Abstract
Cystic fibrosis (CF) is caused by defects in the CFTR, a cAMP-activated Cl– channel of epithelia. The resulting reduction in epithelial fluid transport creates abnormally viscous secretions from airway mucous glands that may be a major factor in CF pathology. Mouse airways have few mucous glands, and the mouse model of CF exhibits no significant airway disease. Pigs and ferrets, however, have approximately the same number of airway mucous glands as humans. In this issue of the JCI, three independent research groups conclude that changes in airway mucous gland function in CFTR-deficient animals of these species resemble the changes seen in human CF. It is expected, therefore, that these animals will develop lung disease similar to human CF and prove to be valuable models on which to test potential therapies.
The body’s main defense against large inhaled particles is the “mucociliary clearance system.” This system traps particles in a blanket of mucus and then moves the mucus and ensnared particles out of the airways using cilia in the apical membrane of the surface epithelium (1). In the larger airways, the great majority of mucus comes from submucosal glands (2), and mucous secretions from these glands are abnormally viscous in individuals with cystic fibrosis (CF) (3). It is believed that this causes the mucus to be poorly cleared by cilia and it accumulates and provides a home for inhaled microorganisms. An inflammatory cascade ensues that clogs the airways with mucous secretions, bacteria, leukocytes, and plasma transudate. By restoring normal viscosity to CF airway mucous gland secretions, it is to be hoped that much of this pathology can be prevented.
Mouse airways contain few mucous glands, and the mouse model of CF shows little airway pathology (4). However, more direct analyses of the role of mucous glands in the airway pathology of CF are now possible, given the recent development of CFTR-deficient pigs and ferrets (5, 6); both species have good numbers of airway glands. In this issue of the JCI, three independent research groups report on their efforts to understand airway gland function in these new animal models of CF (7–9).
Airway mucous gland secretion in CF
Water accounts for approximately 98% of airway gland mucous secretions, and water flow into the gland lumen is driven by local osmotic gradients generated by active Cl– secretion. This Cl– secretion requires simultaneous activity of Cl– channels (either cAMP or Ca2+ activated) in the apical membrane and K+ channels in the basolateral membrane. In CF secretory responses of airway glands to agents that elevate cAMP are almost completely lost, as the only cAMP-activated Cl– channel in the apical membrane is CFTR (10). Secretory responses to agents, such as substance P, that moderately elevate intracellular Ca2+ concentration ([Ca2+]i) are also quite markedly reduced in CF (11). This is because [Ca2+]i is not elevated sufficiently to have much effect on Ca2+-activated Cl– channels (CaCCs) in the apical membrane, but Ca2+-dependent basolateral K+ channels are opened, thereby hyperpolarizing the apical membrane and increasing the driving force for Cl– exit through constitutively open CFTR. By contrast, responses to cholinergic agents are less affected in CF, because these agents have a larger effect on [Ca2+]i and cause substantial activation of CaCCs (10–12). Finally, in non-CF airway glands, subthreshold doses of cAMP-elevating and Ca2+-elevating agents show synergy; neither alone stimulates gland fluid secretion, but they do so in combination, because one opens CFTR and the other opens basolateral Ca2+-activated K+ channels (10–12). This synergy is lost in CF (10).
Airway mucous gland secretion in the CFTR-deficient pig
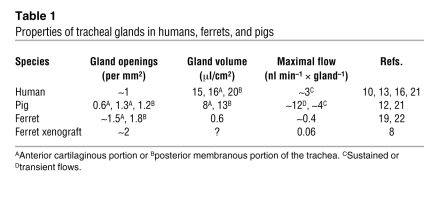
There were several reasons for believing that a CFTR-deficient pig would prove an excellent model of altered gland function in CF. Numbers, size, and maximal flow rates of glands in pig and human tracheas are very similar (Table 1). So too is the specific potency of gland secretagogues: cholinergic agents, such as carbachol or methacholine, elevate [Ca2+]i and are several times more effective at inducing secretion than cAMP-elevating agents, such as forskolin or vasoactive intestinal peptide (VIP); α-adrenergic agents, such as phenylephrine, are least effective; and substance P occupies an intermediate position (10–12). Also, in tracheas of different species and in different generations of human airways, glands disappear as airway diameters become less than about 2 mm (13, 14). Therefore, given that pig and human lungs are similar in size, glands should show a similar distribution along the airways of the two species.
Table 1 .
Properties of tracheal glands in humans, ferrets, and pigs
The CFTR-deficient transgenic pig was generated two years ago (6), and the paper by Joo et al. (7) in this issue of the JCI provides the first comparison of airway gland mucus secretion in CF and normal piglets. Piglets had to be used, as CF pigs die of gut problems shortly after birth. To measure gland secretion, the mucosal surface of a piece of airway wall was coated with paraffin oil, and gland secretions were visualized as spherical droplets forming under the oil. The results were gratifying — CF pig glands behaved very much like human CF glands. The secretory response to forskolin was lost as was the synergy between forskolin and carbachol. The secretory responses to substance P were reduced by approximately 75%. The secretory responses to carbachol were reduced by approximately 40%, a finding largely ascribable to a reduction in gland size in CF piglets. It is not known in human CF whether airway glands are abnormally small at birth, but this should definitely be investigated. Of course, with chronic disease they become markedly hypertrophied (15).
Airway mucous gland secretion in the CFTR-deficient ferret
The paper by Sun et al. (8) describes the overall pathology of the CF ferret. CFTR+/+, CFTR+/–, and CFTR–/– animals were born in the expected Mendelian ratio of 1:2:1, indicating no fetal mortality. However, all CFTR–/– kits died within 4 days of birth, either from meconium ileus or from poor nutrient absorption in the gut. Histological lesions were present in the pancreas, and vasa deferentia were absent or degenerate. There was histological evidence of pneumonia, and blood chemistry was consistent with liver disease.
In humans (and presumably pigs), all airway glands form long before birth (16). In the ferret, by contrast, airway glands form predominantly after birth (17). This, coupled with the neonatal mortality of CF ferrets, caused Sun et al. to resort to the “tracheal xenograft model” to study gland function. Using this approach, tracheas from newly born ferrets were implanted under the skin of immune-deficient mice. After five weeks, differentiated glands had formed. The xenografts were then removed from the mice, and gland secretions were visualized as secretory droplets under oil. As in pigs and humans, the secretory response to forskolin was essentially abolished in the airway glands from CF animals. The response of CF ferrets to methacholine was also reduced (by approximately 50%), as it is in human nasal glands (18).
An important contribution of the study by Sun et al. (8) was the prevention of the neonatal mortality in CF ferrets with a combination of oral ursodeoxycholic acid (to aid fat emulsification) and an oral proton-pump inhibitor (to compensate for the lack of alkaline pancreatic secretions). However, even if CF ferrets achieve adulthood, they may prove less useful than adult CF pigs for studies of how altered airway gland function contributes to airway pathology in CF. Thus, not only do ferret glands develop differently from those in the human, but they are also appreciably smaller than human glands, with lower flow rates (Table 1). There are also differences in the neurohumoral regulation of airway gland secretion between ferrets and humans. In particular, the ferret, like the other carnivore studied, the cat, has airway glands that show large secretory responses to the α-adrenergic agent, phenylephrine (19, 20); responses of pig and human glands to this agent are negligible (20).
Interaction between cAMP and Ca2+ in airway gland cells
The paper by Lee and Foskett (9) concerns the regulation of Cl– secretion in serous cells. These cells line the acini of airway glands and are believed to be the primary source of the liquid component of gland secretions. The authors studied secretory responses of isolated serous acini from pigs and humans, both with and without CF. Simultaneous opening of apical Cl– channels and basolateral K+ channels, in response to secretagogues, resulted in KCl loss and cellular shrinkage, measured by Lee and Foskett using differential interference microscopy. Chloride permeability and [Ca2+]i were measured using fluorescent probes. As expected, the secretory response of non-CF acini to elevation of cAMP was CFTR mediated. Surprisingly, however, the response depended on cAMP-mediated elevation of [Ca2+]i.
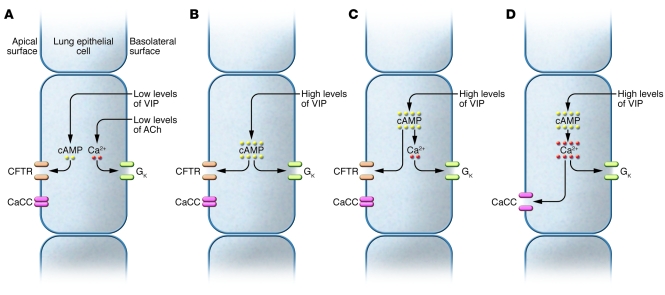
Choi et al. (10) had previously proposed that airway gland serous cells show two types of Cl– (and water) secretion. In the “emergency response,” high levels of acetylcholine activate apical membrane CaCCs and promote fluid flows that are not much altered in CF. By contrast, a low baseline secretion of Cl– is maintained by a synergy between two neurotransmitters: VIP, which raises cAMP and opens CFTR, and low levels of acetylcholine, which elevate [Ca2+]i sufficiently to open basolateral K+ channels but not apical membrane CaCCs (Figure 1A). This baseline secretion is absent in CF. They further proposed that high concentrations of VIP can act without acetylcholine on non-CF glands, because cAMP levels become high enough to open cAMP-activated K+ channels (10) (Figure 1B). Lee and Foskett, however, found that increases in cAMP (induced by either VIP or forskolin) led to increases in [Ca2+]i, and this, not cAMP, was responsible for activation of the basolateral K+ conductance (9) (Figure 1C). The importance of this finding is that pharmacological enhancement of the link between cAMP and [Ca2+]i (or [Ca2+]i and CaCCs) would allow cAMP-elevating mediators such as VIP to stimulate Cl– secretion in CF cells by activating CaCCs (Figure 1D). The authors also showed that high levels of intracellular cAMP (induced using either VIP or forskolin) enhanced the effects of acetylcholine on [Ca2+]i (9). Thus, cAMP-elevating agents, such as phosphodiesterase inhibitors, could potentiate the effects of low-level cholinergic stimulation on CFTR-independent Cl– secretion.
Figure 1. Means by which VIP (or other cAMP-elevating agents) stimulates Cl– secretion by airway gland serous cells.
(A) Low levels of VIP are insufficient to stimulate Cl– secretion but do elevate cAMP sufficiently to open CFTR. Low levels of acetylcholine (ACh) (also subthreshold for secretion) raise [Ca2+]i sufficiently to open basolateral K+ channels (GK). Together, the two agents induce Cl– secretion, as previously suggested by Choi et al. (10). (B) High levels of VIP elevate cAMP sufficiently to activate both Cl– and K+ channels, as previously suggested by Choi et al. (10). (C) cAMP elevates [Ca2+]i, which then activates basolateral K+ channels, as described by Lee and Foskett (9). (D) If the effects of cAMP on [Ca2+]i are somehow enhanced then VIP can induce Cl– secretion in CF cells by activating CaCC, as suggested by Lee and Foskett (9).
Concluding comments
In conclusion, it is possible that airway gland secretions in CF are too concentrated and viscous to be cleared efficiently (3). Thus, anything that returns the viscosity of gland secretions to normal should alleviate the airway symptoms of CF. This hypothesis can now be tested in CFTR-deficient pigs and ferrets, species that (unlike the mouse) have substantial numbers of airway glands. The papers by Sun et al. (8) and Joo et al. (7) in this issue of the JCI are important in that they show that the CF-related reductions in gland fluid secretion in these animals qualitatively resemble those seen in human CF. Of the two species, pig airways and their mucous glands are the most similar to those of humans (Table 1). However, the usefulness of this species is limited by the 100% mortality in the perinatal period, due to gastrointestinal problems that can currently only be overcome surgically (6). In the ferret, by contrast, comparatively simple oral therapies can ensure survival of CFTR-deficient ferrets beyond the perinatal period (8). Thus, for now the ferret would seem the more useful model. The paper by Lee and Foskett (9) is a technical tour de force that provides the novel findings that cAMP elevates [Ca2+]i in airway gland serous cells and potentiates the effects of carbachol on Ca2+ release (9). These findings point to pharmacological ways of enhancing (or inducing) baseline fluid secretion from CF glands without altering neurohumoral input.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(9):3093–3096. doi:10.1172/JCI44235
See the related article beginning on page 3161.
References
- 1.Widdicombe JH, Widdicombe JG. Regulation of human airway surface liquid. Respiration Physiology. 1995;99(1):3–12. doi: 10.1016/0034-5687(94)00095-H. [DOI] [PubMed] [Google Scholar]
- 2.Reid L. Measurement of the bronchial mucous gland layer: A diagnostic yardstick in chronic bronchitis. Thorax. 1960;15:132–141. doi: 10.1136/thx.15.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. . Proc Natl Acad Sci U S A. 2001;98(14):8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36(1):1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, et al. Adeno-associated virus-targeted disruption of the cftr gene in cloned ferrets. J Clin Invest. 2008;118(4):1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers CS, et al. Disruption of the cftr gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo NS, Cho H-J, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120(9):3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. . J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. . J Clin Invest. 2010;120(9):3137–3148. doi: 10.1172/JCI42992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JY, et al. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest. 2007;117(10):3118–3127. doi: 10.1172/JCI31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JY, et al. Substance P stimulates human airway submucosal gland secretion mainly via a cftr-dependent process. J Clin Invest. 2009;119(5):1189–1200. doi: 10.1172/JCI37284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. Stimulation by carbachol and vasoactive intestinal peptide. J Biol Chem. 2002;277(31):28167–28175. doi: 10.1074/jbc.M202712200. [DOI] [PubMed] [Google Scholar]
- 13.Whimster WF. Number and mean volume of individual submucous glands in the human tracheobronchial tree. Appl Pathol. 1986;4(1–2):24–32. [PubMed] [Google Scholar]
- 14.Widdicombe JH, Pecson IS.Distribution and numbers of mucous glands in the horse trachea. Equine Vet J. 2002346630–633. . 10.2746/042516402776180151 [DOI] [PubMed] [Google Scholar]
- 15.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976;7(2):195–204. doi: 10.1016/S0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 16.Tos M. Development of the tracheal glands in man.Number, density, structure, shape, and distribution of mucous glands elucidated by quantitative studies of whole mounts. Acta Pathol Microbiol Scand. 1966;68(suppl 185):3+. [PubMed] [Google Scholar]
- 17.Leigh MW, Gambling TM, Carson JL, Collier AM, Wood RE, Boat TF. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp Lung Res. 1986;10(2):153–169. doi: 10.3109/01902148609061490. [DOI] [PubMed] [Google Scholar]
- 18.Salinas D, et al. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 2005;19(3):431–433. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- 19.Cho H-J, Joo NS, Wine JJ. Mucus secretion from individual submucosal glands of the ferret trachea. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L124–L136. doi: 10.1152/ajplung.00049.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo NS, Wu JV, Krouse ME, Saenz Y, Wine JJ. Optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L458–L468. doi: 10.1152/ajplung.2001.281.2.L458. [DOI] [PubMed] [Google Scholar]
- 21.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197 pt 3:361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson NP, Venning L, Kyle H. Quantitation of the secretory cells of the ferret tracheobronchial tree. J Anat. 1986;145:173–188. [PMC free article] [PubMed] [Google Scholar]