Abstract
Background
Brucellosis is veterinary and human health problem.
Methods
A 13-year-old wild caught multiparous and an 8-year-old colony-born nulliparous baboon had stillbirths in the second trimester of pregnancy. Culture isolates from both postpartum uteruses were characterized using traditional biochemical analysis, PCR, and multilocus sequencing.
Results
The isolates morphologically resembled Brucella although their phenotypic characteristics were not consistent with any currently described species. The isolates represent a novel lineage within the genus with unique alleles, not previously seen in surveys of greater than 300 isolates representing the known diversity of the genus, present at 5/9 loci examined.
Conclusions
The described cases are to the best of our knowledge the first presentation of a naturally acquired Brucella infection in non-human primates associated with stillbirths from the same colony where Brucella seropositivity in the baboons was described 45 years ago. The organism appears to represent a previously undescribed Brucella species.
Keywords: brucella, non-human primates, stillbirth
Introduction
Brucellosis is caused by various species of Brucella, a gram-negative, facultative intracellular rod in the family Brucellaceae. The following species have been described to date: Brucella abortus, Brucella melitensis, Brucella suis, Brucella ovis, Brucella canis, and Brucella neotomae with two additional species from marine mammals, Brucella ceti and Brucella pinnipedialis, described recently [8]. In humans, brucellosis can be caused by B. abortus, B. melitensis, B. suis, and rarely B. canis or marine mammal Brucella [5, 8, 9, 10, 14]. The description of Brucella infection in non-human primates (Papio spp) has been sparse [11] and was initially performed at Southwest Foundation for Biomedical Research (SFBR) 45 years ago [12, 13]. There is no published work on the genotyping and phylogenetic position of Brucella isolated from non-human primates. We report for the first time the culture and genotypic characterization of a novel Brucella isolate associated with two cases of stillbirth and retained placenta in baboons.
Case reports
Two female baboons, a 13-year-old wild caught multiparous and an 8-year-old colony-born nulliparous were enrolled in Institutional Animal Care Committee (IACUC) approved study protocols associated with animal breeding at the Southwest National Primate Research Center (SNPRC).
The 13-year-old baboon (index case) had been captured in Tanzania and imported by Paradise Exports (Arusha, Tanzania) in 1999 and had three previous pregnancies while at the SNPRC, including one abortion/stillbirth. The fourth pregnancy was not remarkable. At the gestational age of 117 days (dGA, term 180 dGA) it had a stillborn fetus without placenta. A cervical swab was taken for culture, and a blood sample for white blood cell count, biochemical evaluation, and serum banking (stored at −80°C) was taken. The culture was positive for Brucella spp., Staphylococcus epidermidis, and Escherichia coli. External evaluation of the animal revealed an enlarged uterus and subsequent ultrasound examination confirmed a retained placenta. The fetal characteristics were within normal limits for this species at this gestational age. Maternal serology testing validated for B. abortus in cattle (Brewers’ Diagnostic Kits, Hynson, Westcott and Dunning Inc., Baltimore, MD, USA) was performed on the banked serum at the Texas State-Federal Animal Laboratory and showed a positive reaction.
All 16 adult female baboons from the same harem cage as the index case had cervical cultures negative for Brucella spp. One had a stillbirth/abortion (culture negative for Brucella) and another one had a preterm birth within 2 months after the stillbirth in the index case. Three animals delivered live infants, of which one was delivered preterm. One infant died at the age of 2 months of fulminate pneumonia (lung culture was negative for Brucella spp).
The second baboon (from a different location) presented with weight loss and hypothermia at 98 dGA. Ultrasound evaluation showed a fetus with signs of severe hypoxia as indicated by the slow fetal heart rate and small for gestational age fetal size, the animal had a stillbirth 5 days later. The mother was euthanized 1 month after the stillbirth due to poor reproductive prognosis. At necropsy an enlarged uterus with pus-like discharge was noticed. A swab of the uterine content was submitted for culture. This animal was seronegative for the Brucella testing described above.
Identification of Brucella isolates
Both culture isolates were screened using traditional biochemical analysis [1]. The organism showed morphology typical of Brucella, but did not conform to the characteristics of any recognized species of Brucella. The unusual profile included: no requirement for carbon dioxide, no production of hydrogen sulfide, sensitivity to the dyes thionin and basic fuchsin at 1:100,000, agglutination only with mono-specific anti-A serum, and only partial susceptibility to Tbilisi phage at 104 ×RTD.
AMOS (B. abortus, B. melitensis, B. ovis, and B. suis) PCR with additional species-specific primers [3, 4, 7] confirmed that the isolates were Brucella species because of the generation of the specific 180 bp amplicon. However, both isolates failed to produce any of the expected PCR products that would have helped identify it as a known species.
Methanol killed culture cell isolate from the index case were used to sequence a discriminatory fragment of the 16S rDNA. Based on BLAST analysis, this strain was found to have 99% identity (429/430 bp) with strains of other Brucella spp. deposited in GenBank.
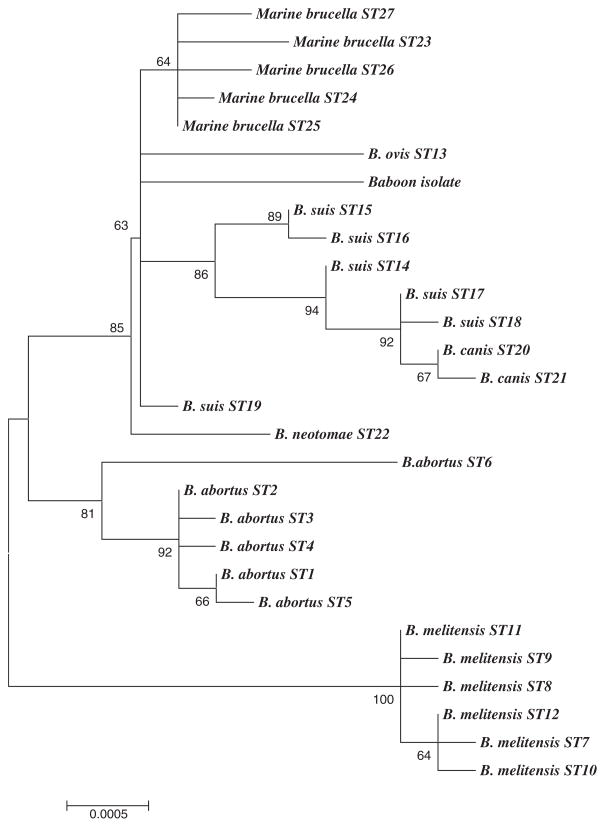
To characterize the Brucella-like isolate in relation to currently described Brucella groups, a recently described multilocus sequencing approach was applied to one of the isolates [15]. This technique involves sequencing of fragments of nine distinct genetic loci and has been shown to be an effective way to identify isolates as members of any of the extant Brucella species. In agreement with the identification of the isolate as a member of the genus Brucella, all nine gene fragments were readily amplified from the strain by PCR. However, sequencing revealed unique alleles, not previously seen in surveys of greater than 300 isolates representing the known diversity of the genus, at 5/9 loci. Construction of an unrooted phylogenetic tree based on the concatenated gene sequences obtained from the baboon isolate in comparison with equivalent sequences from 27 sequence types identified previously [15] shows the isolate is not closely allied to any of the currently described Brucella species (Fig. 1).
Fig. 1.
Unrooted phylogenetic reconstruction of the relationship between the baboon isolate and Brucella Sequence Types previously described [15]. The tree was constructed with concatenated data of nine genetic loci (4396 bp) using the neighbor-joining approach implemented in the MEGA3.1 package. The Jukes–Cantor model was used to determine genetic distances. The percentage bootstrap confidence levels of internal branches were calculated from 1000 resamplings of the original data.
Discussion
Brucellosis has been recognized as not only a veterinary medical, but also a human health problem [10]. Brucella remains the most common cause of laboratory-acquired infections and is abundant in wildlife [10]. Although several primate species have shown antibodies to Brucella, only a single Brucella melitensis positive culture in Papio spp. has been reported [11]. Our data suggest that the genetic difference separating the baboon isolate in our study from any other species is equal or greater than that which separates other extant species such as Brucella neotomae or B. ovis. Based on this analysis, it appears that the isolate might justifiably be characterized as a new species within the genus Brucella.
In humans Brucella is able to survive for prolonged periods in Brucella-containing vacuoles in macrophages and non-professional phagocytes (e.g., HELA cells, trophoblasts) [2]. The virulent B. abortus, for example, transits from autophagosomes to the endoplasmic reticulum of the trophoblasts where actual bacterial multiplication occurs [2, 6]. There is insufficient information to determine whether infection by this organism is a cause of stillbirth in the cases described above. To the best of our knowledge, the experimental infection of non-human primates with Brucella was only performed in non-pregnant animals [12]. The pathogenesis of this new Brucella isolate in non-human primates and identification of the natural host of this new Brucella species requires further study. Interestingly, eight SFBR baboons with seropositivilty for Brucella exhibited no evidence of disease during a 6-year observation period [12, 13].
Conclusion
This is to the best of our knowledge the first presentation of a naturally acquired Brucella infection in non-human primates in association with stillbirth. The organism appears to represent a previously undescribed Brucella species.
Acknowledgments
Brucella research at the VLA is supported by the UK Department of Environment, Food and Rural Affairs (Defra). The authors are thankful Dr. Lenarduzzi (Texas State-Federal Animal Laboratory) for serology evaluation and Drs. Patrice Frost, Stephanie Butler, and Michelle Leland for clinical support. We acknowledge the support for the SNPRC infrastructure under which the baboon colony is maintained, and for the SNPRC pathology laboratories which is provided by NIH grant P51 RR013986, Facilities Improvement Grant C06 RR15456 supported the construction of cages in which the baboons were housed.
References
- 1.Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. Paris, France: INRA; 1988. [Google Scholar]
- 2.Anderson TD, Cheville NF. Ultrastructural morphometric analysis of Brucella abortus-infected trophoblasts in experimental placentitis. Bacterial replication occurs in rough endoplasmic reticulum. Am J Pathol. 1986;124:226–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–6. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bricker BJ, Ewalt DR, Olsen SC, Jensen AE. Evaluation of the Brucella abortus species-specific polymerase chain reaction assay, an improved version of the Brucella AMOS polymerase chain reaction assay for cattle. J Vet Diagn Invest. 2003;15:374–8. doi: 10.1177/104063870301500413. [DOI] [PubMed] [Google Scholar]
- 5.Corbel MJ. Recent advances in brucellosis. J Med Microbiol. 1997;46:101–3. doi: 10.1099/00222615-46-2-101. [DOI] [PubMed] [Google Scholar]
- 6.Detilleux PG, Deyoe BL, Cheville NF. Penetration and intracellular growth of Brucella abortus in non-phagocytic cells in vitro. Infect Immun. 1990;58:2320–8. doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewalt DR, Bricker BJ. Validation of the abbreviated Brucella AMOS PCR as a rapid screening method for differentiation of Brucella abortus field strain isolates and the vaccine strains, 19 and RB51. J Clin Microbiol. 2000;38:3085–6. doi: 10.1128/jcm.38.8.3085-3086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int J Syst Evol Microbiol. 2007;57(Pt 11):2688–93. doi: 10.1099/ijs.0.65269-0. [DOI] [PubMed] [Google Scholar]
- 9.McDonald WL, Jamaludin R, Mackereth G, Hansen M, Humphrey S, Short P, Taylor T, Swingler J, Dawson CE, Whatmore AM, Stubberfield E, Perrett LL, Simmons G. Characterization of a Brucella sp. strain as a marine-mammal type despite isolation from a patient with spinal osteomyelitis in New Zealand. J Clin Microbiol. 2006;44:4363–70. doi: 10.1128/JCM.00680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappas G, Papadimitriou P, Christou L, Akritidis N. Future trends in human brucellosis treatment. Expert Opin Investig Drugs. 2006;10:1141–9. doi: 10.1517/13543784.15.10.1141. [DOI] [PubMed] [Google Scholar]
- 11.Pinkerton ME. Bact Proc. Vol. 67. Philadelphia: WB Saunders; 1967. Bacteremia in Wild Baboons; p. 67. [Google Scholar]
- 12.Pinkerton ME. Brucella. In: Fiennes RNT-W, editor. Pathology of Simian Primates. Part II. Infectious and Parasitic Diseases. S. Karger; Basel, New York: 1972. pp. 289–93. [Google Scholar]
- 13.Ratsen JJ, Pinkerton ME, Kalter SS, Guilloud NB. Antobodies in non-human primates to selected bacterial antigens. Z Versuchtierk. 1969;Bd 11:146–54. [PubMed] [Google Scholar]
- 14.Sohn AH, Probert WS, Glaser CA, Gupta N, Bollen AW, Wong JD, Grace EM, McDonald WC. Human neurobrucellosis with intracerebral granuloma caused by a marine mammal Brucella spp. Emerg Infect Dis. 2003;9:485–8. doi: 10.3201/eid0904.020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whatmore AM, Perrett LL, MacMillan AP. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 2007;7:1–15. doi: 10.1186/1471-2180-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]