Abstract
We have shown that “Reversed Chloroquine molecules” constructed from chloroquine-like and resistance “reversal agent”-like cores, can be powerful drugs against malaria (J. Med. Chem., 2006, 49, 5623–5). Several Reversed Chloroquines are now presented which probe parameters governing the activities against chloroquine-resistant and chloroquine-sensitive malaria strains. The design is tolerant to linker and reversal agent changes, but a piperazinyl group adjacent to the quinoline, at least for the group of compounds studied here, may be detrimental.
Keywords: Antimalarials, Dose-Response Relationship, Drug Evaluation, Erythrocytes/parasitology, Plasmodium falciparum, drug effects
INTRODUCTION
In terms of human suffering, malaria is clearly the most important parasitic disease. Furthermore, the worldwide burden of malaria is increasing – in part due to the spread of resistance to most of the drugs that were once effective, inexpensive, and safe.1 Among these drugs, chloroquine (CQ) had been the prime therapy for nearly half a century. CQ was safe, effective, remarkably inexpensive, and could be administered to pregnant women and infants. Unfortunately, P. falciparum, the cause of the most deadly malaria, is now CQ-resistant (CQR) in most endemic regions. The continuing spread of CQR, as well as resistance to alternative drugs has helped fuel a strong increase in incidence and consequence of malaria worldwide.1
In considering new antimalarial drug candidates, it seemed to us that CQ’s safety and economic advantages are simply too strong to abandon. Others have sought CQ modifications to combat drug resistance.2, 3 We began a program to use CQ’s quinoline core, but linked to entities that are known to overcome CQR, postulating that the resulting hybrid molecules might give enough physicochemical flexibility to allow such hybrids to be tailored to produce compounds that retain the beneficial qualities of CQ and that can be combined with a wide range of other drugs, in current use or in development, for combination therapies. This is an important point because it has become generally accepted that combination therapy should be used to delay the emergence of resistance to new antimalarial agents.1, 4, 5
CQ resistance in P. falciparum malaria is strongly associated with mutations in a parasite digestive vacuole (DV) membrane protein, PfCRT; these mutations have been found to be correlated with enhanced CQ export from the DV.6–9 Various molecules, termed reversal agents (RA), have been identified that inhibit this CQ export from the DV in CQR parasites.10–13 One RA pharmacophore may be described as a pair of aromatic rings, often with an aliphatic nitrogen atom a few angstroms removed from the aromatic rings.14
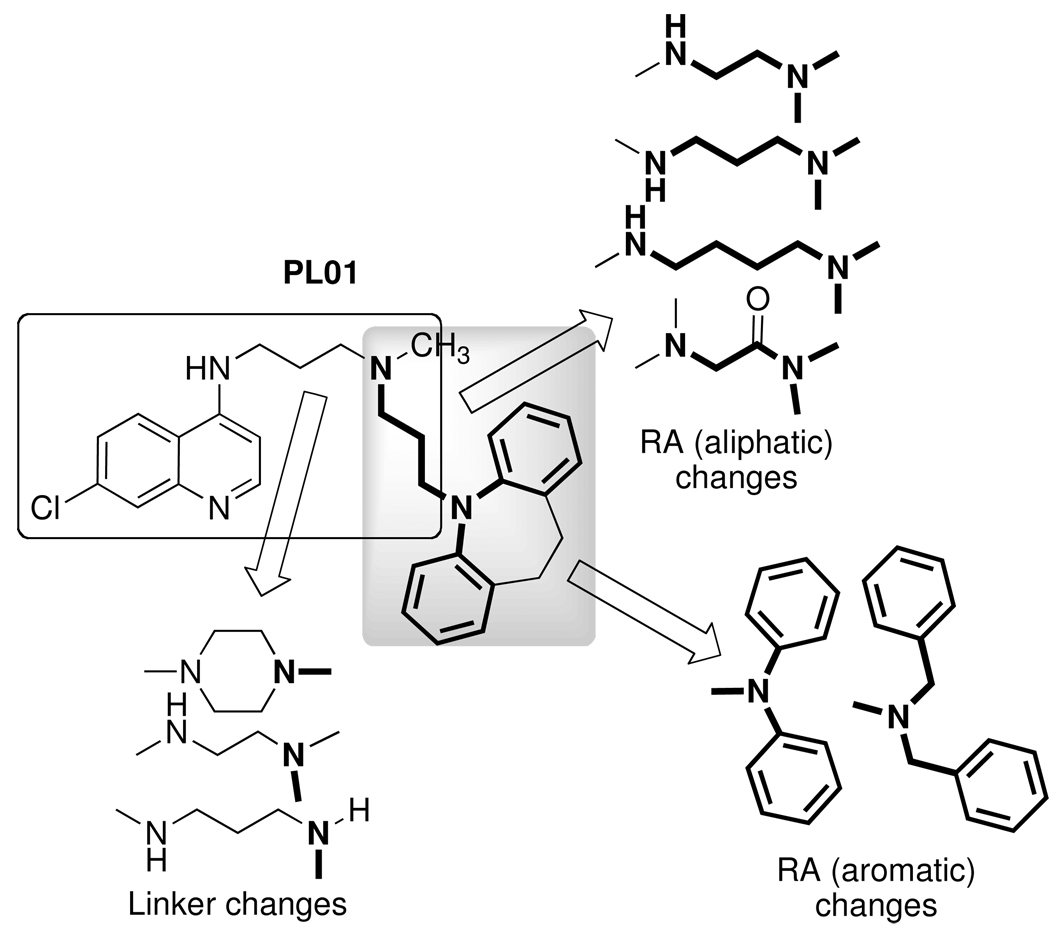
In an earlier publication,15 we showed that it is possible to synthesize a molecule that, conceptually, is a 7-chloro-4-alkylamino-quinoline linked to a reversal agent (RA) via an alkyl group. The RA was envisioned as inhibiting the P. falciparum chloroquine resistance transporter (PfCRT) -associated CQ export from the DV in CQR parasites.10–13 If such an effect were ‘perfect’ (i.e., no drug export, and no other resistance mechanism), then there should be equal efficacy against both CQS and CQR strains. Such a construct would deliver the RA in a 1:1 ratio with the quinoline, lowering the RA dose required if the two were given separately. We termed this conjugate drug a “reversed chloroquine” (RCQ), and showed that our first prototype, 1, has low-nM IC50 values against both CQS (e.g., D6) and CQR (e.g., Dd2) strains of P. falciparum malaria in red cell culture. Further, 1 was able to clear parasitemia from a mouse model to <1% via oral dosing. Encouraging as all this was, 1 is quite lipophilic (ClogP ~ 8.9), and there had been no intentional effort to optimize it against P. falciparum. We therefore undertook to modify the RCQ structure in an effort to delineate the factors that govern efficacy against P. falciparum. Others have also taken up the RCQ approach.19 Herein we report initial variations in the RCQ structure, beginning with the linker and aromatic groups of the RA moiety. The RCQ features evaluated include bridging between the two RA aromatic rings (conversion of the diphenyl to dibenzyl) in addition to some evaluation of the linker length and flexibility between the RA-end and the quinoline (Chart 1).
Chart 1.
Variations in the RCQ structure; the RA portions are shown in bold bonds.
RESULTS AND DISCUSSION
A set of molecules, shown in Table 1, was synthesized as outlined in the Supplementary Material. This set was chosen to be a minimal representation of variants including the linker between the chloroquinoline ring and the RA, as well as varying both the RA aromatics from diphenylamine to dibenzylamine or dibenzylamide, as well as the length of the aliphatic appendage. Thus, the linker was shortened from the imipramine propyl in 1 to an ethyl group, as well as to a piperazine (formally two ethyl linkers). In addition, the methyl attached to the aliphatic N of the RA aliphatic appendage was deleted. Other changes made to the RA moiety included both shortening and lengthening this appendage by one methylene, as well as converting the amine proximal to the aromatic groups into an amide. In each case, the ethyl bridge between the aromatics was removed to give diphenylamino- and dibenzylamino- functionalities. Although we did not produce every possible combination of all these changes, the set was sufficient to examine effects of these structural changes on the activities against CQS and CQR P. falciparum malaria.
Table 1.
RCQ activities against P. falciparum.
| Compound | ClogPa | IC50b (nM) |
IC50 Ratio Dd2/D6 |
Cytotoxicityc (nM) |
||
|---|---|---|---|---|---|---|
| D6 (CQS) |
Dd2 (CQR) |
|||||
| CQ | 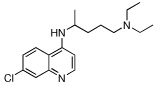 |
5.1 | 7 | 102 | 15 | 12000 |
| 1 | 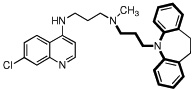 |
8.9 | 3 | 5 | 1.7 | 700 |
| 2 | 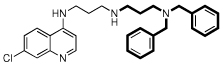 |
7.6 | 23 | 34 | 1.5 | 700 |
| 3 | 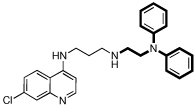 |
7.3 | 26 | 27 | 1.0 | 3700 |
| 4 | 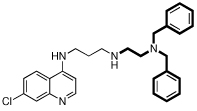 |
7.3 | 2 | 6 | 3.0 | N.D. |
| 5 | 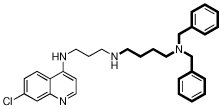 |
7.4 | 8 | 11 | 1.4 | 1300 |
| 6 | 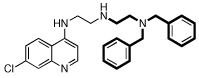 |
7.0 | 10 | 16 | 1.6 | 2200 |
| 7 | 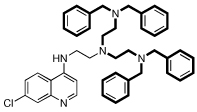 |
11.7 | 2 | 5 | 2.5 | 6200 |
| 8 | 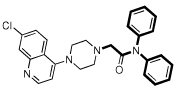 |
5.6 | 49 | 101 | 2.1 | 30000 |
| 9 | 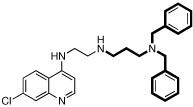 |
7.3 | 7 | 16 | 2.3 | N.D. |
| 10 | 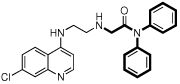 |
5.2 | 5 | 13 | 2.6 | 62000 |
| 11 | 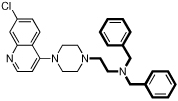 |
7.3 | 52 | 115 | 2.2 | 61000 |
| 12 | 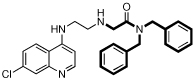 |
5.9 | 14 | 16 | 1.1 | 22000 |
| 13 | 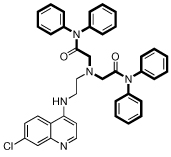 |
8.2 | 30 | 52 | 1.7 | 4300 |
Evaluated using ChemDraw software.
Averages of at least 3 runs (± 15%). The uncertainties are estimated based on weighing uncertainties for the various compounds (which are free-bases and often oils), as well as on variability between determinations that were performed on different weeks.
Cytotoxicities are against mouse spleen lymphocytes. These values are estimated to be ±50%, based on weighing uncertainties for the various compounds (which are free-bases and often oils), as well as on variability between determinations that were performed on different weeks. N.D.: not determined.
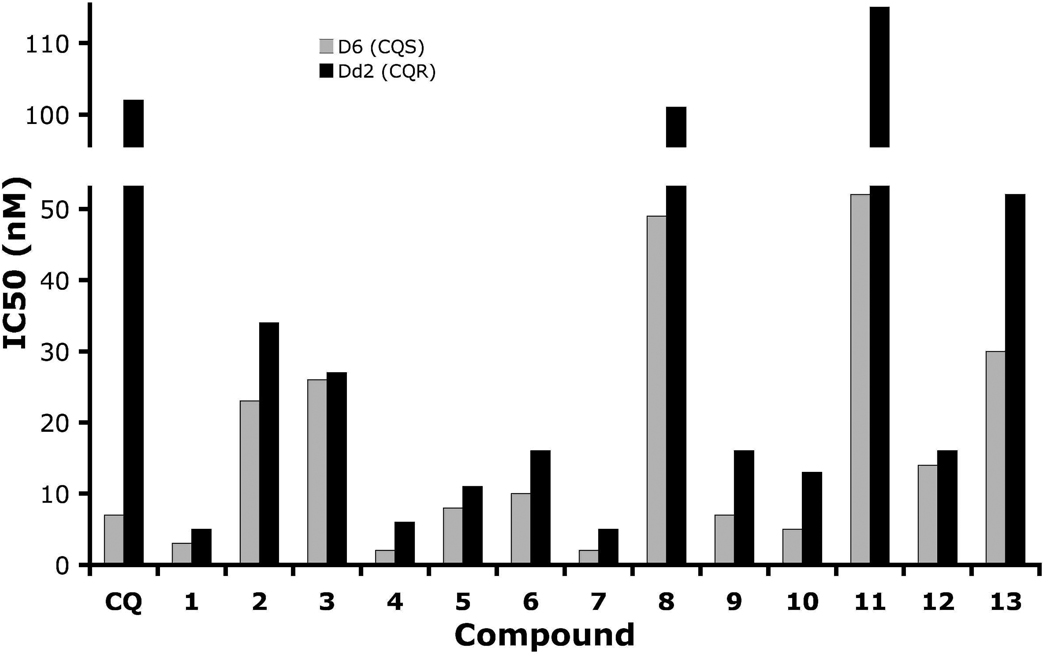
All of the compounds have significant activity (IC50 ≤125 nM; see both Table 1 and Figure 1) against both D6 (CQS) and Dd2 (CQR) P. falciparum malaria strains. The CQR strain generally gives a higher IC50 than the CQS strain, but with the ratio of IC50(CQR)/IC50(CQS) ranging only from about 1 to 3. In fact, of the 11 compounds presented here, 6 have lower IC50 values than does CQ against CQS P. falciparum, and all have a lower ratio of IC50(CQR)/IC50(CQS) than does CQ, by at least a factor of 5. Although the low IC50 values against both types of strain (CQS and CQR) are probably the most important factor, the low strain-sensitivities also contribute to the drug-development process. We conclude that the RCQ design is more general than the single molecule, 1, which was presented in our earlier manuscript on RCQs.15
Figure 1.
The IC50 values for CQ and compounds 1 – 13. The grey bars are for the CQS D6 strain, and the black bars are for the CQR Dd2 strain, of P. falciparum malaria.
However, there are significant differences among the compounds’ effects on the CQS and CQR strains. Comparing 4 to 3, it is seen that the dibenzylamino moiety can be advantageous relative to the diphenylamino group, although this does not infer that the diphenylamino group is inherently bad. 10 is an interesting case, demonstrating that changing the amino alpha to the RA phenyls to an amide is tolerable, giving IC50 values below those of CQ itself, and reduces the ClogP value to almost the same value as CQ. This helps substantially with water solubility, which is needed in developing orally effective drug candidates. Also, the diphenyl to dibenzyl amine advantage noted above (e.g., 3 to 4) is not found for the amides, going from 10 to 12. Also, comparing 2 to 9, or 4 to 6, the IC50 values change by only a small amount when the linker is shortened from 3 to 2 methylenes, at least if the RA portion has a dibenzylamino moiety. With the linker fixed at 3 methylenes, varying the RA aliphatic length also does not make a large difference (IC50s: 4 < 5 < 2) when the RA aromatic portion is dibenyzlamino. 7 and 13 were obtained as side-products during the syntheses of 6 and 10. They constitute an unusual pair, in that they give the lowest and nearly the highest IC50 values, respectively. It was unsurprising that 7 was so effective, insofar as the PfCRT is presumed to be unable to export it to a significant extent, having two RA moieties. The reduced activity of 13 is surprising; the only change is to covert each RA nitrogen proximal to the aromatic groups into an amide, this is seen not to be detrimental in the case of the superior activity of 10. Both 7 and 13 are fairly large and complex molecules, so likely would not be preferred drugs in the context of the Developing World where they are most needed. Compound 12 has a single RA head group, has a much lower ClogP than 13 (6.9 vs. 8.2, respectively), and significantly better IC50 values, comparable to those of its reduced analog, 6.
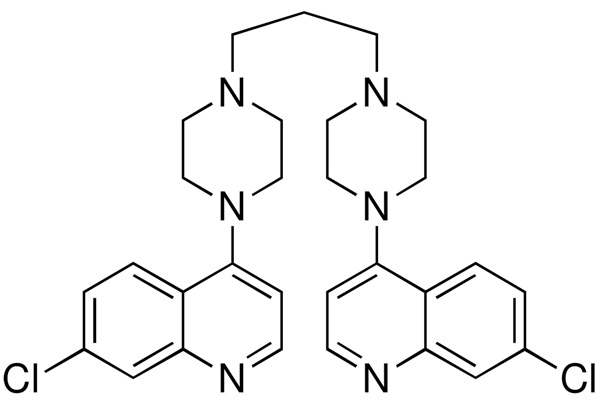
8 and 11 each have a piperazine ring alpha to the 7-chloroquinoline ring, as does piperaquine (Figure 2), a drug that has been in use for some time and has been reported to have an IC50 < 10 nM against the D6 strain.16 This appears to be at odds to the rather higher IC50 values against even D6 presented by 8 and 11. Others have explored arylpiperazines as an antimalarial scaffold, but focused on CQS/CQR cross-reactivity rather than maximizing potency.17 However, piperaquine is a ‘bisquinoline’, and the presence of two 7-chloroquinoline moieties is perhaps the major contributor to its stronger efficacy. This may be balanced by the lack of proton on the nitrogen at the quinoline 4-position, as has been pointed out by others.18
Figure 2.
Piperaquine (1,3-bis-[4-(7-chloroquinolyl-4)-piperazinyl-1]-propane).
Toxicity is an important consideration in any drug development program, and so we provide cytotoxicity data in Table 1. Given the strong potencies of the compounds against malaria, the cytotoxicities are encouraging, especially for 10 – 12. In fact, compound 10 has the combination of high efficacy and low cytotoxicity for a ‘therapeutic index’ (cytotoxicity/efficacy) of 12,000 for D6, and 4800 for Dd2. For comparison, these values are far superior to our calculated ‘therapeutic index’ values for CQ: 1,700 for D6, and only 120 for Dd2. Such numbers, in addition to its low ClogP (approximating that of CQ, and indicating relatively high water solubility) suggest that 10 and 12 could prove to be possible starting points, leading to further progress in the drug development process.
In conclusion, linking any of several reversal agent-like moieties to a 4-amino-7-chloroquinoline yields good activity against CQS or CQR P. falciparum malarias, so that there is considerable flexibility available to the drug designer.
Supplementary Material
Acknowledgment
The authors thank the following for supporting this research: The Medical Research Foundation of Oregon (Grant 0530), and the National Institutes of Health (Grants AI067837 & AI072923) to DHP, as well as a grant from the Murdock Charitable Trust for the NMR instruments.
Abbreviations
- CQ
chloroquine
- CQR
chloroquine-resistant
- CQS
chloroquine-sensitive
- DV
digestive vacuole
- PfCRT
P. falciparum chloroquine resistance transporter
- RA
reversal agent
- RCQ
reversed chloroquine
Footnotes
Supporting Information Available: General Experimental Methods; Compound characterizations: 1H & 13C NMR data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 2.Yearick K, Ekoue-Kovi K, Iwaniuk DP, Natarajan JK, Alumasa J, de Dios AC, Roepe PD, Wolf C. Overcoming drug resistance to heme-targeted antimalarials by systematic side chain variation of 7-chloro-4-aminoquinolines. J. Med. Chem. 2008;51:1995–1998. doi: 10.1021/jm800106u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekoue-Kovi K, Yearick K, Iwaniuk DP, Natarajan JK, Alumasa J, de Dios AC, Roepe PD, Wolf C. Synthesis and antimalarial activity of new 4-amino-7-chloroquinolyl amides, sulfonamides, ureas and thioureas. Bioorg. Med. Chem. 2009;17:270–283. doi: 10.1016/j.bmc.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA, White NJ. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- 5.Schellenberg D, Abdulla S, Roper C. Current Issues for Anti-Malarial Drugs to Control P. falciparum Malaria. Curr. Mol. Med. 2006;6:253–260. doi: 10.2174/156652406776055168. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Paguio M, Roepe PD. The antimalarial drug resistance protein Plasmodium falciparum chloroquine resistance transporter binds chloroquine. Biochemistry. 2004;43:8290–8296. doi: 10.1021/bi049137i. [DOI] [PubMed] [Google Scholar]
- 7.Bennett TN, Kosar AD, Ursos LM, Dzekunov S, Singh Sidhu AB, Fidock DA, Roepe PD. Drug resistance-associated pfCRT mutations confer decreased Plasmodium falciparum digestive vacuolar pH. Mol. Biochem. Parasitol. 2004;133:99–114. doi: 10.1016/j.molbiopara.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Kirk K. The malaria parasite's chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004;21:1938–1949. doi: 10.1093/molbev/msh205. [DOI] [PubMed] [Google Scholar]
- 9.Ginsburg H. Should chloroquine be laid to rest? Acta. Trop. 2005;96:16–23. doi: 10.1016/j.actatropica.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Martin SK, Oduola AM, Milhous WK. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 11.Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987;238:1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- 12.van Schalkwyk DA, Walden JC, Smith PJ. Reversal of chloroquine resistance in Plasmodium falciparum using combinations of chemosensitizers. Antimicrob. Agents Chemother. 2001;45:3171–3174. doi: 10.1128/AAC.45.11.3171-3174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millet J, Torrentino-Madamet M, Alibert S, Rogier C, Santelli-Rouvier C, Mosnier J, Baret E, Barbe J, Parzy D, Pradines B. Dihydroethanoanthracene derivatives as in vitro malarial chloroquine resistance reversal agents. Antimicrob. Agents Chemother. 2004;48:2753–2756. doi: 10.1128/AAC.48.7.2753-2756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharjee AK, Kyle DE, Vennerstrom JL, Milhous WK. A 3D QSAR pharmacophore model and quantum chemical structure--activity analysis of chloroquine(CQ)-resistance reversal. J. Chem. Inf. Comput. Sci. 2002;42:1212–1220. doi: 10.1021/ci0200265. [DOI] [PubMed] [Google Scholar]
- 15.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A Chloroquine-like Molecule Designed to Reverse Resistance in Plasmodium falciparum. J. Med. Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vennerstrom JL, Ellis WY, Ager AL, Jr, Andersen SL, Gerena L, Milhous WK. Bisquinolines. 1. N,N-bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J. Med. Chem. 1992;35:2129–2134. doi: 10.1021/jm00089a025. [DOI] [PubMed] [Google Scholar]
- 17.Molyneaux CA, Krugliak M, Ginsburg H, Chibale K. Arylpiperazines displaying preferential potency against chloroquine-resistant strains of the malaria parasite Plasmodium falciparum. Biochem. Pharmacol. 2005;71:61–68. doi: 10.1016/j.bcp.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Warhurst DC, Craig JC, Adagu IS, Guy RK, Madrid PB, Fivelman QL. Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and resistant blood-stages of Plasmodium falciparum. Role of beta-haematin inhibition and drug concentration in vacuolar water- and lipid-phases. Biochem. Pharmacol. 2007;73:1910–1926. doi: 10.1016/j.bcp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 19.October N, Watermeyer ND, Yardley V, Egan TJ, Ncokazi K, Chibale K. Reversed chloroquines based on the 3,4-dihydropyrimidin-2(1H)-one scaffold: synthesis and evaluation for antimalarial, beta-haematin inhibition, and cytotoxic activity. ChemMedChem. 2008;3:1649–1653. doi: 10.1002/cmdc.200800172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.