Abstract
Obesity and related metabolic conditions are of epidemic proportions in most of the world, affecting both adults and children. The accumulation of lipids in the body in the form of white adipose tissue in the abdomen is now known to activate innate immune mechanisms. Lipid accumulation causes adipocytes to directly secrete the cytokines interleukin (IL) 6 and tumor necrosis factor α (TNFα), but also monocyte chemoattractant protein 1 (MCP-1), which results in the accumulation of leukocytes in fat tissue. This sets up a chronic inflammatory state which is known to mediate the association between obesity and conditions such as cardiovascular disease, type 2 diabetes, and cancer. There is also a substantial literature linking inflammation with risk for depression. This includes the observations that: 1. People with inflammatory diseases such as multiple sclerosis, cardiovascular disease, and psoriasis have elevated rates of depression; 2. Many people administered inflammatory cytokines such as interferon α develop depression that is indistinguishable from depression in non-medically ill populations; 3. A significant proportion of depressed persons show upregulation of inflammatory factors such as IL-6, C-reactive protein, and TNFα; and 4) Inflammatory cytokines can interact with virtually every pathophysiologic domain relevant to depression, including neurotransmitter metabolism, neuroendocrine function, and synaptic plasticity. While many factors may contribute to the association between inflammatory mediators and depression, we hypothesize that increased adiposity may be one causal pathway. Mediational analysis suggests a bi-directional association between adiposity and depression, with inflammation possibly playing an intermediary role.
Keywords: Obesity, inflammation, adiposity, depression, risk factors, cytokines, adipocytokines, fatty acids, lipids
1.0 Introduction
Obesity is of epidemic proportions in the U.S. and in many other parts of the world (Bornstein et al., 2008; Cumurcu et al., 2009; Wilborn et al., 2005). Not only are the rates of obesity and related metabolic conditions such as cardiovascular and liver disease, dyslipidemias, and type 2 diabetes on the rise in adults; children and adolescents are also increasingly affected (Ben-Sefer et al., 2009; MacPhee, 2008; Wang and Lobstein, 2006). In fact, more than 1 billion people are overweight worldwide, with more than 300 million meeting the definition of obesity (Shoelson et al., 2007; World Health Organization, 2010).
In parallel with the rise in obesity has been an increase in associated endocrine (e.g., diabetes), metabolic (e.g., dyslipidemias), and other medical disorders (e.g., cardiovascular disease, hepatic steatosis [fatty liver disease], and certain forms of cancer) (Bellentani and Marino, 2009; Golden et al., 2009; Hevener and Febbraio, 2010) as well as inflammation. For comprehensive reviews of the relationships between obesity, metabolic syndrome (MetS), and inflammation, see Shoelson (Shoelson et al., 2007) Sutherland et al. (Sutherland et al., 2004), and Dandona et al. (Dandona et al., 2005). Emerging literature suggests that inflammation, as measured by elevated inflammatory cytokines and other inflammatory markers, may also represent both a cause and consequence of depression. As we will argue below, increasing evidence implicates obesity, high fat diets, and obesity-induced inflammation in the causal pathway for depression in some people. As well, evidence suggests that prior depression may increase risk for the subsequent development of adiposity. Therefore, depression and lipid accumulation in adipose tissue may form a mutually-enhancing dyad, in much the same way as body fat and other medical diseases. This paper will review the evidence for the association between adiposity and depression and will suggest that inflammation may serve as a possible causal link between these two common conditions.
2.0 Adiposity as a Risk Factor for Both Inflammation and Depression
2.1 Why Obesity? The Adaptive Value of Energy Retention in the Form of Lipids
Although modern constructs of obesity suggest that increasing body fat is “bad” from both a metabolic and cosmetic standpoint, the ability to absorb and retain high levels of energy stores has adaptive value (Wells, 2006). Fat deposition begins in the late gestational period and early infancy and contributes to infant fitness and survival (Kennaugh and Hay, Jr., 1987; Wells, 2006). Fat deposition increases again in adolescence and early adult life, particularly in females, which enhances the capacity to reproduce (Wells, 2006). Under conditions of unpredictable food sources, including the effects of seasons and periodic famines, fat deposition enhances the capacity to survive, reproduce and maintain survival of offspring (Wells, 2006). In particular, the capacity of humans to deposit large fat stores relative to many other species has been hypothesized to account, in part, for the ability to range well out of the typical temperate zones of primates (Wells, 2006). This ability to adapt to seasonal variations in energy availability has had extremely high adaptive value through most of human history. However, this general capacity for energy retention in the form of fat creates problems in the face of easy access to high calorie food. So, our ability to “forage and retain” calories appears to contribute to the propensity toward obesity in the human species. As noted by Wells (Wells, 2006), “the capacity to accumulate fat has therefore been a major adaptive feature of our species, but is now increasingly maladaptive in the modern environment where fluctuations in energy supply have been minimized, and productivity is dependent on mechanization rather than physical effort.”
2.2 Adipose Tissue
Body fat traditionally has been thought of as a simple depository of accumulated excess calories in the diet. However, fat in the form of adipose tissue is understood as a complex and multifaceted organ system (Mathieu et al., 2009). There are two forms of adipose tissue in the body. The first is so-called brown adipose tissue (BAT)(also known as brown fat), which contains a large number of mitochondria and is highly metabolically active. A major purpose of BAT is to generate body heat (Enerback, 2009). Brown adipocytes have higher concentrations of uncoupling protein 1 (UCP-1, also known as thermogenin) in the inner membrane of the mitochondria. UCPs alter the permeability of the inner mitochondrial membrane and allow proton leakage into the intermembranous space. Heat is generated by the uncoupling of the mitochondrial respiratory chain (Kozak et al., 1988; Muzzin, 2002; Nicholls et al., 1978). Brown adipose tissue is present in significant quantities in humans only in neonates (Fruhbeck, 2008; Vazquez-Vela et al., 2008).
The second type of fat stored in the body is white adipose tissue (WAT) or white fat, which is the main site of long-term storage of fat in the body. WAT, particularly in the form of abdominal obesity, is the main contributor to diseases such as type 2 diabetes, cardiovascular disease, and certain forms of cancer associated with obesity (Calabro and Yeh, 2008; Despres et al., 2008; Hevener and Febbraio, 2010). WAT serves several important roles in the body. The most obvious is the role of WAT as a storage site for triglyceride storage and release of fatty acids (Vazquez-Vela et al., 2008). In this role, it breaks down complex triglycerides into glycerol and free fatty acids, which are used in energy metabolism. Adipocytes in WAT also secrete a variety of hormones, inflammatory factors such as cytokines (referred to as adipocytokines), and other proteins (Vazquez-Vela et al., 2008) as described in greater detail below.
2.3 Lipids and Inflammation
2.3.1 Components of the Inflammatory Response
Inflammation is a complex and coordinated response of the body to a range of noxious stimuli. This can include infectious agents, such as bacteria or virus; however, the inflammatory response can also occur in response to other external or internal cues, including components of damaged or diseased tissues. For the purpose of the present discussion, inflammation is an immune response that largely derives from activation of the innate immune system. The innate immune response represents the initial and non-specific responsiveness of the body to infection, thereby providing immediate protection. Exposure to a pathogen (i.e., foreign protein) elicits a rapid, local cellular response, releasing a variety of factors including histamine, leukotrienes, prostaglandins, and chemokines (e.g., chemokine [CC-motif] ligands [CCL] 1–28). This response initiates the cardinal signs of inflammation, including redness and heat due to local vasodilation, edema, produced by extravasation of fluid from the vascular compartment, and pain. Chemokines (chemotactic cytokines) and related proteins function as chemoattractants for leukocytes (e.g., lymphocytes, monocytes). Local inflammation initially activates naïve T-lymphocytes, generating T-helper (CD4+) and cytotoxic (CD8+) cells (Harrington et al., 2005; Stockinger et al., 2007). Other leukocytes including macrophages are attracted to the site of local inflammation, leading to the release of inflammatory factors, particularly cytokines, which include interleukins (IL-1α, IL-1β, IL-2 – 35), interferons (IFNα, β, γ, and ω), and tumor necrosis factor (e.g., TNFα). Many of these cytokines are pro-inflammatory (e.g., IL-1α/β, IL-6), although some serve anti-inflammatory (e.g., IL-10, transforming growth factor-β [TGFβ]) and anti-apoptotic (e.g., IL-9) roles.
The generation of the initial inflammatory response depends on the recognition of specific pathogen-associated molecular patterns (PAMPs) associated with groups of microorganisms by a range of pattern recognition receptors. The prototypical gram-negative bacteria endotoxin protein is lipopolysaccharide (LPS). PAMPs, but also endogenous proteins from injured tissues activate - like receptors (TLRs), which are membrane-spanning pattern-recognition receptors (TLR1–TLR10 in humans) (Cook et al., 2004). Activation of TLRs produces transductional activation of nuclear factor kappa B (NF-kB) as well as the mitogen-activated protein (MAP) kinase cascade including p38, JNK and ERK 1/2 pathways, resulting in an increased expression of cytokines (IL-1α and IL-1β), chemokines and other inflammatory mediators (Billack, 2006) (Hansson and Edfeldt, 2005)(Figure 1). IL-1 activation of IL-1 receptors induces the transcription of other proinflammatory (IL-6 and TNFα, which can also increase IL-1 expression) and anti-inflammatory (IL-10) cytokines. Activation of inflammatory signaling pathways also stimulates the synthesis of nitric oxide via specific nitric oxide synthases derived from endothelial cells (eNOS or NOS III), peripheral lymphocytes (inducible nitric oxide synthase [iNOS] or NOS II), and neuronal tissue (nNOS or NOS I) by activation of NADPH oxidase and NF-κB (Parul et al., 2007; Wu et al., 2008) (Wu et al., 2008; Parul et al., 2007). iNOS is induced by several cytokines including IFNγ and TNFα secreted in response to local inflammation. Nitric oxide (NO) serves a variety of functions, including vasodilation and neurotransmission; however, as a component of the immune system, NO is converted to S-nitrosothiols and related derivatives which function as free radicals that are toxic to invading organisms (Parul et al., 2007; Hughes, 2008) (Figure 1). Of significance to the later discussion, oxidized (“minimally modified”) low density lipoproteins (LDL) can also activate TLRs (specifically TLR4) leading to immune activation, including increased expression of the cytokines TNFα, MIP-2 (a mouse analogue to IL-8), and monocyte chemoattractant protein 1 (MCP-1; also known as cytokine (CC-motif) ligand 2 [CCL2]), a potent tissue chemotactic factor for activated monocytes/macrophages (Miller et al., 2005c). Immune activation by LDL is an important mediating link between LDL and atherosclerosis (Hansson and Edfeldt, 2005). TLR3/4 activation also leads to cholesterol accumulation in cells via inhibition of the lipid-X receptor (LXR) – ABCA1 complex efflux mechanism (Hansson and Edfeldt, 2005).
Figure 1. Intracellular mechanisms mediating the immune response: The role of toll-like receptors.
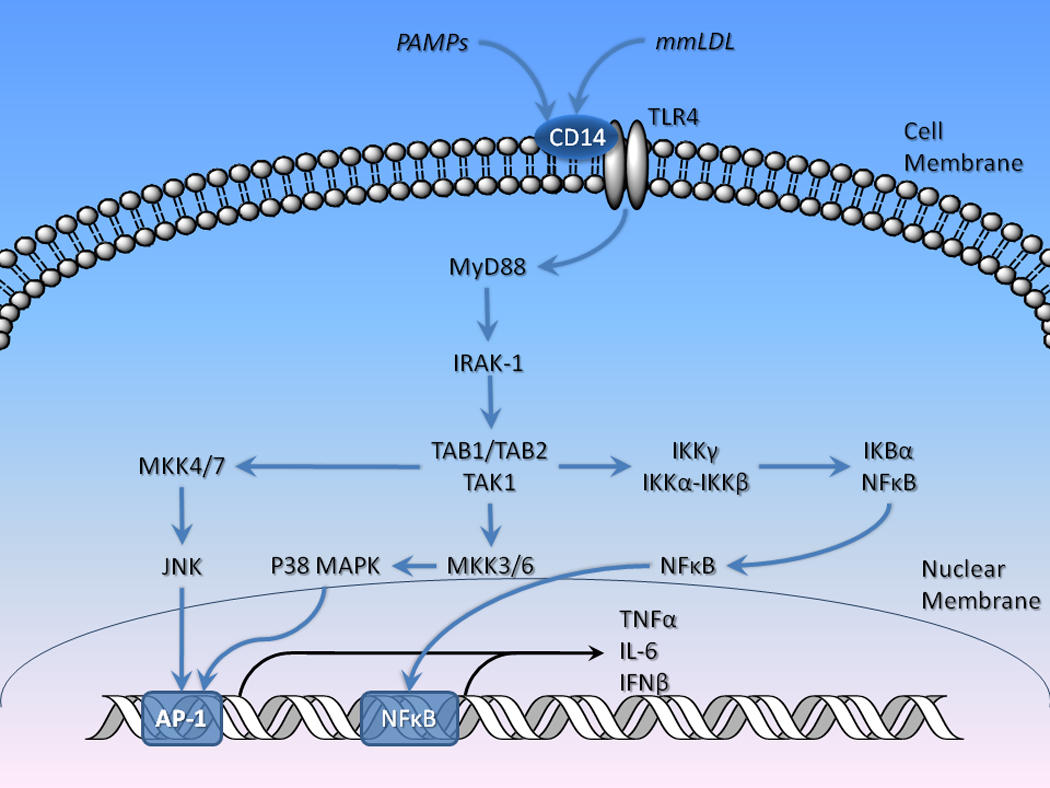
The generation of the initial inflammatory response depends on the recognition of specific molecular patters (PAMPs) associated with microorganisms or injured host tissues by pattern recognition receptors such as toll-like receptors (TLRs) and signal transduction cascades linked to important intracellular mediators of immune response including JNK, p38 MAPK, and NF-κB. Activation of TLRs links to a common transductional molecule, myeloid differentiation primary response gene 88 (MYD88), which, in turn, activates mitogen-activated protein kinase kinase kinase 7-interacting proteins (TAB1–4) and mitogen-activated protein kinase kinases(MKK) that activate P38 MAPK and JNK, which interact with AP-1, resulting in transcriptional activation of cytokine genes. TAB proteins also interact with the IKKγ – NF-κB complex, resulting in the release of the transcriptional factor NF-κB.
Activation of the innate immune response eventually leads to the stimulation of T cells including T-helper cells which in turn contribute to the activation and maturation of B-cells. Mature B-cells including plasma B-cells, memory B-cells, B-1, B-2 subsequently respond with targeted phagocytosis and the generation of antibodies that target the pathogen in a specific manner (Montecino-Rodriguez and Dorshkind, 2006). This activation of acquired or adaptive immunity ultimately culminates in the ability to “remember” a prior exposure to a pathogen resulting in a more specific and rapid immune response to subsequent pathogen exposures.
Depending on the magnitude and/or extent of the inflammatory response, cytokines can enter the peripheral circulation and travel to the liver, inducing up- and down-regulation of a large number of acute phase reactants. Up-regulated proteins suppress microbe growth, increase coagulation, and both activate and suppress the inflammatory response. Key among these for the present discussion are the pentraxins; these include the so-called short pentraxin, C-reactive protein (CRP) and serum amyloid P-component (produced mainly by the liver) and the long pentraxin, pentraxin-3 (PTX3), produced by neuronal and other tissues (Livija et al., 2009). PTX3 is a pattern-recognition protein, which is up-regulated by both cytokines and activation of TLRs. It has been shown to be up-regulated in peripheral tissues in people with depression without known inflammatory disease (Shelton et al., 2003).
Dietary lipids, including polyunsaturated fatty acids (PUFAs) play significant roles in immune activation in the body. Fatty acids are long-chain esters of carboxylic acid, and exist in either saturated or unsaturated forms, depending on the presence or absence of double bonds in their structures. PUFAs contain multiple double bonds in their structures and are divided into n-3, -6, and -9 (also known as omega) fatty acids, depending on the position of the first double bond (IUPAC-IUB Commission on Biochemical Nomenclature, 1978). N-3 fatty acids are found in plants and fish and are the main constituents of fish oil used as a dietary supplement. PUFAs are incorporated into cell membranes and are metabolized into lipid signaling molecules and other compounds (Galli and Calder, 2009). Specific tissue types have particularly high concentrations of lipids, including brain and certain immune cells. PUFAs have complex roles in modulating immune responses. For example, arachidonic acid, a major n-6 fatty acid, is metabolized by cyclooxygenase (COX) enzymes to form eicosanoids and related compounds (e.g., thromboxanes and prostaglandins), which have predominantly pro-inflammatory effects (Rao and Knaus, 2008) (with the exception of anti-inflammatory lipoxins [Serhan, 2009[). By contrast, the major n-3 fatty acids such as eicosapentaenoic acid (EPA) and docsahexanoid acid (DHA) give rise to specific anti-inflammatory molecules, including resolvins, protectins, and maresins (Galli and Calder, 2009; Serhan, 2009), which serve as cellular inflammatory stop signals. Early in the inflammatory process, inflammatory lipid derivatives such as the prostaglandins and leukotrienes predominate; however, with time, there is a shift to anti-inflammatory mechanisms. These mechanisms reduce the production of cytokines, block intracellular mechanisms involved in inflammatory signaling and leukocyte trafficking (Serhan, 2009). The anti-inflammatory benefits of aspirin have to do with the fact that it acetylates COX-2 and increases the synthesis of these anti-inflammatory mediators (Serhan et al., 2002). For example, a lipidomic analysis of exudates obtained from mice treated with aspirin and DHA showed an increase in production of resolvins, which inhibit human microglial expression of cytokines in physiologically-relevant concentrations (Serhan et al., 2002). These data suggest that the anti-inflammatory effects of aspirin, including its benefits in endothelial dysfunction in cardiovascular disease, may be mediated via these mechanisms.
2.3.2 White Adipose Tissue as an Endocrine Organ: Adipocytokines and Related Molecules
Adipocytes in WAT are far from inert storage cells. In fact, they produce a wide range of hormone and immune factors (Tilg and Moschen, 2008; Tilg and Moschen, 2006) (Figure 2). Important among these are the adipocytokines (adipokines), which are cytokines that are produced mainly by adipose tissue. These include resistin, and visfatin, but also IL-6 and TNFα, which are thought to link obesity and both inflammation in general and inflammatory disorders in particular (Tilg and Moschen, 2006). In fact, the increase in fat in adipocytes increases the production of chemokines, including MCP-1. MCP-1 attracts leukocytes including macrophages and other cell types including T lymphocytes, and dendritic cells to adipose tissue (Carr et al., 1994; Xu et al., 1996). Both adipose tissue itself and immune cells produce cytokines such as IL-1, IL-6, and TNFα, although macrophages are considered the primary source of inflammatory cytokines from adipose tissue (Tilg and Moschen, 2006). Indeed, WAT contains large numbers of macrophages, and activated macrophages also produce chemoattractant proteins, which lead to further accumulation of white blood cells and production of cytokines (Kanda et al., 2006). Adipocytokines and related cytokine molecules produced by adipose tissue, particularly WAT, result in general immune activation by mechanisms noted earlier and, ultimately, can contribute to immune-related disorders (Tilg and Moschen, 2006). Obesity is generally associated with an increased inflammatory response, with excess production of adipocytokines, chemokines, cytokines, and acute phase proteins such as CRP, along with inhibition of protective molecules such as leptin and adiponectin, which contribute to common inflammatory diseases including type 2 diabetes and cardiovascular disease (Wellen and Hotamisligil, 2005). Interestingly, abdominal WAT (particularly intra-abdominal fat) produces a greater effect on systemic inflammation than other sites of WAT accumulation, possibly related to the relationship of intra-abdominal WAT to the portal circulation (Shoelson et al., 2007). This is thought to occur, in part, as a result of cellular hypoxia that results from expansion of adipose tissue via compromised vasculature (Stuart, I et al., 2009). The partial pressure of oxygen in most tissues is about 50 mmHg; lean WAT has been measured at 47.8 mmHg O2, while WAT in obese mice was shown to be 15.2 (Trayhurn et al., 2008; Ye et al., 2007). Tissue hypoxia has been shown to be associated with many of the effects described in greater detail below, including increased expression of IL-1β, IL-6, and visfatin and reduced adiponectin (Stuart, I et al., 2009). Intra-abdominal WAT can be particularly insidious since it may be increased in many people who are not overtly obese, and yet still contribute to inflammatory allostatic load. In fact, abdominal fat, not other measures of obesity such as body mass index (BMI), which simply reflects the ratio of weight in kilograms to height in meters squared, is much more predictive of diseases such as type 2 diabetes and cardiovascular disease (Shoelson et al., 2007).
Figure 2. Adiposity and inflammation.
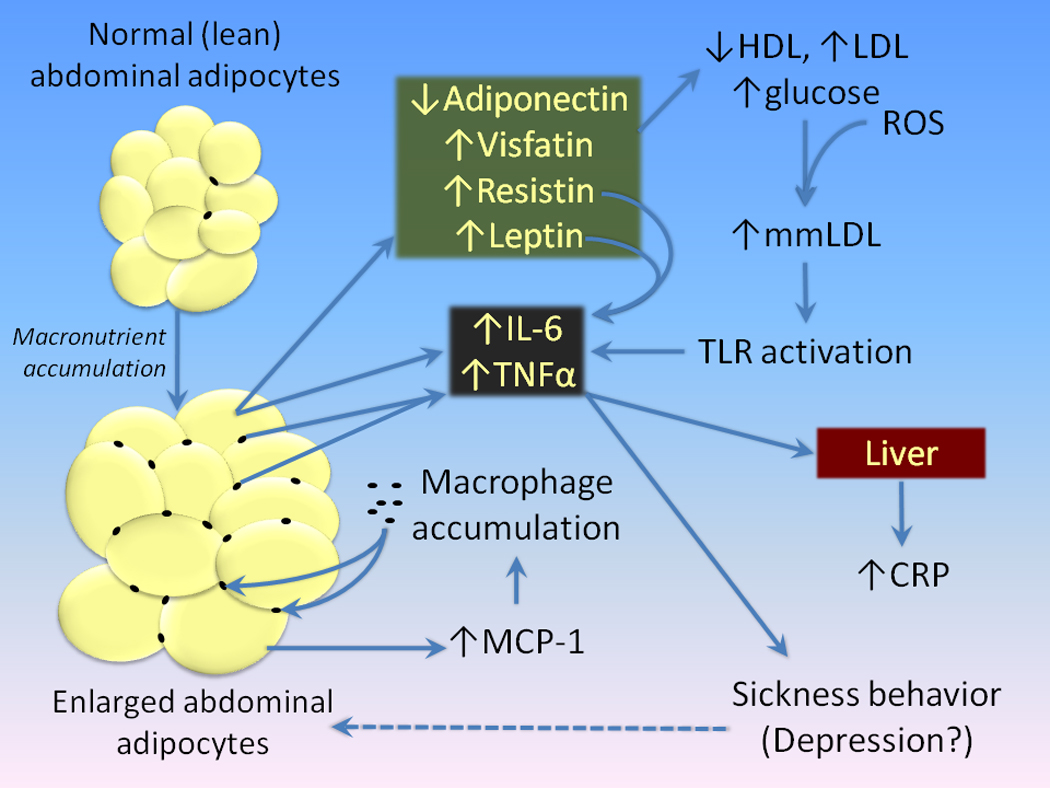
High caloric intake in the diet leads to increased accumulations of lipids in adipocytes. Increased lipid content results in an increased release of MCP-1 (CCL2), a chemoattractant that increases the infiltration of macrophages into adipose tissue. Both adipocytes and macrophages release inflammatory mediators such as IL-6 and TNFα into the peripheral circulation. This, coupled with adverse effects on adipocytokines and related molecules such as leptin, resistin, visfatin, and adiponectin is thought to mediate the relationship between accumulation of adipose tissue and conditions such as dyslipidemias and diabetes. Low density lipoprotein cholesterol is oxidized to form minimally-modified LDL’s (mmLDL), which also can activate toll-like receptors, further stimulating the production of cytokines. Increases in peripheral cytokines may, then, lead to depression. However, depression may also enhance the accumulation of body fat, further aggravating the process of adipose-induced inflammation.
A variety of adipocytokines and related molecules that are produced by WAT are involved in the regulation of dietary intake, lipid distribution, and energy homeostasis (for a review, see Frübeck (Fruhbeck, 2008) and Bays et al. (Bays et al., 2008). For example, leptin, a member of the type I cytokine superfamily (Lago et al., 2007; Lago et al., 2009), is involved in the regulation of energy acquisition (eating) and expenditure in the body, acting primarily via the CNS (Vazquez-Vela et al., 2008). Leptin receptors in the hypothalamus regulate satiety, the perception of dietary sufficiency. Leptin levels increase in obesity, which is related to loss of sensitivity to feedback regulation via leptin receptors (Considineet al., 1996). It also has significant interactions with immune response, and is involved in the modulation of white blood cell response; this includes T-cell activation and a shift to Th1 cytokine production (Lago et al., 2007; Lago et al., 2009). Leptin also is increased by IL-1, IL-6, and LPS (Lago et al., 2007). Therefore, although leptin is generally thought of as a protective factor regarding obesity, the elevated levels found in obese individuals may contribute to the inflammatory state.
Adiponectin is another peptide that is involved in a range of metabolic processes that are produced by WAT. For example, it increases fatty acid oxidation, reduces the synthesis of glucose in the liver and is involved in the feedback sensitivity of insulin receptors (Lago et al., 2007; Lago et al., 2009). Adiponectin levels are reduced in obese persons and increase in response to weight loss (Lago et al., 2007). This mechanism, in part, explains the improvements in glucose metabolism associated with weight reduction, since adiponectin is protective against insulin resistance (Tilg and Moschen, 2006). Adiponectin has a predominantly inhibitory role in Th1 immune responses. For example, it inhibits macrophage IL-6 and TNFα production and increases systemic production of the anti-inflammatory cytokine IL-10 (Tilg and Moschen, 2006). This process occurs via activation of the two subtypes of adiponectin receptors, which activate the transcriptional factor peroxisomeproliferator-activated receptor-α (PPARα) (Tilg and Moschen, 2006). Alternatively, under certain states such as LPS activation adiponectin may have pro-inflammatory effects (Tilg and Moschen, 2006). Resistin is another adipocytokine that is produced by WAT but also is found in blood monocytes (Tilg and Moschen, 2006). It contributes to a positive inflammatory feedback system in which the secretion of resistin by adipose tissue is increased by IL-1, IL-6, and TNFα, but it also increases the production of these same cytokines by macrophages in a NF-κB – dependent manner (Silswal et al., 2005; Tilg and Moschen, 2006). Resistin is thought to play an important role in obesity-induced insulin resistance and endothelial dysfunction leading to cardiovascular disease (Tilg and Moschen, 2008).
Adipose tissue also secretes anti-inflammatory cytokines including IL-10 and IL-1 receptor antagonist (IL-1Ra) (Juge-Aubry et al., 2005; Juge-Aubry et al., 2003). However, the role of these factors, particularly IL1Ra, is highly complex (Feve and Bastard, 2009). Increases in adipose tissue in humans, particularly abdominal fat, has been consistently shown to increase IL-1Ra expression (Fain, 2006; Somm et al., 2006; Juge-Aubry et al., 2003; Cartier et al., 2009; Fain, 2006). Given its role in antagonizing IL-1 receptors, it would be expected to exert a simple anti-inflammatory action, which might have a positive effect in obesity-induced inflammation. In fact, in islet cells in the pancreas, IL-1Ra enhances insulin release and sensitivity and improves glycemic control via IL-1 blockade (Ehses et al., 2009; Larsen et al., 2009). However, IL-1Ra knockout mice have reduced fat mass, related to an increase in energy expenditure and a reduction in adipogenesis (Somm et al., 2005). IL-1Ra alters extrapancreatic insulin sensitivity and glucose metabolism leading to a diabetes-type phenotype (Somm et al., 2006). IL-1Ra null mice have increased insulin sensitivity and lower glucose levels in contrast to wild type (Somm et al., 2006; Matsuki et al., 2003). IL-1Ra is higher in older adults with MetS compared to non-affected controls (Stenholm et al., 2009). IL-1Ra antagonizes the action of leptin in the hypothalamus in rodents and is thought to be involved in leptin resistance in obesity (Meier et al., 2002). The ultimate role of IL-1Ra in weight regulation, leptin and insulin sensitivity remains controversial (Feve and Bastard, 2009). Of relevance to the current discussion, IL-1Ra has been assessed in clinically depressed populations; the majority of studies have found it increased in depression, effects that were maintained even after adjusting for BMI (Howren et al., 2009).
2.3.3 Metabolic Consequences of Lipid Accumulation: Obesity-Associated Inflammatory Diseases
A number of conditions that represent the major sources of morbidity and mortality in the modern world such as type 2 diabetes, cardiovascular disease, and cancer are known to be associated with obesity and have recently been reformulated as inflammatory diseases. For example, the inflammation associated with the accumulation of intra-abdominal fat is associated with progressive resistance to the effects of insulin, ultimately leading to type 2 diabetes. The chronic inflammatory state and changes in leptin, adiponectin, and resistin associated with obesity is thought to be associated with this process (Tilg and Moschen, 2008; Tilg and Moschen, 2006). In fact, obese individuals with type 2 diabetes have increase peripheral inflammatory factors including IL-6, TNFα, and CRP relative to obese individuals without diabetes (Tilg and Moschen, 2008; Tilg and Moschen, 2006). The mechanisms for obesity- and cytokine-induced insulin resistance appears to be, in part, related to mechanisms discussed for intracellular immune activation described earlier. Specifically, activation of JNK and IKKβ – NF-κB signaling cascades appear to be involved in the development of insulin resistance (Shoelson et al., 2007; Tilg and Moschen, 2008; Tilg and Moschen, 2006). This occurs, in part, via the activation of pattern recognition receptors (e.g. TLRs) by fatty acids and, in particular, oxidized low density lipoproteins (also known as minimally modified LDLs [mmLDL]), which act primarily through TLR4 (Miller et al., 2005b). TLR activation of JNK leads to phosphorylation of insulin receptor substrate-1 (IRS-1), a signal transduction factor associated with insulin receptors and insulin-like growth factor receptors, which disrupts insulin cellular signaling leading to insulin resistance. Dissociation of NF-κB from IKKβ leads to transcriptional activation of a variety of gene products that may also contribute to insulin resistance (Shoelson et al., 2006).
A similar inflammatory process links obesity with cardiovascular disease. High LDL cholesterol and serum triglycerides have been thought in the past to lead to cardiovascular disease via simple deposition in small vessels. However, the process of atherosclerosis is now understood to be more complex and involve inflammatory factors leading to endothelial dysfunction in small vessels (Van Gaal et al., 2006). For example, small dense LDL can transit via fenestrations in the endothelial lining of vessels and enter the subendothelial space, leading to a local inflammatory response. This, coupled with platelet aggregation and the activation of other pro-coagulation factors, leads to progressive occlusion of vessels, leading to a variety of conditions such as cardiovascular disease, peripheral artery disease, and, in men, erectile dysfunction (Van Gaal et al., 2006). Obesity is often associated with a variety of factors such as smoking, increased levels of LDL cholesterol, abnormalities of glucose metabolism, and hypertension to increased risk of endothelial dysfunction leading to cardiovascular disease (Van Gaal et al., 2006).
Although metabolic diseases like type 2 diabetes are commonly associated with obesity (that is, elevated total body mass), the contribution of diet to inflammatory diseases is more complex. For example, the phenomenon of so-called “normal weight obesity” (defined as a BMI <25 kg/m2 and a percent body fat ≥ 66th gender-specific percentile) is associated with signs of inflammation similar to that found in typical obesity (Marques-Vidal et al., 2009; Stenholm et al., 2009). A recent large study in Switzerland found normal weight obesity in 5.4% of women but <3% of men. Women with normal weight obesity had blood pressure, lipid levels, fasting hyperglycemia, CRP, and liver enzyme levels that were greater than that found in a lean sample and similar to an overweight group. These results indicate that body adiposity rather than weight or BMI may be the critical factor in inducing inflammation.
3.0 Inflammation and Depression
To this point we have discussed the inflammatory effects of lipid accumulation in adipose tissue. However, are adiposity and depression linked, and do the inflammatory effects of adipose tissue contribute to depression risk? In this section we will discuss the association between inflammation and depression, and then discuss specific linkages between fat accumulation and depression risk.
Pioneering work by Maes (Maes, 2008), Miller (Miller et al., 2009), Irwin (Irwin and Miller, 2007b), and many others over the last 20 years has demonstrated that a significant subset of depressed patients show evidence of innate immune system activation. Some of the very earliest findings, dating to the late 1980’s, suggested alterations in circulating levels of specific subtypes of lymphocytes such as T-helper and natural killer cells; however, these results were not consistently replicated (Irwin and Miller, 2007a). However, subsequent research has shown that a sizeable subset of depressed patients have evidence of activation of innate immunity in the absence of obvious underlying medical causes (Miller et al., 2009). These include the pro-inflammatory cytokines and their soluble receptors in peripheral blood and cerebrospinal fluid (CSF), along with increased acute phase proteins (e.g., CRP), chemokines, cell adhesion molecules, and other inflammatory mediators such as prostaglandins (Levine et al., 1999a; Miller et al., 2009; Raison et al., 2006). The most consistently replicated findings include elevations of serum IL-6, TNFα, and CRP (Raison et al., 2006; Miller et al., 2009; Dowlati et al., 2010; Howren et al., 2009). A number of studies have also shown higher IL-1β (Kaestner et al., 2005; Levine et al., 1999b; Schlatter et al., 2004; Thomas et al., 2005; Yang et al., 2007), although this has been less consistently replicated. Elevations in inflammatory mediators are seen across the life span, including elderly depressed patients both with and without co-existing medical diseases (Andrei et al., 2007; Tiemeier et al., 2003).
3.1 Sickness Behavior: Animal and Human Models of Depression
So-called “sickness behavior” represents an adaptive response of animals seen during the course of an infection (Hart, 1988; Weidenfeld and Yirmiya, 1996; Pollak and Yirmiya, 2002; Dantzer et al., 2008; Dantzer, 2009) (Figure 3). In humans, sickness behavior comprises fever, malaise, fatigue, muscle and joint aches, and reduced appetite (Dantzer et al., 2008; Dantzer, 2009). However, peripheral immune activation in both humans and other vertebrates can induce sickness symptoms that show considerable overlap with depression, including depressed mood, reduced social interaction, and sleep disturbance (as discussed in greater detail below) (Capuron et al., 2009). Hence, inflammatory-induced sickness behaviors include a number of features that are consonant with the human syndrome of major depression.
Figure 3. Sickness behavior: Mechanisms mediating activation of brain cytokine signaling by peripheral cytokines.
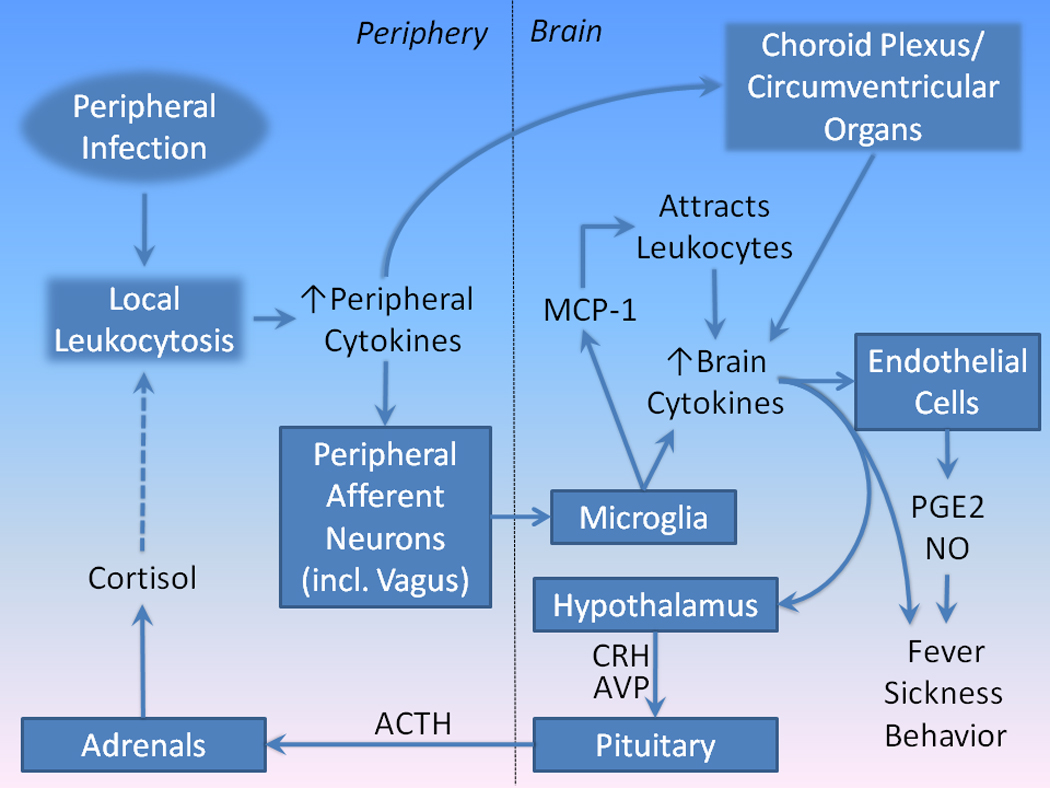
(Adapted from (Dantzer, 2009). The invasion of peripheral tissues by pathogens sets up a local inflammatory response using innate immune cytokine mechanisms. Peripheral inflammation activates brain inflammatory responses by several pathways. First, locally released cytokines activate afferent neurons (e.g., the vagus nerve) that innervate the affected body region. Afferent neurons, then, activate cytokine release from microglia in brain. Activated microglia also produce MCP-1, which attract leukocytes from the peripheral circulation. These leukocytes further increase the local inflammatory response in brain. Second, cytokines released peripherally travel via the circulation and activate leukocytes in the choroids plexus and circumventricular organs, setting up a local immune response, including the diffusion of cytokines into brain tissues. Brain regional cytokine release stimulates endothelial cells in small blood vessels to release PGE2 and NO. The combined effects of cytokines, PGE2, and NO in brain are thought to generate sickness behavior. Cytokines, particularly IL-1β, stimulate the release of CRH and AVP from paraventricular nucleus of the hypothalamus, which activates the release of ACTH from the pituitary, which travels to the adrenals, stimulating the release of cortisol. The latter inhibits immune activation, limiting the score of inflammation both peripherally and centrally.
The molecular mechanisms underlying this response involve innate immune activation, including IL-1α, IL-1β, IL-6, and TNFα (Dantzer et al., 2008; Dantzer and Kelley, 2007a). These induced cytokines, then, set up the local immune response to the invading pathogen. Although the initial immune activation may be primarily peripheral, as in the case of infection, these peripheral immune responses can access and ultimately influence the brain. Because cytokines are too large to freely pass through the blood brain barrier, much attention has been paid to how cytokine signals access the brain; several pathways have been described (Figure 3). For example, local release of cytokines can activate afferent neurons (including the vagus nerve) innervating the infected tissues; these afferent neurons, in turn, lead to activation of microglia resulting in brain production of cytokines. A second route is a humoral pathway by which cytokines can activate leukocytes in the choroid plexus and circumventricular organs, setting up a local immune response, resulting in diffusion of cytokines into brain through leaky regions in the blood brain barrier (Dantzer, 2009). The circumventricular regions include the organum vasculosum of the lamina terminalis, the subfornical organ, the median eminence and the area postrema. These areas have a rich vasculature in which the capillary endothelial cells lack tight gap junctions, which allows the diffusion of large molecules such as the cytokines (Nguyen et al., 2002). Of note, peripheral cytokines can also cross the blood brain barrier via saturable active transport molecules (Quan and Banks, 2007). Regional CNS cytokines stimulate endothelial cells in small blood vessels to release prostaglandin E2 (PGE2) and nitric oxide (NO), which appear to be important mediators of the brain-based symptoms of the immune response such as fatigue and lethargy (Dantzer, 2009).
An additional pathway by which peripheral inflammation can be communicated to the brain includes the release of MCP-1 by activated microglia. MCP-1 recruits peripherally activated monocytes which can directly enter the brain and subserve central inflammatory responses (D'Mello et al., 2009). As noted previously, this role of MCP-1 in attracting activated macrophages to relevant tissues also appears to be important to the accumulation of macrophages in adipose tissue as well as areas of vascular injury (e.g. atherosclerotic plaques) (see below). In rodents, cytokine activation of sickness behavior has been shown to be mediated by IL-1β (Anforth et al., 1998); however, this response appears to be augmented by co-expression of IL-6 (Bluthe et al., 2000b) and TNFα (Bluthe et al., 2000a). Inflammatory mediators also stimulate the preoptic nuclei of the hypothalamus to induce fever (Romanovsky et al., 2005). Therefore, peripheral immune activation acts indirectly to set up a brain inflammatory response. As will be discussed in greater detail later, antagonism of peripheral mediators of inflammation may also reduce the central inflammatory response and related behavioral features (Krishnan et al., 2007; Tyring et al., 2006a).
Cytokines, primarily IL-1β, also stimulate the release of corticotrophin releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus (Figure 3). CRH stimulates the release of adrenocorticotrophic hormone (ACTH) from the pituitary, which travels via the systemic circulation to the adrenals where it induces the release of cortisol. Cortisol inhibits and, therefore, limits the local inflammatory response, both peripherally and centrally. This occurs, in part, by inhibition of IL-1, and other inflammatory cytokines such as TNFα and IL-6 (DeRijk et al., 1997). Curiously, however, whereas TNFα is very sensitive to cortisol-induced downregulation, IL-6 is relatively resistant at typical physiological levels (DeRijk et al., 1997). This is significant since elevated IL-6 has been a consistent finding in studies in depressed patients, despite concomitant elevation of cortisol (Irwin and Miller, 2007a).
Sickness behavior has been consistently shown with immune activation in non-human vertebrates (Dantzer, 2009; Dantzer and Kelley, 2007). One striking example comes from a study of infusion of IFNα in non-human primates (Felger et al., 2007). In this study, Felger and colleagues (Felger et al., 2007) administered recombinant human IFNα or saline to rhesus monkeys in a counterbalanced fashion over 4 weeks. IFNα infusion was associated with increased anxiety-like behaviors and decreased environmental exploration; in a subset of monkeys, IFNα administration was also linked to huddling behavior, a behavior also observed after chronic administration of the monoamine depleting drug, reserpine, as well CRH. IFNα infusion was associated with elevations in plasma IL-6, ACTH, and cortisol, which diminished over time. Curiously, the time-dependent decreases in ACTH, cortisol, and IL-6 were more pronounced in socially dominant than in submissive animals. Similar results have been found in humans, as discussed below (Capuron et al., 2003b)
3.1.1 Sickness Behavior in Humans
Immune activation in humans shows a pattern of symptoms consistent with sickness behavior in animal models. Symptoms such as fatigue, malaise, anorexia, and sleep disturbance are common in the context of immune activation (Dantzer and Kelley, 2007); this can be seen as part of the core symptoms in certain diseases such as rheumatoid arthritis, but also as part of the response to treatments. For example, cancer chemotherapy agents such as paclitaxel induce immune responses (e.g., IL-6, IL-8, and IL-10), which correlate with symptoms such as fatigue (Pusztai et al., 2004). However, is sickness behavior really analogous to depression? The question about the relationship between sickness behavior induced by immune activation and human depression was addressed recently by Capuron et al. (Capuron et al., 2009) Administration of immune factors such as IFNα and IFNβ, TNFα, IL-1, and IL6 as well as immune activators such as LPS had previously been shown to induce depressive-like signs and symptoms (Capuron et al., 2002a; Capuron et al., 2009; Capuron and Miller, 2004; Matrisciano et al., 2009). In this study, 20 persons who were being treated with INFα for malignant melanoma were compared with 28 medically healthy persons with major depression. All participants were evaluated with the Structured Clinical Interview for DSM Axis I Disorders (First et al., 2002) and the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). IFNα-treated participants could not have a current diagnosis of major depression or substance-related disorder at baseline; none had a history of any anxiety disorder and only 5 of 20 IFNα-treated patients had a history of depression. The IFNα-treated patients were followed weekly for 12 weeks. Nine of the 20 (45%) IFNα-treated patients developed depressive signs and symptoms sufficient to meet criteria for major depression. Comparing the depressed IFNα-treated patients and the medically-healthy depressed patients, there were no mean differences in the HRSD total score (21.3 vs. 20.4 respectively) or in the severity of the following HRSD items: depressed mood, psychic anxiety, hypochondriasis, agitation, somatic anxiety, and impairment of work and activities. IFNα-treated patients showed more psychomotor retardation and weight loss than the medically-healthy depressed sample. The latter exceeded the IFNα sample in only two categories: feelings of guilt and thoughts of suicide (Capuron et al., 2009). These results indicate that the depression induced by immune challenge emulates, and in the case of some features exceeds, that seen in depression in medically-healthy persons. Also consistent with this view are observations that aspects of sickness behavior can be reversed or prevented by the use of antidepressant treatments (as discussed further below) (Musselman et al., 2001; Yirmiya et al., 2001; Capuron et al., 2002; Raison et al., 2007)
3.2 Associations Between Inflammatory Mediators and Depression in Medically Ill Persons
Patients with a variety of inflammatory diseases, including certain forms of cancer (Musselman et al., 2001b), rheumatoid arthritis (Zautra et al., 2004), and multiple sclerosis (Gold and Irwin, 2006), have been shown to have increased rates of depression. Depression in these patients also appears to be associated with increased inflammatory cytokine levels. For example, several studies have shown that depressed cancer patients show higher levels of IL-6 than either normal controls or non-depressed cancer patients. (Jehn et al., 2006; Lutgendorf et al., 2008; Musselman et al., 2001b; Soygur et al., 2007) The severity of depression in some cancer patients has also been shown to be correlated with IL-6 levels; for example, Jacobson et al. (Jacobson et al., 2008) found a high degree of correlation between IL-6 level and depression as measured by the HRSD (r=0.68) in cancer patients. The same association between pro-inflammatory factors, including both IL-6 and CRP, and depression has been shown for patients with cardiovascular disease (Miller et al., 2005a). Of significance for the discussion of the relationship between adiposity and depression risk below, this same study showed an association between body mass and depression, which partially accounted for the correlation between CRP and IL-6 and depression. Depressed subjects had a significantly greater mean BMI (BMI=30.5) compared to controls (BMI=25.9) and BMI was positively related to levels of CRP (r = 0.62) and IL-6 (r = 0.63). This is consistent with the hypothesis articulated below that there may be a link between body adiposity, inflammation, and depression risk. In general, pro-inflammatory cytokines may mediate the relationship between many diseases and depression risk.
3.3 Mechanisms by Which Immune Activation Leads to Depression
3.3.1 Activation of Indoleamine 2,3-dioxygenase: Effects on Serotonin and Kynurenines
Depression has been linked to the function of monoamines (serotonin, norepinephrine, and dopamine) for decades, owing in large part to the fact that effective antidepressant drugs consistently target these transmitters (Maas, 1975; Maas, 1978; Schildkraut, 1995). Depletion of monoamines (serotonin and norepinephrine) has been shown to induce depressive signs and symptoms in some drug-free patients (Delgado et al., 1994) and to rapidly reverse the effects of antidepressants leading to depressive relapse (Delgado et al., 1990; Delgado et al., 1991; Delgado et al., 1993; Delgado et al., 1999).
Cytokine signaling has been shown to profoundly influence central monoamine synthesis, metabolism, and cellular transit, particularly that of serotonin. Serotonin is synthesized from tryptophan by tryptophan hydroxylase (TH) and aromatic amino acid decarboxylase (AAAD) (Figure 4). The amount of serotonin synthesized in brain is highly dependent on tryptophan availability (Delgado et al., 1990). Depletion of tryptophan rapidly leads to reduced central serotonin levels (Delgado et al., 1990). Under normal physiological conditions, the idoleamine 2,3-dioxygenase (IDO) (and the related liver enzyme tryptophan 2,3-dioxygenase) pathway competes with tryptophan metabolism by TH. Activation of the IDO pathway metabolizes tryptophan to kynurenine and, ultimately, to quinolinic acid (QUIN) (among other byproducts)(Stone and Darlington, 2002). This is thought to deplete brain tryptophan and reduce the amount of serotonin (Schrocksnadel et al., 2006; Schwarcz and Pellicciari, 2002).
Figure 4. The effects of innate immune activation on serotonin and kynurenines.
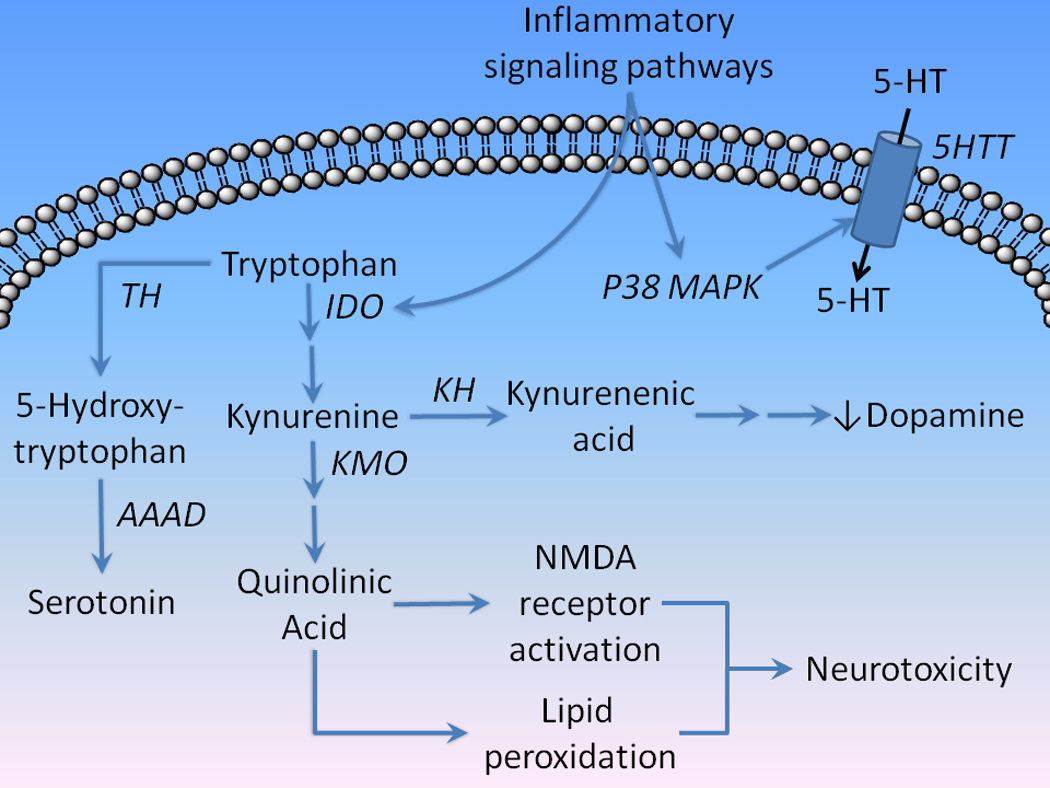
Inflammatory signaling pathways activate indoleamine 2,3-dioxygenase (IDO) which converts tryptophan to the neuroactive metabolite intermediary kynurenine. IDO activation is thought to compete with tryptophan hydroxylase (TH) metabolism of serotonin (5-HT). Kynurenine is then metabolized by kynurenine hydroxylase to kynurenic acid, which activates α7 nicotinic receptors leading to inhibition of the presynaptic release of dopamine. However, kynurenine is also metabolized via the kynurenine 3-monooxygenase (KMO) pathway to QUIN, a potent activator of NMDA receptors and lipid peroxidation, which is thought to underlie the neurotoxic effects of IDO activation. Inflammatory signals also activate P38 MAPK, which phosphorylates the serotonin transport (5HTT), increasing the uptake of serotonin. (AAAD=aromatic amino acid decarboxylase)
Multiple inflammatory signaling pathways activate IDO, particularly those associated with TNFα (Popov et al., 2006) and IFNγ. (Takikawa et al., 1999). These include signal transducer and activator of transcription 1a (STAT1a), interferon regulatory factor-1 (IRF1), NF-κB, and p38 mitogen activated protein kinase (p38 MAPK) (Miller et al., 2009). Peripheral administration of LPS in mice has been shown to activate IDO and cause depressive-like behavior, including increased duration of immobility in the forced-swim and tail suspension tests (O'Connor et al., 2009). Inhibition of inflammation-induced IDO induction by the tetracycline antibiotic minocycline or direct IDO inhibition by the IDO antagonist, 1-methyltryptophan, prevents depression-like behaviors in LPS-treated mice. These results indicate that the activation of IDO contributes to the depressogenic effects of immune activation (O'Connor et al., 2009).
The effect of immune activation on tryptophan availability and kynurenine synthesis has also been tested in humans. In one study (Capuron et al., 2002b), cancer patients undergoing treatment with either IL-2 or IFNα experienced reduced serum tryptophan at one week and one month of therapy compared to baseline. Depressive symptoms were positively correlated with the degree of decrease in tryptophan concentrations during treatment. In a second (Capuron et al., 2003a), patients with malignant melanoma were given IFNα therapy; plasma kynurenine and the kynurenine/tryptophan ratio increased in all patients. Those patients who developed depression had higher kynurenine and lower tryptophan levels than those without depression. Patients receiving IFNα therapy show increases in IFNα, IL-6 and MCP-1 and decreases in the primary metabolite of serotonin 5-hydroxy indoleacetic acid (5-HIAA) in CSF; 5-HIAA levels had the strongest association with depressive signs and symptoms (Raison et al., 2009a). In that study, CSF IL-6 but not IFNα or MCP-1 concentrations correlated significantly with 5-HIAA level. These results indicate that the development of depressive symptoms in patients undergoing cytokine therapy could be mediated by a reduced availability of tryptophan and, ultimately, serotonin in brain by activation of IDO.
When serotonin is released from presynaptic terminals, the signal is rapidly inactivated through reuptake via the high-affinity serotonin transporter (5HTT) (Blakely and Berson, 1992). Many antidepressant drugs block this transporter and lead to sustained synaptic serotonin signaling. Activation of p38 MAPK by both IL-1β and TNFα has been shown to lead to phosphorylation of the 5HTT and increased uptake of serotonin (Figure 4)(Zhu et al., 2005). 5HTT trafficking to the cell surface has also been shown to be dependent on the activity of p38 MAPK (Samuvel et al., 2005). In a related study, IFNα was shown to increase MAPK phosphorylation and both the expression and activity of 5HTTs in an immortalized line of T lymphocytes, effects that were reversed with a MAPK-selective inhibitor (Tsao et al., 2008). Similar results were also shown in leukocytes obtained from depressed patients compared with controls (Tsao et al., 2006). In this study blood samples were obtained from 20 drug-free persons with depression and 22 controls and leukocytes were extracted at baseline and after treatment with fluoxetine. At baseline, mRNA expression of IL1β, IL-6, IFNγ, TNFα, and the 5HTT were higher in the depressed patients than controls. The mRNA expressions of IFNγ and the 5HTT diminished after fluoxetine treatment. Taken together, these studies suggest that cytokines may reduce serotonin signaling via at least two mechanisms: metabolism of tryptophan to kynurenine by activation of IDO and p38 MAPK – dependent increase in expression and phosphorylation of 5HTTs resulting in increased synaptic uptake of serotonin.
The relevance of the effects of immune activation on serotonin to depression is also supported by studies that have examined the effects of serotonergic antidepressants in patients treated with IFN and other immunotherapeutics. In one study (Musselman et al., 2001a), 40 patients treated with malignant melanoma treated with IFNα-2b were randomly assigned to concomitant treatment with the serotonin reuptake inhibitor paroxetine or placebo for 12 weeks. Only 11% of the paroxetine-treated patients developed depression as opposed to 45% of the placebo group. A reanalysis of the data from this study found that symptoms of depression such as depressed mood, anxiety, cognitive dysfunction, and pain were improved with paroxetine, while fatigue and anorexia were not helped (Capuron et al., 2002a). The beneficial effects of serotonin reuptake inhibitors on depressive symptoms induced by immunotherapy (e.g., IFN treatment) has been supported by most ((Capuron et al., 2002)Gleason et al., 2007; Hauser et al., 2002; Kraus et al., 2008; Laguno et al., 2004; Levenson and Fallon, 1993; Loftis et al., 2004; Morasco et al., 2007; Raison et al., 2007; Sammut et al., 2002; Schaefer et al., 2005; Schramm et al., 2000) but not all (Morasco et al., 2007) studies. However, the overwhelming evidence suggests that concomitant treatment with serotonin reuptake inhibitors can either reverse or prevent immunotherapy-induced depressive symptoms.
In addition to its potential effects on tryptophan and serotonin availability, IDO activation yields several neuroactive intermediates that may also be involved in depression. For example, kynurenine is metabolized by kynurenine hydroxylase to kynurenic acid (KYN-A), which antagonizes α7 nicotinic acetylcholine receptors (Figure 4) (Schwarcz and Pellicciari, 2002). α7 receptor blockade leads to reduced striatal dopamine release (Amori et al., 2009; Rassoulpour et al., 2005), which is also seen in the brains of depressed persons (Dunlop and Nemeroff, 2007). A second byproduct of IDO activity is QUIN, which is produced by activation of kynurenine 3-monooxygenase (KMO). In addition to contributing to lipid peroxidation, QUIN is a potent activator of N-methyl-D-aspartic acid (NMDA) receptors and the release of glutamate, all of which can lead to excitotoxicity. (Jang et al., 2010) This mechanism has been implicated in the pathophysiology of conditions such as Alzheimer’s Disease, Huntington’s Disease, Parkinson’s Disease, amyotrophic lateral sclerosis, and human immunodeficiency virus-related dementia (Brew et al., 2007; Guillemin et al., 2005; Kwidzinski and Bechmann, 2007; Mosley et al., 2006; Owe-Young et al., 2008; Sas et al., 2007; Vamos et al., 2009; Zadori et al., 2009). Therefore, IDO activation may contribute to the pathophysiology of depression by several mechanisms, including depletion of tryptophan and serotonin, reduced striatal release of dopamine, and NMDA receptor-glutamate-dependent excitotoxicity. It should be noted that KYN-A reduces glutamate release, which might be expected to antagonize the excitotoxic effects of QUIN via NMDA receptors. However, immune activation increases kynurenine 3-monooxygenase activity, shifting the metabolism of kynurenine away from KYN-A and toward QUIN (Connor et al., 2008).
The administration of IFNα therapy has been shown to significantly shift the ratios of kynurenine and both tryptophan and kynurenine metabolites in humans. In one study, 16 patients with chronic hepatitis C without depression at baseline were treated with IFNα and showed increases in depression scores and the kynurenine to tryptophan ratio, consistent with enhanced IDO activation (Wichers et al., 2005). The kynurenine to KYN-A ratio also increased, and the changes in the measure of depression severity correlated with the change in the ratio. This was taken to indicate that IFNα infusion resulted in not only IDO activation but also a diversion from KYN-A, which is relatively neuroprotective, to neurotoxic metabolites of kynurenine such as QUIN.
A recent study supports the concept of a shift from tryptophan to both kynurenine and neuroactive metabolites by exogenous immunotherapy. Raison et al. (Raison et al., 2009b) conducted a study in which they measured tryptophan, kynurenine, KYN-A, QUIN, cytokines, chemokines, and soluble cytokine receptors in the CSF and blood of 27 patients with hepatitis C; 16 of whom were receiving IFNα treatment and 11 of whom were not. The immunotherapy markedly increased both blood and CSF kynurenine; CSF kynurenine was associated with a significant increase in both KYN-A and QUIN, but did not change CSF tryptophan concentrations in spite of reduced plasma tryptophan levels. Kynurenine and QUIN levels were correlated with depressive signs and symptoms and CSF IFNα, soluble TNF receptor 2, and MCP-1 (CCL2). These data strongly indicate that IFNα infusion resulted in activation of IDO resulting in increases in both kynurenine and the putative neurotoxin QUIN, and that these changes were associated with increases in depressive symptoms.
As with the role of pro-inflammatory cytokines in general discussed earlier, activation of inflammatory factors related to obesity also appears to induce the IDO – kynurenine pathway. For example, plasma tryptophan concentrations have been shown to be reduced in obese individuals (Breum et al., 2003). Specifically, obese persons have a significantly higher kynurenine to tryptophan ratio relative to lean controls, signifying IDO activation (Brandacher et al., 2006; Breum et al., 2003). Nevertheless, significant weight reduction via change in diet (Breum et al., 2003) or bariatric surgery (Brandacher et al., 2006) has not been shown to restore tryptophan balance. This in part may be related to a relative lack of change in inflammatory factors after rapid weight loss (Brandacher et al., 2006), although more delayed responses may be seen (Tziomalos et al., 2010). Therefore, the immune activation found in obesity may contribute to the same diversion of tryptophan metabolism from serotonin to kynurenine, which could contribute to depression.
3.3.2 Effects of Immune Activation on Dopamine Dynamics
Reduced prefrontal and straital dopamine activity is thought to underlie symptoms of depression such as diminished motivation, psychomotor slowing, fatigue, and lack of response to rewarding stimuli (anhedonia) (Dunlop and Nemeroff, 2007; Salamone and Correa, 2009) Patients undergoing immunotherapy commonly experience fatigue and lethargy even if they do not experience a full major depressive episode (Capuron et al., 2002a; Capuron et al., 2009; Dantzer and Kelley, 2007). IFNα therapy has also been shown to produce significant motor slowing on neuropsychological assessment, which correlated with symptoms of fatigue and depression (Majer et al., 2008). Positron emission tomography imaging studies in humans undergoing IFNα therapy show increased striatal resting state glucose metabolism (Capuron et al., 2007; Juengling et al., 2000). This is similar to Parkinson’s disease, a condition associated with reduced striatal dopamine and increased striatal resting state metabolic activity (Spetsieris et al., 2005). Infusion of the dopamine precursor levodopa reduces striatal activity, which is associated with improvements in motor signs in Parkinson’s patients (Feigin et al., 2001). Treatment with pro-dopaminergic agents such as levodopa or psychostimulants improve fatigue symptoms in patients with Parkinson’s disease, cancer, systemic HIV, and those undergoing IFNα therapy (Breitbart et al., 2001; Lou et al., 2003; Schwartz et al., 2002).
There is also evidence from animal studies implicating reduced dopamine turnover with immune activation. For example, IFNα administration in rhesus monkeys has been associated with reduced CSF level of a metabolite of dopamine, homovanillic acid, suggesting reduced turnover of dopamine. This was also associated with huddling behavior, considered a behavioral analogue of human anxiety and depression (Felger et al., 2007). Similarly, whole brain homogenates from mice given species-specific IFNα for five days showed significantly reduced dopamine and its metabolite, 3,4-dihydroxyphenylacetic acid, which was associated with motor slowing (Shuto et al., 1997). Together, these data suggest that immune activation, particularly exogenous administration of IFNα, is associated with reduced dopamine neurotramission, and may account for abnormalities in dopamine activity in depression (Miller, 2009).
Given the shift from KYN-A to QUIN synthesis, the α7 receptor blockade by KYN-A may not be a sufficient explanation for the hypodopaminergic state associated with depression. However, there are alternative mechanisms by which immune activation may alter dopamine transmission. For example, peripheral administration of IFNα to rats reduced brain concentrations of tetrahydrobiopterin (BH4), a co-factor for tyrosine hydroxylase, the rate limiting enzyme in dopamine, epinephrine, and norepinephrine synthesis from tyrosine (Kitagami et al., 2003). The relationship between interferons and BH4 is complex. BH4 synthesis is increased by interferons (Gilchrist et al., 2003; Shi et al., 2004; Fujiwara et al., 2004; Amri et al., 2007). However, BH4, which is a cofactor for NOS-dependent synthesis of NO, has also been shown to be degraded following IFNα stimulation of NO synthesis, leading to reduced catecholamine synthesis (Kitagami et al., 2003). This effect was reversed by the NO synthase inhibitor NG-monomethyl L-arginine (Kitagami et al., 2003). A similar effect on BH4 degradation and catecholamine synthesis has been reported in sympathetic neurons following peripheral administration of IL-6 (Li et al., 2003).
Another mechanism that may alter brain dopamine dynamics is the effect of immune activation on the dopamine transporter (DAT). DAT is the principal mechanism for terminating dopamine synaptic signaling. Phosphorylation of DAT by MAPK kinase (MEK) regulates trafficking of DAT, increasing DAT surface expression and uptake of dopamine in a MAPK dependent manner in both rat synaptosomal and human embryonic kidney (HEK) cell lines (Moron et al., 2003). The co-administration of MAPK inhibitors was shown to decrease DA uptake. Together, these studies suggest that immune activation in the CNS may also alter dopamine dynamics, which may contribute to some of the cardinal symptoms of both sickness behavior and depression, such as low energy, reduced motivation, and decreased responsiveness to rewarding stimuli.
3.3.3 Immune Activation and the Regulation of the Hypothalamic-Pituitary-Adrenal Axis
Overactivation and impaired feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis are some of the most consistently replicated findings in patients with depression (Gillespie and Nemeroff, 2005; Gold and Chrousos, 2002; Pace et al., 2007a; Plotsky et al., 1998). A variety of factors, particularly stress, increase the release of CRH and arginine vasopressin (AVP) from the paraventricular nucleus (PVN) of the hypothalamus (Lolait et al., 2007). CRH and AVP subsequently stimulate the release of adrenocorticotropic hormone (ACTH) from the pituitary, which travels via the peripheral circulation to the adrenals and enhances the secretion of cortisol. Cortisol functions as a feedback regulator of the HPA axis, acting via glucocorticoid receptors (GR) in the hypothalamus, pituitary, and other brain regions. A significant subset of depressed persons show: 1. Elevations in the 24-hour excretion of cortisol in the urine; 2. Elevations in plasma cortisol and adrenocorticotropic hormone (ACTH); 3. Elevations in CSF levels of CRH; 4. Failure of feedback inhibition of cortisol secretion by the cortisol analog dexamethasone; and 5. A blunted ACTH but normal cortisol secretion to exogenously administered corticotrophin releasing hormone (CRH) (Gillespie and Nemeroff, 2005). These results are taken as evidence of reduced availability of receptors for CRH and cortisol. Reduced GRs are thought to mediate the failure of feedback inhibition of secretion of cortisol on HPA axis activity (Gillespie and Nemeroff, 2005).
As noted earlier, cytokines, primarily IL-1β, stimulate the release of CRH from the hypothalamic PVN (Miller et al., 2009). A single administration of exogenous IL-1 has been shown to produce sustained increases in expression of CRH, the CRH receptor CRH-R1, and AVP for up to three weeks in rats (Schmidt et al., 2003). Some research has indicated that the CRH and AVP response to specific types of immune challenges may be different. In one study, peripheral administration of an adjuvant (mycobacterium butyricum) that induced arthritis in rats resulted in increased AVP but decreased CRH expression. Acute LPS administration raised the expression of both AVP and CRH. However, both types of immune challenges increased ACTH and corticosterone levels (Grinevich et al., 2002).
The relationships between elevations in peripheral cytokine levels and HPA axis activation in humans have yielded somewhat inconsistent results. For example, Capuron et al. (Capuron et al., 2003b) evaluated the relationship between initial HPA response to IFNα infusion in patients undergoing treatment for malignant melanoma. Heightened ACTH and cortisol responses to the first IFNα infusion were associated with an increased risk of depression, suggesting that a sensitivity of the HPA axis to immune challenge was associated with vulnerability to depression. However, chronic immune activation has not been reliably associated with HPA axis activation in either humans or animals (Capuron et al., 2003b; Miller et al., 2009), although elevated nocturnal cortisol levels have been shown (Bower et al., 2005; Raison et al., 2010; Rich et al., 2005).
A variety of aspects of immune activation appear to disrupt GR distribution and function, which may account for failure of feedback inhibition of the HPA axis (glucocorticoid resistance) and elevated nocturnal plasma cortisol concentrations (Figure 5). In fact, reduced responsiveness of GR to cortisol has been hypothesized to be part of the pathophysiology of depression (Pariante et al., 1995) as well as inflammatory disorders (Pace et al., 2007a). Under normal physiological conditions, GR is maintained in an inactive state by binding to chaperone proteins, including heat shock proteins 70 (hsp70) and 90 (hsp90) as well as FK506 binding proteins 51 and 52 (Pratt et al., 2006; Tatro et al., 2010) (Figure 5). On binding of glucocorticoids such as cortisol, GR dissociates from chaperone proteins and translocates to the nucleus where it functions as a regulator of gene transcription primarily through its interactions with glucocorticoid response elements (GRE) in promoters of genes (Kumar and Thompson, 2005). GR transcriptional activity is terminated on unbinding of glucocorticoids and re-association with chaperones.
Figure 5. Disruption of gluocorticoid receptor (GR) function by immune activation.
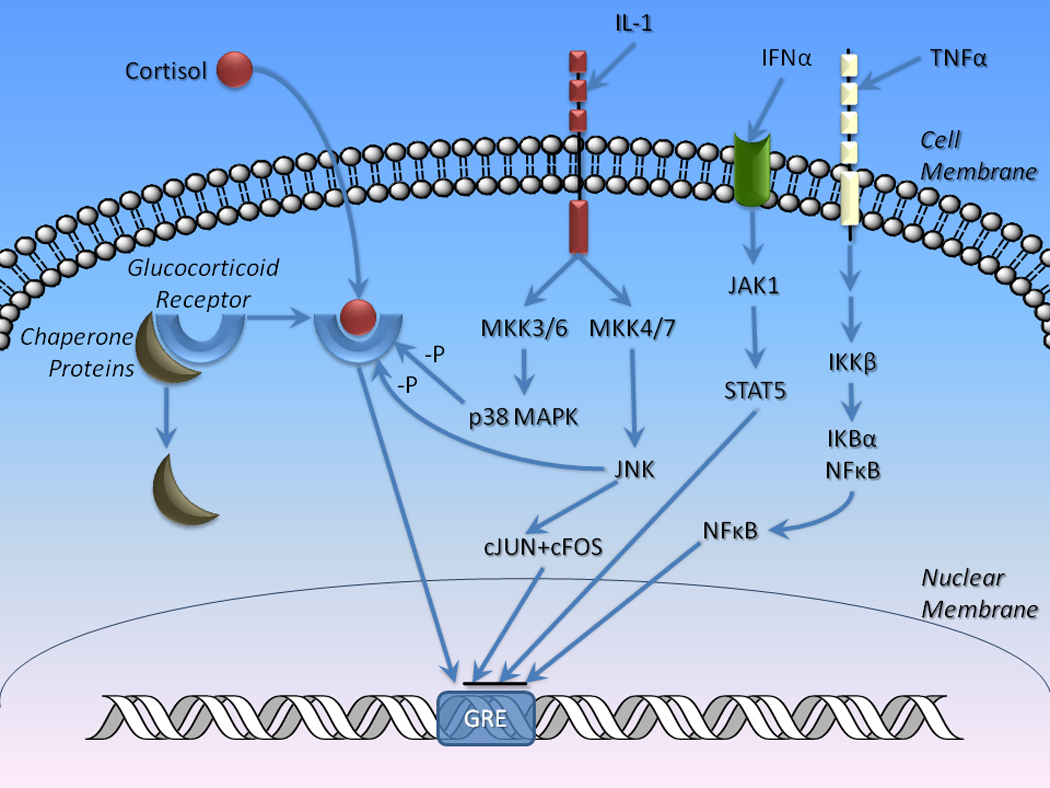
Under normal conditions, GR is maintained in an inactive state in the cytosol bound to chaperone proteins, such as hsp70, hsp90, and FK506 binding proteins 51 and 52. The binding of the glucocorticoid receptor to cortisol causes the dissociation of GR from chaperone proteins allowing it to interact with gluococorticoid response elements (GREs) in promoter regions of relevant genes resulting in gene transcription. A variety of cytokines including IL-1, TNFα, and IFNα can disrupt this process. For example, several mechanisms inhibit transcriptional activation of GREs by GR. These include: 1. IL-1 receptor activation of mitogen activated protein kinases (MKKs), leading to phosphorylation of JNK and activation of cJUN; 2. Activation of INF receptors leading to activation of Janus kinase-1 (JAK1) and STAT5; and 3. TNF receptor activation of IκB kinase β (IKKβ) results phosphorylation and dissociation of the IKBα – NF-κB complex. Il-1-induced MKK activation of P38 MAPK also results in GR phosphorylation, which interferes with nuclear translocation.
A wide range of studies have suggested that cytokines including TNFα, IL-1α, IL-1β, IL-2, IL-6, and IFNα inhibit GR function leading to glucocorticoid resistance [for comprehensive reviews, see Pace et al. (Pace et al., 2007b; Pace and Miller, 2009)] (Figure 5). For example, TNFα binds to its receptor and triggers a signal transduction cascade that activates IκB kinase β (IKKβ or IKK2) which, in turn, phosphorylates the IκB – NF-κB dimer, releasing NF-κB. The latter, then translocates to the nucleus where it interferes with GR binding to GRE segments via protein-protein interactions (McKay and Cidlowski, 1999). Similarly, IFNα, along with other cytokines, binds to types I and II cytokine receptors resulting in phosphorylation of Janus kinase-1 (Jak1) which activates STAT5, which, like NF-κB, inhibits GR-GRE interactions through protein-protein interactions in the nucleus (Hu et al., 2009; Rogatsky and Ivashkiv, 2006). Finally, IL-1 receptor activation results in mitogen activated protein kinase kinase (MKK)3/6 and MKK4/7 activation. These complexes activate p38 MAPK and c-Jun N-terminal kinases (JNK), which can phosphorylate GR impeding its nuclear translocation. JNK also phosphorlates cJUN, which associates with cFOS to form the AP-1 complex, which also interferes with GR-GRE interactions (Pace et al., 2007a; Pace et al., 2007b).
3.4 Stress and Immunity
Depression is clearly a stress-sensitive disorder, and both early life stress such as abuse (Heim et al., 2008; Heim and Nemeroff, 2001a; Heim and Nemeroff, 2001b) and recent stressors (Hilsman and Garber, 1995) increase risk for depression. The actual causal mechanisms for the relationships between stress and depression are unknown. However, recent research suggests that stress-induced immune activation may play an important role. As an example, peripheral monocytes from healthy volunteers exposed to a public speaking stressor (referred to as the Trier Social Stress Test) and mental arithmetic have increased NF-κB – DNA binding (Bierhaus et al., 2003). Both IL-6 response and NF-κB – DNA binding in response to an acute social stress have also been shown to be increased in persons with depression (Pace et al., 2006). In addition, chronic stressors from a variety of sources appear to enhance immune activation (Miller et al., 2009).
Recent evidence also illuminates the stress – immune activation – depression linkage. Early childhood maltreatment has long been associated with risk for depression (Heim et al., 2008). A recent study by Danese et al. (Danese et al., 2009) attempted to elucidate whether early adversity and subsequent risk are associated with enduring abnormalities in stress-sensitive biological systems. This study was part of a 32-year prospective longitudinal study of 1037 persons in New Zealand assessed at intervals beginning in early childhood. At age 32 years, participants were assessed for the presence of major depression, inflammation (as indicated by CRP >3 mg/L), and the indicators of cardiometabolic risk factors, including hypertension, total cholesterol, reduced HDL, elevated glycated hemoglobin (indicating evidence for altered glucose metabolism), and low maximum oxygen consumption. Those who had been exposed to childhood adversity showed not only increased likelihood of depression, but also elevated CRP and metabolic risk markers. This suggests that early adversity has enduring effects not only on risk for depression but also immune and metabolic dysregulation. These data are also consistent with other studies suggesting a link between early life stress, immune activation, and depression. For example, Pace et al. (Pace et al., 2006) found that male patients with major depression who had early life stressors exhibit enhanced IL-6 response and NF-κB – DNA binding in response to the Trier Social Stress Test in comparison to non-traumatized depressed controls. Of significance is that baseline IL-6 levels in the traumatized sample were at about the same level as the maximal response to the social stressor in the non-traumatized controls, suggesting that traumatized depressed persons have chronic elevation in IL-6 levels. These findings support a link between major depression, early life stress, and chronic immune activation, and may elucidate a causal mechanism for the association of early trauma and subsequent negative health outcomes.
3.5 The Effects of Antidepressant Treatments on Immune Function
Antidepressants appear to inhibit immune activation via several mechanisms. Many studies of antidepressant effects in vitro and in vivo have shown reductions in pro-inflammatory factors such as IL2, IL-6, TNFα, and IFNγ (Basterzi et al., 2005; Bengtsson et al., 1992; Kubera et al., 2000a; Kubera et al., 2000b; Kubera et al., 2000c; Kubera et al., 2001a; Kubera et al., 2001b; Lanquillon et al., 2000; Maes et al., 1999; Mohr et al., 2001; Obuchowicz et al., 2006; Seidel et al., 1995; Sluzewska et al., 1995; Song et al., 1994; Szuster-Ciesielska et al., 2003; Zhu et al., 1994; Zhu et al., 1998) (for a review, see Miller et al. (Miller et al., 2009)), although the variability in the data may be accounted for a number of methodological differences (Muller and Schwarz, 2007). One study also found reductions in TNFα levels in severely depressed patients undergoing electroconvulsive therapy (Hestad et al., 2003). The preponderance of evidence suggests that antidepressant treatment induces a shift from Th1 (pro-inflammatory) to TH2/TH3 (anti-inflammatory) processes (Muller and Schwarz, 2007). For example, several studies have demonstrated a reduction in the ratios of IFNγ to IL-10 (Kubera et al., 2001a; Maes et al., 1999; Szuster-Ciesielska et al., 2003) and IL-4 (Myint et al., 2005), as well as an increase in the Th3 molecule transforming (or tumor) growth factor β1 (Myint et al., 2005). Antidepressants also appear to alter the expression of PGE2 and NO. As noted earlier, brain cytokine release, including IL-1α/β (Anforth et al., 1998), IL-6 (Bluthe et al., 2000b), and TNFα (Bluthe et al., 2000a) stimulate endothelial cells in small blood vessels to release prostaglandin E2 (PGE2) and NO, which are important mediators of sickness behavior (Dantzer, 2009). One study (Yaron et al., 1999) showed that fluoxetine and amitriptyline significantly inhibited NO and PGE2 release in synovial tissue exposed to LPS or IL-1α and TNFα. Interestingly, the effects of antidepressants on pro-inflammatory cytokine and related mechanisms may be direct and may not depend on the effects of these drugs on monoamines. For example, amitriptyline and nortriptyline suppress LPS-induced IL-1β and TNFα release in mixed glial cultures (Obuchowicz et al., 2006), suggesting a direct action of the antidepressants on pro-inflammatory cytokines.
Antidepressants also appear to counteract the adverse effects of cytokines on HPA axis function. Most antidepressant treatments, including antidepressant drugs and electrically-induced seizures, increase signal transduction via protein kinase A (PKA) (Duman et al., 1999; Nestler et al., 1989). Transductional activation of PKA by cyclic AMP causes the PKA tetramer to dissociate into regulatory and catalytic subunits (Shelton, 2007). The latter phosphorylates cyclic AMP response element binding protein (CREB), resulting in translocation to the nucleus it interacts with cyclic AMP response elements (CRE) in the promoter regions of genes, including the gene for GR (Eickelberg et al., 1999; Penuelas et al., 1998). PKA phosphorylates GR, which appears to increase GR – GRE interactions and GRE-dependent gene expression. Enhancement of GR activity may be one of the principal mechanisms for normalization of HPA function by antidepressants (Pariante et al., 1995; Plotsky et al., 1998). Signaling via PKA also appears to negatively interact with both NF-κB and p38 MAPK signaling pathways (Pace et al., 2007a), which would be expected to enhance GR signaling. Notably, defects in PKA expression and signaling relative to controls have been noted in both peripheral and brain tissue samples from depressed persons (Akin et al., 2005; Manier et al., 1996; Pandey et al., 2005; Pandey et al., 2007; Shelton et al., 1996; Shelton et al., 1999; Shelton et al., 2009). Reduced PKA activity has been hypothesized to affect GR expression, which would be normalized by antidepressants that enhance PKA-dependent signal transduction (Shelton, 2007).
3.6 Anti-inflammatory Drugs and Cytokine Antagonists as Antidepressants
3.6.1 COX-2 Inhibitors
In spite of considerable evidence linking immune activation and inflammation with depression, surprisingly little research has examined the direct effects of drugs that act on these systems in depressed patients. One target of investigation has been the antagonists of cyclooxygenase (COX) enzymes (also known as prostaglandin-endoperoxide synthase and prostaglandin G/H synthase). COX enzymes are involved in the conversion of arachidonic acid to prostaglandins and eicosanoids (e.g., thromboxanes), which are important mediators of local inflammatory responses (Rao and Knaus, 2008). Both COX-1 and -2 metabolize arachidonic acid to prostaglandin H2 which is a precursor to prostaglandins, prostacyclin, and thromboxanes. Inhibitors of COX-2 are well-known as systemic anti-inflammatory agents (Rao and Knaus, 2008). COX-2 activity is increased by pro-inflammatory cytokines, particularly IL-6, and itself activates the release IL-1β and TNFα (Muller and Schwarz, 2007). A significant downstream product of COX-2 activation is PGE2, which is a significant mediator of sickness behavior as noted above (Dantzer, 2009). Several studies have shown an increase in prostaglandin secretion, including PGE2 in CSF (Linnoila et al., 1983), serum (Calabrese et al., 1986), and saliva (Nishino et al., 1989; Ohishi et al., 1988) of depressed patients (Muller and Schwarz, 2007). One study also demonstrated increased mitogen-stimulated PGE2 release from whole blood samples in depressed patients compared to controls (Song et al., 1998). Given this evidence, COX-2 inhibition represents a logical target for depression treatment.
The beneficial effects of COX-2 inhibitors in animal models of depression have been documented (Guo et al., 2009; Kumari et al., 2007; Myint et al., 2007). However, one of the earliest observations of an antidepressant effect of COX-2 inhibitors in humans came not in a study of depression per se, but an open-label assessment of the effects of the COX-2 antagonist rofecoxib in patients with osteoarthritis (Collantes-Estevez and Fernandez-Perez, 2003). This study was an investigation of the effects of switching from the COX-2 inhibitor celecoxib to rofecoxib in 2228 patients and was intended to determine moderators of response to rofecoxib switch. Patient characteristics (moderators) identified in multivariate analysis as predictive of a favorable response to rofecoxib included age, obesity, depressive symptoms, co-morbid diabetes, and OA severity. A total of 15% of osteoarthritis patients were determined to be depressed at baseline, which declined to 3% during rofecoxib treatment. This was followed by an open-label study of the effects of the non-selective COX-1 and -2 antagonist acetylsalicylic acid (aspirin) added to fluoxetine, which increased remission rates in depressed patients previously nonresponsive to fluoxetine alone (Mendlewicz et al., 2006). The first controlled clinical trial of a COX enzyme inhibitor was a prospective, double-blind, add-on trial in 40 patients with major depression comparing the COX-2 selective antagonist celecoxib (400 mg. per day) versus placebo added to the norepinephrine reuptake inhibitor antidepressant reboxetine (4–10 mg. per day) for 6 weeks (Muller et al., 2006). Although both groups improved, the celecoxib plus reboxetine group experienced greater improvement in depression scores compared to the reboxetine-alone group. These studies suggest a benefit of COX inhibitors added to antidepressant medications in depression, although monotherapy trials of COX-2 antagonists in depression have not been published.
3.6.2 TNF Receptor Antagonists
In recent years, TNF receptor inhibitors have been developed to treat inflammatory diseases such as rheumatoid arthritis, Chrohn’s disease, and psoriasis, and include monoclonal antibodies (infliximab, adalimumab, golimumab, and certolizumab pegol) and the TNF receptor fusion protein etanercept. Although these treatments have not been tested in a primary depressed sample, the effects of etanercept on depressive symptoms in patients with inflammatory disease have been tested. In one study (Tyring et al., 2006b), 618 patients with moderate to severe psoriasis received double-blind treatment with placebo or 50 mg twice weekly treatment with etanercept, a recombinant DNA construct antagonist of the TNF receptor, or placebo for 12 weeks. Psoriasis is an inflammatory disease affecting the skin and other tissues with a relatively high rate of depression (Akay et al., 2002; Krueger et al., 2001). Efficacy endpoints included measures of fatigue (the Functional Assessment of Chronic Illness Therapy Fatigue [FACIT-F] and depression (the HRSD and the Beck Depression Inventory [BDI]). Patients on etenercept had greater improvements in depression (HRSD and BDI) and fatigue (FACIT-F) than those on placebo (although the differences in depression ratings were relatively modest). Improvements in depression were not particularly associated with reduction in psoriatic plaques or joint pain, suggesting a primary effect of TNF antagonism on depression (Tyring et al., 2006b). This finding was confirmed in subsequent studies in which etanercept was administered to psoriasis patients over a 54 (Dauden et al., 2010) or 96 week period (Krishnan et al., 2007). Similar results on depression were shown in an etanercept study in rheumatoid arthritis patients (Kekow et al., 2010). Recent studies have also suggested a benefit of infliximab, a TNFα monoclonal antibody, on depressive symptoms in patients with inflammatory diseases (Feldman et al., 2008; Lichtenstein et al., 2002). To date, however, there have been no published studies of etanercept, infliximab, or other TNFα antagonists in depressed patients without inflammatory diseases.
3.7 Immune Activation, Oxidative Stress, and Depression
As described earlier, immune activation of white blood cells, neurons, and glia activates the synthesis of reactive chemical species such as NO, superoxide (O2−), and peroxynitrile (ONOO−), which contribute to the cytotoxic effects of pathogens (Nathan and Shiloh, 2000). Under normal circumstances, intracellular oxidative stress is buffered through the reducing action of a number of molecules, particularly glutathione. However, high levels of reactive species contribute to cytotoxicity of host cells leading to local tissue necrosis. In addition, chronic immune activation may lead to conditions of heightened oxidative stress.
Heightened oxidative stress has been hypothesized in depression (Cumurcu et al., 2009). Recent studies have shown not only raised indicators of oxidative stress but also reduced anti-oxidant capacity in persons with depression. In one such study (Cumurcu et al., 2009), total oxidative stress and anti-oxidant capacity, measured using standard colometric methods, were examined in 57 persons with major depression and 40 healthy controls. Measures of total oxidative stress were significantly elevated while antioxidant capacity was lower in the depressed group compared to controls. Measures of oxidative stress were positively correlated and antioxidant capacity inversely associated with depression severity. Successful treatment with the antidepressants sertraline, paroxetine, and citalopram were associated with decreases in oxidative stress and enhanced antioxidant capacity. These results are similar to results from other studies, and implicate increased oxidative and nitrosative stress in depression (Maes, 2008).
4.0 Adiposity and Depression
It is clear from the previous discussion that depression is often associated with a pro-inflammatory state, and that body lipid accumulation in the form of WAT is one potential source of inflammatory cytokines. However, is there a link between adiposity, cytokine activation, and depression? Some studies have not found an association between adiposity, inflammation, and depression risk (Murphy et al., 2009; Olszanecka-Glinianowicz et al., 2009; Pan et al., 2008). Moreover, a recent systematic review suggested that the overall association between obesity in general and depression risk is weak (Atlantis and Baker, 2008). However, a more recent meta-analysis of 15 longitudinal studies showed a bidirectional association between depression and obesity (Luppino et al., 2010). That is, obesity increases risk for depression and prior depression increased the likelihood of obesity over time. Another recent study of 2,547 non-depressed, older adults in the Health, Aging, and Body Composition Study, an ongoing prospective community-based cohort study, supports this observation; (Vogelzangs et al., 2009). Assessments in this study include BMI, percent body fat, abdominal obesity measures (waist circumference, sagittal diameter, and visceral fat), and depression (defined as a Center for Epidemiologic Studies Depression scale 10-item score ≥10 at any annual follow-up over 5 years and/or new antidepressant medication use). Over 5 years, both BMI and abdominal obesity predicted onset of depression after adjustment for covariates in men although not in women. However, when BMI and visceral fat were adjusted for each other, only visceral fat was significantly associated with depression onset. The differences between men and women may have to do with the fact that while the women had a higher percentage of body fat, men had more visceral fat at baseline. Other research also supports the significance of visceral (i.e., abdominal) fat. One very large scale epidemiological study found that hip-to-waist ratio, an indicator of abdominal obesity, rather than obesity in general, was associated with risk for depression (Rivenes et al., 2009). Another project conducted a mediational analysis evaluating the relationship between serum inflammatory markers (IL-1β, IL-6, TNFα, MCP-1 [CCL2], and CRP) in 50 physically healthy younger adults with depression and 50 matched non-depressed controls (Miller et al., 2002b). The mediational analysis was conducted in a manner consistent with Baron and Kenny (Baron and Kenny, 1986), in which a mediating role was implied when observed group differences in a variable are attenuated by a co-variate. The depressed sample showed markedly elevated CRP and IL-6 and had a higher body mass index (BMI) than controls. BMI also was independently associated with IL-6 and CRP but not IL-1β. Significantly, when the relationship between depression and both IL-6 and CRP (but not IL-1β) were adjusted for BMI, the results became non-significant. These results support a mediational role for adiposity in the relationship between depression and IL-6 and CRP elevation (Miller et al., 2002b). A separate analysis of the same data (Miller et al., 2003) suggested an even more complex interplay between depression, obesity, and inflammation. This analysis used structural equation modeling (SEM) yielding a maximum likelihood estimation of the relationship among depression, adiposity, leptin, and inflammation (IL-6, CRP). SEM is a statistical method for estimating causal relationships among associated variables. The advantage of SEM in this instance is that it can estimate the extent of interactions between various factors and identify both predicted and latent (unknown) associations. The SEM was conducted over several analytic phases. The first was a confirmatory factor analysis that identified three underlying constructs: depression, adiposity, and inflammation (IL-6), as predicted. In the second phase, structural modeling was performed to determine the relationships between constructs and evaluated six possible models. The best fit model was one referred to as the “joint fit” model, which suggested that the causal pathway was from depression to adiposity to inflammation. The model also suggested that part of the relationship between adiposity and inflammation could be accounted for by the effects of adiposity on leptin (although it should be noted that other adipocytokines and related molecules were not assessed). As noted by Miller and colleagues, the best fit model suggests that depression causes expanded adipose tissue which leads directly to increases in pro-inflammatory biomarkers (e.g., IL-6) and also indirectly via elevated leptin levels (Miller et al., 2003). It should be noted, however, that this model applies to young, physically healthy depressed persons and may not be applicable to other populations, although baseline depression has been linked to subsequent increases in abdominal obesity in older adults as well (Vogelzangs et al., 2008). However, it does support the interplay between adiposity, cytokines, leptin, and depression.
The linkage between leptin adiposity, and depression has been supported by other research (Lu, 2007). Chronic, unpredictable stressors or social defeat, which induce depression-like behaviors, have been shown to reduce leptin levels in rodents (Lu et al., 2006), a state that mimics leptin resistance found in obesity (Considine et al., 1996). Administration of leptin has been shown to reverse the behavioral effects of both acute and chronic stress that are thought to mimic depression (Lu et al., 2006). However, clinical studies in depressed patients have shown highly variable results, with studies showing increased, decreased, or no differences in leptin levels (Deuschle et al., 1996; Antonijevic et al., 1998; Rubin et al., 2002; Jow et al., 2006; Kraus et al., 2001; Atmaca et al., 2002). This is likely to be related to the clinical heterogeneity of depression and the complexity of leptin response. A deficient response to leptin may be associated with either low leptin levels or down-regulation of leptin receptors (Lu, 2007). Reduced signaling via leptin receptors, whether by leptin insufficiency or decreased receptor responsiveness, may contribute to depressive symptoms.
An association between dyslipidemia and depression has also been reported (Nakao and Yano, 2004; Takeuchi et al., 2009a; Takeuchi et al., 2009b; Tyrovolas et al., 2009). For example, in one study, 1190 older (aged 65 to 100 years) men and women with cardiovascular disease were enrolled in a study intended to evaluate the relationships between elevated serum cholesterol (hypercholesterolemia) and both lifestyle and depression status (Tyrovolas et al., 2009). The signs and symptoms of depression were evaluated using the Geriatric Depression Scale (short form) and hypercholesterolemia was defined as total serum cholesterol > 200 mg/dL or use of lipids lowering medication. Hypercholisterolemic individuals had higher prevalence of obesity (43% vs. 25%), hypertension (76% vs. 57%) and diabetes (25% vs. 17%) and had higher levels of depression compared with participants without hypercholesterolemia. These data are supported by previous studies that have demonstrated an increased incidence of depression in persons with metabolic syndrome (MetS) in general and hypercholesterolemia in particular (Nakao and Yano, 2004; Takeuchi et al., 2009a; Takeuchi et al., 2009b). In one health behavior study, high cholesterol levels were positively predicted by four variables: presence of major depression, age, body mass index, and the habit of missing breakfast (Nakao and Yano, 2004). Interestingly, another study evaluated the relationship between baseline MetS and the subsequent development of depression in Japanese men followed for one year (Takeuchi et al., 2009a). MetS is defined as being a cluster of medical conditions typically associated with central (abdominal) obesity. These conditions include dyslipidemia (e.g., elevated total and LDL cholesterol reduced HDL cholesterol, and elevated triglycerides), elevations in fasting blood sugar consistent with insulin resistance (type 2 diabetes), and hypertension (Ford et al., 2002; Grundy et al., 2004; NCEP Expert Panel, 2001). MetS is a known risk factor for the development of cardiovascular and cerebrovascular disease. It is also associated with elevations in pro-inflammatory cytokines, particularly TNFα and altered levels of leptin, adiponectin, and resistin (Grundy et al., 2004; Lara-Castro et al., 2007). In fact, the National Cholesterol Education Program’s Adult Treatment Panel III report (ATP III) includes a pro-inflammatory state as part of the standard definition of MetS (Grundy et al., 2004; NCEP Expert Panel, 2002). Takeuchi et al study (Takeuchi et al., 2009a) evaluated 956 Japanese (mean age, 42.7 years). The objective of the study was to test the temporal relationships between MetS and the development of depression and anxiety, controlling for potential confounding factors like age and lifestyle factors. MetS was diagnosed according to the International Diabetes Federation criteria. Depression and anxiety symptoms were assessed at baseline and at a one year follow-up using the Profile of Mood States (POMS) questionnaire and by clinical interview using the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Baseline MetS was associated with higher rates of new onset depression but not anxiety in the subsequent year (OR 2.14, 95% CI 1.10–4.17). Of the five individual MetS components examined, only waist circumference was significantly related to new-onset depression (OR 2.08, 1.23–3.50). The authors concluded that MetS, particularly waist circumference, is a risk factor for the subsequent development of depression in men. Taken together, these studies suggest a complex and reciprocal relationship between depression, obesity, MetS, and a pro-inflammatory state.
4.1 The Relationship Between Dietary Constituents and Risk for Depression
The last century has seen a dramatic shift in Western diets toward a much higher n-6:n-3 fatty acid ratio (Mischoulon, 2009). This shift in diet is responsible for the increases in diet- and obesity-related medical conditions, as described earlier. Of note is that the rate of depression appears to parallel this trend (Lavori et al., 1993). A variety of observations suggest that there are relationships between specific constituents of diet, particularly n-3 fatty acids, found in large quantities in fish and fish oil extracts. A number of epidemiological studies have found an association between the prevalence of mood disorders, particularly depression, and the annual consumption of fish (for a review, see Kraguljac et al. (Kraguljac et al., 2009)). Although the data have not been completely consistent, the bulk of the evidence supports a protective effect for fish consumption (Kraguljac et al., 2009). For example, one study based in the central Mediterranean region tested the association between level of fish intake and depressive symptoms in older adults. A total of 1,190 men and women over age 65 were assessed for depression severity using Geriatric Depression Scale (GDS) and food intake using the Food Frequency Questionnaire. People in the lowest one third of depression severity were more educated, physically active, and had a higher level of fish consumption. Even a small increase in fish intake was associated with a significant reduction in depression severity. These data suggest that lifestyle habits, especially fish intake, are associated with a substantial reduction in depression risk in older adults. The relationship between fish consumption and depression risk may be clarified by the results of a recent study (Astorg et al., 2008), which found that whereas fish intake in general was associated with a reduction in depression risk, intake of so-called fatty fish (i.e., fish with high n-3 fatty acid content, including anchovy, sea bass, carp, dogfish, eel, halibut, herring, mackerel, mullet, fish, roe, salmon, sardine, trout, and fresh tuna) had a greater effect. The benefit of fatty fish intake was lost in smokers, in whom the intake of fatty fish was actually associated with a higher risk of recurrent episodes of depression over an eight year follow-up period, particularly in women. Fish consumption in general and n-3 fatty acid intake in particular has been associated with positive health benefits for a number of diseases, including cardiovascular disease, diabetes, certain forms of cancer, and arthritis (for reviews, see Mazza et al. (Mazza et al., 2007), Juturi (Juturu, 2008), and Ruxton et al. (Ruxton et al., 2004)). The general health benefits of n-3 fatty acids occur via several mechanisms, including positive effects on platelet aggregation. However, a major benefit appears to result from a reduction in inflammatory allostatic load.
These results are supported by a number of other studies of the relationships between diet, particularly the roles of antioxidants and n-3 fatty acids. A major recent report takes off from earlier observations of a possible lower incidence of depression in the Mediterranean region (Birt et al., 2003) and the general health benefits of a Mediterranean diet pattern (MDP). Traditional Mediterranean diets tend to be high in both antioxidants and n-3 fatty acids relative to other Western diets. Sánchez-Villegas et al. (Sanchez-Villegas et al., 2009) conducted a large-scale assessment of the association between adherence to the MDP and the incidence of depression in a sample 10,094 healthy persons in Spain. A baseline assessment was conducted that used a validated 136-item item food frequency questionnaire, from which was derived relative adherence to the MDP. The study also evaluated the relative consumption of foods in several groupings: vegetables, fruit and nuts, cereal, legumes, fish and other seafood, monounsaturated- to saturated-fatty-acid ratio, alcohol consumption, meat or meat products. The main outcome measure was the incidence of depression over a mean of 4.4 years in people free of depression at baseline. Taking the lowest level of adherence to the MDP as the “control” condition, adjusted hazard ratios for depression for the highest categories of adherence to the MDP ranged from 0.49 (95% confidence intervals 0.44 – 0.77) to 0.74 (95% CI = 0.57–0.98), suggesting a strong protective effect for the MDP. A significant protective effect was found for fruits and nuts, legumes, and unsaturated to saturated fatty acid ratio, while whole fat dairy and meat consumption showed an adverse effect. The MDP is generally associated with a number of health benefits, including anti-inflammatory effects and improvements in endothelial function, cardiovascular health, insulin resistance, and MetS (Chrysohoou et al., 2004; Dai et al., 2008; Esposito et al., 2004; Estruch et al., 2006; Panagiotakos et al., 2007; Salas-Salvado et al., 2008; Tortosa et al., 2007). One study in particular found a strong inverse association between adherence to the MDP and serum IL-6 (and a trend for CRP) levels (Dai et al., 2008). In this study, the investigators administered the Willett food frequency questionnaire to 345 middle-aged male twins and assessed adherence to the MDP. Fasting plasma levels of interleukin-6, C-reactive protein, and other cardiovascular risk factors were measured. A mixed-effects regression analyses showed that adherence to the Mediterranean diet was associated with lower plasma IL-6 after adjustment for total energy intake, other nutritional factors, cardiovascular risk factors, and use of medications and supplements. The overall association of adherence to the diet with IL-6 was much stronger than shared genetic or environmental effects. These results indicate that dietary components, including the ratio of n-3 to n-6 fatty acid intake, are associated with both inflammation and risk for depression.
Other dietary components, particularly simple sugars, have adverse effects on body adiposity. The intake of carbohydrates, particularly in the form of high fructose corn syrup, has increased dramatically in recent decades (Marriott et al., 2009). High fructose corn syrup has been singled out as an important contributor to obesity and related diseases (Marriott et al., 2009; Schaefer et al., 2009; Murphy, 2009; Bray, 2008; Brown et al., 2008). What is undeniable is that the consumption of simple sugars, including high fructose corn syrup, have increased dramatically in the last 40 years (Marriott et al., 2009). High fructose corn syrup is an inexpensive sweetener used in a variety of processed foods, particularly sugared soft drinks (Bray, 2008; Brown et al., 2008). The corn syrup content of sweeteners has more than doubled since 1978 (Marriott et al., 2009). Overall intake of carbohydrates has increased by approximately 42% in the U.S. over this period (Marriott et al., 2009). The unique contribution of fructose to obesity, in contrast to overall carbohydrate loading, has been called into question (White, 2009). However, fructose may be particularly problematic with regard to obesity-related conditions. Body weight in rats is disproportionately higher with equivalent caloric intake of fructose than sucrose (Bocarsly et al., 2010). High fructose intake contributes markedly to dyslipidemia and insulin resistance (Tappy and Le, 2010; Figlewicz et al., 2009; Angelopoulos et al., 2009). The differences between fructose and other sugars appear to be related, in part, to the differential extraction rate of liver on fructose. The extraction of absorbed fructose by the liver is very high as compared to other sugars such as glucose (Schaefer et al., 2009). Therefore, in contrast to other simple sugars, fructose does not contribute substantially to satiety signaling (Moran, 2009) or increases in insulin (Basciano et al., 2005). Fructose loading in the liver increases glycogen and is ultimately metabolized to triglycerides, which are incorporated into very low density lipoproteins. As such, fructose is highly lipogenic (Basciano et al., 2005). Whether in the form of fructose or other simple sugars, high carbohydrate intake is associated with adiposity and associated medical conditions.
4.2 n-3 Fatty Acid Supplementation for Depression
Given the data suggesting a beneficial effect of n-3 fatty acid intake on risk for depression, clinical trials testing the benefits of n-3 supplementation are a logical extension. A number of the earliest studies of n-3 supplementation in mood disorders were in bipolar disorder, suggesting a beneficial effect in depression but not mania (Freeman et al., 2006; Stoll et al., 1999). Subsequent studies in both bipolar and non-bipolar depression yielded highly variable results; however, the designs of these studies were highly variable and many included small sample sizes while others did not use a placebo control (for a review, see Kraguljac et al. (Kraguljac et al., 2009) or Mischoulon (Mischoulon, 2009)). Some of the variability of these data may be related to findings of differential immunoregulatory effects of the two major constituents of n-3 fatty acid extracts EPA and DHA, suggesting that purified EPA might be a better alternative (Maes et al., 2007).
Peet and Horrobin (Peet and Horrobin, 2002) conducted a randomized, placebo-controlled, study of ethyl-EPA at 1, 2, or 4 gm. per day as add-on therapy for 70 persons with major depression who had not responded to an adequate trial of an antidepressant. The 1 mg. per day, but not 2 or 4 mg./day doses showed significantly higher response rates compared to the placebo group (50% versus 29% response). Although preliminary, these data not only indicate that adjunctive EPA may be beneficial in persons with depression not responsive to adequate therapy, but that there may be an optimal dose range for EPA. These results have generally been supported by other studies of ethyl-EPA in depressed samples (Frangou et al., 2006; Mischoulon et al., 2010; Nemets et al., 2002). As an example, Nemets et al. (Nemets et al., 2002) added ethyl-EPA 1 gm. twice per day or placebo to ongoing antidepressant therapy for four weeks in a group of 30 persons with major depression. The patients treated with ethyl-EPA showed greater reduction in depression scores over the treatment period, again supporting a beneficial effect of EPA in depression.
4.3 Are Diet and Adiposity Risk Factors for Childhood Depression?
4.3.1 Adiposity, Inflamamtion, and Obesity-Related Diseases in Childhood
As noted earlier, as with adults, the shifts in diet and reduction of physical activity has led to high rates of overweight and obesity in children and adolescents (Ben-Sefer et al., 2009; James, 2008; Wang and Lobstein, 2006). In fact, there has been an increase of inflammatory diseases associated with overweight in children such as type 2 diabetes, hepatic steatosis (fatty liver disease), cardiovascular disease, endothelian dysfunction, and MetS typically thought of as diseases of adults in the past (Alisi et al., 2009; Chiarelli and Marcovecchio, 2008; Eyzaguirre and Mericq, 2009; Golden et al., 2009; Molleston et al., 2002; Nanda, 2004; Roberts, 2003; Schuster, 2009; Semiz et al., 2008). The expansion of adipose tissue in childhood is thought to dramatically increase the risk for obesity in adult life, setting a lifetime pattern of overweight, inflammatory allostatic loading, and illness.
In fact, recent research has demonstrated that overweight children show increased evidence of inflammation just like their adult counterparts (Tam et al., 2010; Warnberg and Marcos, 2008(Steene-Johannessen et al., 2010)). For example, McMurray and colleagues (McMurray et al., 2007) compared inflammatory responses to exercise in overweight youth with normal weight controls. Participants engaged in 10 two minute periods of exercise above the anaerobic threshold; pre- and post-test blood samples were obtained. At baseline, both leukocyte and IL-6 levels were higher in overweight children and increased further with exercise. Monocytes increased in overweight children and remained elevated two hours post-exercise, whereas natural killer, CD4, and CD8 cells declined. These results are consistent with the hypothesis that childhood obesity is associated with a chronic pro-inflammatory state that may be worsened in the short term by high-intensity exercise (McMurray et al., 2007). This has even been confirmed in non-obese children, suggesting that the association between increasing adiposity and indications of inflammation are not limited to more extreme levels of overweight (McVean et al., 2009). These results are also consistent with other research showing increased pro-inflammatory cytokines and alterations in adipocytokines and related molecules associated with obesity in children and adolescents (Jeffery et al., 2008; Sacheck, 2008; Semiz et al., 2008), where the most consistent findings have included high levels of CRP and reduced adiponectin (Korner et al., 2007; Saltevo et al., 2007; Shin et al., 2008; Winer et al., 2006).
The association between altered adiponectin levels and indicators of inflammation was illustrated in a study by Winer et al. (Winer et al., 2006) which examined the relationships between adiponectin and markers of inflammation and adiposity. A sample of 589 obese children and adolescents were administered an oral glucose tolerance test coupled with baseline measures of adiponectin, lipid profile, CRP, IL-6, and leptin. The results showed associations between low adiponectin levels and elevated CRP and aspects of MetS such as reduced LDL and elevated triglycerides, even when controlling for confounding variables such as body mass, sex, ethnicity, and insulin sensitivity. This suggests that altered adiponectin levels may represent an important link between diet-associated obesity and inflammatory biomarkers, and may occur in a manner that is not entirely dependent on increases in body mass.
4.3.2 Adiposity and Depression in Children and Adolescents
The rates of depression and suicide among both children and adults have increased over the last century (Barnes et al., 1986; Klerman et al., 1985; Maughan et al., 2005). Although many factors undoubtedly have contributed to this trend, one important variable may be increases in adiposity (McElroy et al., 2004), which have largely paralleled this trend. The bulk of evidence suggests a bi-directional relationship between adiposity and depression, such that being overweight increases risk for depression and that being depressed enhances risk of elevated adiposity (McElroy et al., 2004; Miller et al., 2002a). In fact, prior depression in childhood is a relatively strong predictor of the subsequent development of obesity, MetS, and inflammatory diseases in adult life (Goodwin et al., 2009; Hasler et al., 2004). One prospective cohort study (Hasler et al., 2004) followed young adults over a period of 20 years and showed that not only was depression associated with being overweight, depression in general and depression with atypical features in particular was related to weight gain over the follow-up period. Another prospective cohort study (Pulkki-Raback et al., 2009) evaluated 921 males and females in childhood (mean age 12) and again in adult life (mean age 33) and found that symptoms of depression in childhood predicted the development of MetS and that MetS in children predicted depression in females. This suggests a bi-directional relationship between risks for depression and MetS at least for women, although, as discussed earlier, there appears to be a positive association between MetS and level of depression in both men and women (Miettola et al., 2008; Takeuchi et al., 2009a; Takeuchi et al., 2009b). The bi-directionality of the relationship between depression and adiposity is logical. Obesity negatively impacts self-esteem based on cultural aspects of beauty and desirability (Reeves et al., 2008). However, depression also may contribute to risk for depression via effects on physical activity, sleep, and eating behavior (Reeves et al., 2008). However, we would posit that the linkage between depression, adiposity, and inflammation in children is an important area for further research.
Alternatively, do interventions targeting diet and adiposity affect depression risk? One intriguing result has come from a study by Nemets and colleagues (Nemets et al., 2006) of n-3 fatty acid supplementation in children with depression. In this study, depressed children between the ages of 6 and 12 were randomized to receive either 1000 mg. of n-3 fatty acids or a matched placebo containing safflower oil (which contains 74% linoleic acid, an n-6 fatty acid and no n-3) for 16 weeks. The group treated with n-3 fatty acids showed a statistically significant reduction in depression scores compared to placebo. Notably, this effect was relatively slow to accumulate, being evident from weeks 8 to 16. However, the overall effect was relatively robust by endpoint; 70% of children treated with n-3 fatty acids achieved response criteria (defined as a reduction of 50% or more on the Childhood Depression Inventory [CDI]) and 40% achieved a standard definition of remission (CDI < 29). Although the overall sample size was small (N=28), the results are very encouraging and suggest a role for dietary intervention to treat or prevent depression in children.
5.0 The Role of Inactivity in Inflammation and Depression
Adiposity is a major contributor to inflammatory allostatic load and cardiometabolic diseases, but it is by no means the only issue. For example, relative physical inactivity appears to be an important causal factor. Although physical inactivity is strongly associated with obesity, it appears to contribute to risk for inflammation and depression that is relatively independent of adiposity (Bruunsgaard, 2005). Large population-based studies have consistently shown an inverse relationship between physical activity and markers of systemic inflammation (Beavers et al., 2010). Sustained exercise training in both adults and children appears to reduce markers of inflammation, although acute exercise appears to transiently increase inflammation (Ploeger et al., 2009; Buford et al., 2009). It should be noted, however, that even a single bout of exercise improves insulin sensitivity via an increase in insulin-induced translocation of glucose transporters to myocyte cell membranes (Frosig and Richter, 2009). Sustained physical exercise also has been shown to reduce the risk of cardiometabolic diseases (Janiszewski and Ross, 2009). Therefore, adiposity alone is not a sufficient explanation for inflammatory load in the body. In fact Church (Church, 2009) has posited a “low fitness phenotype,” comprising poor diet, overweight, and a sedentary lifestyle. As stated by Church, “there are likely some metabolic disadvantaged individuals with intrinsically low fitness and low oxidative capacity that are also prone to being sedentary due to easy fatigability. For these individuals, there exists a worst case scenario of a sedentary lifestyle superimposed on metabolically disadvantaged muscle resulting in the exaggerated CVD and metabolic risk.” We would also suggest that this is likely to be worsened by the presence of depression. In fact, as noted earlier, structural equation modeling of the relationship between depression, obesity, and inflammation yielded a best fit model which indicated that depression leads to expanded adipose tissue which leads directly to increases in pro-inflammatory biomarkers (e.g., IL-6) in a young, physically healthy sample (Miller et al., 2003).
The relationship between depression, inactivity, adiposity, and inflammation has also been supported by a recent report from the English Longitudinal Study of Ageing (Hamer et al., 2009a). The study sample included 3609 community dwelling older males and females (mean age of 60.5±9.2 years). Depressive symptoms were assessed using the 8-item Center for Epidemiologic Studies Depression (CES-D) scale) along with health behaviors (smoking, alcohol use, and physical activity), body weight, and central adiposity at baseline and at a 2 year follow-up. Elevated depressive symptoms were identified in 12.7% of the sample at baseline and 6.1% at follow-up. Baseline CES-D score was associated with increased CRP and fibrinogen measured 2 years later. Mediational analysis showed a direct association of depressive symptoms on CRP and indirect mediating effects through behavioral risk factors. The fibrinogen effect was not directly related to depression and was entirely explained through indirect mediating effects on health behaviors. When individual health behaviors were examined, a strong protective effect was shown for physical activity on both baseline and follow-up depressive symptoms. Moderate and vigorous physical exercise ≥ 1 time per week reduced depression risk at baseline (OR = 0.60 and 0.44 respectively) and at 2 years (OR = 0.49 and 0.32) (Hamer et al., 2009a). A subsequent analysis showed that physical activity contributed a level of risk for depression and elevated CRP that was independent of other lifestyle determinants (Hamer et al., 2009b). Together, these data suggest a strong interplay of several factors including depression, obesity, inactivity, and inflammation. What is likely to be the case is that each of these factors interacts with the others. For example, a causal path from inactivity to depression could be posited given the relationship between a sedentary lifestyle with both obesity and inflammation. However, depression also may lead to inactivity, as well as other negative lifestyle factors such as smoking and excessive alcohol use that contribute to risk for obesity and inflammation.
6.0 Depression, Immune Dysregulation, and Adiposity: Summary and Conclusions
To this point we can conclude: 1. Many people with medical conditions that are associated with increases in inflammation such as certain form of cancers, rheumatoid arthritis, multiple sclerosis, psoriasis, and cardiovascular disease have elevated rates of depression; 2. Patients with these conditions who are depressed have increased markers of inflammation such as CRP and IL-6 compared to non-depressed persons with the same conditions; 3. Many ostensibly medically healthy persons with depression show elevations in inflammatory markers (e.g., IL-6 and CRP) in the absence of obvious inflammatory disease, consistent with a Th1 immune response; 4. Many of the symptoms of depression (e.g., fatigue, anhedonia, low motivation, HPA axis activation) are similar to symptoms seen in the context of immune activation (sickness behavior); 5. Administration of inflammatory cytokines such as IFNα for the treatment of diseases such as hepatitis C and malignant melanoma induce depressive symptoms in previously asymptomatic individuals; this parallels sickness behavior in animal models but it is almost indistinguishable from clinical depression; 6. Blockade of mediators of depression such as TFNα reduce symptoms of depression in patients with inflammatory disease; 7. Many of the cellular mechanisms involved in immune activation (e.g., serotonin and dopamine depletion and NMDA receptor activation) are related to hypothesized pathophysiological mechanisms for depression. The preponderance of evidence suggests that inflammatory mediators are involved in the generation of depression in certain individuals.
The causes for the activation of the immune system in depression are undoubtedly complex (Maes, 2008) and remain unknown. However, increasing evidence suggests a possible causal link between adiposity, particularly central (abdominal) obesity and both inflammation and depression. Obesity leads to increases in inflammatory cytokines and adipocytokines, and changes in related molecules such as leptin and adiponectin, which may contribute to the development of depression in vulnerable individuals. However, the relationship appears to be bidirectional, such that prior depression seems to increase the risk for subsequent adiposity (Miller et al., 2002a). Clearly, more research is needed to examine the complex interplay between depression, inflammation, and adiposity, an effect that may be related in part to effects of depression on physical activity (Hamer et al., 2009a; Hamer et al., 2009b). However, the interactions between these systems offer unique opportunities for targeted treatment and prevention strategies, particularly in children and adolescents. For example, there is a pressing need to investigate strategies to disrupt inflammatory signaling in persons with depression who show indication of a pro-inflammatory state (e.g., those with elevated CRP). Research on approaches that alter diet, increasing the intake of n-3 fatty acids, or reducing weight in depressed persons or populations at risk of developing depression (e.g., children of depressed parents) are also needed.
This review has focused on adiposity and inflammation as possible contributing factors for depression. However, several salient facts must be noted. Depression is a complex and multifaceted disease with many contributing factors. This review is not intended to suggest that inflammation or adiposity contribute to depression in all or even most affected people. For example, sickness behavior that lies at the heart of the link between inflammation and depression is often associated with weight loss, not weight gain (Kelley et al., 2003; Tisdale, 2009). In fact, in the context of weight loss associated with cancer (cachexia), IL-6 levels are associated with severe muscle wasting, particularly in the terminal state (Tisdale, 2009). Inflammatory cytokines have been hypothesized to be the proximal causal path mediating the link between cancer cachexia and depression (Illman et al., 2005; Menzies et al., 2005). However, this has implications for the pathways linking inflammatory cytokines and depression. Specifically, there are likely to be multiple paths to increased inflammatory cytokines, including adiposity on one side, or muscle-wasting diseases such as cancer on the other, which may contribute to depression risk. Further, in some people, depression itself may contribute to increased inflammatory cytokines in a manner that is independent of other inflammatory conditions in the body.
Acknowledgements
This work was supported by National Institutes of Health Grant Award Numbers MH073630, MH01741, and MH52339 (RCS), and MH069124, MH075102, HL073921, MH020018 (AHM), and NIH/National Center for Research Resources (NCRR) General Clinical Research Center Grant M01RR00039. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Abbreviations
- 5-HIAA
5-hydroxy indoleacetic acid
- 5HTT
serotonin transporter
- AAAD
aromatic amino acid decarboxylase
- ABCA1
ATP-binding cassette transporter-1
- ACTH
adrenocorticotropic hormone
- AP1
activator protein 1
- ATP III
National Cholesterol Education Program’s Adult Treatment Panel III Report
- AVP
arginine vasopressin
- BDI
Beck Depression Inventory
- BH4
tetrahydrobiopterin
- BMI
body mass index
- CCL
chemokine (CC-motif) ligands
- CD4+
t-helper lymphocytes
- CD8+
cytotoxic lymphocytes
- CDI
Childhood Depression Inventory
- CNS
central nervous system
- COX
cyclooxygenase
- CRE
cyclic AMP response element
- CREB
cyclic AMP response element binding protein
- CRH
corticotrophin releasing hormone
- CRP
c-reactive protein
- CSF
cerebrospinal fluid
- DAT
dopamine transporter
- DHA
docsahexanoid acid
- EPA
eicosapentaenoic acid
- ERK
extracellular signal-regulated kinases
- FACIT-F
Functional Assessment of Chronic Illness Therapy Fatigue
- GDS
Geriatric Depression Scale
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HDL
high density lipoprotein
- HEK
human embryonic kidney
- HPA
hypothalamic-pituitary-adrenal
- HRSD
Hamilton Rating Scale for Depression
- hsp70
heat shock protein 70
- hsp90
heat shock protein 90
- IDO
idoleamine 2,3-dioxygenase
- IFN
interferon
- IKK
IκB kinase
- IL
interleukin
- IL-1Ra
interleukin 1 receptor antagonist
- iNOS (NOS II)
inducible nitric oxide synthase
- IRF1
interferon regulatory factor-1
- IRS-1
insulin receptor substrate-1
- JAK1
Janus kinase-1
- JNK
c-Jun N-terminal kinases
- KMO
kynurenine 3-monooxygenase
- KYN-A
kynurenic acid
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein kinas
- MCP-1
monocyte chemoattractant protein 1
- MDP
Mediterranean diet pattern
- MEK
MAPK kinase
- MetS
metabolic syndrome
- MKK
mitogen activated protein kinase kinase
- mmLDL
minimally-modified low density lipoproteins
- MYD88
myeloid differentiation primary response gene 88
- n-3
omega-3 fatty acid
- n-6
omega-6 fatty acid
- n-9
omega-9 fatty acid
- NF-κB
nuclear factor kappa B
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- PAMP
pathogen-associated molecular patterns
- PGE2
prostaglandin E2
- PKA
protein kinase A
- POMS
Profile of Mood States
- PPARα
peroxisomeproliferator-activated receptor-α
- PTX
pentraxin
- PUFA
polyunsaturated fatty acid
- PVN
paraventricular nucleus
- QUIN
quinolinic acid
- STAT1a
signal transducer and activator of transcription 1a
- TAB
mitogen-activated protein kinase kinase kinase 7-interacting proteins
- TH
tryptophan hydroxylase
- Th1
T-helper cell 1 (innate) immunity
- Th2
T-helper cell 2 immunity
- TLR
toll-like receptor
- TNFα
Tumor necrosis factor alpha
- TGFβ
transforming growth factor beta
- UCP-1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akay A, Pekcanlar A, Bozdag KE, Altintas L, Karaman A. Assessment of depression in subjects with psoriasis vulgaris and lichen planus. J. Eur. Acad. Dermatol. Venereol. 2002;16:347–352. doi: 10.1046/j.1468-3083.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- Akin D, Manier DH, Sanders-Bush E, Shelton RC. Signal transduction abnormalities in melancholic depression. Int. J. Neuropsychopharmacol. 2005;8:5–16. doi: 10.1017/S146114570400478X. [DOI] [PubMed] [Google Scholar]
- Alisi A, Manco M, Panera N, Nobili V. Association between type two diabetes and non-alcoholic fatty liver disease in youth. Ann. Hepatol. 2009;8 Suppl 1:S44–S50. S44–S50. [PubMed] [Google Scholar]
- Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri M, Aissa SA, Belguendouz H, Mezioug D, Touil-Boukoffa C. In vitro antihydatic action of IFN-gamma is dependent on the nitric oxide pathway. J Interferon Cytokine Res. 2007;27:781–787. doi: 10.1089/jir.2007.0003. [DOI] [PubMed] [Google Scholar]
- Andrei AM, Fraguas R, Jr., Telles RM, Alves TC, Strunz CM, Nussbacher A, Rays J, Iosifescu DV, Wajngarten M. Major depressive disorder and inflammatory markers in elderly patients with heart failure. Psychosomatics. 2007;48:319–324. doi: 10.1176/appi.psy.48.4.319. [DOI] [PubMed] [Google Scholar]
- Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur. Cytokine Netw. 1998;9:279–288. [PubMed] [Google Scholar]
- Angelopoulos TJ, Lowndes J, Zukley L, Melanson KJ, Nguyen V, Huffman A, Rippe JM. The effect of high-fructose corn syrup consumption on triglycerides and uric acid. J Nutr. 2009;139:1242S–1245S. doi: 10.3945/jn.108.098194. [DOI] [PubMed] [Google Scholar]
- Antonijevic I, Murck H, Frieboes R-M, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. Journal of Psychiatric Research. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, Alessandri JM, Galan P, Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins, Leukotrienes Ess. Fatty Acids. 2008;78:171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int. J. Obes. (Lond) 2008;32:881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Gecici O, Firidin B. Serum Leptin and Cholesterol Values in Suicide Attempters. Neuropsychobiology. 2002;45:124–127. doi: 10.1159/000054950. [DOI] [PubMed] [Google Scholar]
- Barnes RA, Ennis J, Schober R. Cohort analysis of Ontario suicide rates, 1877–1976. Can. J. Psychiatry. 1986;31:208–213. doi: 10.1177/070674378603100305. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basterzi AD, Aydemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, Goka E. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert. Rev Cardiovasc. Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim. Acta. 2010 doi: 10.1016/j.cca.2010.02.069. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD) Ann. Hepatol. 2009;8 Suppl 1:S4–S8. [PubMed] [Google Scholar]
- Bengtsson BO, Zhu J, Thorell LH, Olsson T, Link H, Walinder J. Effects of zimeldine and its metabolites, clomipramine, imipramine and maprotiline in experimental allergic neuritis in Lewis rats. J. Neuroimmunol. 1992;39:109–122. doi: 10.1016/0165-5728(92)90180-s. [DOI] [PubMed] [Google Scholar]
- Ben-Sefer E, Ben-Natan M, Ehrenfeld M. Childhood obesity: current literature, policy and implications for practice. Int. Nurs. Rev. 2009;56:166–173. doi: 10.1111/j.1466-7657.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006;70(102):1–10. doi: 10.5688/aj7005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt C, Bille-Brahe U, Cabecadas M, Chishti P, Corcoran P, Elgie R, van Heeringen K, Horte LG, Marchi AG, Ostamo A, Petridou E, Renberg ES, Stone DH, Wiik J, Williamson E. Suicide mortality in the European Union. Eur. J. Public Health. 2003;13:108–114. doi: 10.1093/eurpub/13.2.108. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE. Molecular biology of serotonin receptors and transporters. Clin. Neuropharmacol. 1992;15 Suppl 1 Pt A:351A–352A. doi: 10.1097/00002826-199201001-00182. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 2000a;12:4447–4456. [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiolk. Behav. 2000b;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: Increased body weight, body fat and triglyceride levels. Pharmacol Biochem. Behav. 2010 doi: 10.1016/j.pbb.2010.02.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinol. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Brandacher G, Winkler C, Aigner F, Schwelberger H, Schroecksnadel K, Margreiter R, Fuchs D, Weiss HG. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes. Surg. 2006;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- Bray GA. Fructose: should we worry? Int. J Obes. (Lond) 2008;32 Suppl 7:S127–S131. doi: 10.1038/ijo.2008.248. S127–S131. [DOI] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Kaim M, Funesti-Esch J. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch. Intern. Med. 2001;161:411–420. doi: 10.1001/archinte.161.3.411. [DOI] [PubMed] [Google Scholar]
- Breum L, Rasmussen MH, Hilsted J, Fernstrom JD. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. Am. J. Clin. Nutr. 2003;77:1112–1118. doi: 10.1093/ajcn/77.5.1112. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Halman M, Catalan J, Sacktor N, Price RW, Brown S, Atkinson H, Clifford DB, Simpson D, Torres G, Hall C, Power C, Marder K, Mc Arthur JC, Symonds W, Romero C. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin. Trials. 2007;2:e13. doi: 10.1371/journal.pctr.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Dulloo AG, Montani JP. Sugary drinks in the pathogenesis of obesity and cardiovascular diseases. Int. J Obes. (Lond) 2008;32 Suppl 6:S28–S34. doi: 10.1038/ijo.2008.204. S28–S34. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl. Physiol Nutr Metab. 2009;34:745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, Subichin S, Krupp NE. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr. Hypertens. Rep. 2008;10:32–38. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J. Affect. Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacol. 2002a;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry. 2003a;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacol. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am. J. Psychiatry. 2003b;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry. 2002b;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl. Acad. Sci. USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A, Bergeron J, Poirier P, Almeras N, Tremblay A, Lemieux I, Despres JP. Increased plasma interleukin-1 receptor antagonist levels in men with visceral obesity. Ann. Med. 2009:1–8. doi: 10.1080/07853890903022801. [DOI] [PubMed] [Google Scholar]
- Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur. J. Endocrinol. 2008;159(Suppl 1):S67–S74. doi: 10.1530/EJE-08-0245. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The Attica study. J. Am. Coll. Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Church T. The Low-fitness Phenotype as a Risk Factor: More Than Just Being Sedentary? Obesity. 2009;17:S39–S42. doi: 10.1038/oby.2009.387. [DOI] [PubMed] [Google Scholar]
- Collantes-Estevez E, Fernandez-Perez C. Improved control of osteoarthritis pain and self-reported health status in non-responders to celecoxib switched to rofecoxib: results of PAVIA, an open-label post-marketing survey in Spain. Curr. Med. Res. Opin. 2003;19:402–410. doi: 10.1185/030079903125001938. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O'Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci. Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: Impact of antidepressant treatment. Psychiatry Clin. Neurosciences. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauden E, Griffiths C, Ortonne JP, Kragballe K, Molta C, Robertson D, Pedersen R, Estojak J, Boggs R. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J. Eur. Acad. Dermatol. Venereol. 2010 doi: 10.1111/j.1468-3083.2009.03321.x. (in press) [DOI] [PubMed] [Google Scholar]
- Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch. Gen. Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Heninger GR, Gelenberg AJ, Charney DS. Monoamines and the mechanism of antidepressant action: effects of catecholamine depletion on mood of patients treated with antidepressants. Psychopharmacol. Bull. 1993;29:389–396. [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol. Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR, Charney DS. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch. Gen. Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Licinio J, Krystal JH, Heninger GR, Charney DS. Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol. Bull. 1991;27:321–330. [PubMed] [Google Scholar]
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, Paciotti G, Gold PW, Sternberg EM. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1beta (IL-beta), IL-6, and tumor necrosis factor-alpha (TNFalpha) production in humans: High sensitivity of TNFalpha and resistance of IL-6. J. Clin. Endocrinol. Metab. 1997;82:2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- Despres JP, Arsenault BJ, Cote M, Cartier A, Lemieux I. Abdominal obesity: the cholesterol of the 21st century? Can. J. Cardiol. 2008;24 Suppl D:7D–12D. doi: 10.1016/s0828-282x(08)71043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum CD, Heuser I. Plasma Leptin in Depressed Patients and Healthy Controls. Horm Metab Res. 1996;28:714–717. doi: 10.1055/s-2007-979885. [DOI] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.09.033. (in press) [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl. Acad. Sci. U. S. A. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, Block LH. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J. Biol. Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Enerback S. The Origins of Brown Adipose Tissue. N. Engl. J. Med. 2009;360:2021–2023. doi: 10.1056/NEJMcibr0809610. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martínez-González M, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E. Effects of a Mediterranean-Style diet on cardiovascular risk factors. Ann. Int. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre F, Mericq V. Insulin resistance markers in children. Horm. Res. 2009;71:65–74. doi: 10.1159/000183894. [DOI] [PubMed] [Google Scholar]
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. 443–477. [DOI] [PubMed] [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, Moeller JR, Eidelberg D. Metabolic correlates of levodopa response in Parkinson's disease. Neurology. 2001;57:2083–2088. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Gottlieb AB, Bala M, Wu Y, Eisenberg D, Guzzo C, Li S, Dooley LT, Menter A. Infliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2008;159:704–710. doi: 10.1111/j.1365-2133.2008.08727.x. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on Rhesus Monkeys: A nonhuman primate model of cytokine-induced depression. Biol. Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feve B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Ioannou G, Bennett JJ, Kittleson S, Savard C, Roth CL. Effect of moderate intake of sweeteners on metabolic health in the rat. Physiol Behav. 2009;98:618–624. doi: 10.1016/j.physbeh.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br. J. Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. 46–50. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr., Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Frosig C, Richter EA. Improved Insulin Sensitivity After Exercise: Focus on Insulin Signaling. Obesity. 2009;17:S15–S20. doi: 10.1038/oby.2009.383. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol. Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. 1–22. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Mori K, Kaneko YS, Nakashima A, Nagasaka A, Itoh M, Ota A. Tetrahydrobiopterin biosynthesis in white and brown adipose tissues is enhanced following intraperitoneal administration of bacterial lipopolysaccharide. Biochim. Biophys. Acta. 2004;1670:181–198. doi: 10.1016/j.bbagen.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann. Nutr. Metab. 2009;55:123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- Gilchrist M, Hesslinger C, Befus AD. Tetrahydrobiopterin, a critical factor in the production and role of nitric oxide in mast cells. J Biol Chem. 2003;278:50607–50614. doi: 10.1074/jbc.M307777200. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom. Med. 2005;67 Suppl 1:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Gleason OC, Fucci JC, Yates WR, Philipsen MA. Preventing relapse of major depression during interferon-alpha therapy for hepatitis C--A pilot study. Dig. Dis. Sci. 2007;52:2557–2563. doi: 10.1007/s10620-006-9729-5. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Neurol. Clin. 2006;24:507–519. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J. Clin. Endocrinol. Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Sourander A, Duarte CS, Niemela S, Multimaki P, Nikolakaros G, Helenius H, Piha J, Kumpulainen K, Moilanen I, Tamminen T, Almqvist F. Do mental health problems in childhood predict chronic physical conditions among males in early adulthood? Evidence from a community-based prospective study. Psychol. Med. 2009;39:301–311. doi: 10.1017/S0033291708003504. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Harbuz M, Ma XM, Jessop D, Tilders FJ, Lightman SL, Aguilera G. Hypothalamic pituitary adrenal axis and immune responses to endotoxin in rats with chronic adjuvant-induced arthritis. Exp Neurol. 2002;178:112–123. doi: 10.1006/exnr.2002.8022. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox. Res. 2005;7:103–123. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 2009;612:54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: The English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009b;34:1050–1055. doi: 10.1016/j.psyneuen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de OC, Demakakos P. Persistent depressive symptomatology and inflammation: to what extent do health behaviours and weight control mediate this relationship? Brain Behav. Immun. 2009a;23:413–418. doi: 10.1016/j.bbi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Edfeldt K. Toll to be paid at the gateway to the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2005;25:1085–1087. doi: 10.1161/01.ATV.0000168894.43759.47. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D, Rossler W, Angst J. The associations between psychopathology and being overweight: a 20-year prospective study. Psychol. Med. 2004;34:1047–1057. doi: 10.1017/s0033291703001697. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol. Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001a;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001b;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinol. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J. ECT. 2003;19:183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Febbraio MA. The 2009 Stock Conference Report: Inflammation, obesity and metabolic disease. Obes. Rev. 2010 doi: 10.1111/j.1467-789X.2009.00691.x. (in press) [DOI] [PubMed] [Google Scholar]
- Hilsman R, Garber J. A test of the cognitive diathesis-stress model of depression in children: academic stressors, attributional style, perceived competence, and control. Journal of Personality & Social Psychology. 1995;69:370–380. doi: 10.1037//0022-3514.69.2.370. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav. Immun. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MN. Chemistry of nitric oxide and related species. Methods Enzymol. 2008;436:3–19. doi: 10.1016/S0076-6879(08)36001-7. 3–19. [DOI] [PubMed] [Google Scholar]
- Illman J, Corringham R, Robinson D, Jr., Davis HM, Rossi JF, Cella D, Trikha M. Are inflammatory cytokines the common link between cancer-associated cachexia and depression? J Support. Oncol. 2005;3:37–50. [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007b;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids (recommendations 1976) J. Lipid Res. 1978;19:114–128. [PubMed] [Google Scholar]
- Jacobson CM, Rosenfeld B, Pessin H, Breitbart W. Depression and IL-6 blood plasma concentrations in advanced cancer patients. Psychosomatics. 2008;49:64–66. doi: 10.1176/appi.psy.49.1.64. [DOI] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity: the size of the problem. J. Intern. Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Jang S, Jeong HS, Park JS, Kim YS, Jin CY, Seol MB, Kim BC, Lee MC. Neuroprotective effects of (−)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res. 2010;11313C:25–33. doi: 10.1016/j.brainres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Janiszewski PM, Ross R. The Utility of Physical Activity in the Management of Global Cardiometabolic Risk. Obesity. 2009;17:S3–S14. doi: 10.1038/oby.2009.382. [DOI] [PubMed] [Google Scholar]
- Jeffery AN, Murphy MJ, Metcalf BS, Hosking J, Voss LD, English P, Sattar N, Wilkin TJ. Adiponectin in childhood. Int. J Pediatr. Obes. 2008;3:130–140. doi: 10.1080/17477160801954538. [DOI] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. Journal of Affective Disorders. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacol.k. 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52:1104–1110. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry CE, Somm E, Pernin A, Alizadeh N, Giusti V, Dayer JM, Meier CA. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. doi: 10.1016/j.cyto.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Juturu V. Omega-3 fatty acids and the cardiometabolic syndrome. J Cardiometab. Syndr. 2008;3:244–253. doi: 10.1111/j.1559-4572.2008.00015.x. [DOI] [PubMed] [Google Scholar]
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, Arolt V, Cassens U, Rothermundt M. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J. Affect. Disord. 2005;87:305–311. doi: 10.1016/j.jad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa Ki, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin. Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, Pedersen R, Robertson D, Freundlich B, Sato R. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann. Rheum. Dis. 2010;69:222–225. doi: 10.1136/ard.2008.102509. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kennaugh JM, Hay WW., Jr. Nutrition of the fetus and newborn. West. J. Med. 1987;147:435–448. [PMC free article] [PubMed] [Google Scholar]
- Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Lavori PW, Rice J, Reich T, Endicott J, Andreasen NC, Keller MB, Hirschfield RM. Birth-cohort trends in rates of major depressive disorder among relatives of patients with affective disorder. Arch. Gen. Psychiatry. 1985;42:689–693. doi: 10.1001/archpsyc.1985.01790300057007. [DOI] [PubMed] [Google Scholar]
- Korner A, Kratzsch J, Gausche R, Schaab M, Erbs S, Kiess W. New predictors of the metabolic syndrome in children--role of adipocytokines. Pediatr. Res. 2007;61:640–645. doi: 10.1203/01.pdr.0000262638.48304.ef. [DOI] [PubMed] [Google Scholar]
- Kozak LP, Britton JH, Kozak UC, Wells JM. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988;263:12274–12277. [PubMed] [Google Scholar]
- Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS. Efficacy of omega-3 Fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacol. Bull. 2009;42:39–54. [PubMed] [Google Scholar]
- Kraus MR, Schafer A, Schottker K, Keicher C, Weissbrich B, Hofbauer I, Scheurlen M. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57:531–536. doi: 10.1136/gut.2007.131607. [DOI] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollm+ñcher T. Low Leptin Levels but Norma Body Mass Indices in Patients with Depression or Schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, Chiou CF, Patel V, Jahreis A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br. J. Dermatol. 2007;157:1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x. [DOI] [PubMed] [Google Scholar]
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch. Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Jaworska-Feil L, Lason W, Scharpe S, Maes M. Suppressive effect of TRH and imipramine on human interferon-gamma and interleukin-10 production in vitro. Pol. J. Pharmacol. 2000a;52:481–486. [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Zieba A, Dudek D, Nowak G, Maes M. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol. J. Pharmacol. 2000b;52:237–241. [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van BD, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J. Clin. Psychopharmacol. 2001a;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Holan V, Basta-Kaim A, Roman A, Shani J. Prolonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depression. J. Affect. Disord. 2001b;63:171–178. doi: 10.1016/s0165-0327(00)00182-8. [DOI] [PubMed] [Google Scholar]
- Kubera M, Simbirtsev A, Mathison R, Maes M. Effects of repeated fluoxetine and citalopram administration on cytokine release in C57BL/6 mice. Psychiatry Res. 2000c;96:255–266. doi: 10.1016/s0165-1781(00)00184-0. [DOI] [PubMed] [Google Scholar]
- Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem. Mol. Biol. 2005;94:383–394. doi: 10.1016/j.jsbmb.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Kumari B, Kumar A, Dhir A. Protective effect of non-selective and selective COX-2-inhibitors in acute immobilization stress-induced behavioral and biochemical alterations. Pharmacol. Rep. 2007;59:699–707. [PubMed] [Google Scholar]
- Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J. Mol. Med. 2007;85:1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- Lago F, Gomez R, Gomez-Reino JJ, Dieguez C, Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009;34:500–510. doi: 10.1016/j.tibs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Laguno M, Blanch J, Murillas J, Blanco JL, Leon A, Lonca M, Larrousse M, Biglia A, Martinez E, Garcia F, Miro JM, de PJ, Gatell JM, Mallolas J. Depressive symptoms after initiation of interferon therapy in human immunodeficiency virus-infected patients with chronic hepatitis C. Antivir. Ther. 2004;9:905–909. [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacol. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavori PW, Warshaw M, Klerman G, Mueller TI, Leon A, Rice J, Akiskal H. Secular trends in lifetime onset of MDD stratified by selected sociodemographic risk factors. J. Psychiatr. Res. 1993;27:95–109. doi: 10.1016/0022-3956(93)90054-6. [DOI] [PubMed] [Google Scholar]
- Levenson JL, Fallon HJ. Fluoxetine treatment of depression caused by interferon-alpha. Am. J. Gastroenterol. 1993;88:760–761. [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiol. 1999a;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiol. 1999b;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J. Neurochem. 2003;86:774–783. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm. Bowel. Dis. 2002;8:237–243. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch. Gen. Psychiatry. 1983;40:405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- Livija D, Barbara B, Cecilia G, Yeny Martinez de la Torre, Alberto M. Pentraxins: Multifunctional proteins at the interface of innate immunity and inflammation. BioFactors. 2009;35:138–145. doi: 10.1002/biof.21. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Socherman RE, Howell CD, Whitehead AJ, Hill JA, Dominitz JA, Hauser P. Association of interferon-alpha-induced depression and improved treatment response in patients with hepatitis C. Neurosci Lett. 2004;365:87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS, III, O'Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou JS, Kearns G, Benice T, Oken B, Sexton G, Nutt J. Levodopa improves physical fatigue in Parkinson's disease: a double-blind, placebo-controlled, crossover study. Mov. Disord. 2003;18:1108–1114. doi: 10.1002/mds.10505. [DOI] [PubMed] [Google Scholar]
- Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr. Opin. Pharmacol. 2007;7:648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl. Acad. Sci. U. S. A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG. Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. Archives of General Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, III, Cole S, Sood AK, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas JW. Biogenic amines and depression. Biochemical and pharmacological separation of two types of depression. Arch. Gen. Psychiatry. 1975;32:1357–1361. doi: 10.1001/archpsyc.1975.01760290025002. [DOI] [PubMed] [Google Scholar]
- Maas JW. Clinical and biochemical heterogeneity of depressive disorders. Ann. Intern. Med. 1978;88:556–563. doi: 10.7326/0003-4819-88-4-556. [DOI] [PubMed] [Google Scholar]
- MacPhee M. Global childhood obesity: how to curb an epidemic. J. Pediatr. Nurs. 2008;23:1–4. doi: 10.1016/j.pedn.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Minireview. Neuro. Endocrinol. Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Bosmans E. Why fish oils may not always be adequate treatments for depression or other inflammatory illnesses: docosahexaenoic acid, an omega-3 polyunsaturated fatty acid, induces a Th-1-like immune response. Neuro. Endocrinol. Lett. 2007;28:875–880. [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De JR, Bosmans E, Scharpe S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacol. 1999;20:370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav. Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manier DH, Eiring A, Shelton RC, Sulser F. Beta-adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacol. 1996;15:555–561. doi: 10.1016/S0893-133X(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Pecoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, Vollenweider P. Normal weight obesity: Relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc. Dis. 2010 doi: 10.1016/j.numecd.2009.06.001. (in press) [DOI] [PubMed] [Google Scholar]
- Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, Ruberto A, Tatarelli R, Nicoletti F, Girardi P, Shelton RC. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J. Psychiatr. Res. 2009;43:247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Iervolino AC, Collishaw S. Time trends in child and adolescent mental disorders. Curr. Opin. Psychiatry. 2005;18:381–385. doi: 10.1097/01.yco.0000172055.25284.f2. [DOI] [PubMed] [Google Scholar]
- Mazza M, Pomponi M, Janiri L, Bria P, Mazza S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Progr. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31:12–26. doi: 10.1016/j.pnpbp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry. 2004;65:634–651. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr. Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- McMurray RG, Zaldivar F, Galassetti P, Larson J, Eliakim A, Nemet D, Cooper DM. Cellular immunity and inflammatory mediator responses to intense exercise in overweight children and adolescents. J. Investig. Med. 2007;55:120–129. doi: 10.2310/6650.2007.06031. [DOI] [PubMed] [Google Scholar]
- McVean JJ, Carrel AL, Eickhoff JC, Allen DB. Fitness level and body composition are associated with inflammation in non-obese children. J. Pediatr. Endocrinol Metab. 2009;22:153–159. doi: 10.1515/jpem.2009.22.2.153. [DOI] [PubMed] [Google Scholar]
- Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 Receptor Antagonist Serum Levels Are Increased in Human Obesity: A Possible Link to the Resistance to Leptin? J Clin Endocrinol Metab. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Menzies H, Chochinov HM, Breitbart W. Cytokines, cancer and depression: connecting the dots. J Support. Oncol. 2005;3:55–57. [PubMed] [Google Scholar]
- Miettola J, Niskanen LK, Viinamaki H, Kumpusalo E. Metabolic syndrome is associated with self-perceived depression. Scand. J. Prim. Health Care. 2008;26:203–210. doi: 10.1080/02813430802117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am. J. Cardiol. 2005a;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol. 2002a;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol. 2002b;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 2005b;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 2005c;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- Mischoulon D. Update and Critique of natural remedies as antidepressant treatments. Obstetrics Gynecol. Clin. North Am. 2009;36:789–807. doi: 10.1016/j.ogc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, Smith J, Beaumont EC, Dahan LE, Alpert JE, Nierenberg AA, Fava M. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J. Clin. Psychiatry. 2010 doi: 10.4088/JCP.08m04603. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Islar J, Hauser SL, Genain CP. Treatment of depression is associated with suppression of nonspecific and antigen-specific T(H)1 responses in multiple sclerosis. Arch. Neurol. 2001;58:1081–1086. doi: 10.1001/archneur.58.7.1081. [DOI] [PubMed] [Google Scholar]
- Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am. J. Gastroenterol. 2002;97:2460–2462. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Moran TH. Fructose and satiety. J Nutr. 2009;139:1253S–1256S. doi: 10.3945/jn.108.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco BJ, Rifai MA, Loftis JM, Indest DW, Moles JK, Hauser P. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients with hepatitis C. J. Affect. Disord. 2007;103:83–90. doi: 10.1016/j.jad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson's disease. Clin. Neurosci. Res. 2006;6:261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Horton NJ, Burke JD, Jr., Monson RR, Laird NM, Lesage A, Sobol AM. Obesity and weight gain in relation to depression: findings from the Stirling County Study. Int. J. Obes. (Lond) 2009;33:335–341. doi: 10.1038/ijo.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP. The state of the science on dietary sweeteners containing fructose: summary and issues to be resolved. J Nutr. 2009;139:1269S–1270S. doi: 10.3945/jn.108.097964. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001a;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am. J. Psychiatry. 2001b;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Muzzin P. The uncoupling proteins. Ann. Endocrinol. (Paris) 2002;63:106–110. [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Myint AM, Steinbusch HW, Goeghegan L, Luchtman D, Kim YK, Leonard BE. Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression. Neuroimmunomodulation. 2007;14:65–71. doi: 10.1159/000107420. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yano E. Relationship between major depression and high serum cholesterol in Japanese men. Tohoku J. Exp. Med. 2004;204:273–287. doi: 10.1620/tjem.204.273. [DOI] [PubMed] [Google Scholar]
- Nanda K. Non-alcoholic steatohepatitis in children. Pediatr. Transplant. 2004;8:613–618. doi: 10.1111/j.1399-3046.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl. Acad Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCEP expert panel. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: A controlled, double-blind pilot study. Am. J. Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Duman RS. Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J. Neurochem. 1989;53:1644–1647. doi: 10.1111/j.1471-4159.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl. 1978;32:89–93. doi: 10.1007/978-3-0348-5559-4_9. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am. J. Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- Obuchowicz E, Kowalski J, Labuzek K, Krysiak R, Pendzich J, Herman ZS. Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. Int. J. Neuropsychopharmacol. 2006;9:27–35. doi: 10.1017/S146114570500547X. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biol. Psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Kocelak P, Janowska J, Semik-Grabarczyk E, Wikarek T, Gruszka W, Dabrowski P. Is chronic inflammation a possible cause of obesity-related depression? Mediators. Inflamm. 2010 doi: 10.1155/2009/439107. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owe-Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, Armati PJ, Crowe SM, Brew BJ. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J. Neurochem. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann. N. Y. Acad. Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007a;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, & Immunity. 2007b;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, Qi Q, Gu W, Pang X, Liu H, Lin X. The association of depressive symptoms with inflammatory factors and adipokines in middle-aged and older Chinese. PLoS One. 2008;3:e1392. doi: 10.1371/journal.pone.0001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Zampelas A, Toussoulis D, Stefanadis C. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: The ATTICA Study. J. Am. Coll. Nutr. 2007;26:32–38. doi: 10.1080/07315724.2007.10719583. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, Conley RR. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacol. 2005;30:1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int. J. Neuropsychopharmacol. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Nemeroff CB, Miller AH. Glucocorticoid receptors in depression. Isr. J Med. Sci. 1995;31:705–712. [PubMed] [Google Scholar]
- Parul T, Prashant T, Luv K, Vinod S. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007;51:443–452. doi: 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyleicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- Penuelas I, Encio IJ, Lopez-Moratalla N, Santiago E. cAMP activates transcription of the human glucocorticoid receptor gene promoter. J. Steroid Biochem. Mol Biol. 1998;67:89–94. doi: 10.1016/s0960-0760(98)00097-1. [DOI] [PubMed] [Google Scholar]
- Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc. Immunol Rev. 2009;15:6–41. 6–41. [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for 'depression due to a general medical condition', immunotherapy and antidepressive treatment. Int. J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, Domann E, Raven EL, Dehus O, Hermann C, Eggle D, Debey S, Chakraborty T, Kronke M, Utermohlen O, Schultze JL. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin Invest. 2006;116:3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Murphy M, Harrell M. Chaperoning of glucocorticoid receptors. Handb. Exp. Pharmacol. 2006;172:111–118. doi: 10.1007/3-540-29717-0_5. [DOI] [PubMed] [Google Scholar]
- Pulkki-Raback L, Elovainio M, Kivimaki M, Mattsson N, Raitakari OT, Puttonen S, Marniemi J, Viikari JS, Keltikangas-Jarvinen L. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol. 2009;28:108–116. doi: 10.1037/a0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav. Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol. Psychiatry. 2009a;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-[alpha] effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010 doi: 10.1038/mp.2008.58. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2009b doi: 10.1038/mp.2009.116. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, Zajecka JM, Bruno CJ, Henderson MA, Reinus JF, Evans DL, Asnis GM, Miller AH. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment. Pharmacol. Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008;11:81S–110S. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Reeves GM, Postolache TT, Snitker S. Childhood obesity and depression: connection between these growing problems in growing children. Int. J. Child Health Hum. Dev. 2008;1:103–114. [PMC free article] [PubMed] [Google Scholar]
- Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin. Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- Rivenes AC, Harvey SB, Mykletun A. The relationship between abdominal fat, obesity, and common mental disorders: Results from the HUNT Study. J. Psychosom. Res. 2009;66:269–275. doi: 10.1016/j.jpsychores.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Roberts EA. Nonalcoholic steatohepatitis in children. Curr. Gastroenterol. Rep. 2003;5:253–259. doi: 10.1007/s11894-003-0028-4. [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1–12. doi: 10.1111/j.1399-0039.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front. Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Rhodes ME, Czambel RK. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Research. 2002;113:255–268. doi: 10.1016/s0165-1781(02)00263-9. [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Reed SC, Simpson MJ, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J. Hum. Nutr. Diet. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Sacheck J. Pediatric obesity: an inflammatory condition? JPEN J. Parenter. Enteral Nutr. 2008;32:633–637. doi: 10.1177/0148607108324876. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite. 2009;53:422–425. doi: 10.1016/j.appet.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Salvado J, Fernandez-Ballart J, Ros E, Martinez-Gonzalez MA, Fito M, Estruch R, Corella D, Fiol M, Gomez-Gracia E, Aros F, Flores G, Lapetra J, Lamuela-Raventos R, Ruiz-Gutierrez V, Bullo M, Basora J, Covas MI for the PREDIMED Study Investigators. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008;168:2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- Saltevo J, Vanhala M, Kautiainen H, Laakso M. Levels of adiponectin, C-reactive protein and interleukin-1 receptor antagonist are associated with the relative change in body mass index between childhood and adulthood. Diab. Vasc Dis. Res. 2007;4:328–331. doi: 10.3132/dvdr.2007.060. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bethus I, Goodall G, Muscat R. Antidepressant reversal of interferon-alpha-induced anhedonia. Physiol. Behav. 2002;75:765–772. doi: 10.1016/s0031-9384(02)00677-7. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch. Gen. Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- Sas K, Robotka H, Toldi J, Vecsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr. 2009;139:1257S–1262S. doi: 10.3945/jn.108.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Schwaiger M, Garkisch AS, Pich M, Hinzpeter A, Uebelhack R, Heinz A, van BF, Berg T. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J. Hepatol. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. 1965. J. Neuropsychiatry Clin. Neurosci. 1995;7:524–533. doi: 10.1176/jnp.7.4.524. [DOI] [PubMed] [Google Scholar]
- Schlatter J, Ortuno F, Cervera-Enguix S. Monocytic parameters in patients with dysthymia versus major depression. J. Affect Disord. 2004;78:243–247. doi: 10.1016/S0165-0327(02)00316-6. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Aguilera G, Binnekade R, Tilders FJ. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience. 2003;116:275–283. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- Schramm TM, Lawford BR, Macdonald GA, Cooksley WG. Sertraline treatment of interferon-alfa-induced depressive disorder. Med. J. Aust. 2000;173:359–361. doi: 10.5694/j.1326-5377.2000.tb125687.x. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Schuster DP. Changes in physiology with increasing fat mass. Semin. Pediatr. Surg. 2009;18:126–135. doi: 10.1053/j.sempedsurg.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol. Nurs. Forum. 2002;29:E85–E90. doi: 10.1188/02.ONF.E85-E90. [DOI] [PubMed] [Google Scholar]
- Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Cytokine production and serum proteins in depression. Scand. J. Immunol. 1995;41:534–538. doi: 10.1111/j.1365-3083.1995.tb03604.x. [DOI] [PubMed] [Google Scholar]
- Semiz S, Rota S, Ozdemir O, Ozdemir A, Kaptanoglu B. Are C-reactive protein and homocysteine cardiovascular risk factors in obese children and adolescents? Pediatr. Int. 2008;50:419–423. doi: 10.1111/j.1442-200X.2008.02615.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. J. Thromb. Haemost. 2009;7 Suppl 1:44–48. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC. The molecular neurobiology of depression. Psychiatric Clin North America. 2007;30:1–11. doi: 10.1016/j.psc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Hal Manier D, Lewis DA. Protein kinases A and C in post-mortem prefrontal cortex from persons with major depression and normal controls. Int. J. Neuropsychopharmacol. 2009;12:1223–1232. doi: 10.1017/S1461145709000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Liang S, Liang P, Chakrabarti A, Manier DH, Sulser F. Differential expression of pentraxin 3 in fibroblasts from patients with major depression. Neuropsychopharmacol. 2003;29:129–132. doi: 10.1038/sj.npp.1300307. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Manier DH, Sulser F. cAMP-dependent protein kinase activity in major depression. Am. J. Psychiatry. 1996;153:1037–1042. doi: 10.1176/ajp.153.8.1037. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Manier DH, Peterson CS, Ellis TC, Sulser F. Cyclic AMP-dependent protein kinase in subtypes of major depression and normal volunteers. Int. J. Neuropsychopharmcol. 1999;2:187–192. doi: 10.1017/S1461145799001509. [DOI] [PubMed] [Google Scholar]
- Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem. Biophys. 2004;41:415–434. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- Shin JY, Kim SY, Jeung MJ, Eun SH, Woo CW, Yoon SY, Lee KH. Serum adiponectin, C-reactive protein and TNF-alpha levels in obese Korean children. J. Pediatr. Endocrinol. Metab. 2008;21:23–29. doi: 10.1515/JPEM.2008.21.1.23. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterol. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-[alpha] and IL-12 in macrophages by NF-[kappa]B-dependent pathway. Biochem. Biophysical Res. Comm. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann. N. Y. Acad. Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, Theander-Carrillo C, Juge-Aubry CE, Dayer JM, Nicklin MJ, Meda P, Rohner-Jeanrenaud F, Meier CA. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia. 2006;49:387–393. doi: 10.1007/s00125-005-0046-x. [DOI] [PubMed] [Google Scholar]
- Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J. Affect. Disord. 1994;30:283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, De JR, Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J. Affect. Disord. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Soygur H, Palaoglu O, Akarsu ES, Cankurtaran ES, Ozalp E, Turhan L, Ayhan IH. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1242–1247. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Moeller JR, Dhawan V, Ishikawa T, Eidelberg D. Visualizing the evolution of abnormal metabolic networks in the brain using PET. Computerized Med. Imaging Graphics. 2005;19:295–306. doi: 10.1016/0895-6111(95)00011-e. [DOI] [PubMed] [Google Scholar]
- Steene-Johannessen J, Kolle E, Reseland JE, Anderssen SA, Andersen LB. Waist circumference is related to low-grade inflammation in youth. Int. J Pediatr. Obes. 2010 doi: 10.3109/17477160903497035. (in press) [DOI] [PubMed] [Google Scholar]
- Stenholm S, Koster A, Alley DE, Visser M, Maggio M, Harris TB, Egan JM, Bandinelli S, Guralnik JM, Ferrucci L. Adipocytokines and the metabolic syndrome among older persons with and without obesity - the InCHIANTI Study. Clin Endocrinol (Oxf) 2010 doi: 10.1111/j.1365-2265.2009.03742.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch. Gen. Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Stuart WI, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab. Syndr. Relat Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- Szuster-Ciesielska A, Tustanowska-Stachura A, Slotwinska M, Marmurowska-Michalowska H, Kandefer-Szerszen M. In vitro immunoregulatory effects of antidepressants in healthy volunteers. Pol. J Pharmacol. 2003;55:353–362. [PubMed] [Google Scholar]
- Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009b;35:32–36. doi: 10.1016/j.diabet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Nakao M, Nomura K, Inoue M, Tsurugano S, Shinozaki Y, Yano E. Association of the metabolic syndrome with depression and anxiety in Japanese men: a 1-year cohort study. Diabetes Metab. Res. Rev. 2009a;25:762–767. doi: 10.1002/dmrr.1041. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv. Exp. Med. Biol. 1999;467:553–557. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes. Rev. 2010 doi: 10.1111/j.1467-789X.2009.00674.x. (in press) [DOI] [PubMed] [Google Scholar]
- Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Kaul M, Achim CL. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2010 doi: 10.1016/j.brainres.2009.06.036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O'Brien JT. Increase in interleukin-1beta in late-life depression. Am. J. Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiol. 2003;14:103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale MJ. Cancer cachexia. Curr. Opin. Gastroenterol. 2009 doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nuñez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean Diet Inversely Associated With the Incidence of Metabolic Syndrome. Diabetes Care. 2007;30:2957–2959. doi: 10.2337/dc07-1231. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem. 2008;114:267–276. doi: 10.1080/13813450802306602. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Chen CC, Bai CH, Wu SR. Cytokines and serotonin transporter in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Lin CF, Wu HT, Wu SR, Tsai WH. Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J. Psychopharmacol. 2008;22:753–760. doi: 10.1177/0269881107082951. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006a;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006b;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Tyrovolas S, Lionis C, Zeimbekis A, Bountziouka V, Micheli M, Katsarou A, Papairakleous N, Metallinos G, Makri K, Polychronopoulos E, Panagiotakos DB. Increased body mass and depressive symptomatology are associated with hypercholesterolemia, among elderly individuals; results from the MEDIS study. Lipids Health Dis. 2009;8:10. doi: 10.1186/1476-511X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziomalos K, Dimitroula HV, Katsiki N, Savopoulos C, Hatzitolios AI. Effects of lifestyle measures, antiobesity agents, and bariatric surgery on serological markers of inflammation in obese patients. Mediators. Inflamm. 2010 doi: 10.1155/2010/364957. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamos E, Pardutz A, Klivenyi P, Toldi J, Vecsei L. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J. Neurol. Sci. 2009;283:21–27. doi: 10.1016/j.jns.2009.02.326. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman ATF, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BWJH. Depressive symptoms and change in abdominal obesity in older persons. Arch. Gen. Psychiatry. 2008;65:1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Brenes GA, Newman AB, Satterfield S, Yaffe K, Harris TB, Penninx BW. An onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry. 2010 doi: 10.4088/JCP.08m04743blu. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J Pediatr. Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Warnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr. Opin. Lipidol. 2008;19:11–15. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Yirmiya R. Effects of bacterial endotoxin on the glucocorticoid feedback regulation of adrenocortical response to stress. Neuroimmunomodulation. 1966;3:352–357. doi: 10.1159/000097295. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC. The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol. Rev. Camb. Philos. Soc. 2006;81:183–205. doi: 10.1017/S1464793105006974. [DOI] [PubMed] [Google Scholar]
- White JS. Misconceptions about high-fructose corn syrup: is it uniquely responsible for obesity, reactive dicarbonyl compounds, and advanced glycation endproducts? J Nutr. 2009;139:1219S–1227S. doi: 10.3945/jn.108.097998. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, Wollschlager M, Seyal AA, Weiss R, Burgert TS, Caprio S. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2006;91:4415–4423. doi: 10.1210/jc.2006-0733. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Fact sheet: Obesity and overweight. 2010 http://www.who.int/mediacentre/factsheets/fs311/en/
- Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol. 2008;217:207–214. doi: 10.1002/jcp.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J. Leukoc. Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- Yang K, Xie G, Zhang Z, Wang C, Li W, Zhou W, Tang Y. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust. N. Z. J Psychiatry. 2007;41:266–273. doi: 10.1080/00048670601057759. [DOI] [PubMed] [Google Scholar]
- Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999;42:2561–2568. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Zadori D, Klivenyi P, Vamos E, Fulop F, Toldi J, Vecsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J. Neural Transm. 2009;116:1403–1409. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Yocum DC, Villanueva I, Smith B, Davis MC, Attrep J, Irwin M. Immune activation and depression in women with rheumatoid arthritis. J. Rheumatol. 2004;31:457–463. [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bengtsson BO, Mix E, Ekerling L, Thorell LH, Olsson T, Link H. Clomipramine and imipramine suppress clinical signs and T and B cell response to myelin proteins in experimental autoimmune neuritis in Lewis rats. J. Autoimmun. 1998;11:319–327. doi: 10.1006/jaut.1998.0209. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bengtsson BO, Mix E, Thorell LH, Olsson T, Link H. Effect of monoamine reuptake inhibiting antidepressants on major histocompatibility complex expression on macrophages in normal rats and rats with experimental allergic neuritis (EAN) Immunopharmacol. 1994;27:225–244. doi: 10.1016/0162-3109(94)90019-1. [DOI] [PubMed] [Google Scholar]


