Abstract
Unidirectional proton transport in bacteriorhodopsin is enforced by the switching machinery of the active site. Threonine 89 is located in this region, with its O—H group forming a hydrogen bond with Asp-85, the acceptor for proton transfer from the Schiff base of the retinal chromophore. Previous IR spectroscopy of [3-18O]threonine-labeled bacteriorhodopsin showed that the hydrogen bond of the O—D group of Thr-89 in D2O is strengthened in the K photocycle intermediate. Here, we show that the strength and orientation of this hydrogen bond remains unchanged in the L intermediate and through the M intermediate. Furthermore, a strong interaction between Asp-85 and the O—H (O—D) group of Thr-89 in M is indicated by a shift in the C⩵O stretching vibration of the former because of 18O substitution in the latter. Thus, the strong hydrogen bond between Asp-85 and Thr-89 in K persists through M, contrary to structural models based on x-ray crystallography of the photocycle intermediates. We propose that, upon photoisomerization of the chromophore, Thr-89 forms a tight, persistent complex with one of the side-chain oxygens of Asp-85 and is thereby precluded from participating in the switching process. On the other hand, the loss of hydrogen bonding at the other oxygen of Asp-85 in M may be related to the switching event.
Bacteriorhodopsin (BR), a membrane protein found in Halobacterium salinarum, is a light-driven proton pump with a retinal chromophore (1–3). The retinal binds covalently to Lys-216 through a protonated Schiff base. Absorption of light by the all-trans form of the chromophore triggers a cyclic reaction that comprises a series of intermediates, designated as the J, K, L, M, N, and O states (1–3). Structural changes in these intermediate states cause proton translocation across the membrane. The proton is translocated via Asp-96, the Schiff base, Asp-85, and Glu-204 (or the Glu-204–Glu-194 region) (2, 3).
Unidirectional proton transport in BR is enforced by a switch in the connectivity of the Schiff base, although little is understood of the mechanism. Mutation studies have provided important information. It is particularly noted that the proton pumping ability is retained upon replacement of Asp-96 and Glu-204 with neutral residues (4). Therefore, even though protons are transferred to and from Asp-96 and Glu-204 (or a release group containing Glu-204) during the photocycle (2, 3), Asp-96 and Glu-204 cannot be actively involved in the switch (5). Thus, it is likely that the switch mechanism is rather local.
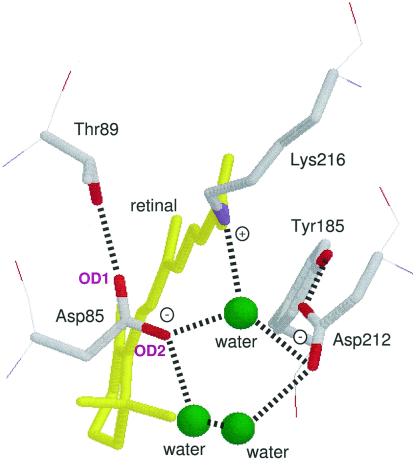
Recent structural studies of BR by electron cryo-microscopy (6, 7) and x-ray crystallography (8–11) motivate renewed spectroscopic studies of the proton pump mechanism in BR. In particular, improved crystallographic resolution has allowed modeling of internal water molecules (10, 11), which had previously been extensively studied by Fourier-transform IR (FTIR) spectroscopy (5, 12). The structural models of BR show that two negatively charged carboxylates, Asp-85 and Asp-212, are located at similar distances from the retinal Schiff base (Fig. 1) (6–11). It is thus an interesting and important question why the Schiff base proton is transferred only to Asp-85. The structures also show that the Schiff base is connected to Asp-85 and Asp-212 through hydrogen bonds of a water molecule. In each case, the aspartate oxygen is also hydrogen-bonded to another water molecule (Fig. 1). There are additional hydrogen bonds for the other oxygen of each of the aspartates; one is between Asp-85 and the O—H group of Thr-89, whereas the other is between Asp-212 and the O—H group of Tyr-185. These hydrogen bonds must play important roles in the switch mechanism.
Figure 1.
Structure of the Schiff base region in BR. This is the side view of Protein Data Bank structure 1C3W (10), which has a resolution of 1.55 Å. The membrane normal is approximately in the vertical direction of this figure. Dotted lines represent supposed hydrogen bonds. The O—H group of Thr-89 forms a hydrogen bond with an oxygen atom of the side chain of Asp-85 (OD1), whereas two water molecules are within the hydrogen bonding distance of the other oxygen (OD2).
Recently, low-temperature x-ray diffraction structures also have been reported for the K (13), L (14), and M intermediates (15–17). Low-temperature and time-resolved x-ray crystallography have been regarded as “ultimate tools” for photoreceptive proteins, such as BR (13–17) and photoactive yellow protein (18, 19), because they can provide “snapshots” of the protein in action. However, the switching machinery can be highly refined, and the current limited accuracy in determining atomic positions makes it difficult to locate hydrogen bonds. In addition, in the case of photoactive yellow protein, the local structural change reported by time-resolved x-ray crystallography (19) has been questioned by spectroscopic measurements (20, 21), suggesting that the photocycle in crystals differs from that in solution. Thus, both spectroscopic and diffraction studies are required for better understanding of the molecular mechanisms.
Polarized FTIR spectroscopy can characterize hydrogen bonds in terms of both vibrational frequencies and dipole orientations (22–24). In BR specifically, our highly accurate measurement of K minus BR difference spectra throughout the whole mid-IR (4,000–700 cm−1) region provided direct assessment of hydrogen bonds by probing O—H and N—H stretching vibrations (25). Furthermore, combined studies of isotope-labeled and mutant BR can lead to identification of particular vibrational bands. In fact, by use of 3-18O-threonine-labeled BR and mutation of individual threonine residues, we showed that among 18 threonines in BR, only three, Thr-89 (26), Thr-17, and Thr-121 (or Thr-90) (27), change their hydrogen bonds in the K intermediate.
For Thr-89 specifically, our previous study of the K minus BR IR spectra of 3-18O-threonine-labeled BR showed an H—D exchangeable O—H group. In BR, the relatively low O—D stretching frequency (2,506 cm−1) indicates a strong hydrogen bond (26). In K, a still lower stretching frequency (2,466 cm−1) corresponds to an even stronger hydrogen bond. In fact, an extraordinarily strong hydrogen bond is indicated in K because the frequency is lower than seen in neat secondary alcohols (26, 28). Given this unusual interaction, it was expected that the relaxation of K would be accompanied by weakening of the hydrogen bond of the O—H group of Thr-89 (26). Indeed, in an FTIR study of the T89C mutant, with a cysteine S—H as a hydrogen-bonding probe (29), the S—H stretching vibrations showed that the hydrogen bond of the S—H group at position 89 was very strong in BR, further strengthened in K, and lost in M. A broken hydrogen bond in M is also supported by x-ray crystallographic results for the M intermediate (17). However, detailed structural changes in photocycle intermediates have to be examined by polarized FTIR spectroscopy of the wild type.
In the present study, we extended highly accurate measurements of 3-18O-threonine-labeled BR to the intermediates, L and M. L is (i) the relaxation product of the K state and (ii) the precursor for the primary proton transfer. M includes all of the intermediates with a deprotonated Schiff base, before and after the switch. However, the M intermediate that we observe at 230 K corresponds to the late M after the switch because it is in the equilibrium with the subsequent N intermediate (24). The results are unexpected: the strengthened hydrogen bond in K persists in L and through M, in contrast to the behavior seen in the FTIR study of T89C and x-ray crystallography of the M intermediate. Moreover, the observation of an isotope effect of [3-18O]threonine on the C⩵O stretch of Asp-85 strongly suggests that the protonated oxygen of Asp-85 is the hydrogen bond acceptor for Thr-89 in the M state. On the basis of the present results, we propose a cluster model of the Asp-85–Thr-89 region from BR to M.
Materials and Methods
Samples were prepared as described (26). Polarized FTIR spectroscopy was applied as described (23–25). A 120-μl aliquot of the sample in 2 mM phosphate buffer (pH 7) or 2 mM borate buffer (pH 10) for the L minus BR or M minus BR spectrum, respectively, was dried on a BaF2 window with a diameter of 18 mm. After hydration by 1 μl of D2O or H2O, the sample was placed in a cell and then mounted in an Oxford DN-1704 cryostat, oriented at various tilt angles (0°, 17.8°, 35.7°, and 53.5°). The film was illuminated with >500 nm of light for 1 min at 273 K to obtain the light-adapted state of BR.
Further illumination with >600 nm of light at 170 K for 1 min converted BR to the L intermediate. Subsequent measurements were done after warming up to 273 K to relax the L intermediate back to the original BR state. On the other hand, illumination with >500 nm of light at 230 K for 1 min converted BR to the M intermediate. Because the M intermediate reverted to BR upon illumination with 420 nm of light (by use of interference filter) for 1 min, as evidenced by the same but inverted spectral shape, cycles of alternating illumination with >500 nm of light and 420 nm of light were repeated a number of times. The difference spectrum was calculated from spectra constructed with 128 interferograms before and after the illumination. The L minus BR spectrum is the average of 3–8 measurements, whereas the M minus BR spectrum is the average of 24 measurements.
Results
Assignment of the O—D Stretch Bands to Threonine Side Chains in L and M.
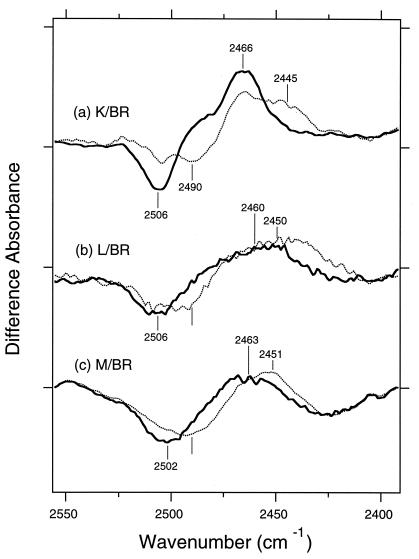
The solid and dotted lines in Fig. 2a show the K minus BR difference spectra in D2O of the unlabeled and 3-18O-threonine-labeled BR, respectively. The window-tilting angles were at 53.5°, which reproduced the previous ones (26). The sharp peak-pair at 2,506 cm−1 (−) and 2,466 cm−1 (+) was thus assigned to the O—D stretching vibration of a threonine in BR and K, respectively. The subsequent mutation study revealed that these bands originate from Thr-89 (26). Because the O—D stretches of neat secondary alcohols, model systems of threonine, are located at 2,680 to 2,480 cm−1 (26), this result indicates that the O—D group of Thr-89 is strongly hydrogen-bonded in BR, and the hydrogen bond is further strengthened upon K formation. The lower frequency (2,466 cm−1) than those of neat secondary alcohols implies that the hydrogen bond of the O—D group is very strong in K. The result is consistent with the result for the S—H group of Cys-89 in the K intermediate of the T89C mutant (29). The angles of the dipole moments of the O—D stretches relative to the membrane normal were calculated to be 21° in BR and 29° in K (26).
Figure 2.
The K minus BR (a), L minus BR (b), and M minus BR (c) difference spectra of unlabeled (thick solid lines) and 3-18O-threonine-labeled (dotted lines) BR in the 2,555–2,390 cm−1 region. The sample was hydrated with D2O, and the window tilting angles are 53.5°. These spectra were normalized at the negative 1,202-cm−1 band, indicating that the same numbers of BR molecules are converted to intermediates. One division of the y axis corresponds to 0.002 absorbance unit.
Fig. 2b shows L minus BR spectra. The solid line shows a broader feature than that of the K minus BR spectrum (Fig. 2a), but there is a similar peak-pair at 2,506 cm−1 (−) and 2,460 cm−1 (+). Both the positive and negative bands exhibit isotope shifts in the 3-18O-threonine-labeled BR (dotted line in Fig. 2b), indicating that the peak-pair originates from the O—D stretch of threonine. Fig. 2c shows M minus BR spectra. The solid line shows a peak-pair at 2,502 cm−1 (−) and 2,463 cm−1 (+) that is similar to those in the K minus BR and the L minus BR spectra. Again, both the positive and negative bands exhibit isotope shifts in the 3-18O-threonine-labeled BR (dotted line in Fig. 2c), indicating that the peak-pair originates from the O—D stretch of threonine.
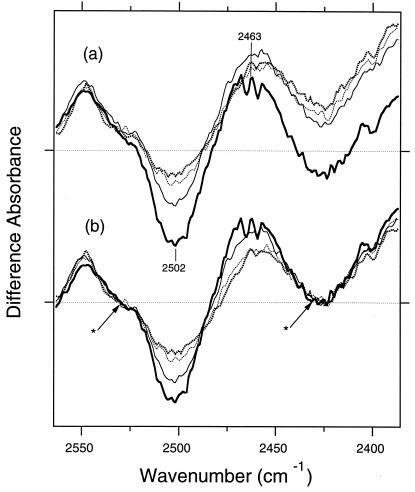
The fact that the 2,506 (−)/2,466 (+) cm−1 bands of Thr-89 in the K minus BR spectrum also are seen in the L minus BR and M minus BR spectra suggests that the bands in L and M also originate from the O—D stretch of Thr-89. Although a common band does not necessarily indicate the same origin, it is supported by the results of dichroic measurements. The tilt-angle dependence of the L minus BR and M minus BR spectra was similar to that of the K minus BR spectra. Fig. 3a shows the M minus BR spectra of unlabeled BR at various tilting angles. Because other spectral components highly overlap the threonine bands, we attempted to isolate them as follows. Fig. 2c shows that the threonine bands that shift upon 18O labeling lie between 2,530 and 2,430 cm−1. Therefore, we subtract a straight line from each spectrum in Fig. 3a so that the absorbance of spectrum is reduced to zero at 2,530 and 2,430 cm−1. Such normalized spectra (Fig. 3b) indicate that both positive and negative bands increase upon tilting of the sample window. This tendency, also seen in the L minus BR spectra (data not shown), is similar to that in the K minus BR spectra (26). Thus, the angle of dipole moment of the O—D group relative to the membrane normal is considerably smaller than the magic angle (54.8°) throughout K, L, and M, as well as BR (26). The similar frequencies and angles strongly suggest that the threonine band in Fig. 2 originates from Thr-89. Next, we tested this tentative assignment by use of mutants as we did previously (26, 27).
Figure 3.
The M minus BR difference spectra in the 2,565–2,385 cm−1 region. The sample was hydrated with D2O. The window tilting angles are 0° (thick dotted lines), 17.8° (thin dotted lines), 35.7° (thin solid line), and 53.5° (thick solid line). One division of y axis corresponds to 0.001 absorbance unit. The raw spectra are shown in a, whereas b shows spectra normalized so that in each case the absorbance at 2,530 and 2,430 cm−1 (asterisks) was reduced to zero.
Location of the Threonine Side Chain in L and M at Position 89 by Use of Mutants.
In the present study, we tested four mutants: two are at position 89 (T89A and T89S) and two are at other locations (T46V and T205V). Spectral analysis of these mutants in the low-frequency region (1,820–750 cm−1) showed that L and M are formed as in the wild type (data not shown). In the low-frequency region, the L minus BR and M minus BR spectra of T205V were identical to those of the wild type, whereas there are some alterations in T46V. The L minus BR and M minus BR spectra of T89S were closer to those of the wild type than those of T89A. This fact suggests that in L and M, as in K (26), the O—H group of Ser-89 in T89S plays an important role similar to that of the O—H group of Thr-89 in the wild type.
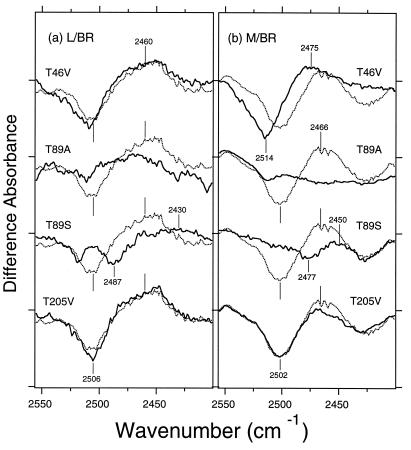
Fig. 4 compares the L minus BR (a) and M minus BR (b) spectra of T46V, T89A, T89S, and T205V (solid lines) with that of the wild type (dotted lines) in the 2,555–2,400 cm−1 region. Both the negative 2,506-cm−1 and positive 2,460-cm−1 bands are clearly present in the L minus BR spectra of T46V and T205V, as in the wild type (dotted line) (Fig. 4a). In contrast, neither the negative 2,506-cm−1 band nor the positive 2,460-cm−1 band was observed for the mutants of Thr-89 [T89A (b) and T89S (c)]. These results strongly suggest that the O—D stretching vibration of threonine in the L minus BR spectrum originates from Thr-89. The O—D stretches of serine in T89S are at 2,481 cm−1 and 2,430 cm−1 for BR and the K intermediate, respectively (26). A similar spectral feature in L (negative 2,487-cm−1 and positive 2,430-cm−1 bands) also supports the conclusion that the bands in question (Fig. 2b) originate from Thr-89. Thus, we conclude that the 2,506 (−)/2,460 (+) cm−1 bands in the L minus BR spectrum originate from Thr-89. This means that the strong hydrogen bond of the O—D group of Thr-89 in K is not relaxed in L, but persists in L.
Figure 4.
The L minus BR (a) and M minus BR (b) difference spectra in the 2,555–2,400 cm−1 region of the wild-type (dotted lines) and the indicated mutant (solid lines) proteins. The sample was hydrated with D2O, and the window tilting angles are 53.5°. One division of y axis corresponds to 0.0015 absorbance unit.
Fig. 4b shows that in M minus BR, both the negative 2,502-cm−1 and positive 2,466-cm−1 bands are identical in T205V to those of the wild type (dotted line). In T46V, spectral upshift of the 2,502 (−)/2,466 (+) cm−1 bands was observed to 2,514 (−)/2,475 (+) cm−1, suggesting that the 2,502 (−)/2,466 (+) cm−1 bands do not originate from Thr-46, although the mutation affects the frequency of the O—D stretch of threonine in question. In contrast, the negative band at 2,506 cm−1 is identical in T46V to the wild type in the case of the K minus BR (26) and L minus BR (Fig. 4a) spectra. Thus, the O—D frequency in T46V is identical to that of the wild type in BR, K, and L, whereas significant upshift occurs in M. In T89A, the 2,502 (−)/2,466 (+) cm−1 band is diminished, suggesting that the bands originate from Thr-89, as in K and L. The small 2,477 (−)/2,450 (+) cm−1 bands in T89S also support the assignment of the O—D stretch to position 89, whose features were also observed in K (26) and L (Fig. 4a). Thus, the mutation study suggested that the threonine residue in question (the 2,506 (−)/2,466 (+) cm−1 bands) is Thr-89 in both the L and M intermediates (Fig. 4). Because the frequency of the intermediates (2,466 cm−1) is lower than seen in neat secondary alcohols (26, 28), it is concluded that the strong hydrogen bond in K persists in L and M.
It is surprising that the strong hydrogen bonding structure of Thr-89 formed after photoisomerization does not change even in the M that is formed after the switch in the connectivity of the Schiff base from the extracellular side of the proton transport pathway to the intracellular side. The similar orientation of the dipole moment of the O—D stretch relative to the membrane normal in K, L, and M, in addition to the identical frequencies, strongly suggests that the hydrogen bonding interaction is not changed in the transitions between these states. In particular, the results suggest that the hydrogen bond acceptor for the O—H (O—D) of Thr-89 remains the side-chain oxygen of Asp-85. However, it is noted that the present experiment does not identify the hydrogen bonding acceptor. Structural changes during the photocycle may lead to new hydrogen bonding partners. Therefore, we should be careful in the assignment of the vibrational bands. More direct experimental evidence on the hydrogen bonding acceptor thus is required.
Isotope Effect of the Threonine Label on the C⩵O Stretching Vibration of Asp-85.
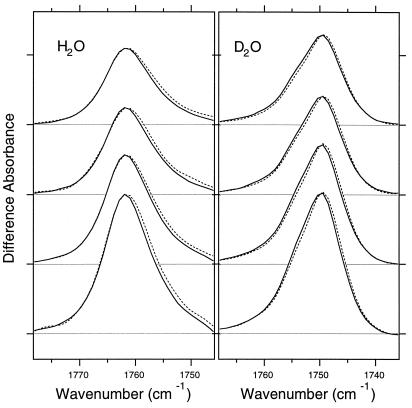
Fig. 5 shows the M minus BR spectra of the unlabeled (solid line) and 3-18O-threonine-labeled (dotted line) BR in the frequency region of the C⩵O stretch of protonated carboxylic acids. Strong positive bands originate from C⩵O stretching vibration of Asp-85 because of protonation in the transition from L to M (30). A high frequency such as 1,762 cm−1 indicates that the C⩵O group is free from hydrogen bonding (31). In H2O, the higher frequency side of the peak (1,775–1,762 cm−1) is identical between labeled and unlabeled BR, whereas 3-18O-threonine-labeled BR exhibits a shift to lower frequency on the low-frequency side of the peak (1,762–1,750 cm−1). The net effect is a broader band in 3-18O-threonine-labeled BR than in unlabeled BR. In addition, both the peak and centered wave numbers are lowered in 3-18O-threonine-labeled BR. The isotope effect is more clearly observed in D2O (Fig. 5 Right), in which the spectral width was almost identical, but entire bands are clearly downshifted in 3-18O-threonine-labeled BR. Such an isotope effect is not observed for the vibrational bands of the chromophore (C⩵C and C⩵C stretching, and hydrogen out-of-plane vibrations), peptide backbone (amide-A vibration, 3,400–3,200 cm−1; amide-I vibration, 1,700–1,600 cm−1), and water molecules (2,750–2,600 cm−1) (data not shown). Thus, the isotope effect of threonine on the C⩵O stretch of Asp-85 is highly specific, implying that the C⩵O group of Asp-85 is vibrationally coupled with a threonine side chain.
Figure 5.
The M minus BR difference spectra of unlabeled (solid lines) and 3-18O-threonine-labeled (dotted lines) BR in the 1,780–1,730 cm−1 region, typical frequencies of the C=O stretching vibration of protonated carboxylic acids. The sample was hydrated with H2O (Left) and D2O (Right), and the window was tilted at 0°, 17.8°, 35.7°, and 53.5° (from top to bottom). One division of y axis corresponds to 0.002 absorbance unit.
If the C⩵O group of the protonated Asp-85 in M were directly hydrogen bonded with the O—H group of threonine, its C⩵O stretching vibration would be expected to appear at low frequency. However, the frequency at 1,762 cm−1 indicates that the C⩵O group is free from hydrogen bonding, and possibly present in a hydrophobic environment (31). This means that the C⩵O group of Asp-85 cannot be the hydrogen bonding acceptor of the threonine. However, the absence of an isotope effect on the vibrations of peptide backbone and water molecules suggests that the isotope effect of threonine on the C⩵O stretching vibration of Asp-85 is through direct hydrogen bonding interaction. This suggests that it is the protonated oxygen atom of Asp-85 that engages in the direct hydrogen bond with Thr-89.
The isotope effect of the threonine side chain on the C⩵O stretching vibration of Asp-85, which is not seen in the vibrations of the chromophore, peptide backbone, or water molecules, implies that Asp-85 strongly interacts with a threonine side chain. The partner is likely to be Thr-89, because structural studies (6–11) show that the Thr-89 and Asp-85 side chains are hydrogen bonded in BR, with the oxygens 2.7 Å apart (10). The oxygens of the other threonine side chains are all more than 7 Å from the side-chain oxygens of Asp-85 in BR. According to the structure of M in the wild type (17), the nearest threonine oxygen from side-chain oxygens of Asp-85 is Thr-89 at 5.1 Å, whereas the second nearest one is Thr-55 at 8.4 Å. According to the structure of M in D96N (15) and E204Q (16), the nearest threonine oxygen from side-chain oxygens of Asp-85 is Thr-89 at 3.5 and 4.2 Å, respectively, whereas the second nearest one is Thr-55 at 6.9 and 6.3 Å, respectively. Thus, the hydrogen bond donor of Asp-85 is presumably the O—H (O—D) group of Thr-89.
Discussion
Proposed Structural Model of the Asp-85–Thr-89 Region.
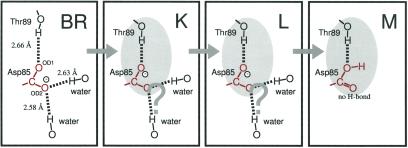
We previously concluded that (i) the O—H (O—D) group of Thr-89 forms a strong hydrogen bond with one of the side-chain oxygens of Asp-85 in BR, and (ii) the hydrogen bond is further strengthened in K (26). The present results provide additional important information on the local structure in the Asp-85–Thr-89 region, namely (iii) the strengthened hydrogen bond in K persists in L, and (iv) Thr-89 remains strongly hydrogen bonded to Asp-85 through M, where the C—O—H (C—O—D) group (not the C⩵O group) of protonated Asp-85 is the hydrogen bond acceptor. On the basis of these results, we propose the structural model of the Asp-85–Thr-89 region from BR through M shown in Fig. 6.
Figure 6.
Structural changes of the Asp-85–Thr-89 region on the basis of the present FTIR results. The distances represent those between two oxygen atoms in 1C3W (10). The shaded oval represents the cluster structure in the Asp-85–Thr-89 region. According to the present scheme, OD1 accepts the proton from the retinal Schiff base in M, whereas OD2 loses hydrogen bonds with water molecules.
In BR, one of the side-chain oxygens of Asp-85 (OD1) is the hydrogen bond acceptor of Thr-89, whose O—O distance is reported to be 2.66 Å (10). The O—H and O—D stretching frequencies of Thr-89 are located at 3,378 and 2,506 cm−1, respectively (26). Another oxygen (OD2) of Asp-85 accepts two hydrogen bonds of water molecules (Fig. 6), one of which further accepts the hydrogen bond of the protonated Schiff base (the N—H group) (10).
Upon photoisomerization, the hydrogen bond between OD1 and the O—H group of Thr-89 becomes stronger, even stronger than those observed in neat secondary alcohols. The stronger hydrogen bond in K may involve a shortened distance between the atoms. However, a straighter bond could result in strengthening without changing the distance. In any case, strong interaction between Asp-85 and Thr-89, as shown by an shaded oval in Fig. 6, constitutes a structural element of the K intermediate. In other words, photoisomerization is accompanied by formation of a “cluster structure” in the Asp-85–Thr-89 region.
In the K to L transition, the Thr-89–Asp-85 hydrogen bond strength is not changed as shown in the present study, indicating that the local structure in the Asp-85–Thr-89 region is preserved at this stage. On the other hand, substantial changes in the stretching vibrations of water molecules are probably involved in this transition (5). In the L to M transition, the Schiff base proton is transferred to Asp-85, specifically to OD1. Even so, the hydrogen bond between OD1 and the O—H group of Thr-89 remains very strong. This fact suggests that the negative charge of Asp-85 does not play a substantial role in the hydrogen bond with the O—H (O—D) group of Thr-89. Whereas the hydrogen bonding does not change for OD1 with Thr-89, dramatic change occurs for OD2 in M. The hydrogen bond donors of OD2 have disappeared in M, which must be caused by the movement of the water molecules. Nevertheless, the cluster structure in the Asp-85–Thr-89 region remains stable through M, and the relaxation of this tight hydrogen bond must occur in subsequent processes.
Previous mutation studies suggested that the BR “switch” that enforces vectorial proton motion is located in the vicinity of the retinal Schiff base (4). The present work sets additional constraints on the switching mechanism. One of the key conclusions is that the Asp-85–Thr-89 region does not actively participate in “switching” because the hydrogen bonding interaction between the two amino acids is persistently strong over a wide time scale (from 10−12 to 10−3 sec). Because the Thr-89–Asp-85 side of the active site shows no structural changes from K through M, the switch mechanism must be located elsewhere.
Structural Contradiction with X-Ray Crystallography of Photointermediates.
The present IR study provides experimental evidence that the interaction between the side chains of Asp-85 and Thr-89 is stronger in K, L, and M than in BR. These results seem at variance with structures of photointermediates based on x-ray crystallography. Recently, x-ray crystallographic structures have been reported for the K (13), L (14), and M (17) intermediates of the wild type and the M intermediates of the D96N (15) and E204Q (16) mutants, with features contrary to the present results.
Table 1 shows the change in distance between side-chain oxygens of Asp-85 and Thr-89 in these crystallographic structures. According to the structures of BR and K (13), the distance between the side-chain oxygens of Asp-85 and Thr-89 increases by more than 1 Å to a distance of 3.8 Å in K. This does not agree with the FTIR result, in which the hydrogen bond of Thr-89 is strengthened without a change in orientation (26). According to the x-ray structure of the K intermediate, the O—H group of Thr-89 may form a hydrogen bond with the peptide carbonyl of Asp-85 because the distance becomes 2.9 Å in K (13). However, the angle (55°) between the O—O vector and the membrane normal does not coincide with that (29°) between the dipole moment of the O—D stretch and the membrane normal in the IR study (26). The IR results are most consistent with continued hydrogen bonding between the O—H group of Thr-89 and the side chain oxygen of Asp-85. Similarly, the x-ray structure of the L intermediate reported an increase in this distance by 1.4 Å (14), which contradicts the present FTIR results.
Table 1.
The distance between the side-chain oxygens of Asp-85 and Thr-89 and the angle between the O—O vector and the membrane normal
| Intermediate | Sample | Distance, Å;
angle, °
|
PDB | |
|---|---|---|---|---|
| BR | Intermediate | |||
| K | Wild type | 2.8; 32 | 3.8 (+1.0); 44 | 1QKP (13) |
| L | Wild type | 2.8; 32 | 4.2 (+1.4); 46 | 1EOP (14) |
| M | D96N | 2.8; 31 | 3.5 (+0.7); 43 | 1C8S (15) |
| M | E204Q | 2.6; 31 | 4.2 (+1.6); 46 | 1F4Z (16) |
| M | Wild type | 2.6; 31 | 5.1 (+2.5); 44 | 1CWQ (17) |
Table 1 also shows the change in distance upon M formation of the wild-type (17) and the D96N (15) and E204Q (16) mutant proteins. The O—O distance between Asp-85 and Thr-89 increases to more than 5 Å in the wild type, whereas it increases to 3.5 and 4.2 Å in D96N and E204Q, respectively. Such observations also contradict the present results. Interestingly, according to the M structure of the wild type, the nearest hydrogen bonding acceptor of the O—H group of Thr-89 is the Schiff base nitrogen (3.1 Å away) (17). In D96N and E204Q, the Schiff base nitrogen is located at 3.3 Å (15) and 3.0 Å (16) from the oxygen of Thr-89, respectively. These structures of M suggest that the deprotonated Schiff base may be the hydrogen bonding acceptor in M. However, a hydrogen bond between Thr-89 and the Schiff base would not explain the present results in terms of (i) the similar frequencies and orientations of the O—D stretch of Thr-89 among K, L, and M and (ii) the isotope effect of Thr-89 on the C⩵O stretch of Asp-85. Therefore, the FTIR evidence favors the continued interaction of Thr-89 with the side-chain oxygen of Asp-85 through M (Fig. 6).
This study showed (i) a very strong hydrogen bond of Thr-89 in M and (ii) OD1 of Asp-85 as the hydrogen bonding acceptor. Although the former conclusion is clear, other hydrogen bond acceptors cannot be excluded for the latter such as the peptide carbonyl oxygen of Asp-85, the Schiff base nitrogen, or a water molecule. However, none of the M structures can explain the very strong hydrogen bond of Thr-89 because the distances to the possible hydrogen bonding acceptors including OD1 of Asp-85 are all greater than 3.0 Å (15–17). Thus, the present FTIR study may caution that the current atomic structures of photointermediates might not be sufficiently accurate for such detailed structural changes. The photocycle in the restricted three-dimensional crystal might cause different structural changes, as has been implicated for photoactive yellow protein (20, 21) and BR (32).
Conclusions
We have characterized the O—D stretching vibration of Thr-89 during the functional processes of BR by polarized FTIR spectroscopy of isotope-labeled and mutant proteins. On the basis of the vibrational frequency and orientation, and the isotope effect on Asp-85, we proposed a structural model in the Asp-85–Thr-89 region. The persistence of this cluster structure through the M intermediate precludes the participation of Thr-89 in the switch mechanism of the pump. The present interpretation, which significantly differs from those based on x-ray crystallography of photointermediates, points to the importance of fine-grained structural details in the functional mechanism of BR. Further complementary use of spectroscopy and diffraction is needed to improve our understanding of light-driven proton pumping by BR.
Acknowledgments
We thank R. Needleman for providing the mutant strains. This work was supported by Japanese Ministry of Education, Culture, Sports and Science Grants 10206206, 10358016, and 11480193 (to H.K.) and by National Institutes of Health Grant GM 36810 (to J.H.).
Abbreviations
- BR
bacteriorhodopsin
- FTIR
Fourier transform IR
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mathies R A, Lin S W, Ames J B, Pollard W T. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- 2.Lanyi J K. J Struct Biol. 1998;124:164–178. doi: 10.1006/jsbi.1998.4044. [DOI] [PubMed] [Google Scholar]
- 3.Haupts U, Tittor J, Oesterhelt D. Annu Rev Biophys Biomol Struct. 1999;28:367–399. doi: 10.1146/annurev.biophys.28.1.367. [DOI] [PubMed] [Google Scholar]
- 4.Krebs M P, Khorana H G. J Bacteriol. 1993;175:1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandori H. Biochim Biophys Acta. 2000;1460:176–190. doi: 10.1016/s0005-2728(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 6.Grigorieff N, Ceska T A, Downing K H, Baldwin J M, Henderson R. J Mol Biol. 1996;259:393–421. doi: 10.1006/jmbi.1996.0328. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuoka K, Hirai T, Murata K, Miyazawa A, Kidera A, Kimura Y, Fujiyoshi Y. J Mol Biol. 1999;286:861–882. doi: 10.1006/jmbi.1998.2529. [DOI] [PubMed] [Google Scholar]
- 8.Essen L, Siegent R, Lehmann W D, Oesterhelt D. Proc Natl Acad Sci USA. 1998;95:11673–11678. doi: 10.1073/pnas.95.20.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato H, Takeda K, Tani K, Hino T, Okada T, Nakasako M, Kamiya N, Kouyama T. Acta Crystallogr D. 1999;55:1251–1256. doi: 10.1107/s090744499900503x. [DOI] [PubMed] [Google Scholar]
- 10.Luecke H, Schobert B, Richter H-T, Cartailler J P, Lanyi J K. J Mol Biol. 1999;291:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]
- 11.Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch J P, Landau E M, Pebay-Peyroula E. Struct Fold Des. 1999;15:909–917. doi: 10.1016/s0969-2126(99)80118-x. [DOI] [PubMed] [Google Scholar]
- 12.Maeda A, Kandori H, Yamazaki Y, Nishimura N, Hatanaka M, Chon Y-S, Sasaki J, Needleman R, Lanyi J K. J Biochem (Tokyo) 1997;121:399–406. doi: 10.1093/oxfordjournals.jbchem.a021602. [DOI] [PubMed] [Google Scholar]
- 13.Edman K, Nollert P, Royant A, Belrhali H, Pebay-Peyroula E, Hajdu J, Neutze R, Landau E M. Nature (London) 1999;401:822–826. doi: 10.1038/44623. [DOI] [PubMed] [Google Scholar]
- 14.Royant A, Edman K, Ursby T, Pebay-Peyroula E, Landau E M, Neutze R. Nature (London) 2000;406:645–648. doi: 10.1038/35020599. [DOI] [PubMed] [Google Scholar]
- 15.Luecke H, Schobert B, Richter H-T, Cartailler J P, Lanyi J K. Science. 1999;286:255–261. doi: 10.1126/science.286.5438.255. [DOI] [PubMed] [Google Scholar]
- 16.Luecke H, Schobert B, Cartailler J-P, Richter H-T, Rosengarth A, Needleman R, Lanyi J K. J Mol Biol. 2000;300:1237–1255. doi: 10.1006/jmbi.2000.3884. [DOI] [PubMed] [Google Scholar]
- 17.Sass H J, Büldt G, Gessenich R, Hehn D, Neff D, Schlesinger R, Berendzen J, Ormos P. Nature (London) 2000;406:649–653. doi: 10.1038/35020607. [DOI] [PubMed] [Google Scholar]
- 18.Genick U K, Soltis S M, Kuhn P, Canestrelli I L, Getzoff E D. Nature (London) 1998;392:206–209. doi: 10.1038/32462. [DOI] [PubMed] [Google Scholar]
- 19.Genick U K, Borgstahl G E, Ng K, Ren Z, Pradervand C, Burke P M, Srajer V, Teng T Y, Schildkamp W, McRee D E, et al. Science. 1997;275:1471–1475. doi: 10.1126/science.275.5305.1471. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstenn G, Vuister G W, Mulder F A, Dux P E, Boelens R, Hellingwerf K J, Kaptein R. Nat Struct Biol. 1998;5:568–570. doi: 10.1038/823. [DOI] [PubMed] [Google Scholar]
- 21.Kandori H, Iwata T, Hendriks J, Maeda A, Hellingwerf K J. Biochemistry. 2000;39:7902–7909. doi: 10.1021/bi000357f. [DOI] [PubMed] [Google Scholar]
- 22.Earnest T N, Roepe P, Braiman M S, Gillespie J, Rothschild K J. Biochemistry. 1986;25:7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- 23.Hatanaka M, Kandori H, Maeda A. Biophys J. 1997;73:1001–1006. doi: 10.1016/S0006-3495(97)78133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandori H. J Am Chem Soc. 1998;120:4546–4547. [Google Scholar]
- 25.Kandori H, Kinoshita N, Shichida Y, Maeda A. J Phys Chem B. 1998;102:7899–7905. [Google Scholar]
- 26.Kandori H, Kinoshita N, Yamazaki Y, Maeda A, Shichida Y, Needleman R, Lanyi J K, Bizounok M, Herzfeld J, Raap J, Lugtenburg J. Biochemistry. 1999;38:9676–9683. doi: 10.1021/bi990713y. [DOI] [PubMed] [Google Scholar]
- 27.Kandori H, Kinoshita N, Yamazaki Y, Maeda A, Shichida Y, Needleman R, Lanyi J K, Bizounok M, Herzfeld J, Raap J, Lugtenburg J. Proc Natl Acad Sci USA. 2000;97:4643–4648. doi: 10.1073/pnas.080064797. . (First Published April 11, 2000; 10.1073/pnas.080064797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin-Vien D, Colthup N B, Fateley W G, Grasselli J G. The Handbook of Characteristic Frequencies of Organic Molecules. San Diego: Academic; 1991. [Google Scholar]
- 29.Kandori H, Kinoshita N, Shichida Y, Maeda A, Needleman R, Lanyi J K. J Am Chem Soc. 1998;120:5828–5829. [Google Scholar]
- 30.Maeda A. Isr J Chem. 1995;35:387–400. [Google Scholar]
- 31.Dioumaev A K, Braiman M S. J Am Chem Soc. 1995;117:10572–10574. [Google Scholar]
- 32.Subramaniam S, Henderson R. Nature (London) 2000;406:653–657. doi: 10.1038/35020614. [DOI] [PubMed] [Google Scholar]