Abstract
Chagas disease, caused by Trypanosoma cruzi, is a wide spread infection in Latin America. Currently, only 2 partially effective and highly toxic drugs, i.e., benznidazole and nifurtimox, are available for the treatment of this disease and several efforts are underway in the search for better chemotherapeutic agents. Here, we have determined the trypanocidal activity of 2,3-diphenyl-1,4-naphthoquinone (DPNQ), a novel quinone derivative. In vitro, DPNQ was highly cytotoxic at a low, micromolar concentration (LD50 = 2.5 μM) against epimastigote, cell-derived trypomastigote, and intracellular amastigote forms of T. cruzi, but not against mammalian cells (LD50 = 130 μM). In vivo studies on the murine model of Chagas disease revealed that DPNQ-treated animals (3 doses of 10 mg/kg/day) showed a significant delay in parasitemia peak and higher (up to 60%) survival rate 70 days post-infection, when compared to control group (infected, untreated). We also observed a 2-fold decrease in the parasitemia between the control group (infected, untreated) and the treated group (infected, treated). No apparent drug toxicity effects were noticed in the control group (uninfected, treated). In addition, we determined that DPNQ is the first competitive inhibitor of T. cruzi lipoamide dehydrogenase (TcLipDH) thus far described. Our results indicate that DPNQ is a promising chemotherapeutic agent against T. cruzi.
Chagas disease, or American trypanosomiasis, caused by the protozoan parasite Trypanosoma cruzi, is one of the most deadly infectious diseases in Latin America (Dias et al., 2002). Recent reports suggest that Chagas disease is a potentially emergent public health concern in the United States due to the increased immigration from Latin American countries where the disease is endemic (Leiby et al., 1999, 2002; Andrade et al., 2004; Diaz, 2007; Kirchhoff and Pearson, 2007; Young et al., 2007). The only commercially available drug, benznidazole, is effective in the acute or early chronic phase of the infection (de Andrade et al., 1996; Andrade et al., 2004), but shows a much decreased performance among infected individuals during the late chronic phase and can cause severe side effects (Urbina and Docampo, 2003). Recently, the emergence of T. cruzi strains resistant to benznidazole has also been reported (Murta and Romanha, 1998). Moreover, thus far, no vaccine is available to prevent or treat Chagas disease (Minoprio, 2001; Martin and Tarleton, 2004; Garg and Bhatia, 2005). These facts clearly emphasize the urgent need of new therapeutic approaches against this parasite.
Quinones and their derivatives possess anticancer, antibacterial, antimalarial, and antifungal activities (Kim et al., 2004; Tasdemir et al., 2006; Verma, 2006; Brondani et al., 2007; Ui et al., 2007). Their biological activity is related to the acceptance of 1 and/or 2 electrons to form the corresponding radical anion or dianion species as well as the acid-base properties of the compounds (O’Brien, 1991). Studies performed by Salmon-Chemin et al. (2001) showed the anti-trypanosomal activity of 1,4-naphthoquinone (NQ) derivatives with alkylamine side chains at the C2- and C3- positions. The present study revealed that the most potent trypanocidal NQs acted as subversive substrates for T. cruzi lipoamide dehydrogenase (TcLipDH). We recently synthesized a novel quinone, derivative 2,3-diphenyl-1,4-naphthoquinone (DPNQ), and tested it for trypanocidal activity and toxicity for mammalian cells. Here, we report on the anti-trypanosomal action of DPNQ against epimastigote, cell-derived trypomastigote, and intracellular amastigote forms of T. cruzi in vitro and in vivo.
MATERIALS AND METHODS
Reagents and compounds
DPNQ was synthesized according to the method previously described (Montoya et al., 2005; Shanmagasundarama et al., 2005). The compound was dissolved in dimethyl sulfoxide (DMSO) and filtered sterile using a 0.22-μm filter (Sigma-Aldrich, St. Louis, Missouri). DL-Dihydrolipoamide was prepared by the reduction of DL-lipoamide (BDH Chemicals, VWR International GmbH, Darmstadt, Germany) with sodium borohydride (Reed et al., 1958). The reactive oxygen species (ROS) reagent (5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate, carboxy-DCFDA), and the DNA dye (4′, 6-diamidino-2-phenylindole dihydrochloride, DAPI) were purchased from Invitrogen (Carlsbad, California), and Vectashield was obtained from Vector Laboratories (Burlingame, California).
Parasite cultures
Epimastigote forms of T. cruzi (Y strain) were grown in liver infusion-tryptose (LIT) medium (Camargo, 1964). Mammalian cell-derived trypomastigote forms of T. cruzi (Y strain) were obtained from infected LLC-MK2 cell (American Type Culture Collection-ATCC, Manassas, Virginia) monolayers as described (Andrews and Colli, 1982).
In vitro assay with epimastigote forms
The assay was performed in a 96-well tissue culture microplate (Axygen, Union City, California) at drug concentrations of 33, 11, 3.3, 1.1, and 0.36 μM. As negative controls, we used parasites incubated with LIT medium alone (control 1) or LIT medium plus 3% DMSO (control 2). Epimastigotes (1 × 105 cells) in 100 μl of LIT medium were placed in each well and incubated at 28 C with the medium alone or drug for 1, 3, or 5 days. After incubation, the number of living parasites in each sample was determined with a hematocytometer. Each experiment was performed in triplicate and repeated 3 times. The results were expressed as percentage of survival of epimastigotes present in the sample.
Free radical formation assays
The assays were performed by setting up a 96-well tissue culture microplate, as described above, with 2 drug concentrations (33 and 11 μM) and 2 controls, 1 positive control (800 μM H2O2 plus parasites) and 1 negative control (LIT medium plus parasites). After addition of epimastigotes (1 × 105 cells) in 100 μl of LIT medium to each well, the microplate was incubated at 28 C for 24 hr. Then, 2 μl of 60 nM ROS reagent were added to each sample and the microplate was placed into a Fluoroskan fluorescent microplate reader (LabSystems, Thermo) for 350 min at 28 C. The amount of fluorescence emitted was recorded in every sample at 5-min intervals, starting at time 5 min.
In vitro infection experiments
The in vitro effect of DPNQ on T. cruzi-infected host cells was studied using 2 approaches, i.e., first, by treating the infected cells and, second, by treating the trypomastigotes prior to infecting the cells. In both cases, all experiments were performed 3 times in triplicate. Briefly, sterile 12-mm coverslips (Thermo Fisher Scientific, Worcester, Massachusetts) were placed into a 24-well plate. In each well, 5 × 104 LLC-MK2 cells were cultured for 24 hr, in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS), penicillin and streptomycin, under 5% CO2 atmosphere, at 37 C. In our first experiment, the monolayer of LLC-MK2 cells was exposed to trypomastigotes (1:20, host cell/parasite ratio), for 2 hr at 37 C. The non-adherent parasites were removed by 5 consecutive washes with 1 ml phosphate-buffered saline (PBS). The infected cells were treated with DPNQ concentrations of 100, 33, and 3.3 μM for 24 hr at 37 C. In the second approach, the trypomastigotes were incubated with 100, 33, and 3.3 μM DPNQ for 1 hr prior to host-cell infection. After treatment, the parasites were washed twice with PBS and the cells were infected (1:20, host cell/parasite ratio) for a period of 2 hr at 37 C. Subsequent to infection, non-adherent parasites were removed by washing twice with PBS. In both experimental approaches, the medium was removed and the cells were fixed with methanol for 30 min (300 μl/well), followed by 2 washes with ice-cold PBS, and 2 hr incubation at room temperature with PBS. The DAPI dye solution (1 mg/ml) was prepared at 1:1,000 dilution in PBS. Cells were stained with 300 μl of DAPI solution for 5 min at room temperature. After incubation, cells were washed twice with PBS and additional 500 μl PBS were added while slides were prepared. The cover slips were mounted onto the slides using 10 μl of Vectorshield mounting solution. In both procedures, host cell and parasite nuclei were stained with DAPI and cells were analyzed by microscopy using an LSM5 Pascal Zeiss confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, New York). In each sample from either approach, a total of 100 infected and uninfected cells were analyzed.
LipDH inhibition assay
Recombinant T. cruzi lipoamide dehydrogenase (TcLipDH) was cloned and expressed as described (Schoneck et al., 1997). Inhibition of recombinant TcLipDH by DPNQ was measured in 1 ml of 50 mM potassium phosphate, 1 mM EDTA, pH 7.0, at 25 C. The reaction mixture contained 1 mM NAD, 65 to 520 μM dihydrolipoamide (Boehringer Mannheim, Ingelheim, Germany), and 20 or 40 μM inhibitor. The reaction was initiated by the addition of the enzyme, and the absorption increase at 340 nm was followed. The inhibitor Ki and Ki′ constants for mixed type inhibition were derived from the Line weaver-Burk (double reciprocal) plot. Intercept on the vertical axis = (1 + I/Ki′)/V; intercept on the base line = (1+ I/Ki′)/Km (1+ I/Ki), where I = inhibitor concentration; Km = Michaelis-Menten constant for dihydrolipoamide (135 μM; here determined as 150 μM); V = maximum activity obtained from the intersection (1/V) with the y-axis for the reaction without inhibitor.
Oxidase assay
The oxidase activity of LipDH was followed in 1 ml of 50 mM potassium phosphate, 1 mM EDTA, pH 7.0, at 25 C containing 200 μM NADH and 300 mU TcLipDH (Lohrer and Krauth-Siegel, 1990) in the absence and presence of 40 μM DPNQ. The absorbance decrease was followed at 340 nm.
In vivo activity of DPNQ in the murine T. cruzi infection model
Three groups of 5 C3H/HeN (Harlan Sprague Dawley, Inc., Indianapolis, Indiana) female mice weighing from 20 to 22 g were used. Two groups (infected, treated; and infected, untreated) were inoculated intraperitoneally (i.p.) with 104 trypomastigotes, and 1 group remained uninfected (control for drug toxicity). Twenty-four hr post-infection the treatment with DPNQ was initiated, given 1 daily dose i.p. for 3 consecutive days, and 2 more doses with interval of 1 day in between. The treatment protocol was based on the limited amount of DPNQ available. The administrated dose was 10 mg DPNQ/kg body weight. The parasitemia was measured by mouse tail bleeding as described elsewhere (Brener, 1979). The use of animals in this research has complied with all relevant federal guidelines and institutional policies.
Statistical analyses
Statistical analyses were performed using the Statistical Analysis System (SAS Version 9.1.3, SAS Institute Inc., Cary, North Carolina) software and Prism software (GraphPad Software Inc., La Jolla, California). To test for the effects of dose for the in vitro infectivity experiments, the 1-way analysis of variance (ANOVA) was performed. If the dose effect was significant, then Tukey’s post-hoc procedure was used to see where the differences lay. For the other experiments, the General Linear Mixed Model Analysis was performed to test for the treatment effect, the time effect, and the treatment-by-time interaction. With significant results, Tukey’s post-hoc procedure was used to see were the differences rest. The t-test was performed to test for a linear dose effect and the LD50 estimated, using a dose-response curve with the logit transformation of the “percent infected cells” as the response and the log (dose + 1) as the predictor variable fitted in regression analysis, with repeated measures using the general linear mixed model analysis. All tests were performed at the 0.05 level of significance (P).
RESULTS
In vitro effect of DPNQ on T. cruzi epimastigotes
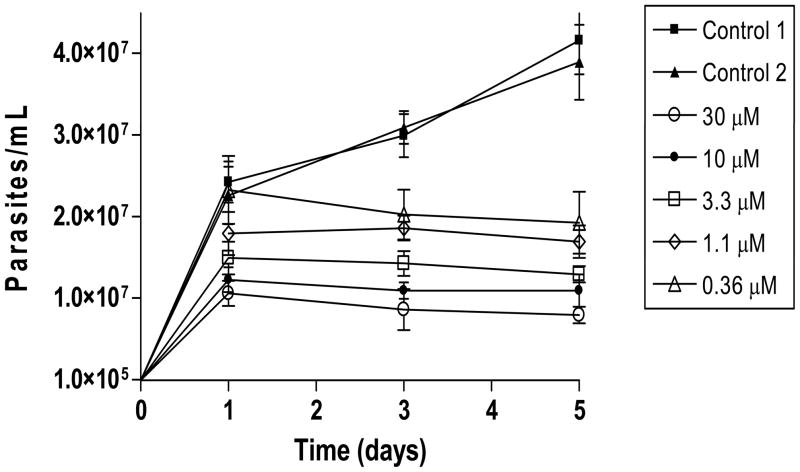
First, we tested the effect of DPNQ on non-infective epimastigote forms of T. cruzi. As shown in Figure 1, overall there was a significant decrease in growth as a function of the incubation time at all drug concentrations tested. Furthermore, we observed a significant treatment-by-day interaction (P-value < 0.0001). For instance, parasites treated with 30 and 10 μM DPNQ for 24 hr showed a decrease in growth of 84% and 49%, respectively, as compared to the control group 1 (untreated, LIT medium). After 5 days of treatment, the decrease in growth varied from 81% (30 μM) to 54% (0.36 μM). It is worth pointing out that addition of 3% DMSO to the LIT medium in the control group 2 had no effect in parasite growth when compared to control group 1.
Figure 1.
Growth curve of Trypanosoma cruzi epimastigotes treated with DPNQ. The experiment was performed 3 times in triplicate. The values are the means of the number of epimastigotes per ml with the corresponding standard deviations. The estimated drug LD50 was 2.5 μM. Control 1, untreated parasites incubated in LIT medium alone; Control 2, parasites in LIT medium plus 3% DMSO.
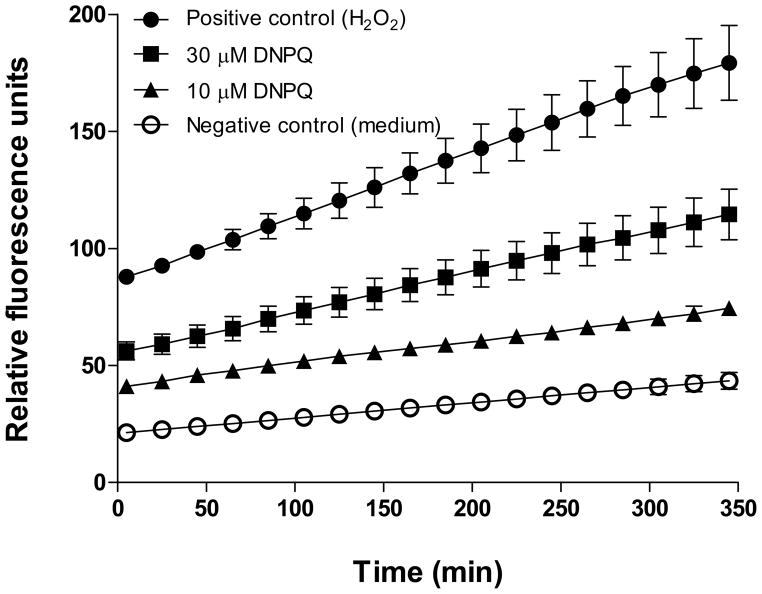
Studies with mammalian cells showed that DPNQ at higher concentrations induces formation of free radicals and apoptosis (K. Garza et al., unpubl. obs.). Based on these data, we tested the formation of free radicals and genomic DNA fragmentation by epimastigote forms of T. cruzi following a 24-hr treatment with 10 and 30 μM DPNQ. Our results showed a considerable production of oxidative radicals (Fig. 2), but no genomic DNA fragmentation (data not shown), indicating that the toxic effect of DPNQ on epimastigotes is not due to apoptosis, but rather to the formation of free radicals. At the beginning of the measurements (time 5 min), parasites treated with 30 μM DPNQ had approximately 64% of the free radicals produced by the positive control (parasites treated with 800 μM H2O2) (Fig. 2). Similar concentration of DPNQ did not induce detectable generation of free radicals by mammalian cells (K. Garza, unpubl. obs.).
Figure 2.
Formation of free-radicals by epimastigotes treated with DPNQ for 24 hr. High number of relative fluorescence units indicates high level of production of oxidative radicals. There was a significant treatment-by-time interaction, P < 0.0001.
In vitro effect of DPNQ on T. cruzi trypomastigotes
In vitro infectivity experiments were carried out to determine the action of DPNQ on trypomastigotes, the infective forms for mammalian cells. These experiments showed a 41% decrease in infected cells at 33 μM concentration (Fig. 3A). Both experimental approaches (infection prior to treatment, and treatment followed by infection) yielded a very similar percentage of infected cells and number of intracellular amastigotes per cell. For both experimental approaches, the estimated LD50 calculated using t-test and linear regression analysis was 2.5 μM. Moreover, the Tukey’s post-hoc analysis showed a significant difference between the treatments with 100, 33, 3.3, and 0 μM DPNQ (P = 0.05).
Figure 3.
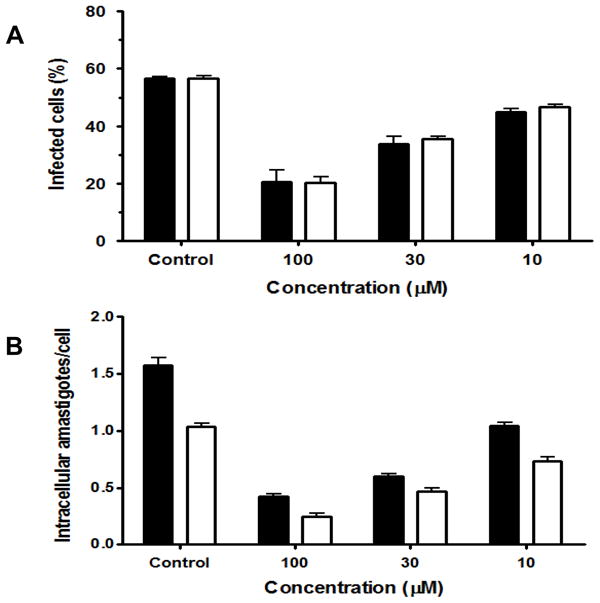
Effect of DPNQ on the in vitro infection of LLC-MK2 cells by Trypanosoma cruzi trypomastigotes. (A, B) Percentage of infected cells and number of intracellular amastigotes per infected cell, respectively. Black bars, infected host cells treated with DPNQ 24 hr post-infection (treatment 1); and white bars, parasites treated with DPNQ for 1 hr prior to host-cell infection (treatment 2). The estimated drug LD50 was 2.5 μM. These experiments were performed 3 times in triplicate. The values are the means of the percentage of infected cells for (A) and the means of the number of amastigotes per infected cell for (B) with the corresponding standard deviations, as indicated above the bars.
In vivo activity of DPNQ in the murine T. cruzi infection model
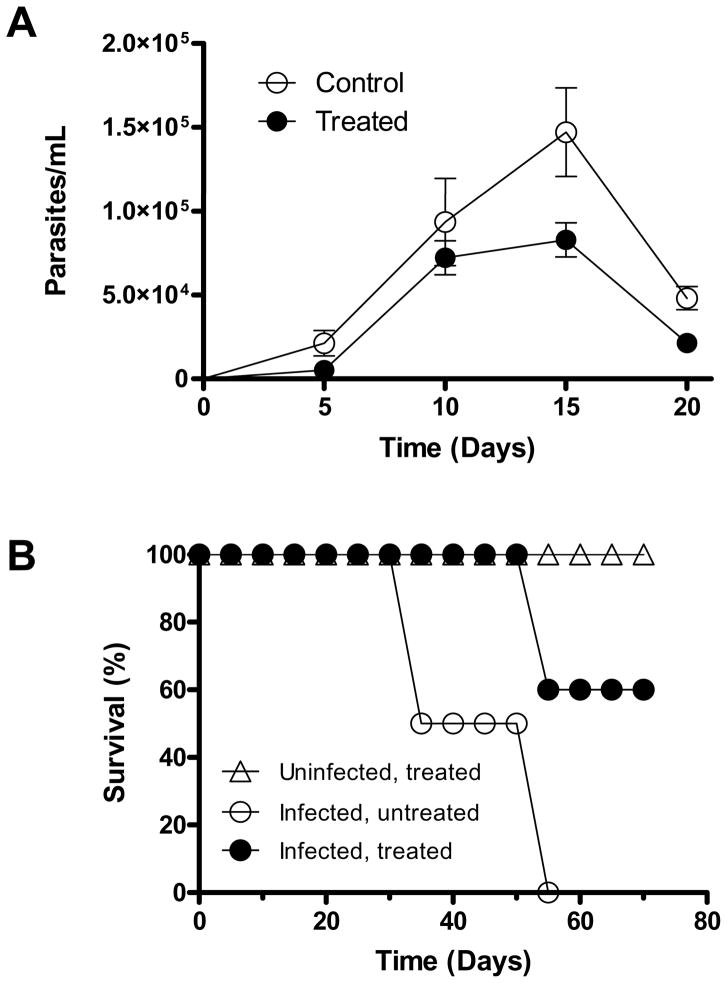
The promising results in the in vitro infection experiment encouraged us to study DPNQ in the murine model of Chagas disease. The in vivo experiments consisted of 3 groups of 5 C3H/HeN female mice. The groups were categorized as: (1) experimental group (infected, treated); (2) control group for infection (infected, untreated); and (3) control for drug toxicity (uninfected, treated). The DPNQ-treated group showed a 24 hr delay in the appearance of the parasitemia with respect to the control, untreated group. Also, during the experiment, the parasitemia was 2-fold lower in the group treated with DPNQ (Fig. 4A). A significant difference was observed between the groups by comparing the average parasitemia across the days. In Figure 4B, we observed no survivors in the control (infected, untreated) group 55 days post-infection (PI). In the case of the treated group, 60% survival was observed until day 70 PI when the experiment was terminated. The control group for DPNQ toxicity (non-infected, treated) did not show any phenotypic or behavioral changes, suggesting a very low toxicity of this compound to the animals.
Figure 4.
In vivo activity of DPNQ in C3H/HeN mice infected with Trypanosoma cruzi. (A, B) Parasitemia and survival curves, respectively. Control, uninfected and treated with 10 mg/kg DPNQ (control of drug toxicity). Similar profiles were verified in 2 independent experiments. The values depicted in (A) are the mean of 5 determinations with standard deviations as indicated. The statistical analysis using the General Linear Mixed Model analysis with square root transformation showed that the parasitemia of the treated group is significantly lower than that of the control group (P = 0.0237).
Inhibition of TcLipDH by DPNQ
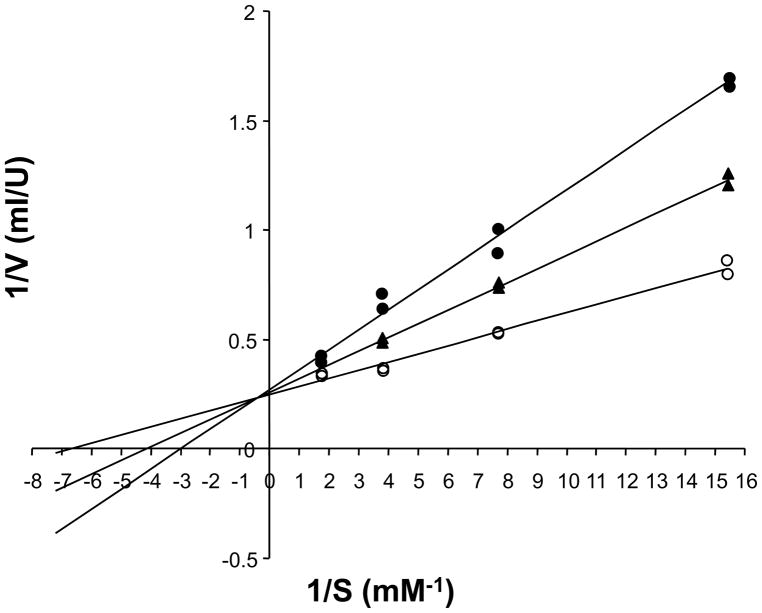
Since naphthoquinones have been shown to act as subversive substrates of lipoamide dehydrogenase (LipDH) (Blumenstiel et al., 1999), we tested whether DPNQ would have the same effect on the TcLipDH. As shown in Figure 5, DPNQ is a competitive inhibitor of the parasite enzyme. Nevertheless, the lines in the Lineweaver-Burk plot did not intersect exactly the y-axis. Thus, assuming a mixed-type inhibition, a Ki-value of 29 μM and a Ki′-value of 455 μM were calculated. From these data we suggest that DPNQ is a competitive inhibitor of TcLipDH with additional very weak binding to the enzyme-substrate complex. The Ki-value of 29 μM may appear rather high. However, considering the Km-value of the enzyme for dihydrolipoamide is 130 μM (150 μM in the current series of assays), this means that DPNQ is an efficient inhibitor of the enzyme. To our knowledge, DPNQ is the first competitive inhibitor of any LipDH known. Usually, naphthoquinones act as subversive substrates (Blumenstiel et al., 1999), but do not inhibit LipDH. Therefore, we also studied whether DPNQ is reduced by the enzyme. This does not appear to be the case. In a total volume of 1 ml, the assays contained 200 μM NADH and 2 μl TcLipDH (150 U/ml). In the absence of DPNQ, a delta Abs340 nm/min of 0.015 was observed (LipDH catalyzed oxidation of NADH by molecular oxygen). This oxidized activity of TcLipDH of 1.2 U/ml is about 1% of the lipoamide dehydrogenase activity. Addition of 40 μM DPNQ increased the delta Abs/min only slightly to 0.02. The delta Abs/min of 0.005 yields a volume activity of 0.4 U/ml for DPNQ reduction. Higher concentrations of DPNQ could not be used because the compound precipitates in the buffered solution, and 5% DMSO in the buffer is the maximum of organic solvent that can be used without inhibition of the enzyme.
Figure 5.
Lineweaver-Burk plot of the competitive inhibition of Trypanosoma cruzi lipoamide dehydrogenase by DPNQ. Enzyme kinetics were measured at 25 C, at a constant concentration of 1 mM NAD. The dihydrolipoamide concentration was varied in the absence (○), or presence of 20 μM (▲), or 40 μM (●) DPNQ.
DISCUSSION
During the past 2 decades, no considerable progress has been achieved in the treatment of Chagas disease. Because of the toxic effects, variable efficacy, and length of treatment, chemotherapy of Chagas remains unsatisfactory (Croft et al., 2005; Castro et al., 2006). Currently, specific inhibitors of ergosterol biosynthesis, cruzipain, and trypanothione reductase are under pre-clinical studies. Despite several efforts, so far, no human vaccine is available to prevent Chagas disease, indicating that chemotherapy is the only option (Croft et al., 2005; Rakotomanga et al., 2005). In this regard, the ideal chemotherapeutic agent should target essential molecules or metabolic pathways of the parasite, with limited toxicity for the host. DPNQ seems to fulfill these requirements. In our study, the novel naphthoquinone DPNQ showed excellent results in vitro and very promising outcome in the in vivo studies. Our results clearly demonstrated that DPNQ has selective toxicity, since the LD50 (2.5 μM) for epimastigotes and the in vitro infection experiments are 2 orders of magnitude lower than the LD50 (130 μM) for mammalian cells (K. Garza unpubl. obs.). In addition, the in vivo toxicity studies demonstrated no severe side effects for the animals.
It has been shown earlier that trypanosomes are more sensitive to oxidative stress than their mammalian hosts (Docampo and Moreno, 1984). During evolution, these parasites developed a specific thiol redox metabolism based on the trypanothione system. In addition, they possess LipDH, an enzyme that is structurally and mechanistically closely related to trypanothione reductase (TR). In vitro studies with several naphthoquinones revealed that the compounds act as subversive substrates of T. cruzi trypanothione reductase and lipoamide dehydrogenase (Salmon-Chemin et al., 2001). As shown here, DPNQ is a competitive inhibitor of TcLipDH, but the concentration that is lethal for the parasites is an order of magnitude lower than the Ki for TcLipDH. Also, DPNQ showed only marginal inhibition on T. cruzi TR (data not shown). Thus, the high trypanocidal activity of DPNQ may be due to an enrichment of this drug in the parasite or because it has additional cellular target(s). Naphthoquinones can affect a wide variety of cellular functions (Wu et al., 2005). For instance, the 2-hydroxynaphthoquinone atovaquone exerts its antimalarial effect by inhibiting the electron transport chain of Plasmodium falciparum (Kessl et al., 2004; Mather et al., 2005).
In sum, based on the promising results obtained here, new studies are underway aiming at the synthesis of new DPNQ derivatives, which could be more specific and effective for the treatment of Chagas disease.
Acknowledgments
This study was supported by the Research Initiative for Scientific Enhancement (RISE) Program at the University of Texas at El Paso, El Paso, Texas (UTEP), NSF Advance Program grant 0245071, NIH/NCRR grant 5G12RR008124, and the Deutsche Forschungsgemeinschaft (SFB544, project B3, L.K.-S.). RAM was supported by grant # 2S06GM00812-37 from the NIH/MBRS/SCORE Program. LEM was supported by the National Institute of Health (MBRS-SCORE 2S06 GM-801233 and NIAID U54 Al057156) and the Robert A. Welch Foundation. We are thankful to the Biomolecule Analysis Core Facility (BACF) and Statistical Consulting Laboratory (SCL) at the Border Biomedical Research Center, UTEP, both supported by NIH/NCRR grant # 5G12RR008124. We are indebted to Professor Alan Fairlamb and Dr. Deuan Jones (University of Dundee, Dundee, Scotland) for testing DPNQ against trypanothione reductase, and Dr. Igor Almeida (UTEP) for his critical review of the manuscript.
LITERATURE CITED
- Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, Covas DT, Silva LS, Andrade JG, Travassos LR, Almeida IC. Short report: Benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. American Journal of Tropical Medicine and Hygiene. 2004;71:594–597. [PubMed] [Google Scholar]
- Andrews NW, Colli W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. Journal of Protozoology. 1982;29:264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- Blumenstiel K, Schoneck R, Yardley V, Croft SL, Krauth-Siegel RL. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem Pharmacol. 1999;58:1791–1799. doi: 10.1016/s0006-2952(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Brener Z. Present status of chemotherapy and chemoprophylaxis of human trypanosomiasis in the Western Hemisphere. Pharmacological Therapy. 1979;7:71–90. doi: 10.1016/0163-7258(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Brondani DJ, Nascimento CR, de MMDR, Lima Leite AC, de Souza IA, Bieber LW. Synthesis and antitumour activity of the Primin (2-methoxy-6-n-pentyl-1,4-benzoquinone) and analogues. Medical Chemistry. 2007;3:369–372. doi: 10.2174/157340607781024410. [DOI] [PubMed] [Google Scholar]
- Camargo EP. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Revista Instituto Medical Tropical Sao Paulo. 1964;12:93–100. [PubMed] [Google Scholar]
- Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Human Experimantal Toxicology. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- Croft SL, Barrett MP, Urbina JA. Chemotherapy of trypanosomiases and leishmaniasis. Trends in Parasitology. 2005;21:508–512. doi: 10.1016/j.pt.2005.08.026. [DOI] [PubMed] [Google Scholar]
- de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: A review. Memorias Instituto Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Diaz JH. Chagas disease in the United States: A cause for concern in Louisiana? Journal of the Louisiana State Medical Society. 2007;159:21–23. 25–29. [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Reviews of Infectious Disease. 1984;6:223–238. doi: 10.1093/clinids/6.2.223. [DOI] [PubMed] [Google Scholar]
- Garg N, Bhatia V. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Review of Vaccines. 2005;4:867–880. doi: 10.1586/14760584.4.6.867. [DOI] [PubMed] [Google Scholar]
- Kessl JJ, Hill P, Lange BB, Meshnick SR, Meunier B, Trumpower BL. Molecular basis for atovaquone resistance in Pneumocystis jirovecii modeled in the cytochrome bc(1) complex of Saccharomyces cerevisiae. Journal of Biological Chemistry. 2004;279:2817–2824. doi: 10.1074/jbc.M309984200. [DOI] [PubMed] [Google Scholar]
- Kim YM, Lee CH, Kim HG, Lee HS. Anthraquinones isolated from Cassia tora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. Journal of Agriculture and Food Chemistry. 2004;52:6096–6100. doi: 10.1021/jf049379p. [DOI] [PubMed] [Google Scholar]
- Kirchhoff LV, Pearson RD. The emergence of chagas disease in the United States and Canada. Current Infectious Disease Report. 2007;9:347–350. doi: 10.1007/s11908-007-0053-9. [DOI] [PubMed] [Google Scholar]
- Leiby DA, Fucci MH, Stumpf RJ. Trypanosoma cruzi in a low- to moderate-risk blood donor population: Seroprevalence and possible congenital transmission. Transfusion. 1999;39:310–315. doi: 10.1046/j.1537-2995.1999.39399219290.x. [DOI] [PubMed] [Google Scholar]
- Leiby DA, Herron RM, Jr, Read EJ, Lenes BA, Stumpf RJ. Trypanosoma cruzi in Los Angeles and Miami blood donors: Impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002;42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- Lohrer H, Krauth-Siegel RL. Purification and characterization of lipoamide dehydrogenase from Trypanosoma cruzi. European Journal of Biochemistry. 1990;194:863–869. doi: 10.1111/j.1432-1033.1990.tb19480.x. [DOI] [PubMed] [Google Scholar]
- Martin D, Tarleton R. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunology Reviews. 2004;201:304–317. doi: 10.1111/j.0105-2896.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Mather MW, Darrouzet E, Valkova-Valchanova M, Cooley JW, McIntosh MT, Daldal F, Vaidya AB. Uncovering the molecular mode of action of the antimalarial drug atovaquone using a bacterial system. Journal of Biological Chemistry. 2005;280:27458–27465. doi: 10.1074/jbc.M502319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoprio P. Parasite polyclonal activators: New targets for vaccination approaches? International Journal for Parasitology. 2001;31:588–591. doi: 10.1016/s0020-7519(01)00171-0. [DOI] [PubMed] [Google Scholar]
- Montoya J, Varela-Ramirez A, Shanmugasundram M, Martinez LE, Primm TP, Aguilera RJ. Tandem screening of toxic compounds on GFP-labeled bacteria and cancer cells in microtiter plates. Biochemical and Biophysical Research Communications. 2005;335:367–372. doi: 10.1016/j.bbrc.2005.07.086. [DOI] [PubMed] [Google Scholar]
- Murta SM, Romanha AJ. In vivo selection of a population of Trypanosoma cruzi and clones resistant to benznidazole. Parasitology. 1998;116(Pt 2):165–171. doi: 10.1017/s0031182097002084. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chemical and Biological Interactions. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- Rakotomanga M, Saint-Pierre-Chazalet M, Loiseau PM. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrobial Agents and Chemotherapy. 2005;49:2677–2686. doi: 10.1128/AAC.49.7.2677-2686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Leach FR, Koike M. Studies on a lipoic acid-activating system. Journal of Biological Chemistry. 1958;232:123–142. [PubMed] [Google Scholar]
- Salmon-Chemin L, Buisine E, Yardley V, Kohler S, Debreu MA, Landry V, Sergheraert C, Croft SL, Krauth-Siegel RL, Davioud-Charvet E. 2- and 3-substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: Synthesis and correlation between redox cycling activities and in vitro cytotoxicity. Journal of Medical Chemistry. 2001;44:548–565. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- Schoneck R, Billaut-Mulot O, Numrich P, Ouaissi MA, Krauth-Siegel RL. Cloning, sequencing and functional expression of dihydrolipoamide dehydrogenase from the human pathogen Trypanosoma cruzi. European Journal of Biocemistry. 1997;243:739–747. doi: 10.1111/j.1432-1033.1997.00739.x. [DOI] [PubMed] [Google Scholar]
- Shanmugasundarama M, Garcia-Martinez I, Li Q, Estrada A, Martinez NE, Martinez LE. Microwave-assisted solid-phase Dötz benzannulation reactions: A facile synthesis of 2,3-disubstituted-1,4 naphthoquinones. Tetrahedron Letters. 2005;46:7545–7548. [Google Scholar]
- Tasdemir D, Brun R, Yardley V, Franzblau SG, Ruedi P. Antituberculotic and antiprotozoal activities of primin, a natural benzoquinone: In vitro and in vivo studies. Chemical Biodiversity. 2006;3:1230–1237. doi: 10.1002/cbdv.200690124. [DOI] [PubMed] [Google Scholar]
- Ui H, Ishiyama A, Sekiguchi H, Namatame M, Nishihara A, Takahashi Y, Shiomi K, Otoguro K, Omura S. Selective and potent in vitro antimalarial activities found in four microbial metabolites. Journal of Antibiotics (Tokyo) 2007;60:220–222. doi: 10.1038/ja.2007.27. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: Controversies and advances. Trends in Parasitology. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Verma RP. Anti-cancer activities of 1,4-naphthoquinones: A QSAR study. Anticancer Agents and Medical Chemistry. 2006;6:489–499. doi: 10.2174/187152006778226512. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Huang ZS, Bu XZ, Shen YD, Zhang ZL, Xie BF, Liu ZC, Gu LQ, Chan AS. The molecular mechanisms involved in the cytotoxicity of alkannin derivatives. European Journal of Medical Chemistry. 2005;40:1341–1345. doi: 10.1016/j.ejmech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Young C, Losikoff P, Chawla A, Glasser L, Forman E. Transfusion-acquired Trypanosoma cruzi infection. Transfusion. 2007;47:540–544. doi: 10.1111/j.1537-2995.2006.01147.x. [DOI] [PubMed] [Google Scholar]