Abstract
Background
Imaging studies report that hippocampal volume is decreased in major depressive disorder (MDD). A cellular basis for reduced hippocampal volume in MDD has not been identified.
Methods
Sections of right hippocampus were collected in 19 subjects with MDD and 21 normal control subjects. The density of pyramidal neurons, dentate granule cell neurons, glia, and the size of the neuronal somal area were measured in systematic, randomly placed three-dimensional optical disector counting boxes.
Results
In MDD, cryostat-cut hippocampal sections shrink in depth a significant 18% greater amount than in control subjects. The density of granule cells and glia in the dentate gyrus and pyramidal neurons and glia in all cornv ammonis (CA)/hippocampal subfields is significantly increased by 30% –35% in MDD. The average soma size of pyramidal neurons is significantly decreased in MDD.
Conclusion
In MDD, the packing density of glia, pyramidal neurons, and granule cell neurons is significantly increased in all hippocampal subfields and the dentate gyrus, and pyramidal neuron soma size is significantly decreased as well. It is suggested that a significant reduction in neuropil in MDD may account for decreased hippocampal volume detected by neuroimaging. In addition, differential shrinkage of frozen sections of the hippocampus suggests differential water content in hippocampus in MDD.
Keywords: Depression, glia, hippocampus, pyramidal neurons
Preclinical and neuroimaging studies have implicated the hippocampus in the pathophysiology of major depressive disorder (MDD). In addition, plasticity within the hippocampal formation is thought to play a role in neurobiological responses to stress and to antidepressant drug action (Duman et al 1999).
Evidence for a role of the hippocampus in depression comes from magnetic resonance imaging (MRI) studies examining the volume of the hippocampus (Campbell et al 2004). In subjects with MDD or a history of MDD, MRI studies demonstrate reduced volume of the hippocampus (Bremner et al 2000; Frodl et al 2002; MacQueen et al 2003; Mervaala et al 2000; Shah et al 1998; Sheline et al 1996, 1999; Steffens et al 2000; but not in Posener et al 2003; Rusch et al 2001; Vakili et al 2000). It appears that hippocampal atrophy is preferentially seen in older, recurrently depressed subjects or subjects who are refractory to antidepressant medications. Recently, hippocampal volume and function was assessed over the course of illness in younger patients with MDD (MacQueen et al 2003). Recollection memory was diminished in subjects with either a first-episode or multiple episodes of depression; however, hippocampal volume was significantly decreased only in depressed subjects with multiple depressive episodes.
Histopathologic evidence reveals cellular changes in the forebrain in depression (Davidson et al 2002; Rajkowska 2002). In MDD, there are decreases in cortical thickness, neuronal sizes, and neuronal and glial densities in left rostral orbitofrontal cortex and left dorsolateral prefrontal cortex (Rajkowska et al 1999). In left subgenual cortex, a region of the anterior cingulate cortex, Ongur et al (1998) reported a decrease in glial number in familial MDD or bipolar disorder. Studies by Cotter et al (2001, 2002), in both left and right hemispheres, confirm decreases in neuron size and glial density in the dorsolateral prefrontal and anterior cingulate cortex. Finally, Bowley et al (2002) reported a decrease in glial density in the left amygdala in MDD. Thus, both neurons and glia appear to participate in the neuropathology of depression.
Few studies have structurally examined the postmortem human hippocampus in depression. Cellular integrity and apoptosis have been evaluated in the hippocampus in subjects with depression, steroid-treated subjects, and normal control subjects (Lucassen et al 2001; Muller et al 2001). Using semiquantitative methods, these studies reported no significant cell loss in any hippocampal region in any of the subject groups. In most of the subjects with depression, there was evidence for a slight increase in fragmented DNA associated with apoptosis and necrotic neuron death detected in the dentate gyrus, cornu ammonis (CA)1, and CA4 (Lucassen et al 2001). In depression, decreases in astrocytic immunoreactivity for cellular glial fibrillary acidic protein and the neuron-specific phosphoprotein B50 (or GAP-45) were detected in CA1 and CA2 (Muller et al 2001). The authors suggested that apoptosis may only be a minor contributor to volume changes in the hippocampus in depression, whereas patterns of reactive astrogliosis and synaptic reorganization proteins were significantly altered in some hippocampal regions in depression. Other reports of hippocampal changes in mood disorders identified a significant decrease in the density of nonpyramidal neurons in the CA2 region and a reduction in Reelin-positive cell density in the hilus in subjects with bipolar disorder (Benes et al 1998; Fatemi et al 2000).
The purpose of this study was to identify the cellular basis for reductions in hippocampal volume in MDD by the application of direct three-dimensional cell-counting methods in tissue sections to evaluate neurons and glia in the hippocampal formation in MDD. Neuronal and glial densities, as well as neuronal soma size and glial nuclear size, were estimated in hippocampus proper and the dentate gyrus of subjects with MDD compared with age-matched, psychiatrically healthy control subjects. We hypothesized that the size of pyramidal neuron cell bodies would be decreased and the packing density of neurons and glia increased in the hippocampal formation in MDD.
Methods and Materials
Tissues from 19 depressed subjects and 21 age-matched psychiatrically healthy control subjects were obtained at autopsy from the Coroner’s Office of Cuyahoga County, Cleveland, Ohio, USA. An ethical protocol approved by the Institutional Review Board of the University Hospitals of Cleveland was used, and informed written consent was obtained from the next-of-kin for all subjects. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse.
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects (see Tables 1 and 2). A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) to knowledgeable next-of-kin of 15 of the depressed subjects, as previously described (Stockmeier et al 2002). The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) was administered to next-of-kin of the four remaining depressed subjects (First et al 1996). Axis I psychopathology was assessed and consensus diagnosis was reached in conference using information from the interview and medical records. Responses from the 15 subjects evaluated with the SADS-L were also recorded in the SCID, and these subjects met DSM-IV criteria for MDD using information collected with either structured diagnostic interview. Eighteen subjects met DSM-IV criteria for an MDD episode within the last 2 weeks of life, and one subject with depression was in remission. Five depressed subjects were comorbid for panic disorder with agoraphobia; agoraphobia; benzodiazepine abuse; sedative anxiolytic hypnotic related disorder, not otherwise specified; pathologic gambling; or delusional disorder. Two depressed subjects met diagnostic criteria for alcohol abuse at 2 and 24 years before their deaths.
Table 1.
Characteristics of the Control Subjects
| Age / Gender | Smoker | Cause of Death | PMI (Hours) |
Toxicologya (Blood) |
Medicationb | Axis I Diagnosis |
|---|---|---|---|---|---|---|
| 26/M | No | Homicide, gunshot | 13 | Nothing detected | None | No diagnosis |
| 37/M | No | Acute hemorrhagic pancreatitis | 17 | Nothing detected | Amlopidine,b, ranitidineb | No diagnosis |
| 42/M | Prior | Coronary sclerotic heart disease | 20 | Nothing detected | None | No diagnosis |
| 43/M | No | Pulmonary thromboemboli | 23 | Propoxyphene, oxycodone |
Glyburide,b, methylprednisolone,b propoxypheneb |
No diagnosis |
| 46/F | No | Homicide, gunshot | 24 | Nothing detected | Maxitrol | No diagnosis |
| 46/M | No | Hypertensive, hypertrophic, and ischemic cardiomyopathy |
19 | Nothing detected | None | No diagnosis |
| 47/M | Prior | Hypertensive, cardiovascular disease, and diabetes mellitus |
25 | Propoxyphene | insulin | No diagnosis; alcohol abuse 10 years prior |
| 49/F | No | Atherosclerotic heart disease with remote myocardial infarcts and acute myocardial ischemia |
29 | Nothing detected | Ticlopidine,b, aspirin,b, lisinopril,b, lipitor,b insulin,b, nitroglycerin b |
No diagnosis |
| 50/F | Yes | Coronary sclerotic heart disease with remote myocardial infarct |
27 | Nothing detected | None | No diagnosis |
| 52/F | Not known | Coronary sclerotic heart disease with acute thrombotic occlusion of right coronary artery, and acute and remote myocardial infarcts |
12 | Morphine | Atenolol, cimetidine, penicillin | No diagnosis |
| 54/M | Yes | Hypertensive coronary sclerotic heart disease with remote myocardial infarcts and cardiomegaly |
19 | Lidocaine | Digoxin, dipyridamole | No diagnosis |
| 56/M | Yes | Hypertrophic cardiomyopathy with severe coronary atherosclerosis |
25 | Nothing detected | None | No diagnosis |
| 66/M | No | Hypertrophic cardiomyopathy with coronary sclerotic heart disease and myocardial fibrosis |
12 | Lidocaine | None | No diagnosis |
| 67/F | Yes | Acute thrombotic occlusion of coronary artery | 28 | Nothing detected | None | No diagnosis |
| 67/F | Yes | Coronary sclerotic heart disease with myocardial infarct and myocardial rupture |
16 | Nothing detected | Insulin,b aspirinb | No diagnosis |
| 69/M | No | Hemopericardium, hemodiastinum, and left hemothorax |
18 | Nothing detected | None | No diagnosis |
| 70/M | Prior | Hypertrophic and ischemic cardiomyopathy with remote myocardial infarct |
20 | Nothing detected | Lisinopril,b isosorbide,b KCl,b furosemide,bclonazepam,b ipratropiumb |
No diagnosis; alcohol abuse 30 years prior |
| 80/F | No | Hypertensive coronary sclerotic heart disease with remote myocardial infarct |
21 | Nothing detected | Premarin,b provera,b liotrix, KCl (administered in ER),b nadolol, estrace, dipivefrin, medroxyprogesterone, hydrochlorothiazide, hydrocodone, zostrix, |
No diagnosis |
| 82/M | No | Ruptured aneurysmc | 16 | Nothing detected | Levothyroxine | No diagnosis |
| 83/F | No | Ruptured myocardial infarct with hemopericardium | 25 | Nothing detected | Fluoxetineb (for nerves and sleeping) | No diagnosis |
| 84/F | No | Coronary sclerotic heart disease with myocardial fibrosis, myocardial infarcts, and cardiomegaly |
22 | Nothing detected | Methazolamideb ibuprofen, dipivefrin, carbachal, timolol |
No diagnosis |
ER, emergency room; F, female; M, male; PMI, postmortem interval.
Toxicological determinations were performed on all subjects.
Medications prescribed in the last month of life. Other medications listed were prescribed more than 1 month before death.
Coroner’s report on microscopic diagnoses not available for this case only.
Table 2.
Characteristics of the Depressed Subjects
| Age/ Gender |
Smoker | Cause of Death | PMI (Hours) |
Toxicologya (blood) | Medicationb | Axis I Diagnosis | Age of Onset of MDD |
Duration of MDD (Years) |
|---|---|---|---|---|---|---|---|---|
| 30/M | Yes | Suicide, self-inflicted gunshot wound |
18 | Ethanol, .07c | None | MDD (chronic nonmelancholic, nonpsychotic); alcohol abuse 2 years prior |
27 | 3 |
| 34/F | No | Suicide, asphyxia by carbon monoxide |
24 | Ethanol, .12 (urine), carbon monoxide, alprazolam |
Alprazolam,b amoxicillin,b valproic acid,b nitrofurintoin,b trazodone, risperidone, |
MDD (severe, nonpsychotic); Panic disorder with agoraphobia |
14 | 20 |
| 40/F | No | Hypertensive, hypertrophic cardiomyopathy with cardiomegaly and congestive heart failure |
25 | Morphine, codeine, hydrocodone, diphenhydramine |
Temazepam,b fluoxetine,b hydrocodone,b etodolac |
MDD (recurrent, in full remission) sedative, hypnotic, anxiolytic related disorder NOS |
35 | 3 |
| 42/M | No | Suicide, drowning | 20 | Sertraline, ethanol .02, (urine), diphenyhydramine |
Sertralineb | MDD (single episode, severe, nonpsychotic) |
41 | 0.25 |
| 42/M | Not known | Suicide, self-inflicted gunshot wound |
20 | Nothing detected | None | MDD (single episode, nonmelancholic, nonpsychotic) |
42 | 0.5 |
| 46/M | No | Homicide, shotgun | 17 | Nothing detected | None | MDD (single episode, mild) | 45 | 1 |
| 47/M | No | Suicide, self-inflicted gunshot wound |
11 | Ethanol, - .19 | None | MDD (recurrent, moderate, nonpsychotic, nonmelancholic) |
27 | 20 |
| 48/M | No | Suicide, self-inflicted gunshot wound, cut wrists |
21 | Flurazepam | Flurazepam,b lorazepamb | MDD (severe, nonpsychotic, nonmelancholic); alcohol abuse 24 years prior |
36 | 12 |
| 50/F | Yes | Suicide, hanging | 23 | Nothing detected | Clomipramine, ranitidine, fluoxetine, thiothixene |
MDD (psychotic, mood congruent) |
46 | 4 |
| 54/M | Prior | Accidental death, asphyxia by carbon monoxide |
23 | Carbon monoxide, phenobarbital, phenytoin |
Sertralineb | MDD (moderate, chronic) | 51 | 3 |
| 62/M | Yes | Suicide, self-inflicted gunshot wound |
20 | Nothing detected | 6 days of buspirone, lorazepam |
MDD (severe, nonpsychotic, melancholic) |
59 | 3 |
| 63/F | Yes | Hypertrophic cardiomyopathy, with severe coronary atherosclerosis and cardiomegaly |
18 | Lidocaine | Fluoxetineb (quit 2 weeks prior to death), tolbutamide, digoxin, albuterol |
MDD | 55 | 8 |
| 67/F | Yes | Rupture of atherosclerotic aneurysm |
17 | Nothing detected | Doxepin,b alprazolam, nabumetone |
MDD (seasonal, recurrent, nonpsychotic, nonmelancholic); agoraphobia |
37 | 30 |
| 68/M | No | Suicide, asphyxia by carbon monoxide |
4 | Carbon monoxide | None | MDD (single episode, moderate) |
Unknown | Unknown |
| 73/M | No | Suicide, self-inflicted gunshot wound |
18 | Diazepam, codeine | Trazodone, fluoxetine, hydroxyzine, diazepam, nitroglycerine, captopril, furosemide |
MDD (severe, nonpsychotic, nonmelancholic) |
72 | 1 |
| 77/M | No | Suicide, hanging | 27 | Sertraline | Sertralineb | MDD (single episode, severe) | 51 | 26 |
| 78/F | No | Suicide, fall from height | 25 | Nothing detected | Lorazepam | MDD NOS; pathological gambling; delusional disorder |
63 | 15 |
| 82/M | No | Suicide, asphyxia by carbon monoxide |
12 | Carbon monoxide | Furosemide,b levothyroxine,b atenolol,b risperidone,b sertralineb |
MDD (recurrent); benzodiazapine abuse; history of MDD with psychotic symptoms |
25 | 57 |
| 87/F | Prior | Rupture of aortic aneurysm | 24 | Diphenhydramine | Albuterol,b flurazepamb trazodone, lisinopril, omeprazole, propoxyphene, hydrochlorothiazide |
MDD (recurrent, moderate, nonpsychotic, nonmelancholic) |
67 | 20 |
MDD, major depressive disorder; NOS, not otherwise specified; PMI, postmortem interval.
Toxicological determinations were performed on all subjects for all psychotropic medications.
Medications prescribed in the last month of life. Other medications listed were prescribed more than one month before death.
Ethanol was measured in blood (g/dL) unless otherwise indicated.
The depressed subjects consisted of 7 women and 12 men. The deaths of 13 of the 19 depressed subjects were ruled to be suicide by the coroner. Of the subjects with depression, Table 2 reveals that seven had a prescription for an antidepressant drug filled in the last month of life, and the antidepressant drug sertraline was detected postmortem in two of these subjects. Table 2 includes information on whether the depressed subjects were ever treated with an antidepressant drug. That an antidepressant drug was present in the blood of so few depressed subjects or suicide victims has been noted by others as well (Isometsa et al 1994; Marzuk et al 1995; Oquendo et al 1999).
The control subjects, consisting of 9 women and 11 men, did not meet criteria for an Axis I disorder at the time of their deaths and were closely age-matched with the depressed subjects. Two control subjects met diagnostic criteria for alcohol abuse at 10 and 30 years before their deaths.
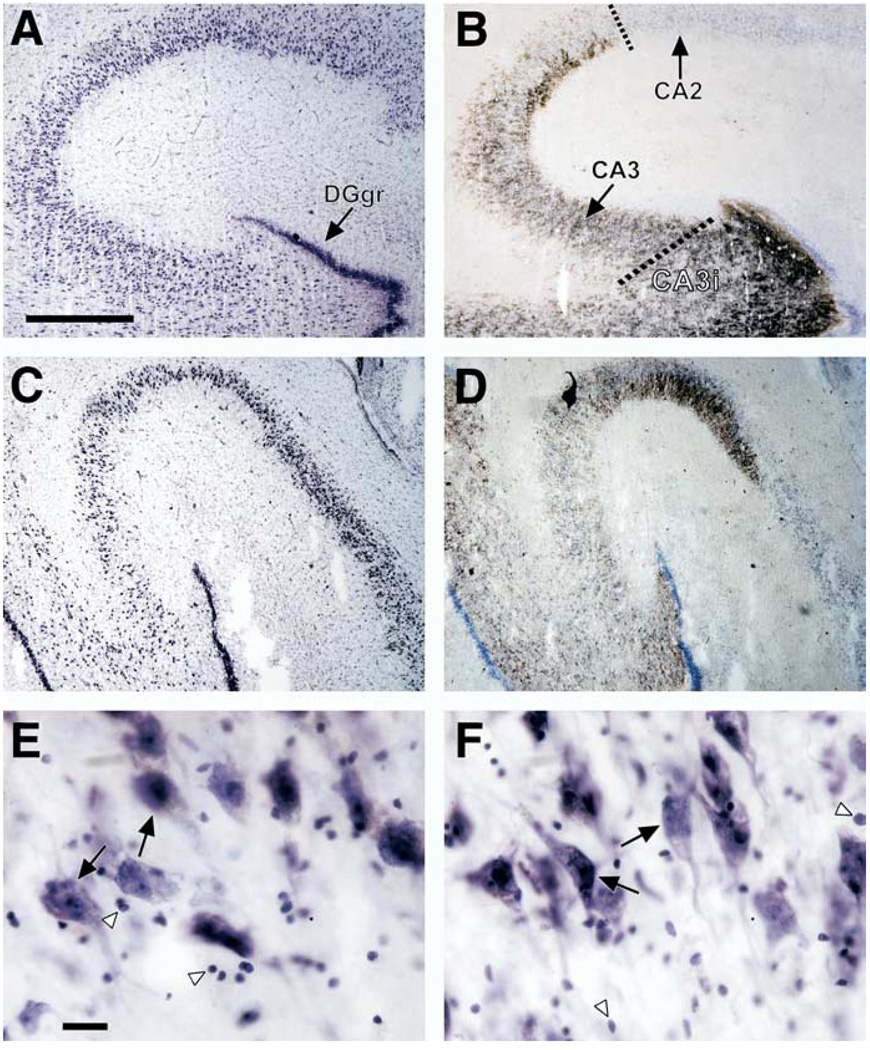
The hippocampal formation was dissected from the right temporal lobe at autopsy. Two coronal cuts were made: at the interface between the anterior and posterior segments of the uncus and at 2 cm posterior to the first cut. The body of the hippocampal formation was dissected, frozen in dry-ice-cooled isopentane, and stored at −80°C. Tissue samples from age-matched pairs of control and depressive subjects were coded throughout all histologic procedures, image processing and morphometric analysis so that laboratory personnel were not aware of the psychiatric diagnoses assigned to the samples. Coded and anonymous blocks of tissue from the two groups of subjects were alternatively selected and sectioned. Care was taken that coded blocks of tissue from both cohorts were sectioned in an alternating manner to avoid a possible difference in histologic treatment of tissue. From the anterior surface of each coded block four frozen sections were cut on an IEC microtome at a setting of 40 µm thickness by the same experienced technician. The sections were thaw-mounted on chrome-alum subbed microscope slides and air dried before staining. Three of these sections were processed for routine staining Nissl substance with cresyl violet. The remaining section was fixed in Millonig’s buffer (Dowlatshahi et al 2000) and processed by the Timm’s sulfide silver method to facilitate detection of hippocampal subregions (Danscher 1981). Regions of the hippocampal formation were identified (Amaral and Insausti 1990). The pyramidal neuron layer of CA3 makes a sharp bend extending toward the hilus of the dentate gyrus and folds back on its own self. In this study, the portion of CA3 extending between CA2 and the sharp bend toward the hilus is termed CA3, and the portion of CA3 extending toward the hilus and enclosed in the granule cell layer is termed CA3-internal (CA3i) (Figure 1).
Figure 1.
Brightfield photomicrographs of coronal sections of the postmortem human hippocampal formation. (A) Cresyl violet–stained section from a 70-year-old male control subject (postmortem interval = 20 hours) and (B) an adjacent section processed by Timm staining. Note the intensely stained granule cell layer of the dentate gyrus (DGgr) in (A) and (B), and the clear demarcation in (B) between hippocampal subfields CA2 and CA3 afforded by the Timm staining. A dashed line identifies the border between CA2 and CA3, and the second dashed line shows the border between CA3 inserted within the dentate gyrus (CA3i) and CA3 external to the dentate gyrus. (C) Cresyl violet–stained section from a depressed 77-year-old man (postmortem interval = 26 hours) and (D) an adjacent section processed by Timm staining. Pyramidal neurons and glial nuclei of CA3 are highlighted (E, Control; F, MDD) with large black arrows and white arrowheads, respectively. The scale bars in (A) and (E) are 750 µm and 25 µm, respectively.
After staining, the section thickness was determined by differential focusing using an oil-immersion high-powered objective. An experienced observer using these criteria focused from the top to the bottom of all sections at the selected points (Gardella et al 2003; Uylings et al 1986). The vertical movement of the microscope stage was measured by a microcator (Heidenhain, Germany). For each section, the thickness was measured at three randomly selected points in the CA areas, avoiding the edges of the section, and mean values were determined (Andersen and Gundersen 1999; Dorph-Petersen et al 2001). Because these three measurements per section in the CA subareas were very similar, no more measurements per section were performed. The coefficient of variance (CV = ± SD/mean) for intrasection thickness was 3% (controls) and 5% (MDD). The CV for intersection thickness was 4% (control) and 5% (MDD). These coefficients of variance are much smaller than the difference in section thickness for the two cohorts (~20%). The differential shrinkage in depressed subjects was in the z axis, because the sections were thaw-mounted on glass slides immediately after cutting.
The number of glia and neurons (pyramidal neurons, granule cell neurons of the dentate gyrus) per volume unit was estimated with the optical disector (Pakkenberg and Gundersen 1988). Cell measurements were made with a 63.5X oil objective (N.A. 1.4). The horizontal x axis and y axis dimensions of the three-dimensional disector counting boxes in CA1–CA3 were 150 × 150 µm, and in the granule cell layer of the dentate gyrus they were 50 × 50 µm. These counting boxes were positioned in a systematic, randomly placed manner in three sections per subject. The counting unit of a cell was the center of the nucleus defined by focusing on the clear nuclear edge and the most clearly defined nuclear chromatin and nucleolus (e.g., Gardella et al 2003; Gundersen et al 1988; Howard and Reed 1998). A nucleolus is present in pyramidal cells but not in glia. Using this counting unit, the height of the counting box was the thickness of the pertinent section at the counting sites (see Results for thickness values in the two cohorts). In each brain in CA1–CA3, 12–15 counting boxes per region per subject were examined, and in the granule cell layer of the dentate gyrus, 7–15 boxes were examined per subject. In all the three-dimensional boxes for CA1, 135 pyramidal neurons and 180 glia were counted on average per subject; for CA3, 135 pyramidal neurons and 210 glia were counted on average per subject. For the granule cell layer of the dentate gyrus, an average of 80 neurons and 38 glia were counted per subject in all three-dimensional boxes. In addition, to correct for the differential shrinkage along the z axis between MDD and control subjects, the probe volumes for cell densities were multiplied by 40 µm, divided by the actual section thickness, so that the height of the counting box became equal to the “section thickness” setting of the cryostat. The cell densities thus were differentially corrected relative to the uncorrected values, that is, relatively more in the MDD cases. The advantage of this correction for calculating cell density is that it assesses density of neurons and glia in the original sections before histologic processing and thus before differential shrinkage of MDD as opposed to control sections. This permits the comparison of cell density between cohorts without the confounding influence of group differences in section thickness.
Somatic size of neurons and glial nuclear size was indicated from projected surface area measurements in the absence of the vertical section design (Gundersen et al 1988; Uylings and van Pelt 2002) applying a 63.5X oil objective (N.A. 1.4).
Least squares adjusted means and SE estimates are presented. The main statistical analysis used was a repeated-measure analysis of variance (ANOVA; SAS PROC Mixed), with diagnosis as a between-subjects effect; CA regions as a within-subjects effect; and age, postmortem interval, tissue pH, and brain weight as covariates (entered separately). Gender was included as a factor in some analyses. Size and density data for neurons and glia in the granule cell layer of the dentate gyrus were analyzed separately from data gathered in the CA regions because the scale of these measures was markedly different from the scale of data from the CA regions. The potential effect of being an active smoker before death, having an antidepressant medication prescription within the last month of life, or of dying by suicide was assessed individually by evaluating the depressives with one of these potential confounds verses the depressives without these confounds. Bonferroni corrections were used to test for statistically significant effects between the two subject groups; a p value of .05 was divided by eight, representing the anatomic variables being assessed (CA neuron density, CA neuron soma size, CA glial density, CA glial nuclear size, DG neuron density, DG neuron soma size, DG glial density, and DG glial nuclear size).
Pearson correlations were calculated to examine potential interactions between age, postmortem interval, tissue pH, age at onset, and the duration of the depressive illness on the eight neuronal and glial density and size measures. A Bonferroni-corrected p value of .00125 was necessary for there to be a statistically significant effect of these variables on neuronal and glial measures.
Results
Age, Postmortem Interval, and Tissue pH
There was no significant difference between subject groups in age, postmortem interval (time between death and freezing tissue), or tissue pH. The average age (years, mean ± SE) of the two groups was 57.9 ± 3.6 (range 26–84) for control and 57.4 ± 3.9 (range: 30–87) for depressive subjects. The average postmortem interval (hours) of the two groups was 20.5 ± 1.1 for control and 19.3 ± 1.2 for depressive subjects. The average pH of cerebellar tissue was 6.5 ± .1 for control and 6.6 ± .1 for depressive subjects.
Section Shrinkage
There was a robust and significant difference between control and depressive patients in the thickness of the sections after histologic processing, regardless of cutting tissue blocks alternatively from control and depressed subjects at the same cryostat setting (40 µm). After histologic processing, sections from the 21 control subjects were 19.5 ± .7 µm thick (mean ± SE), and sections from the 19 subjects with MDD were 16.1 ± .7 µm thick (F = 11.05, df = 38; p < .002). As a group, sections from depressive subjects shrank approximately 18% more than sections from control subjects. No significant difference was detected in section thickness between depressed subjects who died by suicide versus depressed subjects dying form other causes of death (data not shown). For the group of control subjects, there was no significant correlation between section thickness and any of the confounding factors (age, postmortem interval, pH, or brain weight; data not shown). Likewise, for the group of subjects with MDD, there was no significant correlation between section thickness and any of the confounding factors (age, postmortem interval, pH, brain weight, duration of depression, or age at onset of depression; data not shown).
Soma and Nuclear Size
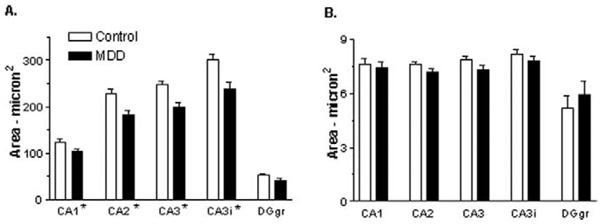
There was a significant effect of diagnosis and of region, but no significant diagnosis by region interaction, on soma size of pyramidal neurons in the CA regions (Table 3, Figure 2A). There was a 17%–21% decrease in the mean soma size of pyramidal neurons in depressed subjects, compared with normal control subjects. In the granule cell layer of the dentate gyrus, there was a statistical trend for an effect of diagnosis, with neuronal soma size decreased by 22% in MDD (Table 3, Figure 2A). There was no significant effect of diagnosis or region on the size of glial nuclei in the CA regions or granule cell layer of the dentate gyrus (Table 3, Figure 2B).
Table 3.
Statistical Analysis of Neurons and Glia in the Hippocampal Formation in Major Depressive Disorder
| Region | F | df | p-Value |
|---|---|---|---|
| CA1–CA3 Pyramidal Neuron Subfields |
|||
| Pyramidal neuron soma size |
|||
| Diagnosis | 14.04 | 38.1 | p = .0006a |
| Region | 172.75 | 38.1 | p < .0001a |
| Diagnosis × region | 2.81 | 38.1 | p = .0522 |
| Pyramidal neuron density | |||
| Diagnosis | 54.47 | 37.6 | p < .0001a |
| Region | 92.28 | 37.5 | p < .0001a |
| Diagnosis × region | 2.14 | 37.5 | p = .1112 |
| Glial nuclear size | |||
| Diagnosis | 1.37 | 37.5 | p = .2492 |
| Region | 12.89 | 37 | p < .0001a |
| Diagnosis × region | .98 | 37 | p = .4144 |
| Glial density | |||
| Diagnosis | 23.93 | 36 | p < .0001a |
| Region | 17.6 | 37.4 | p < .0001a |
| Diagnosis × region | .47 | 37.4 | p = .7069 |
| Dentate Gyrus Granule Cell Layer |
|||
| Neuron size Diagnosis |
7.81 | 38.1 | p = .0081 |
| Neuron density Diagnosis |
15.05 | 38 | p = .0004a |
| Glial nuclear size Diagnosis |
.63 | 38 | p = .4326 |
| Glial density Diagnosis |
13.54 | 38 | p = .0007a |
Statistically significant Bonferroni-adjusted p value ≤ .00625.
Figure 2.
Neuronal soma size (A) and glial nuclear size (B) in the hippocampus of control subjects and subjects with major depressive disorder (MDD). Pyramidal neurons were quantified in hippocampal fields CA1–CA3, and granule cells were quantified in the granule cell layer of the dentate gyrus (DGgr) of 21 control and 19 depressed subjects with the exception of 18 depressed subjects for CA1 and CA2. Values are least squares adjusted means ± SE. (A) There is a significant effect of diagnosis on pyramidal neuron soma size (*p = .0006) in all CA fields and a trend for an effect of diagnosis on granule cell soma size in the dentate gyrus (p = .0081). Pyramidal neuron soma size is decreased by 17%–21%, and granule cell soma size is decreased in the dentate gyrus by 22%. (B) Glial nuclear size was not significantly affected in MDD. CA3i refers to CA3 pyramidal neurons that are inserted within the dentate gyrus.
Cell Density
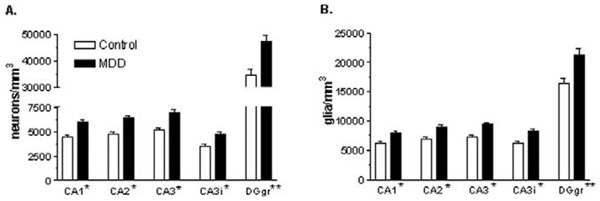
There was a significant effect of diagnosis and of region, but no significant diagnosis by region interaction, on the density of pyramidal neuron cell bodies in the CA regions (Table 3, Figure 3A). After correction for differential shrinkage along the z axis, there was still a significant, 35% –36% increase in the mean density of pyramidal neurons in depressed subjects, compared with normal control subjects. In the granule cell layer of the dentate gyrus, there was still a significant effect of diagnosis, with granule cell density increased by 37% in MDD (Table 3, Figure 3A).
Figure 3.
Neuronal (A) and glial (B) density in the hippocampus of control subjects and subjects with major depressive disorder (MDD). Pyramidal neurons were quantified in hippocampal fields CA1–CA3, and granule cells were quantified in the granule cell layer of the dentate gyrus (DGgr) of 21 control subjects and CA3 and dentate gyrus (DGgr) from 19 depressed subjects. Data in CA1 and CA2 are presented from 18 depressed subjects. Values are least squares adjusted means ± SE. (A) There is a significant effect of diagnosis on pyramidal neuron density in all CA subfields (*p < .0001) and granule cell density in the dentate gyrus (**p = .0004). Pyramidal neuron density is increased by 35%–36% in CA subfields, and granule cell density is increased in the dentate gyrus by 37%. (B) There is a significant effect of diagnosis on glial cell density in all CA pyramidal neuron subfields (*p < .0001) and glial cell density in the granule cell layer of the dentate gyrus (**p = .0007). Glial cell density is increased by 28%–31% in the CA pyramidal neuron subfields and glial cell density is increased in the granule cell layer of the dentate gyrus by 30%.
After correction for differential shrinkage along the z axis, there was still a significant effect of diagnosis and of region, but no significant diagnosis by region interaction, on the density of glia within the pyramidal cell layer of the CA regions (Table 3, Figure 3B). There was a 28%–31% significant increase in the mean density of glia within the pyramidal cell layer of the CA regions in depressed subjects, compared with normal control subjects. In the granule cell layer of the dentate gyrus, there is a significant effect of diagnosis, with glial cell density increased by 30% in MDD (Table 3, Figure 3B).
Other Variables
The potential effect of a number of factors (gender, age, postmortem interval, tissue pH, smoking, suicide, antidepressant drug prescription in the last month of life, duration and age of onset of depression) on neuronal and glial changes in the hippocampal formation in depression was determined. On the basis of covariate analyses, the main findings of increased neuronal and glial density and decreased neuron soma size in depression were not significantly altered when taking into consideration factors such as gender, age, postmortem interval, tissue pH, brain weight, smoking, antidepressant drug prescription in the last month of life, or suicide (data not shown). There are no significant correlations between the age of onset of depression or the duration of the illness and any of the density or size measures (data not shown). When using Bonferroni corrections for multiple comparisons, there were no significant correlations between age, postmortem interval, tissue pH or brain weight, and the neuronal and glial density and size measures.
Discussion
Several imaging studies report that hippocampal volume is decreased in MDD, yet no cellular basis for a reduction in hippocampal volume has been identified. To the authors’ knowledge, this is the first study to evaluate neuronal and glial density and soma and glial nucleus size in postmortem hippocampus in a large cohort of subjects with MDD and age-matched psychiatrically normal control subjects. Three-dimensional cell counting methods were applied to the evaluation of neurons and glia in the human hippocampal formation in major depression. Cryostat sections have been reported to show a uniform shrinkage in the z axis (Gardella et al 2003; Hatton and von Bartheld 1999) allowing the three-dimensional cell-counting technique applied in this study, which used the counting criterion of nuclei centers. After correction for differential shrinkage in the z axis, the density of granule cells in the dentate gyrus and pyramidal neurons in all hippocampal CA subfields is still significantly increased in MDD by approximately 35%. The average soma size of pyramidal neurons is significantly decreased in MDD. In MDD, glial density is significantly increased by about 30% across hippocampal pyramidal subfields and the granule cell layer of the dentate gyrus. The substantial increases noted in neuronal and glial packing density and decrease in neuronal soma size detected in postmortem tissue show per cell a reduced neuropil and can thus be related to the decrease in hippocampal volume noted by structural imaging studies in MDD.
There are a number of strengths to the observations in postmortem tissue presented here regarding increased packing density of neurons and glia in the hippocampal formation in depression. Strengths of this study of postmortem tissue include the large cohorts of control subjects and depressives, the use of retrospective psychiatric assessments of both the control subjects and those with major depressive disorder, the use of a balance of men and women in both cohorts ranging in age over 6 decades, the inclusion of some major depressive subjects not dying by suicide, the use of Timm staining to delineate the CA2 from CA3 subregions of the hippocampus proper, and the use of toxicological screening and reporting of medication histories of all subjects.
A number of potential limitations in this study of hippocampal cellular features in depression deserve mention. Only one rostrocaudal level of the right hippocampal formation was available for examination. Consequently, only data regarding neuronal and glial density are presented, and the total number of these cells throughout the hippocampal formation cannot be assessed. Random sampling of the entire hippocampal formation at regular intervals will be necessary to evaluate potential changes in total numbers of neurons and glia in depression, although Lucassen et al (2001) and Muller et al (2001) reported no massive cell death in the hippocampus in depression. Additional limitations related to this study involve the use of mostly suicide victims in the depressive cohort and the use of many depressed subjects with a history of treatment with antidepressant medications at some time during their lives.
An unexpected observation of this study, related to the measurement of cell density, is the recording of a significant 18% greater z axis shrinkage of hippocampal sections in depressed subjects than in age-matched control subjects. As outlined in the Methods and Materials section, there were no obvious differences in handling of tissue samples that would account for the enhanced shrinkage in depressed subjects. Among several possible causes of this differential shrinkage, one might speculate from this observation that tissue from depressed subjects contains more water. Interestingly, Krishnan et al (1991) reported significantly shortened T1 relaxation times for hippocampus (although not in the thalamus or cortical white matter) in older depressed patients. Shorter T1 relaxation times were interpreted by Krishnan et al (1991) to reflect differences in the content or organizational properties of hippocampal water protons in the depressed patients. Such potential changes in depressed patients in the properties of water detected with MRI may parallel changes in the shrinkage of such tissues processed postmortem. To obtain a fair and statistically conservative comparison of numerical cell density in depressed and control subjects, the technique used for determining cell density was adapted to account for the presence of differential shrinkage in the depressed subjects.
The rank order of pyramidal neuron density between hippocampal subfields was compared with two other studies using nonbiased cell-counting techniques. In agreement with Heckers et al (1991) and Walker et al (2002), the highest density of pyramidal neurons is in CA3, followed by CA2 and CA1. The absolute density values for pyramidal neurons are higher in these two published reports, likely because of the use of formalin-fixed tissues in these studies versus frozen sections in our study. The size of pyramidal neuron soma determined in our study is also in good agreement with that noted by Arnold et al (1995).
Neuronal and glial changes are detected elsewhere in the brain in depression. In contrast to the increase in cell density noted in hippocampus, studies by Ongur et al (1998), Rajkowska et al (1999), Cotter et al (2001, 2002), and Bowley et al (2002) report a decrease in the density or number of glia in various regions of frontolimbic cortex and amygdala in depression. In these studies, changes in glial density in MDD are not consistently shown across all layers in all cortical regions. Varying cortical pathology versus hippocampal pathology is not unexpected considering the unique normal functions and unique contributions of these regions to the psychopathology of depression. Other evidence of dissimilarities between prefrontal cortex and hippocampus in depression comes from the work of Mayberg and colleagues (Kennedy et al 2001; Mayberg et al 2000, 2002). Successful clinical treatment (or even the use of placebo) in depression was associated with an increase in metabolism in prefrontal cortex and a decrease in metabolism in hippocampus.
The different pattern of density change noted in depression in the hippocampus in contrast to frontal cortical areas may be related to a unique reduction in neuropil in the hippocampus in depression. Neuropil consists of the lattice of glial cells and their processes, dendrites, and proximal axons surrounding neuron cell bodies. The hypothesis of neuropil reduction in the hippocampus in MDD is supported by other postmortem studies revealing a decrease in dendritic spine density on neurons and diminished arborization of apical dendrites in the subiculum in a small group of mixed subjects with bipolar disorder or depression (Rosoklija et al 2000) and decreased level of synaptic proteins found in CA4 hippocampal region in bipolar depression (Harrison and Eastwood 2001). Thus, the diminished volume of the hippocampus in depression that some studies have found may be critically determined by a loss in neuropil including dendritic branching, dendritic spine complexity, and glial processes.
Alterations in cell density and soma size in the hippocampal formation in depression may be related or in response to diminished availability of neurotrophic factors in the brain in depression. Supporting evidence for this hypothesis comes from studies in experimental animals in which stress and antidepressant drugs have significant effects on brain-derived neurotrophic factor (BDNF) and related signal transduction systems in brain (Duman et al 1999). There is preliminary evidence the BDNF is the human hippocampus can be regulated by chronic treatment with antidepressant medications. In an immunohistochemical study of subjects with MDD, and others with bipolar disorder or schizophrenia, the immunoreactivity of BDNF, as measured by optical density, is up-regulated in the dentate gyrus and hilus only in subjects taking antidepressant medication (Chen et al 2001). The small number of depressed subjects not taking psychotropic medications in this study prevents determination of whether BDNF is significantly affected in drug-free subjects with MDD, however. In a recent study of the hippocampus, Dwivedi et al (2003) observed a significant reduction in mRNA and protein levels of BDNF in hippocampus in suicide victims with either MDD or other psychiatric disorders. In the Dwivedi et al (2003) study, the decrease in expression of BDNF occurred regardless of antidepressant treatment. To determine a possible role for BDNF in MDD-related changes in cell density, it will be of interest to perform studies on cell counting and BDNF expression in the same subjects.
In conclusion, differential shrinkage in thickness of hippocampal cryostat sections between subjects with MDD and control subjects may be related to a differential content of water in the hippocampus in MDD. The increased cell density in the hippocampus indicates a reduction of neuropil per cell, which may contribute to the volume reduction noted in MRI studies in the hippocampus in MDD. Independent replication of the findings regarding differential shrinkage of sections and increased cell density in subjects with MDD will be an important next step.
Acknowledgments
This study was supported by the National Alliance for Research on Schizophrenia and Depression, and Public Health Service Grant Nos. P20 RR17701, MH63187, MH61578, MH60451, and MH67996. We acknowledge the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also thank the Cuyahoga County Coroner and its staff, Cleveland, Ohio, for their willing assistance.
References
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press; 1990. pp. 711–755. [Google Scholar]
- Andersen BB, Gundersen HJ. Pronounced loss of cell nuclei and anisotropic deformation of thick sections. J Microsc. 1999;196:69–73. [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ, Trojanowski JQ. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc. 2001;204:232–246. doi: 10.1046/j.1365-2818.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen G, Wang JF, Chen B, Young LT. Increased hippocampal supragranular Timm staining in subjects with bipolar disorder. Neuroreport. 2000;11:3775–3778. doi: 10.1097/00001756-200011270-00036. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;56:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS. Differential tissue shrinkage and compression in the z-axis: Implications for optical disector counting in vibratome-, plastic- and cryosections. J Neurosci Methods. 2003;124:45–59. doi: 10.1016/s0165-0270(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: Calibration of the optical disector counting method reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry. 1991;48:1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Bios Scientific Publications. Microscopy Handbook Series, Nr. 41. Oxford: Royal Microscopical Society; 1998. [Google Scholar]
- Isometsa ET, Henriksson MM, Aro HM, Heikkinen ME, Kuoppasalmi KI, Lonnqvist JK. Suicide in major depression. Am J Psychiatry. 1994;151:530–536. doi: 10.1176/ajp.151.4.530. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Doraiswamy PM, Figiel GS, Husain MM, Shah SA, Na C, et al. Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 1991;3:387–391. doi: 10.1176/jnp.3.4.387. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, et al. Hippocampal apoptosis in major depression is a minor event and absent from sub-areas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuk PM, Tardiff K, Leon AC, Hirsch CS, Stajic M, Hartwell N, Portera L. Use of prescription psychotropic drugs among suicide victims in New York City. Am J Psychiatry. 1995;152:1520–1522. doi: 10.1176/ajp.152.10.1520. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Malone KM, Ellis SP, Sackeim HA, Mann JJ. Inadequacy of antidepressant treatment for patients with major depression who are at risk for suicidal behavior. Am J Psychiatry. 1999;156:190–194. doi: 10.1176/ajp.156.2.190. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shi X, Konick L, Overholser JC, Jurjus G, Meltzer HY, et al. Neurokinin-1 receptors are decreased in major depressive disorder. Neuroreport. 2002;13:1223–1227. doi: 10.1097/00001756-200207020-00031. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986;18:19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Pelt J. Measures for quantifying dendritic arborizations. Network Comput Neural Syst. 2002;13:397–414. [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, Crow TJ. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am J Psychiatry. 2002;159:821–828. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]