Abstract
Background
Cardiac dysfunction is associated with neuroanatomic and neuropsychological changes in aging adults with prevalent cardiovascular disease (CVD), theoretically because systemic hypoperfusion disrupts cerebral perfusion, contributing to subclinical brain injury. We hypothesized that cardiac function, as measured by cardiac index, would be associated with pre-clinical brain magnetic resonance imaging (MRI) and neuropsychological markers of ischemia and Alzheimer’s disease in the community.
Methods and Results
Brain MRI, cardiac MRI, neuropsychological, and laboratory data were collected on 1504 Framingham Offspring Cohort participants free from clinical stroke, transient ischemic attack, or dementia (61±9 years; 54% women). Neuropsychological and brain MRI variables were related to cardiac MRI-assessed cardiac index (cardiac output/body surface area). In multivariable-adjusted models, cardiac index was positively related to total brain volume (P=0.03) and information processing speed (P=0.02) and inversely related to lateral ventricular volume (P=0.048). When participants with clinically prevalent CVD were excluded, the relation between cardiac index and total brain volume remained (P=0.02). Post-hoc comparisons revealed that participants in the bottom cardiac index tertile (values<2.54) and middle cardiac index tertile (values between 2.54 and 2.92) had significantly lower brain volumes (P=0.04) than participants in the top cardiac index tertile (values>2.92).
Conclusions
Although observational data cannot establish causality, our findings are consistent with the hypothesis that decreasing cardiac function, even at normal cardiac index levels, is associated with accelerated brain aging.
Keywords: brain, cardiac output, epidemiology, imaging, neuropsychology
Cardiac function indices have been related to neuropsychological impairment1,2 and dementia3 among patients with severe cardiomyopathies. In the absence of end-stage heart disease, less is known about how subclinical cardiac dysfunction affects brain aging. Recent cross-sectional work in referral-based samples of cardiovascular patients free of end-stage heart disease indicates that reduced cardiac output is associated with executive dysfunction4 and increased white matter hyperintensities (WMH).5 These data suggest that even subtle cardiac dysfunction is related to central nervous system (CNS) injury.
The mechanism underlying an association between cardiac function and maladaptive brain aging remains unknown, but reduced systemic perfusion may impact cerebral perfusion homeostasis. Convention suggests auto-regulatory processes augment blood flow to the brain during acute periods of reduced cardiac function,6 but data indicate that auto-regulatory mechanisms are less effective with subclinical or chronic systemic flow reductions.7,8 Animal data demonstrate that lowering cardiac output directly reduces cerebral blood flow,7 and clinical studies indicate that cerebral blood flow increases by more than 50% following heart transplantation.8 Such reduced systemic blood flow appears to influence cerebral blood flow homeostasis and may contribute to subclinical brain injury.
Initial research in this area has been restricted to small, referral samples with clinical cardiovascular disease (CVD). These studies have not systematically adjusted for environmental risk factors known to contribute to both CNS and myocardial injury.1,2,4,5 The proposed cross-sectional investigation aims to enhance knowledge about relations between cardiac function and brain aging by extending prior work to a large, epidemiological cohort, using a more precise cardiac imaging method (MRI) with excellent reproducibility for quantifying cardiac function,9 and simultaneously considering shared risk factors for CNS and myocardial injury. Based on prior animal and clinical research, we hypothesized that MRI-assessed cardiac function is associated with cognitive and neuroimaging markers of pre-clinical Alzheimer’s disease10-13 (i.e., decreased learning/memory, brain volume, and hippocampal volume, and increased lateral ventricular volume) and cerebrovascular disease4,5,14,15 (i.e., decreased executive functioning/information processing, increased WMH) in a community-based cohort of adults free of clinical dementia or stroke.
Methods
Participants
The Framingham Offspring Study design and selection criteria have been described elsewhere.16 Briefly, from 1971-1975, 5124 participants were recruited and have been examined every 4 to 8 years since. The current sample was derived from 3539 participants attending the 7th examination cycle (1998-2001). Participants underwent physical examination, medical history, and laboratory assessment of cardiovascular risk factors. From April 1999 to August 2005, 2182 participants attending the 7th Offspring examination cycle consented to undergo an ancillary neuropsychological and brain MRI study. Those participants who refused or were unable to undergo brain MRI were older and less healthy than those who received brain MRI.17 Of the 2182 participants consenting to the ancillary study, 1537 also consented to undergo cardiac MRI between April 2002 and August 2006, 32 of which were excluded from the present study because of a neurological condition that could significantly alter the brain MRI measures (e.g., dementia, clinical stroke, multiple sclerosis). An additional participant was excluded for missing body surface area data (necessary for cardiac index calculation as described below), yielding 1504 participants for the current investigation. The study protocol was approved by the local Institutional Review Boards, and all participants provided written informed consent prior to assessments.
Clinical covariates were defined at the 7th examination cycle. Systolic blood pressure was the mean of two measurements obtained by a Framingham Heart Study (FHS) physician. Current cigarette smoking (i.e., yes/no within the year prior to examination 7) and medication use were ascertained by self-report. The definition of diabetes mellitus included previous or current fasting blood glucose ≥126 mg/dL or previous or current use of oral hypoglycemic or insulin. Information about prior CVD (i.e., coronary heart disease, heart failure, intermittent claudication – individuals with prevalent stroke were excluded) was obtained via medical histories and physical examinations conducted at the FHS, as well as hospitalization and personal physician records. A panel of three experienced investigators adjudicated CVD events using previously described criteria.18 APOE genotyping was determined by polymerase chain reaction amplification and restriction isotyping.19
Neuropsychological Protocol
As previously described, FHS participants completed a neuropsychological protocol that was in part based on measures administered to the Original Cohort over 25 years ago.20 In more recent cycle examinations, tests were added to assess cognitive domains not adequately captured by the original protocol.20 Measures included in the protocol have strong reliability and validity and are sensitive to cognitive functions mediated by both frontal-subcortical and cortical systems.21,22
For the present study, neuropsychological performances were adjusted for age and education, separately by sex, and these values were expressed in standard deviation units. Natural logarithmic transformations were applied to normalize raw scores with skewed distributions. Next, each variable was regressed onto age and education categories, and residuals from these regressions were standardized using a z-score transformation. Because the protocol is comprised of numerous tests measuring related cognitive domains, a factor analysis was conducted to define composites, using oblique rotation. The data reduction process yielded five neuropsychological factors, and z-score transformations were applied to each of the five factors. The five composites and corresponding tests are as follows: (1) Verbal Memory: Wechsler Memory Scale (WMS) Logical Memory Immediate and Delayed Recall;23 (2) Visuospatial Memory: WMS Visuospatial Memory Immediate and Delayed Recall;23 (3) Verbal Learning: Paired Associate Learning Immediate and Delayed;23 (4) Executive Functioning/Information Processing: Trail Making Test Parts A & B;24 (5) Language/Object Recognition: Hooper Visual Organization Test,25 Boston Naming Test,26 and Wechsler Adult Intelligence Scale-Revised Similarities Subtest.27
Brain MRI Acquisition
The FHS MRI acquisition protocol has been reported in detail elsewhere.17 Briefly, the majority of participants were imaged on a Siemens 1T MR machine using a T2-weighted double spin-echo coronal imaging sequence. Digital information was post-processed by a central laboratory blinded to demographic and clinical information. Quantification was performed with a custom-written computer program operating on a UNIX, Solaris platform. The semi-automated segmentation protocol for quantifying total cranial volume,28 total brain volume29 frontal lobar volume,17 lateral ventricular volume,17 hippocampal volume,30 and WMH29 has been described elsewhere, as has the inter-rater reliabilities for these methods.17,28,31,32 For the present study, intra- and inter-rater reliabilities were consistently above 0.90. Hippocampal data were available for a subset of the sample included in the current analyses (n=696 of 1504 participants).
Cardiac MRI (CMR) Acquisition
The FHS CMR protocol has been reported in detail elsewhere.33,34 Briefly, supine CMR imaging was performed using a Philips 1.5T MR scanner with a 5-element commercial cardiac array coil for radiofrequency signal reception. End-expiratory breath-hold, ECG-gated cine steady state free precession images were acquired in 2-chamber, 4-chamber, and contiguous short axis orientations (temporal resolution 39ms, repetition time=R-R interval, echo time 9ms, flip angle 30°, field of view 400mm, matrix size 208×256, slice thickness 10mm, gap=0). Steady state free precession is advantageous for quantifying cardiac function over conventional MRI sequences because it provides briefer acquisition time and enhanced signal-to-noise ratio.35,36 CMR data analysis was performed by an experienced reviewer blinded to clinical information using dedicated software (EasyVision 5.1, Philips Medical Systems). End-systolic phase was determined as the minimal cross-sectional area of a mid-ventricular slice. End-diastolic volume (EDV) and end-systolic volume (ESV) were computed by summation of disks method with cardiac output calculated as (EDV-ESV) × heart rate with values reflecting L/min. CMR methods yield more precise measurements of EDV, ESV, and stroke volume as compared to echocardiography, and cardiac output measurements are highly reproducible.9 Inter-rater reliability correlation coefficients for EDV and ESV are 0.95 and 0.92, respectively. Intra-observer coefficients of variation for EDV and ESV are 2.6% and 3.5%, respectively.37 Interobserver coefficients of variation for EDV and ESV are 3.5% and 4.8%, respectively.37
Statistical Analysis
To standardize cardiac output values across participants for analytical purposes, cardiac output was divided by body surface area to yield cardiac index measured in L/min/m2. Total brain volume, frontal lobe volume, lateral ventricular volume, hippocampal volume, and WMH were expressed as percent of total cranial volume to adjust for head size, yielding percentages for analyses. WMH was natural log-transformed to normalize its skewed distribution. All models included the following covariates, which were selected based on prior work:38,39 age, sex, systolic blood pressure, cigarette smoking status, diabetes mellitus, hypertension treatment, atrial fibrillation, and prevalent CVD.
Two primary analyses were conducted. First, multivariable-adjusted linear regressions were used to assess relations between cardiac index and each dependent brain MRI measure (WMH, total brain volume, frontal lobar volume, lateral ventricular volume, hippocampal volume) and the five neuropsychological composites (verbal memory, visuospatial memory, verbal learning, information processing/executive functioning, language/perception). Post-hoc tertile analyses were conducted for significant continuous cardiac index and brain aging relations. Second, to assess how low cardiac index relates to brain aging, multivariable-adjusted linear regression was used to assess relations between low cardiac index (i.e., defined40 as <2.5 L/min/m2; n=456) and brain aging markers.
Secondary analyses were performed: (1) excluding participants with prevalent CVD (n=112) and (2) using interaction terms to assess effect modification of relations by sex, age (<60 vs. ≥60 years), and APOE genotype status (ε4 negative vs. ε4 positive41), and then stratifying analyses by these subgroups. Significance was set at p<0.05 for all models and p<0.10 for analyses assessing effect modification. All data were analyzed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
Results
Participant Characteristics
Participant characteristics are provided in Table 1. The mean sample age was 61 years (34-84 years) and 54% were women. Low cardiac index (i.e., <2.5 L/min/m2)40 was present in 30% of the sample (n=457). After exclusion of individuals with prevalent CVD (n=112), low cardiac index was still present in 30% of the sample (n=415). Brain MRI mean and standard deviation data and neuropsychological median, minimum, and maximum data are provided in Table 2.
Table 1. Participant characteristics.
| Clinical Characteristics, units | n=1504 |
|---|---|
| Age at MRI, years | 61±9 |
| Sex, % female | 54 |
| Systolic blood pressure, mm Hg | 125±18 |
| Cigarette smoking, % | 10 |
| Diabetes, % | 9 |
| Atrial fibrillation, % | 3 |
| Hypertension treatment, % | 28 |
| Prevalent CVD, % | 7 |
| Heart failure, % | 6 |
| Coronary artery disease, % | 2 |
| Intermittent claudication, % | 1 |
| Imaging Characteristics, units | |
| Interval from Cycle Exam 7 to brain MRI, years | 0.68±0.65 |
| Interval from brain MRI to cardiac MRI, years | 3.70±1.10 |
| CMR cardiac index, L/min/m2 | 2.77±0.51 |
| Cardiac index tertiles, L/min/m2 | |
| Tertile 1 | <2.54 |
| Tertile 2 | 2.54 to 2.92 |
| Tertile 3 | >2.92 |
| Impaired cardiac index, % | 30 |
Note: Values denoted as percentages or mean±standard deviation; CVD does not include clinical stroke or transient ischemic attack; CVD categories are not mutually exclusive
Table 2. Brain aging descriptive data.
| Characteristic | n=1504 |
|---|---|
| Brain MRI Data (percentage of total cranial volume #) | Mean±SD |
| WMH | 0.09±0.21 |
| Total brain volume | 79.66±3.21 |
| Frontal lobar volume | 36.45±3.33 |
| Lateral ventricular volume | 1.75±1.07 |
| Hippocampal volume † | 0.32±0.05 |
|
| |
| Neuropsychological Data (total correct unless otherwise noted) |
Median
(minimum, maximum) |
| F1: Verbal Memory | |
| WMS Logical Memory Immediate Recall | 12 (2, 21) |
| WMS Logical Memory Delayed Recall | 11 (0, 21) |
| F2: Visuospatial Memory | |
| WMS Visual Reproduction Immediate Recall | 10 (0, 14) |
| WMS Visual Reproduction Delayed Recall | 9 (0, 14) |
| F3: Verbal Learning | |
| WMS Paired Associate Learning Immediate Recall | 14 (3, 21) |
| WMS Paired Associate Learning Delayed Recall | 9 (3, 10) |
| F4: Information Processing/Executive Function | |
| Trail Making Test A, time to completion (minutes) | 0.5 (0.2, 2.3) |
| Trail Making Test B, time to completion (minutes) | 1.2 (0.4, 5.0)* |
| F5: Language/Object Recognition | |
| Hooper Visual Organization Test | 26 (3, 30) |
| WAIS-R Similarities | 17 (2, 25) |
| Boston Naming Test 30-item | 28 (16, 30) |
Note: SD=standard deviation; WMH=white matter hyperintensities
=all brain MRI variables are adjusted for total cranial volume and presented as percentages; WMH is log-transformed
=analyses based on n=695
=higher values denote worse performance; WMS=Wechsler Memory Scale; WAIS-R =Wechsler Adult Intelligence Scale Revised
Cardiac index and brain MRI
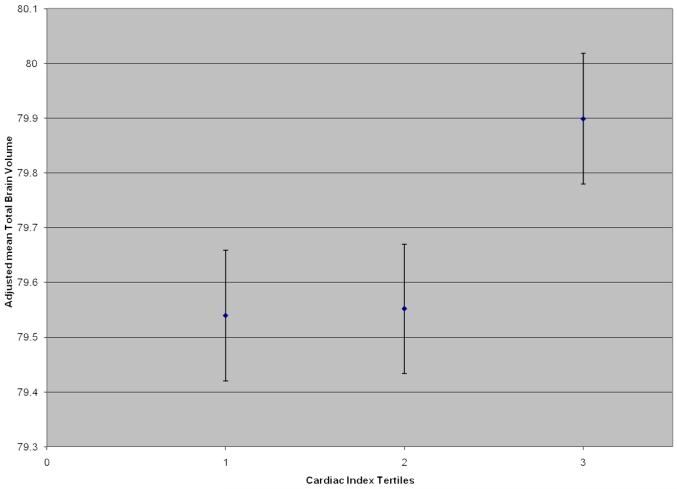
As a continuous measure, cardiac index was positively related to total brain volume (ß=0.30, P=0.03), such that an increase in one standard deviation of cardiac index resulted in an increase of total brain volume (as a percentage of total cranial volume) of 0.30. As a continuous measure, cardiac index was inversely related to lateral ventricular volume (ß=−0.10, P=0.048; i.e., as cardiac index increased one standard deviation, lateral ventricular volume decreased −0.10). In secondary analyses, when participants with clinically prevalent CVD were excluded from analyses, the significant relation between cardiac index and total brain volume remained (ß=0.33, P=0.02); however the relation between cardiac index and lateral ventricular volume was no longer significant (ß=−0.09, P=0.08). Post-hoc, cardiac index tertiles were compared to assess changes in brain volume. The top (or highest) tertile of cardiac index was used as the referent for analysis, as higher values of cardiac index reflect healthier cardiac function. Participants in the middle cardiac index tertile had an average total brain volume (as a ratio value adjusted for total cranial volume) of 0.35 less than those in the top quartile (β=−0.35, P=0.04). Similarly, participants in the bottom cardiac index tertile had a lower mean total brain volume of 0.36 (β=−0.36, P=0.04), as compared to participants in the top tertile, corresponding to an average difference of 1.9 years in brain aging (i.e., 0.19 decrease in total brain volume is observed per increased year in age). The Figure depicts the adjusted mean total brain volume with standard error bars across tertiles of cardiac index. Cardiac index was unrelated to the remaining brain MRI measures when treated as a continuous variable (all P-values>0.17).
Figure.
Mean total brain volume (with standard error bars) adjusted for age, sex, systolic blood pressure, cigarette smoking status, diabetes mellitus, hypertension treatment, atrial fibrillation, and prevalent CVD is depicted by tertile of cardiac index. Tertile 3 is significantly different from tertile 1 (P=0.04) and tertile 2 (P=0.04).
When low cardiac index was related as a dichotomous variable to the brain aging variables, no significant relations were observed between low cardiac index and total brain volume (ß=−0.18, P=0.24) or lateral ventricular volume (ß=0.02, P=0.68). Cardiac index was unrelated to the remaining brain MRI measures when dichotomized into normal and low subgroups (all P-values>0.10). See Table 3 for details on all statistical models relating cardiac index to total brain volume.
Table 3. Cardiac index and total brain volume results.
| Total Brain Volume |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample n=1504 |
Without Prevalent CVD n=1392 |
Analyses Stratified by Sex | Analyses Stratified by Age | |||||||||
| Female n=806 | Male n=698 | <60 years n=711 | ≥60 years n=793 | |||||||||
| β±SE | P | β±SE | P | β±SE | P | β±SE | P | β±SE | P | β±SE | P | |
| Cardiac index, continuous | 0.30±0.14 | 0.03 | 0.33±0.14 | 0.02 | 0.07±0.19 | 0.72 | 0.42±0.20 | 0.03 | 0.47±0.19 | 0.01 | 0.03±0.02 | 0.90 |
| Tertile 1 (bottom/low) | −0.36±0.17 | 0.04 | −0.41±0.17 | 0.02 | −0.26±0.23 | 0.25 | −0.35±0.25 | 0.17 | −0.51±0.24 | 0.03 | −0.16±0.24 | 0.50 |
| Tertile 2 | −0.35±0.17 | 0.04 | −0.34±0.17 | 0.04 | −0.32±0.23 | 0.17 | −0.17±0.24 | 0.47 | −0.19±0.22 | 0.40 | −0.35±0.25 | 0.15 |
| Tertile 3 (top/high) | Referent | Referent | Referent | Referent | Referent | Referent | ||||||
| Low cardiac index | −0.18±0.15 | 0.24 | −0.21±0.15 | 0.17 | −0.05±0.19 | 0.80 | −0.29±0.23 | 0.22 | −0.35±0.22 | 0.11 | −0.01±0.20 | 0.98 |
Note: Cardiac index=(cardiac output/body surface area) with values <2.5 L/min/m2 defined as low cardiac index; low cardiac index n=457; SE=standard error; Models adjusted for age, sex, systolic blood pressure, smoking status, diabetes mellitus, hypertension treatment, atrial fibrillation and prevalent CVD ; beta=change in brain volume as a function of a one standard deviation change in cardiac index; Cardiac index values for tertiles are: <2.54 L/min/m2 for tertile 1 (n=501), 2.54 to 2.92 L/min/m2 for tertile 2 (n=502), and >2.92 L/min/m2 for tertile 3 (n=501).
Cardiac function and neuropsychological variables
Cardiac index as a continuous variable was unrelated to any of the neuropsychological factor scores (all P>0.23). However, low cardiac index (i.e., <2.5L/min/m2) was borderline significantly related to the executive function/information processing factor (ß=−0.10, P=0.06). Post-hoc analyses isolating the individual neuropsychological tests comprising this factor score suggest that low cardiac index was significantly related to poorer performances on Trail Making Test Part A, assessing information processing speed (ß=−0.13, P=0.02). In secondary analyses, when participants with prevalent CVD were excluded, the borderline significant relation between low cardiac index and the information processing/executive function factor was attenuated (ß=−0.11, P=0.05).
Effect modification by age
There was a significant interaction between age and cardiac index in their relation to total brain volume (P=0.01), such that the magnitude of the association was stronger in the adults under 60 years of age as compared to the older adults (Table 3). No additional significant interactions were observed between age and cardiac index in their relation to the brain aging variables.
Effect modification by sex
There was a significant interaction between cardiac index and sex in their relation to total brain volume (P=0.03), such that the magnitude of the association was stronger for men than women (Table 3). No additional significant interactions were observed between sex and cardiac index in their relation to the brain aging variables.
Effect modification by APOE ε4 status
There was a possible interaction between low cardiac index and APOE status in their relation to the visuospatial memory factor (P=0.09), such that the magnitude of the association was stronger for ε4 positive carriers than ε4 negative carriers. No additional significant interactions were observed between APOE ε4 status and cardiac index in their relation to the brain aging variables.
Discussion
In this study of a community dwelling cohort of ambulatory adults, we found that cardiac index was associated with total brain volume and lateral ventricular volume, both markers of accelerated brain aging;17,42 however, when analyses excluded participants with prevalent CVD, only the association between cardiac index and total brain volume remained. The association was stronger for individuals less than 60 years of age, which coincides with a period in the lifespan with reduced risk for abnormal brain changes, possibly allowing the influence of cardiac index on brain health to be more prominent. Individuals in the top tertile of cardiac index had a higher mean total brain volume equivalent to nearly two years of healthy brain aging as compared to those participants in either the middle or bottom tertile of cardiac index. Though cardiac index as a continuous variable was unrelated to neuropsychological performances, when clinical cut-offs were applied, low cardiac index was related to information processing speed, a finding that was modestly attenuated when participants with prevalent CVD were excluded from analyses.
Collectively, these results suggest that even in the absence of prevalent CVD, cardiac index is related to total brain volume, a neuroimaging marker of brain health, and low cardiac index is related to information processing speed. Prior research relating cardiac output to neuroimaging and neuropsychological phenotypes of maladaptive brain aging in clinical cohorts have yielded significant associations.2,4,5 For instance, among patients with severe cardiomyopathies2 reduced cardiac output is related to cognitive impairment. Among elders with prevalent CVD, reduced cardiac output is related to both executive dysfunction4 and WMH.5
The current findings enhance prior research in two important ways. First, these data extend past reports by demonstrating that cardiac output is related to brain aging markers in the absence of clinical CVD in a community-based cohort. Second, based on the cohort under investigation, the point at which cardiac index is significantly related to abnormal brain health appears to differ from the clinical threshold for abnormal cardiac function (i.e., a cardiac index value <2.5 is often used to define impaired cardiac function40). The Figure illustrates that the level at which cardiac index is associated with differences in brain health (defined as total brain volume) appears to be higher than 2.5 (i.e., closer to 2.9), suggesting that a range of normal cardiac index values (i.e., 2.5-2.9) may be related to compromised brain health integrity. These findings require further study, but if replicated, such results may have significant clinical implications, including the early identification of individuals with low (<2.5) or low normal (2.5-2.9) cardiac index for treatment to prevent abnormal brain changes.
The mechanism accounting for associations between cardiac index and markers of brain aging is unknown; however, reduced systemic blood flow may contribute to subclinical brain injury because of its impact on cerebral blood flow homeostasis.43,44 Despite auto-regulatory mechanisms to preserve blood flow to the brain, research in macaque monkeys has demonstrated that lowering systemic blood flow via reduced cardiac output directly reduces cerebral perfusion.7 Similar findings have been reported in heart transplant candidates with severe cardiomyopathies, such that cerebral blood flow values return to healthy levels following restoration of cardiac function.45 Alterations in cerebral blood flow homeostasis can contribute to clinical or subclinical brain injury by propagating or exacerbating microvascular damage or Alzheimer’s disease neuropathology. For instance, alterations in cerebral perfusion lead to microvessel structure changes, expression of vascular cell receptors, alterations in microvessel permeability, and vascular remodeling.46,47 Furthermore, rats develop Alzheimer’s disease-related neuropathology following acute cessation of blood flow, including diffuse beta-amyloid peptide and amyloid precursor protein expression in the hippocampus, entorhinal cortex, and neocortex.48 In transgenic mouse models of Alzheimer’s disease, chronic cerebral hypoperfusion places the brain at risk for amyloid deposition, resulting in neuronal death.49 Thus, reductions in cardiac function and systemic blood flow may lead to subclinical or clinical brain injury by affecting cerebral blood flow homeostasis. Future studies are needed to understand the mechanism(s) accounting for the preliminary epidemiological associations reported here and clinical findings reported elsewhere.2,4,5,44
Beyond the primary findings described above, an unanticipated finding was the number of community-dwelling adults with cardiac index values below standard clinical cut-off criteria. That is, approximately 30% of participants had low resting cardiac index (i.e., values <2.5 L/min/m2).40 When individuals with prevalent CVD were excluded, 30% of participants still had resting cardiac index values below 2.5 L/min/m2. In light of the current observation that cardiac index is associated with cross-sectional markers of accelerated brain aging, such a high proportion of cardiac index values below clinical criteria warrants further investigation.
Our study has a number of strengths, including the large community-based cohort free of clinical dementia and stroke, comprehensive ascertainment of potential confounding variables, an innovative and precise cardiac imaging technique, stringent quality control procedures for measurement of cardiac and brain MRI, and core reading laboratory for processing measurements. However, the present findings must be tempered by several caveats. First, multiple comparisons were made, raising the possibility of a false positive finding. Next, the age and racial makeup of the Framingham Offspring Study is predominantly white, of European descent, and middle-aged to elderly, so the generalizability to other races, ethnicities, and age-groups is unknown. The exclusion of institutionalized individuals and participants with clinical stroke and the inclusion of individuals willing to undergo MRI yielded a generally healthy sample, thereby reducing the likelihood of finding relations that may be present in the general population that includes individuals with cognitive impairment or stroke. The lack of an association between cardiac index and most of the neuropsychological measures may be due to inadequate power. Similarly, the smaller dataset available for analyses relating cardiac index to hippocampal volume may have been insufficiently powered. The observational design limits inferences about causality. The brain MRI data were temporally acquired before the cardiac MRI data, which limits interpretation of directionality. Finally, the cross-sectional design increases the likelihood of residual confounding despite attempts to thoroughly adjust for confounders, including current systolic blood pressure and hypertension medication use, in analytical models. Our results are preliminary and our findings require replication in other samples.
In summary, cardiac index is associated with brain volume, even in individuals without diagnosed prevalent CVD. Though our analyses are based on an observational design and we are unable to establish a causal relation or temporality for the associations observed, we propose that subtle reductions in cardiac index, as well as cardiac index values in the low end of the normal range, may be implicated in accelerating age-related changes in the brain. However, we cannot rule out the possibility that the findings are due to some epiphenomenon. Further investigation into the mechanisms and clinical significance of the association between cardiac index and brain aging is merited.
Acknowledgments
Funding Sources: Research supported by the National Heart Lung Blood Institute’s Framingham Heart Study HC25195; AG027480, AG030962, Paul B. Beeson Career Development Award in Aging Program, Alzheimer’s Association IIRG-08-88733 (ALJ); P30-AG013846 (Boston University Alzheimer’s Disease Core Center); AG08122, NS017950, AG033040, AG16495 (PAW); AG028321 and HL076784 (EJB); AG033193, AG031287 (SS); AG021028 (CD), AG010129 (University of California at Davis Alzheimer’s Disease Core Center); HL070279 (WJM).
Footnotes
Disclosures: The authors have reported no conflicts of interest
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Putzke JD, Williams MA, Rayburn BK, Kirklin JK, Boll TJ. The relationship between cardiac function and neuropsychological status among heart transplant candidates. Journal of Cardiac Failure. 1998;4:295–303. doi: 10.1016/s1071-9164(98)90235-4. [DOI] [PubMed] [Google Scholar]
- 3.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Archives of Internal Medicine. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. Journal of the American Geriatrics Society. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena PR, Schoemaker RG. Organ blood flow protection in hypertension and congestive heart failure. The American Journal of Medicine. 1993;94:4S–12S. [PubMed] [Google Scholar]
- 7.Tranmer BI, Keller TS, Kindt GW, Archer D. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. Journal of Neurosurgery. 1992;77:253–259. doi: 10.3171/jns.1992.77.2.0253. [DOI] [PubMed] [Google Scholar]
- 8.Massaro AR, Dutra AP, Almeida DR, Diniz RV, Malheiros SM. Transcranial Doppler assessment of cerebral blood flow: effect of cardiac transplantation. Neurology. 2006;66:124–126. doi: 10.1212/01.wnl.0000191397.57244.91. [DOI] [PubMed] [Google Scholar]
- 9.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. American Journal of Cardiology. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 10.Cahn DA, Sullivan EV, Shear PK, Marsh L, Fama R, Lim KO, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Structural MRI correlates of recognition memory in Alzheimer’s disease. Journal of the International Neuropsychological Society. 1998;4:106–114. doi: 10.1017/s1355617798001064. [DOI] [PubMed] [Google Scholar]
- 11.Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ., Sr. SPECT and MRI analysis in Alzheimer’s disease: relation to apolipoprotein E epsilon 4 allele. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White KG, Ruske AC. Memory deficits in Alzheimer’s disease: the encoding hypothesis and cholinergic function. Psychonomic Bulletin & Review. 2002;9:426–437. doi: 10.3758/bf03196301. [DOI] [PubMed] [Google Scholar]
- 13.Giesel FL, Hahn HK, Thomann PA, Widjaja E, Wignall E, von Tengg-Kobligk H, Pantel J, Griffiths PD, Peitgen HO, Schroder J, Essig M. Temporal horn index and volume of medial temporal lobe atrophy using a new semiautomated method for rapid and precise assessment. American Journal of Neuroradiology. 2006;27:1454–1458. [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Experimental Neurology. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 17.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 34. National Heart, Lung, and Blood Institute; Bethesda, MD: 1987. [Google Scholar]
- 19.Hixon JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- 20.Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, Beiser A, D’Agostino RB. New norms for a new generation: cognitive performance in the framingham offspring cohort. Experimental Aging Research. 2004;30:333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 21.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford University Press; New York: 2004. [Google Scholar]
- 22.Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- 23.Wechsler D. A standardized memory scale for clinical use. Journal of Psychology. 1945;19:87–95. [Google Scholar]
- 24.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 25.Hooper H. Hooper Visual Organization Test (HVOT) Western Psychological Services; Los Angeles: 1983. [Google Scholar]
- 26.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. Psychological Corporation; New York: 1981. [Google Scholar]
- 28.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. Journal of Computer Assisted Tomography. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 29.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 30.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, Yoshita M, Rosenberg IH, D’Agostino RB, DeCarli C. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65:642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeCarli C, Murphy DG, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B. Lack of age-related differences in temporal lobe volume of very healthy adults. American Journal of Neuroradiology. 1994;15:689–696. [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Archives of General Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 33.Jaffer FA, O’Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, Salton C, Botnar RM, Levy D, Manning WJ. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:849–854. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]
- 34.Salton CJ, Chuang ML, O’Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. Journal of the American College of Cardiology. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 35.Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology. 2001;219:828–834. doi: 10.1148/radiology.219.3.r01jn44828. [DOI] [PubMed] [Google Scholar]
- 36.Fieno DS, Jaffe WC, Simonetti OP, Judd RM, Finn JP. TrueFISP: assessment of accuracy for measurement of left ventricular mass in an animal model. Journal of Magnetic Resonance Imaging. 2002;15:526–531. doi: 10.1002/jmri.10107. [DOI] [PubMed] [Google Scholar]
- 37.Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. Journal of the American College of Cardiology. 2000;35:477–484. doi: 10.1016/s0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 38.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 39.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, D’Agostino RB, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 40.Givertz MM, Colucci WS, LeJemtel TH, Gottlieb SS, Hare JM, Slawsky MT, Leier CV, Loh E, Nicklas JM, Lewis BE. Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation. 2000;101:2922–2927. doi: 10.1161/01.cir.101.25.2922. [DOI] [PubMed] [Google Scholar]
- 41.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson AL, Massaro JM, Larson MG, Wolf PA, Au R, D’Agostino RB, Seshadri S, Lipinska I, Meigs JB, Keaney JF, Jr., Vasan RS, Beiser A, Benjamin EJ, DeCarli C. Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefferson AL, Benjamin EJ. Cardiovascular disease, cognitive decline, and dementia. In: Wahlund LO, Erkinjuntti T, Gauthier S, editors. Vascular cognitive impairment in clinical practice. Cambridge University Press; Cambridge, UK: 2009. pp. 166–177. [Google Scholar]
- 44.Jefferson AL, Holland CM, Tate DF, Poppas A, Csapo I, Cohen RA, Guttman CRG. Atlas-derived perfusion correlates of white matter hyperintensities in patients with reduced cardiac output. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2009.01.011. In press. http://www.sciencedirect.com/dx.doi.org/10.1016/j.neurobiolaging.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 46.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. Journal of Cerebral Blood Flow & Metabolism. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T, Deguchi K, Nagotani S, Zhang H, Sehara Y, Tsuchiya A, Abe K. Cerebral ischemia and angiogenesis. Current Neurovascular Research. 2006;3:119–129. doi: 10.2174/156720206776875902. [DOI] [PubMed] [Google Scholar]
- 48.Pluta R. The role of apolipoprotein E in the deposition of beta-amyloid peptide during ischemia-reperfusion brain injury. A model of early Alzheimer’s disease. Annals of the New York Academy of Sciences. 2000;903:324–334. doi: 10.1111/j.1749-6632.2000.tb06383.x. [DOI] [PubMed] [Google Scholar]
- 49.Aliev G, Smith MA, de la Torre JC, Perry G. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer’s disease. Mitochondrion. 2004;4:649–663. doi: 10.1016/j.mito.2004.07.018. [DOI] [PubMed] [Google Scholar]