Abstract
The endoplasmic reticulum (ER) is the site of maturation for secretory and membrane proteins that together make up about one third of the cellular proteome. Cells carefully control the synthetic output of this organelle to regulate both quality and quantity of proteins that emerge. Here, we synthesize current concepts underlying the pathways that mediate protein degradation from the ER and their deployment under physiologic and pathologic conditions.
Secreted and cell surface proteins are essential mediators of cellular communication with the environment. Their functional properties and levels, particularly in complex metazoan organisms, markedly influence cellular and organismal physiology. Thus, the cell devotes considerable resources to the regulation of secretory and membrane protein biogenesis at the ER. This specialized site of protein manufacture and maturation affords the cell an opportunity to inspect each polypeptide before it is released for transit to the cell surface. This opportunity is utilized in two major ways. First, proteins that fail to mature properly in one way or another are selectively culled to provide quality control [1]. Second, proteins that are deemed unnecessary for the present cellular conditions are also degraded to effect regulatory quantity control [2]. Quality and quantity control both employ similar pathways, are essential for normal cellular homeostasis, and can be corrupted during disease.
Central to both quality and quantity control is protein degradation from the ER. This involves selective recognition of the degradation substrate, targeting to specialized machinery for export to the cytosol, and usually transfer to the ubiquitin-proteasome system for destruction. This series of events, collectively termed ER-associated degradation (ERAD), is a conserved collection of multiple pathways involving dozens of individual components. Detailed descriptions of the machinery and mechanisms of ERAD pathways have been extensively covered elsewhere [3]. Here, we strive to step back from the details and provide a synthesis of emerging concepts. By doing so, our goal is to highlight especially important but poorly understood aspects of this field.
Quality control at the ER
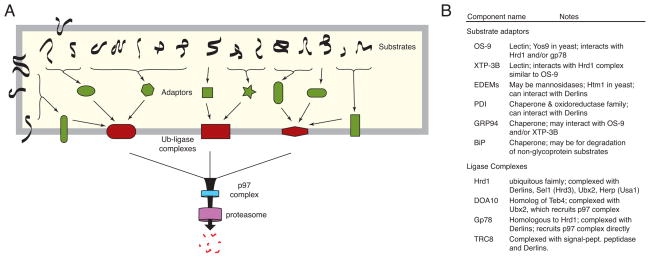
The ERAD of misfolded proteins is best conceptualized as a hierarchical system (Fig. 1). What feeds this hierarchy are literally thousands of potential substrates that vary widely in size, abundance, topology, nature of the folding defect, glycosylation status, and other biophysical parameters. A relatively limited number of factors, often chaperones or lectins, that can be considered adaptors recognize these substrates. The adaptors, together with their bound substrates, are targeted to one of a few membrane-embedded ubiquitin ligase-containing complexes. The membrane complexes facilitate exposure of substrate to the catalytic site of the ubiquitin ligase contained in them, a step that occurs on the cytosolic face of the ER membrane. Once this decisive ubiquitination has occurred, an ATPase-driven mechanism extracts substrates into the cytosol for transfer to the protesasome and may well be the step where multiple ERAD pathways converge. Thus, the key events of ERAD are: (i) substrate recognition, (ii) substrate delivery to the cytosolic site of ubiquitin ligase action, (iii) substrate extraction from the ER, and (iv) delivery to the proteasome for degradation. Of these steps, the first two are the most diversified, tightly controlled, and decisive reactions where substrates are chosen and their fate irreversibly determined by covalent modification with ubiquitin. The steps after substrate ubiquitination are probably common to almost all substrates, and may well be constitutive, rapid, and tightly coupled.
Figure 1. Logical hierarchy of quality control and degradation.
(A) General pyramidal scheme with many substrates, several adaptors, a handful of membrane complexes, and a commonly shared mechanism for substrate extraction and degradation in the cytosol. Substrates vary with regard to topology, post-translational modifications, and nature of the folding defect. These parameters influence the specific pathway(s) available to the substrate. Although not depicted, some substrates might engage a ubiquitin ligase complex directly. There may also be considerable overlap among pathways: substrates could access multiple adaptors, and adaptors might be capable of binding multiple ligase complexes. (B) Several examples of putative adaptors (many of which are chaperones) and ubiquitin ligase complex components are listed.
Substrate recognition during ERAD
Proteins in the ER that fail to fully mature into their final folded structure or assume their proper quaternary structure must be identified and destroyed. Exposure of structural elements that in the mature product would be buried typically distinguishes mature from immature proteins. Immature proteins may expose hydrophobic patches in otherwise soluble domains, hydrophilic residues within a transmembrane segment, unpaired sulfhydryls on normally disulfided bonded cysteines, and sequences (such as a targeting or GPI anchoring signal) that are normally processed. Because chaperones, oxidoreductases, and other protein processing machinery readily recognize such elements [4], identification of immature proteins is conceptually straightforward. The real challenge in ERAD recognition is to distinguish proteins that are unlikely or unable to fold from the far more abundant sea of newly synthesized proteins that are in the process of folding. For glycoproteins, whose folding and degradation are most extensively studied, an answer to this question is beginning to emerge.
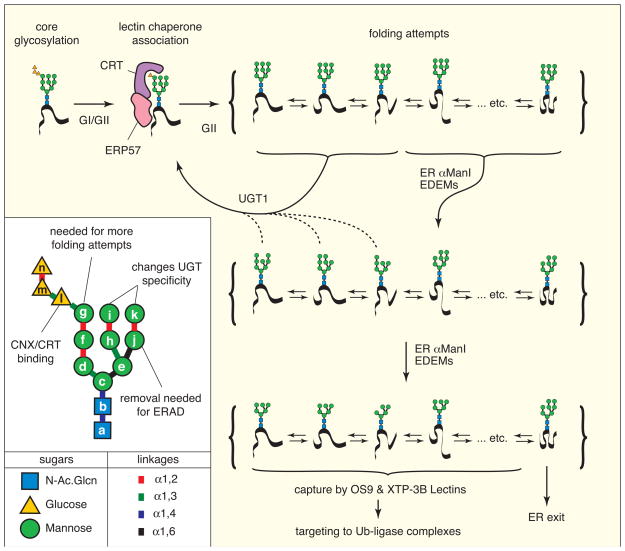
Most nascent proteins entering the ER are co-translationally modified on asparagines in the Asn-X-Thr/Ser sequon (X = not Pro) by an asymmetric, three-branched, 14-hexose glycan [5] (Fig. 2). Asymmetry is apparent in both the sugar composition of the branches and the linkages between the individual hexoses. Various ER-localized glucosidases and mannosidases thus allow the generation of numerous distinct glycan structures [5–8]. ER-resident lectins of differing specificity recognize these distinct glyans [7–11], and each lectin has different interacting partners that can determine outcomes: folding, degradation, or trafficking [12–16]. The activity of at least some of the lectins and glycan-modifying enzymes is sensitive to non-native folding features in the substrate [17]. A dynamic ‘glycan code’ may thus help shape substrate interactions in the ER with biosynthetic, degradative, and trafficking machinery [18]. A plausible series of events illustrating the general steps and key machinery in the decision tree of glycoprotein quality control is shown in Fig. 2, although much further detail awaits discovery.
Figure 2. A working scheme for glycoprotein quality control.
Newly synthesized proteins are core-glycosylated (upper left) with a highly asymmetric 14-hexose glycan (see inset for details). The glucoses are trimmed by glucosidase I and glucosidase II (GI/GII), generating a mono-glucosylated glycan that binds Calreticulin (CRT) or Calnexin, along with an associated oxidoreductase such as ERP57. Upon release, the terminal glucose can be trimmed by GII, preventing re-binding by CRT. During this time, the substrate accesses various possible folding conformations. Depending on the conformation, the substrate can be acted upon by either UGT1 (which re-glucosylates the glycan) or ER mannosidases such as αER-ManI and possibly EDEM family members. Mannose-trimmed glycans can still potentially be re-glucosylated by UGT1 (albeit with lower efficiency) or further de-mannosylated, depending again on the folding status. Removal of the ‘g’ mannose (see inset) irreversibly precludes re-glucosylation, precluding any further folding attempts. The substrate then only has the option of degradation or ER exit. Depending on its folding state, it is thought mannosidases like EDEM family members remove the ‘k’ mannose, exposing the α1,6 linked ‘j’ mannose needed for binding the lectin ERAD adaptors OS9 or XTP-3B. Other lectins such as ERGIC53 facilitate ER export. Note that many substrates have multiple glycans and multiple folding domains, markedly increasing the complexity of these reactions. Note that the precise glycan structures generated by each enzyme and recognized by the different lectins remains to be fully elucidated.
While the concepts that underly quality control of glycoproteins are emerging from the fog, large gaps remain for other substrates. For example, numerous potential ERAD substrates are not glycosylated [19], and even glycoproteins can access degradation pathways independent of ER-resident lectins [20]. How such substrates are inspected and triaged between folding, trafficking, and degradation is unclear. Some components of the ubiquitin ligase complexes may directly recognize misfolded proteins [21]. In addition, if the chaperones involved in folding can interface with the degradation machinery, perhaps prolonged substrate interaction favors degradation.
Indeed, chaperones such as PDI, GRP94, and BiP can associate with ERAD components such as Derlins, OS-9, XTP3-B, and signal peptide peptidase [22–25]. Furthermore, BiP interfaces with numerous co-chaperones containing J-domains, motifs that regulate the ATPase cycle of BiP [26]. Each of these co-chaperones may have different interaction partners, functional properties, and the capacity to recognize non-native structures. These diverse BiP regulatory factors may help control the function of BiP and channel it as a folding or an ERAD factor. It is possible that the relative folding kinetics of a substrate would influence, at least partially, the probability of its recognition by the degradation machinery. How nascent, not yet fully folded polypeptides are distinguished from those that have exhausted their folding options is not known. There must be a committed step that deprives a protein of further folding options and targets it for degradation. This step, which ought to be irreversible, may well coincide with delivery to a membrane-embedded ubiquitin ligase complex. Similarly, proteins that have sustained damage (oxygen radicals, nitrosylation) may rely on yet other recognition systems that may then feed them into an appropriate degradative pathway.
Substrate delivery to the cytosol
Substrates for ERAD must be delivered to one of several membrane-embedded complexes built around an E3-ubiquitin ligase [27–31]. These complexes serve at least three functions. First, they must recognize and bind to the adaptor that has captured an ERAD substrate, thereby serving as a receptor. Second, they must facilitate exposure of substrate to the cytosolic face of the ER membrane, where the E3 ubiquitin ligase active site resides. And third, they must ubiquitinate (more specifically, polyubiquitinate) the substrate. The number of such membrane ubiquitin ligase complexes is at least two (in yeast), with substantial evolutionary expansion in higher eukaryotes such as mammals [27–31]. The need for a greater diversity of ubiquitin ligase complexes presumably reflects the wider range of substrates whose recognition and delivery are dependent on a larger number of adaptor proteins. Consider, for example, the widely different sets of proteins produced by different tissues in higher eukaryotes, necessitating a more specialized and diversified quality control apparatus as well.
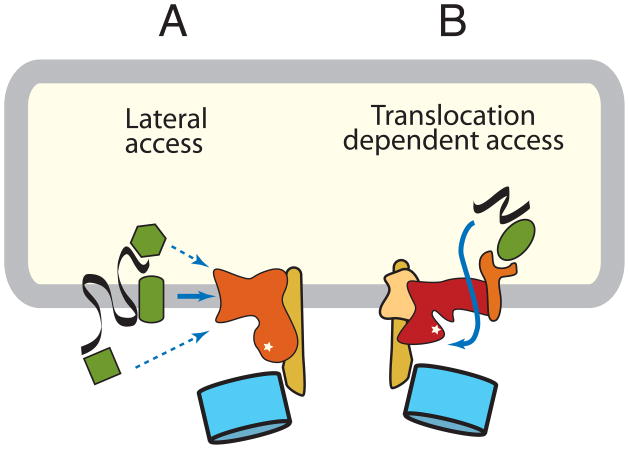
For many integral membrane protein substrates that contain at least a portion of the protein exposed to the cytosol, their initial delivery to the site of ubiquitination is easier to grasp than for lumenal substrates. Potential site(s) for ubiquitination on the substrate can be accessed by lateral diffusion in the plane of the membrane (Fig. 3A). Thus, when the substrate is targeted to the ubiquitin ligase complex, even if via interactions on the lumenal side of the ER, a portion will already be close to the active site of the E3 ligase. Although the cytosolic domain itself need not be ubiquitinated or even positioned correctly, the physical barrier of the membrane is less of an obstacle.
Figure 3. Pathways of substrate ubiquitination.
Membrane ubiquitin ligase complexes mediate substrate access to the catalytic site by two distinct mechanisms. (A) Membrane protein substrates might access the catalytic site by lateral delivery. Recognition and targeting might be mediated by an adaptor in the membrane, cytosol, or lumen. Alternatively, the ubiquitin ligase complex itself could recognize some substrates. (B) Lumenal substrates and some membrane proteins access the catalytic site by a translocation-dependent mechanism. The mechanism or components mediating the key translocation step to provide initial substrate access is unknown, but might involve the ubiquitin ligase itself or an associated membrane protein. In yeast, a complex centered around the Doa10 ubiquitin ligase is probably an example of the first pathway, while a complex containing the Hrd1 ubiquitin ligase is an example of the second pathway. In mammals, many additional similar complexes built around other ubiquitin ligases exist, although their compositions remain to be clearly defined.
By contrast, a wholly lumenal substrate (or membrane protein with no potential ubiquitination sites on the cytosolic domain) must be at least partially translocated across a membrane barrier to access the E3 ligase (Fig. 3B). This dislocation step remains somewhat nebulous and is the subject of considerable contemporary debate. Access of a lumenal hydrophilic segment of polypeptide to the cytosolic environment necessarily requires traversal of the lipid bilayer, presumably via a pore in the membrane, most likely composed of a protein channel. Candidates include Sec61 (the protein-conducting channel used for cotranslational translocation into the ER), Derlin family members, the multi-spanning ubiquitin ligases themselves, or perhaps a complex containing these and/or other membrane proteins (summarized in ref. 32). A case has been made for a means of dislocation that does not involve a protein conducting channel, but rather exploits the mechanism by which lipid droplets form [33]. Given the ability of certain proteins to insert in, and possibly traverse the lipid bilayer spontaneously, with no essential requirement for membrane proteins demonstrated [34], alternatives to protein conducting channels should probably be kept on the table for now. It is a problem akin to the secretion of proteins that lack a discernible signal sequence [35]. Recent studies suggest that at least some instances of non-conventional secretion use autophagy machinery [36, 37]. While the key step of how non-conventional secretion substrates penetrate the lipid bilayer remains unresolved, these observations illustrate the range of components and pathways used by proteins to cross membrane barriers. It may well be that a deeper understanding of non-conventional secretory mechanisms may advance our understanding of dislocation as well. Resolution of this debate will require the establishment of robust in vitro dislocation assays.
Regardless of its identity, the channel through which substrates first access the cytosol must necessarily be part of, or adjacent to the ubiquitin ligase. This would permit efficient ubiquitination, which may act to prevent backsliding and allow the building of a polyubiquitin chain. This polyubiquitin chain, perhaps in combination with the unfolded substrate itself, is then recognized by the p97/Cdc48 complex, a hexameric ATPase containing the accessory proteins Ufd1 and Npl4 [38]. This complex may be pre-recruited to the site of ubiquitination by interaction with either the ubiquitin ligase itself, or one of several associated membrane proteins [39–43]. Such recruitment may favor immediate substrate binding and tight coupling of ubiquitination with subsequent dislocation.
The energy required for extracting the substrate from the membrane comes from the ATPase activity of the p97 complex [38]. Related proteins of this family (e.g., Hsp104 or NSF) indeed harness the energy of ATP hydrolysis to mediate disassembly of otherwise very stable protein assemblies [44], illustrating the power of this class of molecular machines. In some specialized cases, the proteasome itself may provide the ATP-dependent pulling force [45, 46]. Precisely how p97 (or the proteasome) pulls on the chain during ERAD is not clear, nor is it known where the chain resides when it is being extracted. The most widely considered possibility is that for most proteins, extraction from the membrane, or across the membrane in the case of a lumenal substrate, needs a protein-conducting channel. However, demonstration of the presence and identity of such channels has been an experimental challenge. Indeed, a single universal mechanism seems unlikely, given the remarkably wide range of substrates including folded proteins [47] and whole viral particles [48]. Furthermore, the current emphasis on the ER should not distract attention from the possibility that at least some misfolded proteins may be delivered to the endolysosomal system via the secretory pathway, and destroyed by lysosomal proteolysis [49, 50].
Physiologic quantity control
A robust machinery dedicated to disposal of misfolded proteins has allowed the evolution of pathways where this machinery is used for the regulated disposal of unwanted, but not necessarily misfolded, proteins. A key distinction between quality and quantity control would be the criterion used for substrate recognition. Rather than being dependent on maturation status per se, other parameters influence the substrate’s recognition by an adaptor capable of interfacing with the degradation machinery.
The best studied example of physiologic quantity control is probably the regulated degradation of the ER membrane protein HMG-CoA-reductase (HMGR) in response to steroid pathway status [2, 51]. In mammalian cells, the stability of HMGR is inversely regulated by lanosterol, the first sterol generated by the cholesterol biosynthetic pathway [52]. Lanosterol appears to bind to the membrane domain of HMGR, causing it to associate with another membrane protein, Insig1. Insig1 interacts with gp78 [53], a membrane-embedded ubiquitin ligase that is part of a multi-protein complex mediating ERAD of various misfolded proteins. Thus, Insig1 acts as an adaptor for HMGR, recruiting it to the gp78 ubiquitin ligase complex in a sterol-dependent manner and so controls its abundance.
In the analogous yeast system, Hmg2p is also degraded in a regulated manner that is dependent on both a sterol biosynthetic intermediate (in this case, farnesyl pyrophosphate, or FPP [54]) and a ubiquitin ligase complex (in this case Hrd1 [55], a homolog of gp78 that also functions in ERAD). Hrd1 directly recognizes FPP-bound Hmg2p without the need for an adaptor [21]. The yeast Insig1 (called Nsg1) nonetheless regulates Hmg2p by binding to and inhibiting its interaction with Hrd1 [56]. Thus, in both systems, physiologic quantity control is effected by an adaptor that partially regulates (either positively or negatively) access of the substrate to the quality control machinery. Although the specific details and the role of the adaptor have diverged between yeast and mammals, the basic concept is conserved.
While the HMGR/Hmg2p systems are wonderfully instructive examples, physiologic quantity control in the secretory pathway has been less well studied, and the scope of its use is unknown. Given the regulatory importance of cytosolic quality control pathways, there is no reason to expect the analogous ER pathways to be any different. In fact, given the considerable physiologic importance of tightly regulating the levels of secreted hormones, cell surface receptors, ion channels, and other key factors, fine-tuning their export from the ER in response to cellular need is essential for both homeostasis and adequate physiological responses.
Pathogen-directed quantity control
Directly analogous to physiologically regulated quantity control, pathogen-mediated quantity control pathways also selectively regulate the fate of various host factors [57]. A pathogen-encoded protein can serve as an adaptor between a host factor and the quality control degradation machinery. An instructive example in this respect is the human cytomegalovirus (CMV) US2 protein, which selectively targets MHC class I heavy chain (HC) for proteasome-dependent degradation. Recent work suggests that the ubiquitin ligase involved in this degradation pathway is TRC8, which forms a complex with other ER proteins including the lumenal chaperone PDI and the integral membrane protein signal peptide peptidase (SPP) [58]. HC is recruited to this complex in a US2-dependent manner, suggesting that US2 may be serving as an adaptor [59].
Another CMV protein, US11, also targets HC, but by a distinct mechanism that seems to utilize a different subset of the quality control machinery including Derlin1 and SEL1L [60], both of which are conserved ERAD pathway components. Yet another mechanism is used by the HIV-encoded membrane protein Vpu to mediate degradation of CD4. Here, Vpu, after its phosphorylation, recruits a cytosolic ubiquitin ligase complex containing βTrCP [61]. This is surprising because βTrCP is not implicated in ER protein degradation, and it is still unclear if or how ER-localized machinery for extraction of CD4 is utilized. Nonetheless, the involvement of p97 suggests that Vpu indeed acts as an adaptor to interface with at least part of the ERAD machinery [62,63]. And finally, the mK3 protein from murine gamma herpervirus 68 is itself an E3 ubiquitin ligase. By forming a complex with Derlin1 and p97, mK3 seems to form a unique ubiquitin ligase complex for degradation of its HC substrate [64], with the added distinction that ubiquitination may occur on serine/threonine hydroxyls [65].
What is especially interesting about these and other viral degradation pathways is the diversity in engagement of the QC machinery. Indeed, some systems may recruit machinery that is normally not even used for misfolded proteins (such as βTrCP). Thus, as in other areas of cell biology, pathogens have been remarkably instructive in uncovering key players in quality control degradation pathways. For example, several insights have been gained from the US2/US11 systems, including the discoveries of the mammalian Derlins, and identifying potential roles for SPP and TRC8 in ERAD. Continued analysis of these and other pathogen systems is likely to yield additional insights into how quality control and degradation are regulated. Furthermore, because pathogens often exploit only a subset of the components in an ERAD pathway, while short-circuiting other components, they may be especially useful systems for biochemical reconstitution of key sub-reactions.
Stress-induced quality control pathways
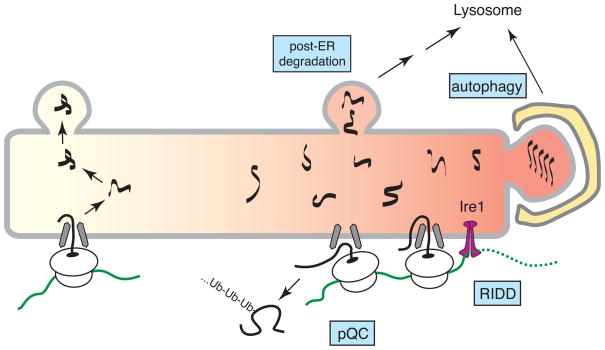
Under particular maladaptive conditions, where ER protein maturation is severely compromised, the constitutive quality control and degradation pathways are likely to be saturated. Over time, the unfolded protein response (UPR) transcriptionally upregulates a wide range of factors that improve ER protein processing capacity (reviewed extensively elsewhere [66]). In the intervening period, rapidly acting mechanisms are needed to minimize substrate burden on the ER. The best known pathway involves translational attenuation due to eIF2α phosphorylation by the ER stress sensor PERK [67]. This effect is general, and not selective to ER substrates. At least four additional ER-selective pathways may facilitate quality and quantity control in a stress-dependent manner to minimize misfolded protein generation or maximize misfolded protein clearance (Fig. 4).
Figure 4. Pathways of stress-dependent quality control.
During particularly severe ER stress, several pathways of quality control that may not operate during normal conditions become important for limiting protein misfolding in the ER. Pre-emptive quality control (pQC) involves reduced translocation of certain protein that are instead routed into the cytosol for degradation. Regulated Ire1-dependent degradation (RIDD) mediates degradation of select ER-bound mRNAs. Some misfolded proteins may be degraded by post-ER pathways involving vesicular trafficking to the lysosome. Autophagy can sequester whole sections of the ER containing misfolded or aggregated proteins.
Almost immediately upon induction of ER stress, one of the stress sensors, an ER resident membrane protein termed Ire1, is activated by autophosphorylation. The primary function of Ire1 is to use its cytosolically disposed nuclease activity to mediate splicing of the mRNA for Xbp1, a key UPR transcription factor. However, Xbp1 mRNA is not Ire1’s only substrate and other mRNAs may be destroyed [68–70]. This would abort production of difficult-to-fold proteins, and might facilitate recovery from ER stress. The mechanisms underlying this regulated Ire1-dependent decay (RIDD) are not clear at present, but may be important in certain highly secretory cell types [69] or for specialized tissue-specific substrates [70].
Another rapidly induced stress-dependent pathway is ‘pre-emptive’ quality control (pQC), where certain proteins are blocked in their initial translocation into the ER lumen and instead routed directly for proteasomal degradation [71,72]. This mechanism of substrate reduction during stress appears to be at least partially selective, depending on features of the signal sequences that mediate the substrate’s translocation [71]. Although the details remain to be worked out, it seems that some signal sequences require lumenal proteins to facilitate efficient translocation of its attached substrate. Because these stimulatory lumenal factors (perhaps chaperones) are otherwise occupied with unfolded proteins during stress, translocation would necessarily be attenuated. The specific pathway by which these translocationally attenuated proteins are ubiquitinated and degraded is not known, but is likely to involve different components than those needed for proteins in the lumen or membrane bilayer of the ER.
RIDD and pQC act to reduce the generation of new substrates during stress. In addition, non-ERAD mechanisms also help remove proteins that are already in the ER at the time of an acute stress. Vesicular trafficking can route proteins to the lysosome, as observed when ERAD pathways are overwhelmed, or for those substrates that perhaps cannot efficiently access ERAD [49, 50, 73]. An intriguing implication of these observations is that there exist mechanisms of discriminating native from non-native proteins in post-ER compartments of the secretory pathway such as the Golgi. Such post-ER quality control pathways should rise to prominence or even appear during ER stress.
And finally, there appear to be mechanisms to rid especially intransigent and aggregated substrates from the ER by bulk degradation of entire sections of the ER by autophagy [74–76]. Misfolded and aggregated proteins, which are more likely to accumulate during severe or prolonged stress, may be segregated to ER sub-domains that are recognized by the autophagy machinery. This pathway could also be employed to control ER abundance under normal homeostatic conditions. At present, the relative contributions of each of these different pathways of protein disposal under either normal or stressed conditions remains unknown. It is possible that different subsets of pathways are utilized differentially in a substrate-specific, cell type-specific, or condition-specific manner to fully accommodate the incredibly wide range of circumstances faced by a cell.
Conclusions and perspective
Quality control and protein degradation from the ER have emerged as a major area of investigation While the general framework of the main steps has changed little, progress has been made on identifying the conserved factors involved. Genetic screens, whole-genome searches, and protein-protein interaction analyses have provided an extensive parts list in both yeast and mammals (e.g., [77]). However, in no model system is it clear which parts fit where, or how they actually function at a molecular level. Thus, one of the most pressing needs in this field is a highly robust substrate that engages only a single pathway amenable to biochemical reconstitution and dissection. Such a reductionist strategy will necessarily miss many interesting nuances, but is essential in defining a set of core mechanistic principles. At the same time we need to extend the horizon to include plants and single celled eukaryotes other than yeast, and explore the similarities and differences with protein quality control in organelles such as mitochondria and chloroplasts. Furthermore, the ER is unlikely to be a single homogeneous compartment. Contiguous with the nuclear envelope, the ER is likely composed of subdomains not only with respect to the presence (rough ER) or absence (smooth ER) of ribosomes, but also with respect to components of the quality control apparatus [78]. The functions of cortical and perinuclear ER are as likely to differ in their functional capacity as do the various subcompartments of the endocytic pathway or the Golgi apparatus. Would these hypothetical ER subcompartments be equipped equally for synthetic and quality control functions? These questions, too, await resolution by experiment.
Lumenal and membrane factors have been almost inaccessible to manipulation, with only limited progress so far in in vitro reconstitution. Greater control over the folding status of the substrate, by a temperature sensitive mutation or interaction with a small molecule would be highly desirable. However, integrity of the membrane vesicles used to study transport processes in vitro is an impediment to achieving these goals. Reconstitution of ERAD may thus require a radically different approach to that used to study other protein translocation processes. Non-vesicular membrane patches, or the creation of an artificial ER equivalent between two chambers, while technically challenging, may be worth investigation.
The physiologic roles of the different pathways, particularly in more complex metazoans, remains unsolved. Showing that a component contributes to ERAD need not imply that its role is limited to it and does not extend into other, perhaps even more important aspects of cellular physiology. At present, there is not even agreement on the number of pathways and their composition. Why are there so many ER-localized ubiquitin ligases? Are they all involved in ERAD, and if so, what is the reason for such a marked expansion of parallel pathways during evolution? It is intriguing to consider the possibility that much of this expansion is not for quality control, but for regulatory quantity control in organisms where tighter control of protein access to extracellular space is desirable.
Acknowledgments
RSH’s laboratory is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health. HLP’s laboratory is supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ramanujan S. Hegde, Email: hegder@mail.nih.gov.
Hidde L. Ploegh, Email: ploegh@wi.mit.edu.
References and recommended reading
* Of special interest
** Of outstanding interest
- 1.Anelli T, Sitia R. Protein quality control in the early secretory pathway. Embo J. 2008;27:315–27. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampton RY, Garza RM. Protein quality control as a strategy for cellular regulation: lessons from ubiquitin-mediated regulation of the sterol pathway. Chem Rev. 2009;109:1561–74. doi: 10.1021/cr800544v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–57. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992;2:227–31. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- 5.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–49. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 6.Hosokawa N, Tremblay LO, Sleno B, Kamiya Y, Wada I, Nagata K, Kato K, Herscovics A. EDEM1 accelerates the trimming of alpha1,2-linked mannose on the C branch of N-glycans. Glycobiology. 2010;20:567–75. doi: 10.1093/glycob/cwq001. [DOI] [PubMed] [Google Scholar]
- **7.Quan EM, Kamiya Y, Kamiya D, Denic V, Weibezahn J, Kato K, Weissman JS. Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol Cell. 32:870–7. doi: 10.1016/j.molcel.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **8.Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M. Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol. 2009;184:159–72. doi: 10.1083/jcb.200809198. These two studies defined a specific glycan structure, the enzyme that likely generates it, and the lectin that recognizes it, for ERAD of glycoproteins in yeast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa N, Kamiya Y, Kato K. The role of MRH domain-containing lectins in ERAD. Glycobiology. 2010 doi: 10.1093/glycob/cwq013. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya Y, Kamiya D, Yamamoto K, Nyfeler B, Hauri HP, Kato K. Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL, and VIP36. J Biol Chem. 2008;283:1857–61. doi: 10.1074/jbc.M709384200. [DOI] [PubMed] [Google Scholar]
- 11.Jakob CA, Chevet E, Thomas DY, Bergeron JJ. Lectins of the ER quality control machinery. Results Probl Cell Differ. 2001;33:1–17. doi: 10.1007/978-3-540-46410-5_1. [DOI] [PubMed] [Google Scholar]
- 12.Daniels R, Kurowski B, Johnson AE, Hebert DN. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell. 2003;11:79–90. doi: 10.1016/s1097-2765(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 13.Appenzeller C, Andersson H, Kappeler F, Hauri HP. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol. 1999;1:330–4. doi: 10.1038/14020. [DOI] [PubMed] [Google Scholar]
- 14.Mikami K, Yamaguchi D, Tateno H, Hu D, Qin SY, Kawasaki N, Yamada M, Matsumoto N, Hirabayashi J, Ito Y, Yamamoto K. The sugar-binding ability of human OS-9 and its involvement in ER-associated degradation. Glycobiology. 2010;20:310–21. doi: 10.1093/glycob/cwp175. [DOI] [PubMed] [Google Scholar]
- 15.Molinari M, Calanca V, Galli C, Lucca P, Paganetti P. Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science. 2003;299:1397–400. doi: 10.1126/science.1079474. [DOI] [PubMed] [Google Scholar]
- 16.Oda Y, Hosokawa N, Wada I, Nagata K. EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science. 2003;299:1394–7. doi: 10.1126/science.1079181. [DOI] [PubMed] [Google Scholar]
- 17.Keith N, Parodi AJ, Caramelo JJ. Glycoprotein tertiary and quaternary structures are monitored by the same quality control mechanism. J Biol Chem. 2005;280:18138–41. doi: 10.1074/jbc.M501710200. [DOI] [PubMed] [Google Scholar]
- 18.Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 2005;15:364–70. doi: 10.1016/j.tcb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- *19.Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell. 2007;28:544–54. doi: 10.1016/j.molcel.2007.09.012. Degradation of a non-glycoprotein was shown to involve the chaperone BiP and Herp, a membrane protein that is part of the Hrd1/Derlin1 ubiquitin ligase complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M. Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol. 2010;188:223–35. doi: 10.1083/jcb.200910042. This study illustrated that a combination of misfolding and topology influence the specific choice of degradation pathway used by a a misfolded substrate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Sato BK, Schulz D, Do PH, Hampton RY. Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol Cell. 2009;34:212–22. doi: 10.1016/j.molcel.2009.03.010. The Hrd1 ubiquitin ligase was shown to be capable of distinguishing folding status of membrane protein substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10:272–82. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Hosokawa N, Wada I, Nagasawa K, Moriyama T, Okawa K, Nagata K. Human XTP3-B forms an endoplasmic reticulum quality control scaffold with the HRD1-SEL1L ubiquitin ligase complex and BiP. J Biol Chem. 2008;283:20914–24. doi: 10.1074/jbc.M709336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Lee SO, Cho K, Cho S, Kim I, Oh C, Ahn K. Protein disulphide isomerase is required for signal peptide peptidase-mediated protein degradation. Embo J. 2010;29:363–75. doi: 10.1038/emboj.2009.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Bernardi KM, Forster ML, Lencer WI, Tsai B. Derlin-1 facilitates the retro-translocation of cholera toxin. Mol Biol Cell. 2008;19:877–84. doi: 10.1091/mbc.E07-08-0755. These four studies, in different ways, provide plausible ways that chaperones physically and functionally interface with degradation machinery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzmann A, Baldes C, Dudek J, Zimmermann R. The heat shock protein 70 molecular chaperone network in the pancreatic endoplasmic reticulum - a quantitative approach. FEBS J. 2007;274:5175–87. doi: 10.1111/j.1742-4658.2007.06039.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453–60. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126:361–73. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Denic V, Quan EM, Weissman JS. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell. 2006;126:349–59. doi: 10.1016/j.cell.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 30.Gauss R, Sommer T, Jarosch E. The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment. Embo J. 2006;25:1827–35. doi: 10.1038/sj.emboj.7601088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehara K, Xie W, Ng DT. Modularity of the Hrd1 ERAD complex underlies its diverse client range. J Cell Biol. 2010;188:707–16. doi: 10.1083/jcb.200907055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebert DN, Bernasconi R, Molinari M. ERAD substrates: Which way out? Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–8. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 34.Brambillasca S, Yabal M, Makarow M, Borgese N. Unassisted translocation of large polypeptide domains across phospholipid bilayers. J Cell Biol. 2006;175:767–77. doi: 10.1083/jcb.200608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 36.Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188:527–36. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol. 2010;188:537–46. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y. Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J Struct Biol. 2006;156:29–40. doi: 10.1016/j.jsb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Ye Y, Shibata Y, Kikkert M, van Voorden S, Wiertz E, Rapoport TA. Inaugural Article: Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A. 2005;102:14132–8. doi: 10.1073/pnas.0505006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lilley BN, Ploegh HL. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A. 2005;102:14296–301. doi: 10.1073/pnas.0505014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballar P, Shen Y, Yang H, Fang S. The role of a novel p97/valosin-containing protein-interacting motif of gp78 in endoplasmic reticulum-associated degradation. J Biol Chem. 2006;281:35359–68. doi: 10.1074/jbc.M603355200. [DOI] [PubMed] [Google Scholar]
- 42.Schuberth C, Buchberger A. Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat Cell Biol. 2005;7:999–1006. doi: 10.1038/ncb1299. [DOI] [PubMed] [Google Scholar]
- 43.Neuber O, Jarosch E, Volkwein C, Walter J, Sommer T. Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat Cell Biol. 2005;7:993–8. doi: 10.1038/ncb1298. [DOI] [PubMed] [Google Scholar]
- 44.White SR, Lauring B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic. 2007;8:1657–67. doi: 10.1111/j.1600-0854.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee RJ, Liu CW, Harty C, McCracken AA, Latterich M, Romisch K, DeMartino GN, Thomas PJ, Brodsky JL. Uncoupling retro-translocation and degradation in the ER-associated degradation of a soluble protein. Embo J. 2004;23:2206–15. doi: 10.1038/sj.emboj.7600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipson C, Alalouf G, Bajorek M, Rabinovich E, Atir-Lande A, Glickman M, Bar-Nun S. A proteasomal ATPase contributes to dislocation of endoplasmic reticulum-associated degradation (ERAD) substrates. J Biol Chem. 2008;283:7166–75. doi: 10.1074/jbc.M705893200. [DOI] [PubMed] [Google Scholar]
- 47.Tirosh B, Furman MH, Tortorella D, Ploegh HL. Protein unfolding is not a prerequisite for endoplasmic reticulum-to-cytosol dislocation. J Biol Chem. 2003;278:6664–72. doi: 10.1074/jbc.M210158200. [DOI] [PubMed] [Google Scholar]
- 48.Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, Helenius A. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131:516–29. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 49.Ashok A, Hegde RS. Selective processing and metabolism of disease-causing mutant prion proteins. PLoS Pathog. 2009;5:e1000479. doi: 10.1371/journal.ppat.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Ng DT. Evasion of endoplasmic reticulum surveillance makes Wsc1p an obligate substrate of Golgi quality control. Mol Biol Cell. 2010;21:1153–65. doi: 10.1091/mbc.E09-10-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–21. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song BL, Javitt NB, DeBose-Boyd RA. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 2005;1:179–89. doi: 10.1016/j.cmet.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Song BL, Sever N, DeBose-Boyd RA. Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell. 2005;19:829–40. doi: 10.1016/j.molcel.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Shearer AG, Hampton RY. Lipid-mediated, reversible misfolding of a sterol-sensing domain protein. Embo J. 2005;24:149–59. doi: 10.1038/sj.emboj.7600498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol. 2001;3:24–9. doi: 10.1038/35050524. [DOI] [PubMed] [Google Scholar]
- 56.Flury I, Garza R, Shearer A, Rosen J, Cronin S, Hampton RY. INSIG: a broadly conserved transmembrane chaperone for sterol-sensing domain proteins. Embo J. 2005;24:3917–26. doi: 10.1038/sj.emboj.7600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaacson MK, Ploegh HL. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5:559–70. doi: 10.1016/j.chom.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **58.Stagg HR, Thomas M, van den Boomen D, Wiertz EJ, Drabkin HA, Gemmill RM, Lehner PJ. The TRC8 E3 ligase ubiquitinates MHC class I molecules before dislocation from the ER. J Cell Biol. 2009;186:685–92. doi: 10.1083/jcb.200906110. The ubiquitin ligase for US2-mediated degradation of MHC heavy chain was identified, suggesting a previously unknown role for this ligase in ERAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loureiro J, Lilley BN, Spooner E, Noriega V, Tortorella D, Ploegh HL. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature. 2006;441:894–7. doi: 10.1038/nature04830. [DOI] [PubMed] [Google Scholar]
- 60.Mueller B, Klemm EJ, Spooner E, Claessen JH, Ploegh HL. SEL1L nucleates a protein complex required for dislocation of misfolded glycoproteins. Proc Natl Acad Sci U S A. 2008;105:12325–30. doi: 10.1073/pnas.0805371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–74. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 62.Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen EA. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology. 2007;4:75. doi: 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magadan JG, Perez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Path. 2010 doi: 10.1371/journal.ppat.1000869. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Ye Y, Lencer W, Hansen TH. The viral E3 ubiquitin ligase mK3 uses the Derlin/p97 endoplasmic reticulum-associated degradation pathway to mediate down-regulation of major histocompatibility complex class I proteins. J Biol Chem. 2006;281:8636–44. doi: 10.1074/jbc.M513920200. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Herr RA, Chua WJ, Lybarger L, Wiertz EJ, Hansen TH. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J Cell Biol. 2007;177:613–24. doi: 10.1083/jcb.200611063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 67.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- *68.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–75. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **70.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–55. doi: 10.1016/j.cmet.2008.03.005. These three studies suggest a broader and regulated role for Ire1 nuclease activity in controlling mRNA abundance, in addition to its known splicing activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell. 2006;127:999–1013. doi: 10.1016/j.cell.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orsi A, Fioriti L, Chiesa R, Sitia R. Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. J Biol Chem. 2006;281:30431–8. doi: 10.1074/jbc.M605320200. [DOI] [PubMed] [Google Scholar]
- 73.Spear ED, Ng DT. Stress tolerance of misfolded carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol Biol Cell. 2003;14:2756–67. doi: 10.1091/mbc.E02-11-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, Yoshimori T. Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem. 2006;281:4467–76. doi: 10.1074/jbc.M509409200. [DOI] [PubMed] [Google Scholar]
- 75.Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol Biol Cell. 2006;17:203–12. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, Schuldiner M. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–7. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell. 2001;12:1711–23. doi: 10.1091/mbc.12.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]