Abstract
The toxicity of the ubiquitous pollutant and endogenous metabolite, acrolein, is due in part to covalent protein modifications. Acrolein reacts readily with protein nucleophiles via Michael addition and Schiff base formation. Potential acrolein targets in protein include the nucleophilic side chains of cysteine, histidine, and lysine residues as well as the free amino terminus of proteins. Although cysteine is the most acrolein-reactive residue, cysteine-acrolein adducts are difficult to identify in vitro and in vivo. In this study, model peptides with cysteine, lysine, and histidine residues were used to examine the reactivity of acrolein. Results from these experiments show that acrolein reacts rapidly with cysteine residues through Michael addition to form M+56 Da adducts. These M+56 adducts are, however, not stable, even though spontaneous dissociation of the adduct is slow. Further studies demonstrated that when acrolein and model peptides are incubated at physiological pH and temperature, the M+56 adducts decreased gradually accompanied by the increase of M+38 adducts, which are formed from intra-molecular Schiff base formation. Adduct formation with the side chains of other amino acid residues (lysine and histidine) was much slower than cysteine and required higher acrolein concentration. When cysteine residues were blocked by reaction with iodoacetamide and higher concentrations of acrolein were used, adducts of the N-terminal amino group or histidyl residues were formed but lysine adducts were not detected. Collectively, these data demonstrate that acrolein reacts avidly with protein cysteine residues and that the apparent loss of protein-acrolein Michael adducts over time may be related to the appearance of a novel (M+38) adduct. These findings may be important in identification of in vivo adducts of acrolein with protein cysteine residues.
Introduction
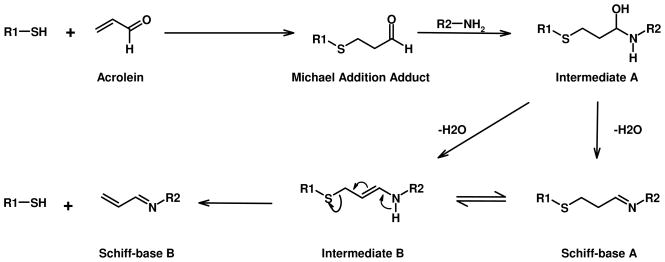
Acrolein is a highly toxic compound generated in the environment by oxidation of organic compounds, including tobacco or fossil fuels. Acrolein is also generated endogenously during lipid peroxidation or from the metabolism of some drugs, such as the antitumor drug cyclophosphamide. Acrolein is the strongest electrophile among the α, β-unsaturated aldehyde series (1, 2). The toxicity of acrolein is related to its ability to deplete glutathione (3), and to its ability to form DNA (4, 5), and protein adducts (1, 2). Potential targets of acrolein in protein include side chains of cysteine, histidine, and lysine residues as well as the free N-terminal amino group. Acrolein reacts with these residues through two basic mechanisms: Schiff-base formation and Michael addition. During Schiff-base formation, the carbonyl carbon of acrolein reacts with the primary amino group of lysine or N-terminal residues to form Schiff-bases after loss of water from an unstable intermediate. During Michael addition, the β-carbon of acrolein reacts with nucleophilic groups to form 1,2-addition with the double bond. The aldehyde group of the Michael addition adducts can further react with other nucleophilic groups to form intermolecular and intramolecular protein-protein (6), DNA-DNA (7, 8) and protein-DNA (9) cross links. More complex adducts can also form when several molecules of acrolein react with same residue, such as formation of the Nε-(3-formyl-3,4-dehydropiperidino)lysine adduct (FDP-lysine) (10), and the Nε-(3-methylpyridinium)lysine (MP-lysine) adduct (11). The FDP-lysine adducts have been detected in arterial tissue using an anti-FDP-lysine antibody (12). The MP-lysine adducts in apolipoprotein A-I have been identified by mass spectrometry (MS), after in vitro incubation of acrolein with apo A-I and in human atherosclerotic lesions using a MP-lysine specific antibody (13).
In general, the sulfhydryl group of cysteine residue is the most reactive nucleophile in proteins (1, 2), which makes it the most likely target for acrolein. Cysteine residues are located at the active site of several proteins and are often involved in the catalytic activity of enzymes. Reduced cysteine residues impart structural stability to proteins by maintaining an appropriate secondary structure, thus the formation of protein-cysteine adducts has broad functional implications. Nevertheless, little is known in regard to acrolein-thiol adducts and their in vivo presence has not been unequivocally demonstrated. Although inactivation of enzymes such as aldose reductase (14) and recently protein tyrosine phosphatase 1B (15) due to modification of cysteine residue has been reported, the general reactivity of acrolein with protein thiols has remained largely unexplored. Furthermore, despite the fact that cysteine is widely accepted as the most likely site of acrolein adduct formation and the implication of such reaction in mechanisms of acrolein toxicity, no in vivo cysteine adducts of acrolein have been identified. In studies of adduct formation in intact proteins, extensive modifications are detected using electrospray ionization MS. However peptide mass fingerprinting studies show few adducts when using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) MS of trypsin hydrolysates. To study these reaction rates, we used model peptides with lysine, hisitidine and cysteine residues to examine the chemistry of this process.
Materials and Methods
Materials
Sequencing grade glutamic-C (Glu-C) was obtained from Roche (Indianapolis, IN). Bovine insulin, ammonium bicarbonate, dithiothreitol (DTT) and oxidized bovine insulin β-chain, in which the sulfhydryl groups were oxidized to sulfonic acids, were obtained from Sigma (St. Louis, MO). Acrolein diethylacetal, boric acid, and borax were obtained from Aldrich (Milwaukee, WI). Synthetic model peptides MGCAEGK, MCAAR, Ac-HKVCD, and Ac-RVCAKH were ordered from AAPPTec, LLC (Louisville, KY). C18 spin columns were obtained from Agilent Technologies (Wilmington, DE) and succinimido-4-fluorobenzoate (SFB) was a gift from Drs. Chin Ng and Junling Li (Department of Radiology, University of Louisville). All solvents were of HPLC grade.
Insulin and oxidized insulin β-chain digestion with Glu-C
Bovine insulin or oxidized bovine insulin β-chain (10 nmol) was dissolved in 50 μL of 250 mM NH4HCO3. A solution of Glu-C (0.5 μg/μL in water) was prepared and 2.5 μl was added to each protein sample. The mixture was incubated at 37 °C for 18 h and the digestion was stopped by adding 50 μL of 5% formic acid. The digested samples were desalted using C18 spin columns, divided into 5 nmol aliquots, dried using a SpeedVac, and stored at −20 °C.
Acylation of N-terminal amino groups with SFB
SFB was dissolved in acetonitrile (ACN) to make a 100 mM solution and then diluted to 5 mM with borate buffer (200 mM, pH 7.7). Peptides from the digestion of 10 nmol of bovine insulin were dissolved in 50 μL of freshly prepared 5 mM SFB reagent and incubated at 37 °C for 30 min. The reaction was stopped by addition of 50 μL of 5% formic acid. The acylated samples were cleaned and treated as described above.
Preparation of acrolein solution
Acrolein diethylacetal was dissolved in 0.1 M HCl to prepare a 40 mM solution (assuming complete conversion of acetal to aldehyde.) The solution was incubated at room temperature for 1 h to generate a 40 mM acrolein solution. The acrolein solution was prepared fresh for all experiments and diluted with 100 mM NH4HCO3 (pH 7.4) as needed. Caution: Acrolein is highly toxic. It needs to be handled in a well ventilated hood with appropriate protection.
Modification of insulin peptides with acrolein
Bovine insulin peptides (5 nmol) obtained from the Glu-C digestion, with or without acylation with SFB, were dissolved in 25 μL of 1 mM DTT in 100 mM NH4HCO3 (pH 7.4) and incubated at 70 °C for 30 minutes. The reduced samples were cooled to room temperature, mixed with 75 μL of 1 mM acrolein in 100 mM NH4HCO3 (pH 7.4), and incubated at 37 °C. Two μL aliquots of the reaction mixture were removed at different time points, immediately diluted with 98 μL ACN/0.1% formic acid (50/50, v/v), and analyzed by electrospray ionization/mass spectrometry (ESI/MS).
Modification of oxidized insulin β-chain peptides with acrolein
The peptides from 5 nmol oxidized bovine insulin β-chain digest were dissolved in 100 μL of 1 mM acrolein in 100 mM NH4HCO3 (pH 7.4), and incubated at 37 °C. Aliquots of 2 μL reaction mixture were removed at different time points, diluted with 48 μL ACN/0.1% formic acid (50/50, v/v), and analyzed by ESI/MS.
ESI/MS and ESI/MS/MS
Samples were analyzed by ESI/MS in positive ion mode and mass resolution of 8000 with a Q-TOF API-US mass spectrometer from Waters (Milford, MA). Samples were infused with a syringe pump at 1 μL/min. Data acquisition lasted for at least 1 min after the signal was stabilized and the spectra were summed, smoothed, and stored. Peak heights of the monoisotopic peaks were used to calculate the percentages of unmodified and modified peptides with the assumption that the peptide modifications do not affect MS analysis of peptides. For MS/MS analysis, the collision energy was adjusted to a level such that the intensities of the precursor ions were decreased by 80 to 90%.
Results
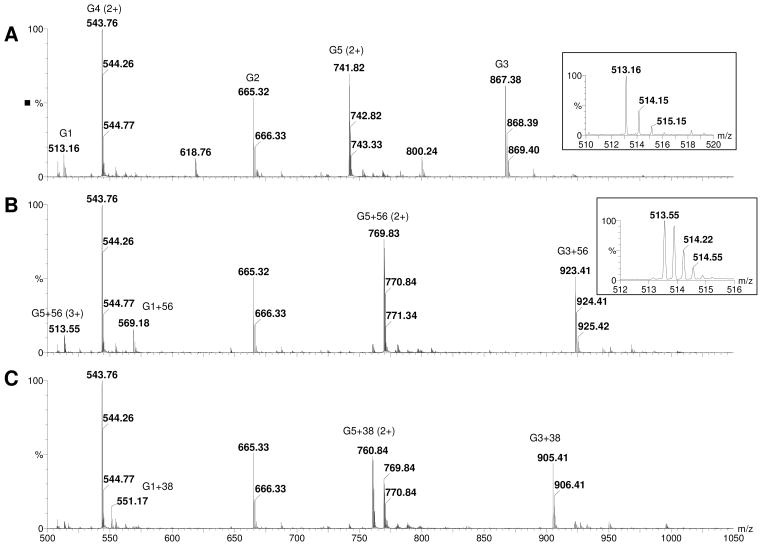
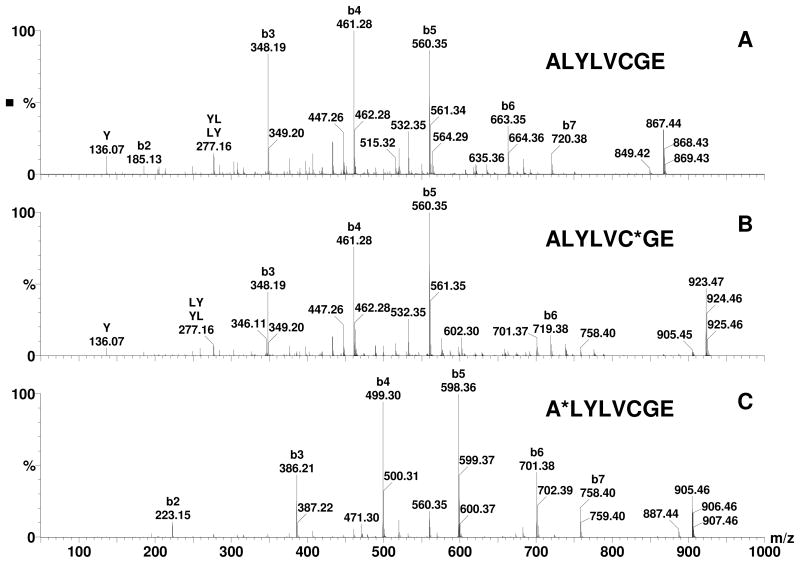
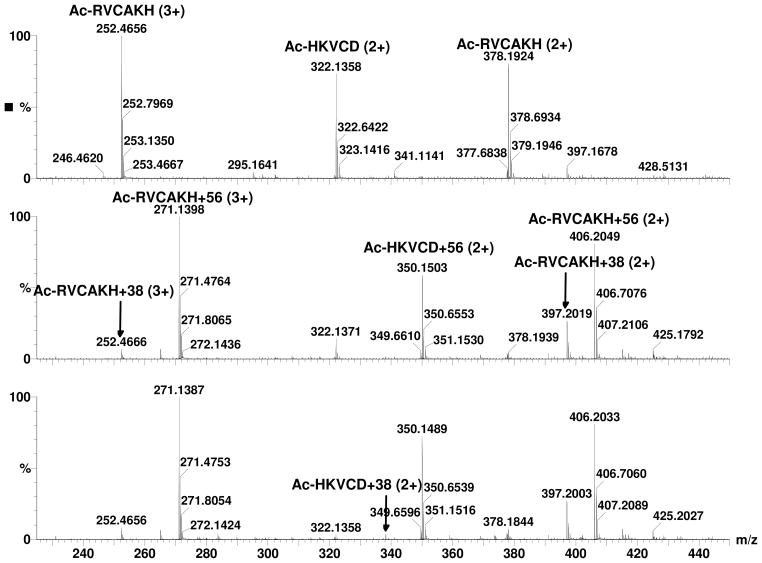
After digestion of bovine insulin with Glu-C and reduction with DTT, 5 peptides were detected as shown in Table 1 and Panel A of Figure 1 and their sequences were confirmed by MS/MS. Peaks with the highest intensities for each peptide (bold font in Table 1) were used for estimating the percentages of unmodified and modified peptides. When these peptides were incubated with acrolein, only minimal modification was observed in peptides G2 and G4 (m/z 665.33 and 543.76 respectively) that did not have a cysteine residue. However, the peaks of peptides possessing a cysteine, namely G1, G3, and G5 (m/z 513.16, 867.38, and 741.82), disappeared within 1 minute (Panel B of Figure 1) and new peaks with a plus 56.03 Da shift (will be referred as M+56 peaks or adducts in the following text. m/z 569.18 and 923.41 are singly charged M+56 peaks of G1 and G3 respectively and m/z 769.83 is the doubly charged M+56 peak of G5) were detected. The peak at m/z 513.55 is the triply charged ion of G5+56 and not the singly charged unmodified G1 (m/z 513.16). MS/MS spectra of G3 and G3+56 are shown in Figure 2 (Panels A and B). In the MS/MS spectrum of G3+56, there was no mass shift for the b-series ions b2 through b5 ions (b1 was not detected), however, a mass shift of +56 Da was observed for b6 and b7 (m/z 719.38 and 776.41 respectively), clearly indicating that acrolein modification occurred at the cysteine residue of G3. Modification of the cysteine residues was also confirmed for G1 and G5 by MS/MS (data not shown). The observed modification of the cysteine residues by acrolein was the result of Michael addition to the sulfhydryl group as this is the only reaction that could account for the 56 Da shift in the mass of the cysteine residues. As the incubation time of acrolein with the peptides was increased, the intensity of the cysteine adduct peaks decreased and the intensity of peaks 18 Da less than the M+56 peaks increased (Panel C of Figure 1 and Figure 3. These peaks will be referred as M+38 peaks or adducts in the following text. The MS/MS spectra of these M+38 peaks matched very well with corresponding Schiff-base adducts at the N-terminus of the peptides. For example, all the b ions, starting from the b2 ion, in the MS/MS spectrum of G3 (Panel A of Figure 2) were shifted by 38 Da (Panel C of Figure 2). Since the only functional group that can be modified in the residues within the b2 ion is the N-terminal amino group, we assign this modification to the formation of Schiff-base adducts at N-terminal end of the peptide. Addition of fresh acrolein at this stage, however, did not produce peaks with an additional 56 Da mass shift (data not shown), indicating that Cys in the M+38 adducts is not free and cyclic adducts were formed (see Discussion).
Table 1.
Peptides from Glu-C digested insulin.
| Peptide | Residue | Sequence | MW* | MH+ | MH22+ |
|---|---|---|---|---|---|
| G1 | α18–21 | NYCN | 512.1689 | 513.16 | |
| G2 | α13–17 | LYQLE | 664.3432 | 665.32 | |
| G3 | β14–21 | ALYLVCGE | 866.4208 | 867.38 | |
| G4 | β22–30 | RGFFYTPKA | 1085.5658 | 1086.55 | 543.76 |
| G5 | β1–13 | FVNQHLCGSHLVE | 1481.7085 | 1482.67 | 741.82 |
Calculated monoisotopic molecular weight.
MH+ and MH22+: Detected monoisotopic peaks. Most abundant peaks are in bold font and intensities of these peaks are used to estimate the percentages of unmodified and modified peptides.
Figure 1. ESI/MS spectra of Glu-C digested insulin before and after incubation with acrolein.
A: before incubation with acrolein, B: incubated with acrolein for 1 minute, and C: incubated with acrolein for 120 minutes. The m/z 513.55 peak in Panel B is the triple charged G5+56 peak not the single charged G1 peak (m/z 515.16) as shown in the insects of the respective panels. Peaks with multiple charges are marked in parentheses and the sequences of the peptides are shown in Table 1.
Figure 2. MS/MS spectra of G3 before and after alkylation by acrolein.
A: G3, B: G3+56, and C: G3+38. Apparent modified residues are marked with ‘*’ on the upper right corner of the residue.
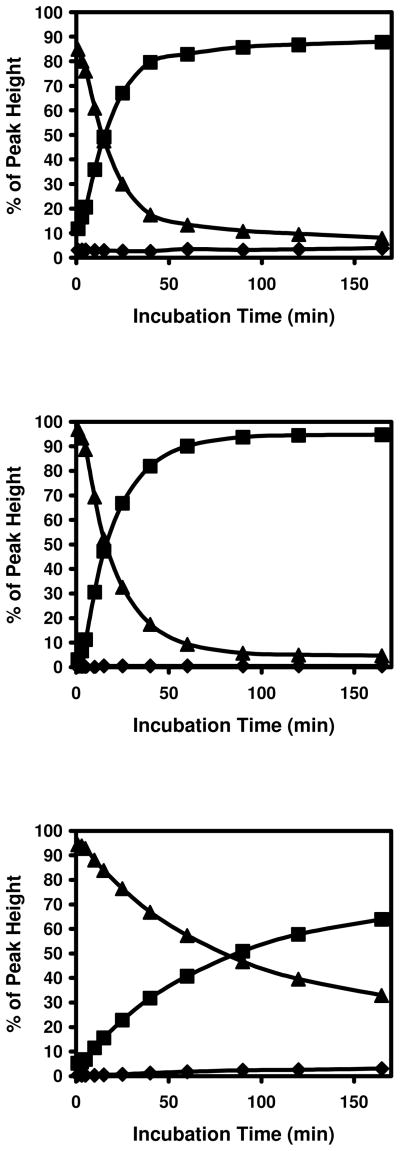
Figure 3. Modification of cysteine containing insulin peptides by acrolein.
Top panel: G1, middle panel: G3, and bottom panel: G5. ◆: unmodified peptides, ▲: modified peptides with +56 Da shift, and ■: modified peptides with +38 Da shift.
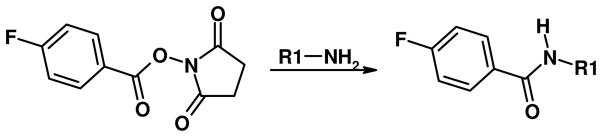
To examine the role of the N-terminal amino groups in this reaction, these groups were blocked with SFB (Scheme 1); a reagent that reacts efficiently with amino groups under aqueous conditions (16, 17). After blocking the amino groups, the peptides were desalted with C18 spin columns and dried using a SpeedVac. The peptides were then reduced with DTT and incubated with acrolein under the same conditions as in the earlier experiments. The results are shown in Figure 4. Alkylation of the amino groups significantly increased the stability of the Michael addition adducts at the cysteine residues. This indicates that the amino groups are directly involved in formation of the M+38 adducts. Nevertheless, the M+38 adducts do not appear to be Schiff-bases formed directly from free acrolein and N-terminal amino groups. If this were the case, Schiff-base adducts would be expected to form in G2 and G4 that lack cysteine residues. However, no M+38 adducts were detected in G2 and G4 peptides (Figure 5). Also, if the formation of Michael addition adducts and Schiff-base adducts are independent reactions, peptides with both modifications (M+94) should exist in peptides with Cys residues (G1, G3, and G5). However, negligible M+94 peaks were detected in any of these peptides.
Scheme 1.
Acylation of amino groups by SFB.
Figure 4. Modification of cysteine containing insulin peptides by acrolein after blocking amino groups with SFB.
Top panel: G3 and bottom panel: G5. ◆: unmodified peptides, ▲: modified peptides with +56 Da shift, and ■: modified peptides with +38 Da shift.
Figure 5. ESI/MS spectra of N-acetyl peptides before and after incubation with acrolein.
Top Panel: before incubation with acrolein, Middle Panel: incubated with acrolein for 1 minute, and Bottom Panel: incubated with acrolein for 120 minutes. Charge status of the peaks are marked in parentheses.
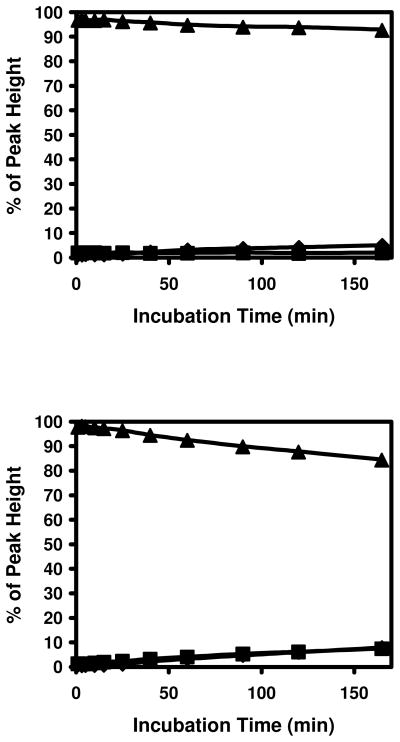
On side chain of lysine residue, there is a free amino group that may also participate in such reactions. To test this possibility, peptides containing cysteine and lysine residues in close proximity were synthesized. The N-terminus was blocked by acetylation, leaving the e–amino group as the only possible site for Schiff base formation. These peptides were incubated with acrolein under same conditions as the peptides from insulin and spectra before and after incubation with acrolein were shown in Figure 5. M+38 peaks of Ac-RVCAKH were observed with M+56 peaks in spectrum after 1 min incubation (Middle Panel). MS/MS spectrum with same collision energy as in MS experiment indicated that the M+38 peaks were partially formed in the collision cell (data not shown) and not from Schiff base formation as the peptides from insulin. The peak intensities of triply charged M, M+56, and M+38 of Ac-RVCAKH were used in Figure 6. As shown in Figure 6, acrolein reacted with the peptide quickly to form the M+56 adducts and the +56 Da adducts were relatively stable. Low levels of M+38 peaks were detected in the spectra and they remained constant through incubation. Even though the lysine residues in these peptides were very close to the cysteine, the M+38 peaks remained stable through incubation, indicating that M+56 adducts does not react with the e–amino group of lysine to form M+38 adducts. Instead the M+56 adducts slowly released acrolein and turned back to free peptides.
Figure 6. Modification of cysteine and lysine containing peptides by acrolein.
Top panel: Ac-HKVCD and bottom panel: Ac-RVCAKH. ◆: unmodified peptides, ▲: modified peptides with +56 Da shift, and ■: modified peptides with +38 Da shift.
Interestingly, the rate of conversion of Michael addition adducts to M+38 adducts decreased with increasing peptide length (Figure 3). The time required for a 50 % decrease in the M+56 peaks was approximately 13.5, 15.5, and 80 min for G1, G3, and G5 respectively. These results suggest that the formation of M+38 adducts is an intra-molecular reaction and the Michael addition adduct at Cys is the intermediate for this reaction. To confirm the role of Michael addition at Cys in the formation of M+38 adducts, the reaction between the peptide and acrolein was stopped after 5 min by acidifying the reaction mixture. Remaining free acrolein and acrolein-DTT adducts were removed with C18 spin columns. After removing the solvent from the peptides and reconstituting them with 100 mM NH4HCO3 buffer, incubation at 37 °C was resumed. Reaction curves similar to those illustrated in Figure 3 were obtained from peptides containing cysteine residues (data not shown). These results clearly show that the Michael addition adduct at Cys was the intermediate in M+38 adduct formation and that the free acrolein is not involved in the secondary formation of the Schiff base.
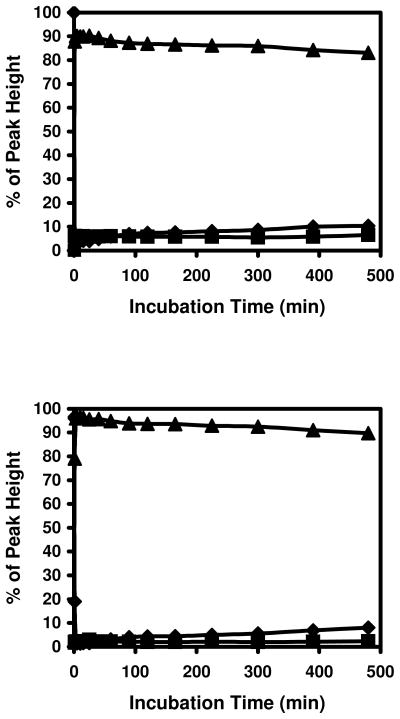
Formation of the M+38 adducts were predominately formed at peptides containing cysteine residues. Little Schiff-base formation was observed in G2 and G4 that lack cysteine residues (top panels in Figure 7). More adducts were formed with oxidized peptides when 1 mM acrolein was used (bottom panels in Figure 7). The MS/MS spectrum of the M+56 peak in oxidized G3 indicated that it was an adduct formed at the N-terminal. Both Michael addition and Schiff-base formation can occur with N-terminal amino groups. Michael addition adducts and intermediates of Schiff-base formation at N-terminus will both introduce 56 Da mass shifts and they are difficult to distinguish by MS/MS spectral changes. However, the M+56 peak in oxidized G3 is most likely a Michael addition adduct, because the intermediate of Schiff-base is expected to convert quickly to the more stable Schiff-base after losing water (M+38). The MS/MS spectrum of the M+56 peak of oxidized G5 indicated that partial alkylation at N-terminal amino group and histidine residues occurred during incubation. Interestingly, more M+38 adduct was found in oxidized G5 than in oxidized G3 (bottom panels in Figure 7). It is possible that the Michael addition adducts in histidine, which does not exist in G3, can act as Michael addition adducts in cysteine to form M+38 adducts at N-terminus.
Figure 7. Modification of insulin peptides without sulfhydryl group by acrolein.
Up left panel: G2, upper right panel: G4, bottom left panel: oxidized G3, and bottom right panel: oxidized G5. ◆: unmodified peptides, ▲: modified peptides with +56 Da shift, and ■: modified peptides with +38 Da shift.
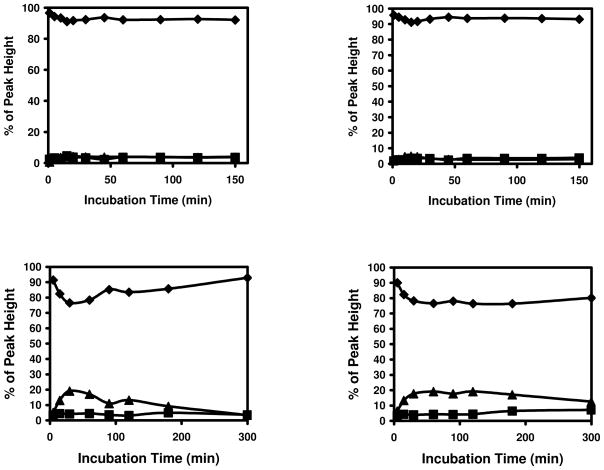
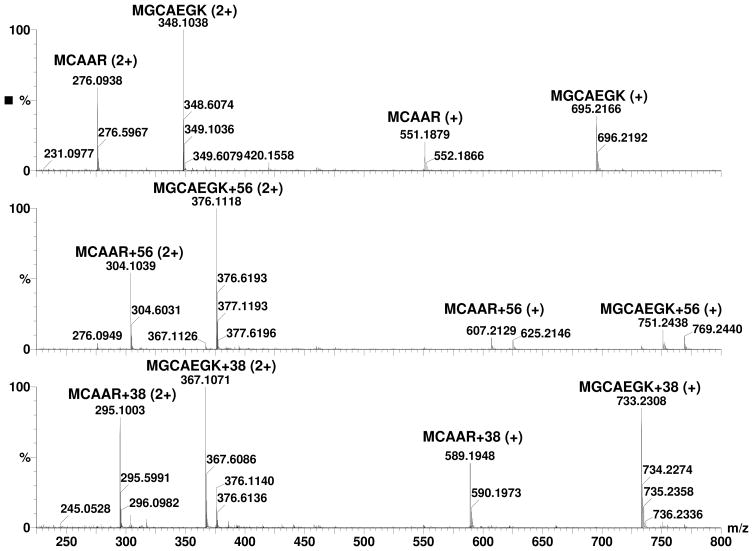
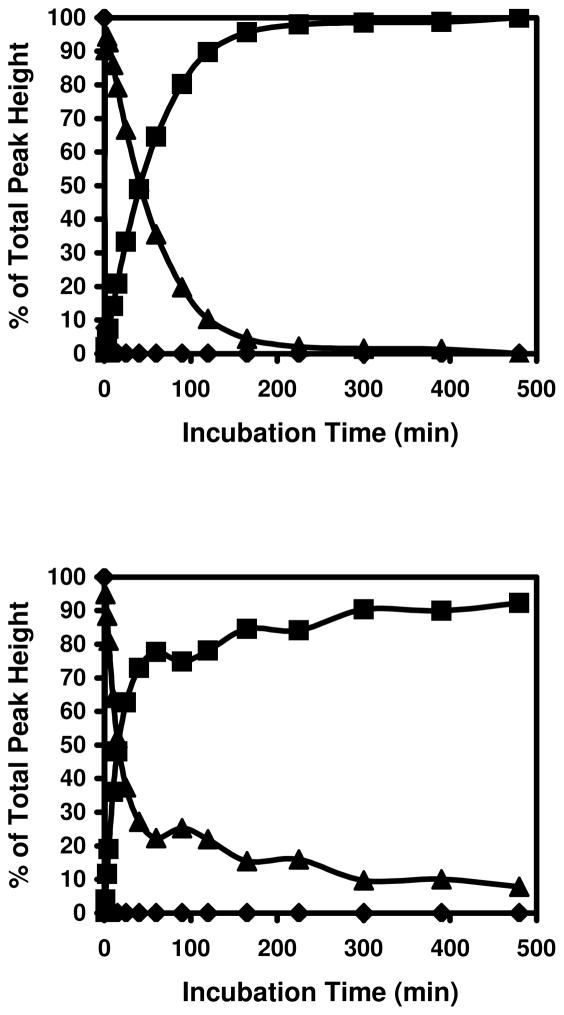
These initial results indicated that Michael addition adducts of cysteine and acrolein can rearrange to form a cyclic adducts with the free amino group at the N-terminus. To assess whether such adduct formation is likely in biological proteins, we examined proteins with cysteine residues close to the N-terminus amino group, because proximity with the N-terminus increases the probability of the formation of this adduct. Our sequence search of the human genome showed that there were 345, 654, 642, 625, 688, and 592 proteins with a cysteine a position 2, 3, 4, 5, 6, and 7, respectively, suggesting the frequent possibility of a cysteine residue being located close enough to the N-terminus to form a cyclic imine adduct. To test this experimentally, we synthesized peptides of sequence – MCAAR and MGCAEGK, which duplicate the N-terminal sequence of human glutathione peroxidase 1 isoform 2 (NP_00572) and thioredoxin reductase 1 isoform 3 (NP_001087240), respectively. The synthesized peptides were reduced and incubated with acrolein and spectra from the peptides before and after incubation with acrolein are shown in Figure 8. As shown in Figure 9, treatment with acrolein readily led to the formation of M+38 peaks in these peptides. These data suggest that the M+38 adduct could be formed with amino acids within the sequence context of natural proteins.
Figure 8. ESI/MS spectra of N-terminal peptides of proteins before and after incubation with acrolein.
Top Panel: before incubation with acrolein, Middle Panel: incubated with acrolein for 1 minute, and Bottom Panel: incubated with acrolein for 120 minutes. Charge status of the peaks are marked in parentheses.
Figure 9. Modification of protein N-terminal peptides by acrolein.
Top panel: MCAAR and bottom panel: MGCAEGK.◆: unmodified peptides, ▲: modified peptides with +56 Da shift, and ■: modified peptides with +38 Da shift.
Discussion
The reaction of α,β-unsaturated aldehydes with nucleophilic side chains of amino acid residues in proteins involves two basic reactions: Michael addition and Schiff-base formation. The reaction rates of α, β-unsaturated aldehydes for these two competing reactions depend on the electrophilicity of the β-carbon (Michael addition) and the carbon at the carbonyl group (Schiff-base formation). In α, β-unsaturated aldehydes, the strong electron withdrawing capability of carbonyl groups polarizes the double bond. This makes the β-carbon more electrophilic and susceptible to Michael addition. At the same time, the contribution of electron from the double bond decreases the electrophilicity of the carbon at the carbonyl group and thus reduces its reactivity towards Schiff-base formation. These factors contribute to the capability of the sulfhydryl in facilitating the M+38 adduct formation at amino groups as shown in Scheme 2.
Scheme 2. Mechanism of Schiff-base adduct formation from Michael addition adduct.
For Schiff-base A, a cyclic product is formed if R2-NH2 is the N-terminal of the same peptide.
In the Michael adducts of acrolein, the carbonyl group is maintained and the conjugated double bond is lost. The loss of electron donation from the double bond increases the reactivity of the carbonyl group towards nearby amino groups to form intermediate A (Scheme 2). Intermediate A can lose water to form either Schiff-base A or the enamine intermediate B which may also form from rearrangement of Schiff-base A. The rearrangement of intermediate B to form the more stable Schiff-base B should produce adducts with linear sequences that match well with the MS/MS spectra of the M+38 adducts (compare Panel C with Panel A and B in Figure 2). However, Schiff-base B has a free sulfhydryl group and should be able to react with acrolein. Failure for M+38 adducts to react with acrolein eliminated Schiff-base B as M+38 adduct. An intra-molecular reaction will produce cyclic Schiff-base A that also has a 38 Da mass shift and does not have free sulfhydryl group, match with the characteristics of the M+38 adducts. Therefore, the M+38 adducts could be assigned the Schiff-base A structure. In MS/MS analysis, the M+38 adducts could rearrange to Schiff-base B before fragmentation to produce MS/MS spectra that match well with Schiff-base B.
Sulfhydryl groups react rapidly with acrolein through Michael addition to form an adduct that is more reactive than the aldehyde group in acrolein and it can react with nearby amino groups to form Schiff-base adducts. Without the formation of the Michael addition adduct, the carbonyl group of acrolein is less reactive and the direct reaction of acrolein with amino groups is much slower (Figure 5). Such reaction might also occur through other residues, such as histidine. However, cysteine residues react with acrolein at much higher rate than other residues and are likely to be the main reaction site in proteins for this reaction.
The rates of acrolein adduct formation at histidine, lysine, and the N-terminal amino groups are much slower, especially for lysine residues. Results from our experiments show that the M+38 adducts are formed exclusively at N-terminal amino groups but not the e-amino group of lysine. When peptides lacking a free sulfhydryl group were incubated with elevated concentration of acrolein (5 mM), many adduct peaks were detected. MS/MS spectra of these peaks indicated that the modifications mainly occurred at the N-terminal amino group or at histidine residues. Little modification was found at lysine residues (unpublished data). This is likely due to the fact that the e-amino group of lysine is much more basic (model pKa 10.4) (18) than His (model pKa 6.3) and the amino groups at the N-terminus (model pKa 8.0). Thus, much smaller fraction of the e-amino group of lysine exists in a unionized state, which is the reactive form of the amine. For small peptides, the pKa values of the functional groups in amino acid residues are very close to their model pKa value, making reactions at lysine residues very unfavorable. However the pKa values of lysine residues in proteins can vary dramatically, depending on their environment in the protein. Schiff-base adducts are known to form at some lysine residues in proteins. For example, the widely used indicator of glycemic exposure, hemoglobin A1c, is formed through Schiff-base formation between glucose and the N-terminal valine residue of hemoglobin beta chain (19). Therefore, the formation of Schiff base subsequent to Michael addition could occur in proteins when a reactive cysteine residue is in close proximity to N-terminal residues. This possibility is supported by our results from peptides of protein N-terminus and peptides generated from insulin. Cysteine residue at 7th position from N-terminal (G5) can readily form the M+38 adduct. Our analysis of human proteins in the gene bank indicate that over 3,500 proteins have a cysteine residue within 7 residues from N-terminal Moreover, our results with two model peptides with the N-terminal sequence of human glutathione peroxidase and thioredoxin indicate that such adduct formation can also occur within the sequence context of native proteins. Such reaction may also happen when lysine residues with lowered pKa are close to cysteine residues. It is expected that such reactions can lead to cross-linking between residues in protein (intramolecular cross-linking) or between two different proteins (intermolecular cross-linking). We will test this possibility in future studies.
To avoid interference from nonvolatile buffers or chelating agents in the ESI/MS analysis, NH4HCO3 buffer without a chelating agent was used in our experiments. The concentration of acrolein in solution might decrease quickly under these conditions, which may be unfavorable for adduct formation via slow reactions. The FDP-lysine and MP-lysine adducts, which were detected with antibodies in tissues (12, 13), were not detected in our experiments. Small M+76 and M+94 (corresponding to mass shift of MP and FDP adducts respectively) peaks were detected when peptides were incubated with a high concentration of acrolein for a long time. It is yet to be determined whether these peaks are FDP or MP adducts or the result of multiple alkylation at different residues. FDP-lysine and MP-lysine were formed when acrolein was incubated with peptides in phosphate buffer and the N-terminal amino groups were acetylated (10, 11). The effect of buffers in adduct formation needs to be investigated and it will be interesting to see whether FDP and MP adducts at N-terminus will form under these conditions.
The sulfhydryl group in Cys has the highest reactivity with acrolein. Acrolein is expected to react mainly with sulfhydryl groups and only under special circumstances with other amino groups (1). However even in vitro identification of acrolein-cysteine adducts in proteins has not been reported until recently (15) and such adducts formed in vivo have not been detected. The failure to detect acrolein-cysteine adducts is attributed to the reversibility of the Michael addition reaction. Our results indicate that acrolein-cysteine adducts can be relatively stable (Figure 4). This observation agrees the apparent stability of S-(3-oxopropyl)-N-acetylcysteine. When it was incubated in phosphate buffer at pH 7.4 and dinitrophenylhydrazine was used to trap released acrolein, only 3% of the adducted acrolein was released after 30 minutes of incubation (20). Nonetheless, we did observe slow reversal of the Michael adduct and in the Ac-HKVCD, free acrolein was released from the peptide to generate a non-adducted peptide. Hence, such adducts formed in the extracellular space or in tissues could potentially liberate free acrolein to modify other proteins, especially if the adducts linger and are not readily degraded or repaired.
A common approach to identify the type and location of modifications in proteins is to digest the protein into peptides and then analyze the peptides by MS. The potential conversion of acrolein-cysteine adducts along with the formation of intra molecular and inter molecular cross-links could make it difficult to detect acrolein-cysteine adducts with this approach. Nevertheless, given the high reactivity of acrolein with cysteine and the high propensity of these adducts to cross link protein could induce dramatic functional changes in proteins that may be important in understanding several pathological and toxicological states associated with high oxidative stress, inflammation or pollutant exposure. Thus, failure to detect acrolein-cysteine adducts does not rule out the possibility that formation of Michael addition adducts with cysteine residues can be and likely is the principal mechanism of acrolein adduct formation. Accordingly, novel approaches will be required in future studies to detect, isolate, quantify, and characterize such adduct formation in vitro and in vivo and then to explore potential mechanisms of their toxicity.
Acknowledgments
We thank Drs. Frederick Benz and Donald Nerland for helpful discussion and suggestions. This study is supported by NIH Grant P01 ES11860 (WMP/AB) and partially supported by University of Louisville, School of Medicine (WMP).
Abbreviations
- DTT
dithiothreitol
- SFB
succinimido-4-fluorobenzoate
- ACN
acetonitrile
- ESI/MS
electrospray ionization/mass spectrometry
- MS/MS
tandem mass spectrometry
- FDP-lysine
Nε-(3-formyl-3,4-dehydropiperidino)lysine
- MP-lysine
Nε-(3-methylpyridinium)lysine
References
- 1.Witz G. Biological interactions of alpha,beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 2.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4- hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez AM, Kozekov ID, Harris TM, Lloyd RS. Formation of inter- and intrastrand imine type DNA-DNA cross-links through secondary reactions of aldehydic adducts. Chem Res Toxicol. 2005;18:1683–1690. doi: 10.1021/tx0500528. [DOI] [PubMed] [Google Scholar]
- 5.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- 7.Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM. DNA interchain cross-links formed by acrolein and crotonaldehyde. J Am Chem Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez AM, Kozekov ID, Harris TM, Lloyd RS. Formation of inter- and intrastrand imine type DNA-DNA cross-links through secondary reactions of aldehydic adducts. Chem Res Toxicol. 2005;18:1683–1690. doi: 10.1021/tx0500528. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz AJ, Lloyd RS. 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J Biol Chem. 2003;278:5970–6. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 11.Furuhata A, Ishii T, Kumazawa S, Yamada T, Nakayama T, Uchida K. N(epsilon)-(3-methylpyridinium)lysine, a major antigenic adduct generated in acrolein-modified protein. J Biol Chem. 2003;278:48658–48665. doi: 10.1074/jbc.M309401200. [DOI] [PubMed] [Google Scholar]
- 12.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O’Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 2005;280:36386–36396. doi: 10.1074/jbc.M508169200. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 15.Seiner DR, Labutti JN, Gates KS. Kinetics and Mechanism of Protein Tyrosine Phosphatase 1B Inactivation by Acrolein. Chem Res Toxicol. 2007;20:1315–1320. doi: 10.1021/tx700213s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietzsch J, Bergmann R, Rode K, Hultsch C, Pawelke B, Wuest F, van den Hoff J. Fluorine-18 radiolabeling of low-density lipoproteins: a potential approach for characterization and differentiation of metabolism of native and oxidized low-density lipoproteins in vivo. Nucl Med Biol. 2004;31:1043–1050. doi: 10.1016/j.nucmedbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, Li F, Chen X. microPET of Tumor Integrin αvβ3 Expression Using 18F-Labeled PEGylated Tetrameric RGD Peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen JE, Vriend G. Optimizing the hydrogen-bond network in Poisson- Boltzmann equation-based pK(a) calculations. Proteins. 2001;43:403–412. doi: 10.1002/prot.1053. [DOI] [PubMed] [Google Scholar]
- 19.Higgins PJ, Bunn HF. Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1981;256:5204–5208. [PubMed] [Google Scholar]
- 20.Ramu K, Fraiser LH, Mamiya B, Ahmed T, Kehrer JP. Acrolein mercapturates: synthesis, characterization, and assessment of their role in the bladder toxicity of cyclophosphamide. Chem Res Toxicol. 1995;8:515–524. doi: 10.1021/tx00046a005. [DOI] [PubMed] [Google Scholar]