually, but in less time than for full 2D acquisitions. A different proposed application would be the use of time-reversal acoustic principles (15) to this phased-array technology as an alternative method for focusing mechanical wave energy to improve MRE.
Conclusions
This work shows that the use of phased-array mechanical transducers for MRE is feasible and can improve MRE stiffness estimates by reducing artifacts due to attenuation and shadowing. The use of multiple drivers improves the uniformity of the induced wave field, thus producing more uniform elastograms. Also, optimization of the timing of the elements of the phased array yields improved stiffness estimates in particular regions of interest. In vivo applications involving characterizing lesions, imaging large organs, and using high-frequency vibrations to improve MRE image resolution are anticipated to benefit from this technique.
Reference
- 1.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 2.Rouviere O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, Ehman RL. MR Elastography of the Liver: Preliminary Results. Radiology. 2006;240(2):440–448. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 3.Sinkus R, Tanter M, Xydeas T, Catheline S, Bercoff J, Fink M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magnetic Resonance Imaging. 2005;23:159–165. doi: 10.1016/j.mri.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 4.Sack I, Beierbach B, Hamhaber U, Klatt D, Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR in Biomedicine. 2008;21(3):265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- 5.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5(4):237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 6.Sarvazyan AP, Skovoroda AR, Emelianov SY, Fowlkes JB, Pipe JG, Adler RS, Buxton RB, Carson PL. Biophysical Bases of Elasticity Imaging. In: Jones JP, editor. Acoustical Imaging. Vol. 21. Plenum Press; New York: 1995. pp. 223–240. [Google Scholar]
- 7.Ophir J, cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrasonic imaging. 1991;13(2):111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 8.Mariappan YK, Rossman PJ, Ehman RL. Magnetic Resonance Elastography with Multiple Drivers; Proceedings of the 13th annual meeting of ISMRM; Miami Beach, Florida, USA. 2005.p. 617. [Google Scholar]
- 9.Zheng Y, Chan QC, Yang ES. Magnetic resonance elastography with twin drivers for high homogeneity and sensitivity; Conf Proc IEEE Eng Med Biol Soc; 2006; pp. 1916–1919. [DOI] [PubMed] [Google Scholar]
- 10.Manduca A, Lake DS, Kruse SA, Ehman RL. Spatio-temporal directional filtering for improved inversion of MR elastography images. Medical Image Analysis. 2003;7(4):465–473. doi: 10.1016/s1361-8415(03)00038-0. [DOI] [PubMed] [Google Scholar]
- 11.Lake D, Manduca A, Mariappan YK, Ehman RL. Directional filtering for multiple driver magnetic resonance elastography data; Proceedings of the 14th annual meeting of ISMRM; Seattle, USA. 2006.14.p. 330. [Google Scholar]
- 12.McCracken PJ, Manduca A, Felmlee JP, Ehman RL. Mechanical transient-based magnetic resonance elastography. Magnetic Resonance in Medicine. 2005;53:628–639. doi: 10.1002/mrm.20388. [DOI] [PubMed] [Google Scholar]
- 13.Glaser Kevin J., Felmlee JP, Ehman RL. Rapid MR elastography using selective excitations. Magnetic Resonance in Medicine. 2006;55(6):1381–1389. doi: 10.1002/mrm.20913. [DOI] [PubMed] [Google Scholar]
- 14.Heywang-Kobrunner SH, Viehweg P, Heinig A, Kuchler C. Contrast-enhanced MRI of the breast: accuracy, value, controversies, solutions. European Journal of Radiology. 1997;24(2):94–108. doi: 10.1016/s0720-048x(96)01142-4. [DOI] [PubMed] [Google Scholar]
- 15.Mariappan Y, Manduca A, Ehman RL. Medical Imaging 2007: Physiology, Function, and Structure from Medical Images. Vol. 6511. SPIE; 2007. Time reversal principles for wave optimization in multiple driver magnetic resonance elastography; pp. 191–199. [Google Scholar]