Abstract
We examined puberty-specific effects on affect-related behavior and on the psychophysiology of defensive and appetitive motivation while controlling for age. Adolescents (N=94, ages=12 and 13 years), viewed 75 pictures (IAPS: pleasant, neutral and aversive) while listening to auditory probes. Startle response and postauricular (PA) reflex were collected as measures of defensive and appetitive motivation respectively. Pubertal status and measures of anxiety/stress reaction and sensation/thrill seeking were obtained. Mid/late pubertal adolescents showed enhanced startle amplitude across all picture valences. A puberty by valence interaction revealed that mid/late pubertal adolescents showed appetitive potentiation of the PA, while pre/early pubertal adolescents showed no modulation of the PA reflex. Mid/late pubertal adolescents also scored significantly higher on measures of sensation/thrill seeking than did their pre/early pubertal peers and puberty moderated the association between psychophysiology and behavioral measures, suggesting that it plays a role in reorganizing defensive and appetitive motivational systems.
Keywords: puberty, startle and post-auricular reflex, behavioral traits, moderation
Introduction
Adolescence is of particular importance in the study of developmental psychopathology. Defined as the interval between the onset of puberty and the transition into adult roles (Steinberg, Dahl, Keating, Kupfer, Masten et al., 2006), it is distinguished by marked and rapid biological, cognitive and emotional changes, and restructuring of children’s social contexts (Adams & Berzonsky, 2003). The transformations that take place during this period constitute improvements in almost every domain we can evaluate (Dahl & Spear, 2004). Despite these improvements, the rates of morbidity and mortality paradoxically double from childhood to adolescence (Grunbaum, Kann, Kinchen, Williams, Ross et al., 2002). In addition to increased mortality, there is an upsurge in psychiatric disorders during adolescence including affective disorders, schizophrenia, eating disorders, and substance use disorders (Cicchetti, Fred, & Toth, 1994).
Noting the health paradox of adolescence, Dahl (2004) argued that problems in emotion regulation and changes in affect-related processes that influence behavioral choices underpin a key dimension of the increased mortality and morbidity during this period. Research is accumulating to support the argument for puberty-associated modifications in brain regions supporting emotion, arousal, response inhibition and the calibration of risks and rewards (Dahl & Spear, 2004). These changes may include increases in the intensity of emotions experienced by adolescents, their sensitivity to emotional influences, and their difficulty in regulating emotions in the face of conflicting demands (Steinberg, 1989). Notably, there is also mounting evidence of a temporal gap between the maturation brain regions involved in behavior regulation (i.e., of dorsal areas of the prefrontal cortex, PFC); and regions controlling emotional processing and behaviors associated with reward/ punishment (ventral-medial areas of the PFC) that have bi-directional connections with the amygdala and the hypothalamus (Conklin, Luciana, Hooper, & Yarger, 2007). Thus, the argument is that experiencing more intense emotions in the context of slowly developing regulatory skills makes adolescents more vulnerable to psychopathology and errors of judgments than children (who experience lower levels of motivation and arousal) or adults (who have stronger regulatory capabilities) (Steinberg et al., 2006).
The neurobiological changes described above may be influenced by both experience and pubertal maturation. Puberty represents a core set of biological processes that are foundational to the adolescent transition. Nonetheless, differentiating puberty-specific effects from experiential effects during adolescence is challenging. The world of adolescence differs from the world of childhood. Social relationships and roles change dramatically. Individuals become increasingly more embedded in their peer groups and spend less time with their families (Eccles, 1999). Relationships with parents are redefined from one of unilateral authority in childhood to one of cooperative negotiation in adolescence (Steinberg et al., 2006). At the same time, emotional relations with friends become more favorable and important, while those with family often become temporarily less positive (Larson & Richards, 1991). To a large extent, these social changes correlate with age, which is also correlated with pubertal stage, thus obscuring the specific role of puberty-specific processes in influencing emotional changes in adolescence. One method of disentangling pubertal and social-experiential changes is to study effects of puberty among adolescents of roughly the same age. Early adolescence provides the opportunity for such an analysis, as early in adolescence it is not difficult to find many children who have and many who have not progressed far in the pubertal transition.
The Role of Puberty
There are a number of reasons to expect that puberty will have significant effects on emotion processing (see for a review, Patton & Viner, 2007). In animal models increases in gonadal steroids are known to exert robust effects on brain areas mediating affect and behavior in ways that underscore not just the effect of hormones but puberty-specific changes in brain receptivity to their influence (Romeo, 2001). Puberty may also be as a unique formative period for future behavioral tendencies. Animal models show that hormonal influences on behavior are important during initial encounters with stimuli, though once behavior patterns are established they become less susceptible to hormonal influences (Fleming & Corter, 1995). Therefore puberty is likely to change behavior-biology associations. For example, predicted relations between serotonin or sexual hormones and patterns of maladaptive behavior have been found only after the pubertal transition (Twitchell, Hanna, Cook, Fitzgerald, & Zucker, 2000). These findings suggest the importance of taking into account pubertal status as a moderator in various models of behavior-physiology relations.
In humans there is also evidence for the role of gonadal steroids in the shaping of social processes such as sexual activity, maternal behavior, social bonding, and social memory (Nelson, Leibenluft, McClure, & Pine, 2005). Sexual hormones play a key role in neural plasticity. For example, neural plasticity in the hypothalamus and the hippocampus is regulated by the ebb and flow of estrogens in females (McEwen, 1994). Both estrogens and androgens influence verbal fluency, spatial abilities, verbal memory and motor skills (McEwen & Alves, 1999). There are many domains aside from sexuality that exhibit puberty-specific maturational changes including sensation seeking, sleep/arousal regulation (Dahl, 2004), appetite and feeding (Barb, Hausman, & Czaja, 2005), mood lability, and risk for affective disorders among girls (Ge, Kim, Brody, Conger, Simons et al., 2003). Taken as a whole, these findings suggest that puberty is not only a useful milestone for studying a specific developmental process within the broader set of transformations of adolescence, but also that pubertal processes may trigger rapid modifications in the intensity, direction and calibration of multiple emotional and/or motivational domains.
The Current Study
The purpose of the present study was to examine the role of puberty in the transformations of two systems that characterize emotional life: defensive and appetitive motivation (Lang, 1995). Changes in these core dimensions of emotion are believed to contribute to the increased morbidity and mortality of adolescence (Spear, 2000). The defensive motivational system supports avoidance of predators and safeguarding of physical integrity; and its neurobiology is common to both pathological anxiety and normative fearfulness (Rosen & Schulkin, 1998). The appetitive motivational system, which underlies reward and primary reinforcers, supports behavior directed toward multiple stimuli such as food, sex, pair bonding, money and drugs of abuse (Sevy, Hassoun, Bechara, Yechiam, Napolitano et al., 2006; Volkow, Fowler, & Wang, 2004; Young, Murphy Young, & Hammock, 2005). The neural bases of these systems and behaviors may significantly change during the adolescent transition (Galvan, 2006; Laviola, Adriani, Terranova, & Gerra, 1999), and these transformations are hypothesized to be linked to the onset of multiple forms of psychopathology and behavior problems (Nelson et al., 2005). Our goal was not only to examine self-reported changes in defensive and appetitive behavior, but also to explore how the pubertal transition would influence the psychophysiology of appetitive and defensive emotions. This in turn would allow us some insight into how puberty might alter the neural bases of these emotion systems. To accomplish this we examined the psychophysiology of emotional processing while controlling for age so that significant findings, if any, could be more directly linked to pubertal status. Below we elaborate on each motivational system and the psychophysiological measures associate with them.
Defensive Motivation: Fear and Anxiety
The period of adolescence is characterized by an increase in stress and socio-environmental challenges (Shirtcliff, Dorshorst, Nguyen, & Pollak, 2006), particularly for adolescents undergoing puberty and those who mature early. These factors are noted by many authors as part of the underlying causes for increased prevalence of stress-related psychopathology during this period, such as anxiety and panic disorders (Pine, Cohen, Gurley, Brook, & Ma, 1998). Related emotional states, such as self-consciousness (Roth, 1999), also increase with the physical changes of puberty. In summary, adolescents as a group are generally more likely to evidence signs of enhanced anxiety and physiological stress reactivity (Spear, 2000). However, it should be noted that some researchers propose that adolescence is characterized by a weak harm-avoidance system and a more active reward-sensation seeking system (See Ernst, Pine, & Hardin, 2006). While this not automatically contradictory with our premise, given the manifold complexity of the neural bases of these systems, there is evidence for puberty-specific effects on anxiety and stress related disorders. For example, more advance pubertal status in girls is associated with more symptoms of depression, anxiety and stress perception (Huerta & Brizuela-Gamino, 2002). Altogether, when epidemiological data and pre-clinical studies are considered, it would appear that adolescents are experiencing higher levels of anxiety and stress and they also find themselves in situations that would trigger intense fears (Greist, 1995). Against this background we target the specific role that pubertal processes may play in increased defensive motivation and anxiety during adolescence.
We examined the emotional modulation of the eye-blink startle reflex in pre/early pubertal and mid/late pubertal adolescents. The eye-blink startle reflex is part of a set of fear and defense motivated behaviors. The startle response has emerged as a useful non-invasive tool for the study of emotion and processing of frightening/negative events in particular. The eye-blink startle magnitude to sudden acoustic probes (specifically the strength of the orbicularis oculi contraction) is augmented during exposure to noxious stimulus (termed startle potentiation) and inhibited during pleasurable ones. Startle potentiation during aversive stimuli is modulated by the amygdala (Davis, 1988), and is intrinsically linked to the neurobiology of fear and anxiety. There is a growing literature on emotion modulated startle in children and adolescents. Startle modulation has been successfully obtained in children using pictures (McManis, Bradley, Berg, Cuthbert, & Lang, 2001). Additionally the startle response differentiates clinically anxious individuals from not affected individuals in studies with children, adolescents and adults (Grillon, Ameli, Foot, & Davis, 1993; Grillon, Dierker, & Merikangas, 1998), and it has been successfully used to differentiate maltreated from not maltreated children (Klorman, Cicchetti, Thatcher, & Ison, 2003). Therefore this is a well validated measure of defensive motivation and processing of aversive events. Finally, in addition to startle we sampled self report measures of anxiety and stress reactivity, and we screened for other forms psychopathology and maladaptive behaviors. Our hypotheses were as follows. (1) Mid/late pubertal adolescents would report increased anxiety and stress when compared to pre/early pubertal adolescents. (2) Mid/late pubertal adolescents would evidence enhanced startle reactivity during aversive stimuli (startle potentiation) compared to pre/early pubertal peers. (3) Startle potentiation during aversive stimuli would relate to individual differences in anxiety and individual differences in sensation seeking. And, (4) puberty would moderate the relation between anxiety related behaviors and fear potentiated startle. Specifically, behavioral measures of anxiety and stress reactivity would link more strongly to fear-potentiated startle in mid/late pubertal than pre/early pubertal adolescents.
Appetitive Motivation: Sensation Seeking and Risk Taking
During adolescence there is a shift toward increased sensation seeking and risk taking that may reflect a larger modification in reward systems and appetitive motivation during this period. Adolescent animals, when compared with adults, are characterized by increased novelty seeking, increased risk-taking behavior as well as elevated levels of impulsivity and restlessness (Laviola, Macri, Morley-Fletcher, & Adriani, 2003). Similarly, human adolescents show increased use of psychoactive drugs, and they engage in more extravagant and reckless behavior, hazardous driving, and unprotected sex when compared to adults (Spear, 2000). Again the hormonal changes of puberty may play a specific role in the increased drive for thrills and sensations; for example, higher levels of testosterone and estradiol are linked to higher sensation seeking in college-age males and females (Daitzman, Zuckerman, Sammelwitz, & Ganjam, 1978). Additionally, one study employing self-report measures showed that higher sensation seeking was positively correlated to pubertal maturation but not to increasing age (Martin, Kelly, Rayens, Brogli, Brenzel et al., 2002). However, there are no data on how the putative effects of puberty on sensation seeking and risk taking may be evident in the psychophysiology of reward systems. Our research questions in this domain pertain to the specific role of puberty in increases in appetitive motivation observed during adolescence. In the present study we examined the postauricular (PA) reflex and its modulation in pre/early pubertal and mid/late pubertal adolescents. The PA reflex is a vestigial muscle response in humans that acts to pull the ear upward and backward (Berzin & Fortinguerra, 1993). It is recorded from electrodes positioned over the postauricular muscle behind the ear (Patuzzi & O’Beirne, 1999). PA reflex magnitude is modulated by emotional stimuli so generally it is larger during pleasant stimuli (termed PA reflex potentiation) and smaller during aversive ones. PA reflex magnitudes are potentiated during pleasant pictures and sounds and inhibited during aversive pictures and sounds (Benning, 2006; Benning, Patrick, & Lang, 2004). PA reflex magnitude is also greater during exposure to happy facial expressions and inhibited during exposure to angry expressions (Hess, Sabourin, & Kleck, 2007).We also examined self-report measures of affect-related behavior such as sensation seeking, behaviors regarding risk taking and thrill seeking and measures of competence, positive emotionality and adaptive functioning. All of these dimensions could be theoretically related to the PA potentiation and evidence transformations during the pubertal transition. Our specific hypotheses in this domain were as follows (1) Mid/late pubertal adolescents would report higher levels of sensation seeking than same age pre/early pubertal peers. (2) As an indication of changes in the neurobiology of reward, relative to pre/early pubertal adolescents, mid/late pubertal adolescents would evidence stronger potentiation of the PA reflex during pleasurable stimuli. (3) PA potentiation during pleasurable stimuli would be related to individual differences in sensation seeking. (4) Puberty would moderate the relationship between sensation seeking behaviors and the psychophysiology of reward/pleasure. Specifically, increases in sensation seeking would be linked more strongly to appetitive potentiation of the PA in mid/late pubertal than pre/early pubertal adolescents.
Methods
Participants
The study involved a total of 94 participants (20 pre/early pubertal females and 22 mid/late pubertal females, 31 pre/early pubertal males and 21 mid/late pubertal males). Participants and their families were recruited through a registry of families who, at the time of their child’s birth, indicated interest in participating in child development studies. This registry is largely composed of middle- to upper-income families and parents with at least college educations. Nearly all the participants were Caucasian (99% Caucasian, 1% other). Given our particular interest in the specific effects of puberty on emotional process, age was strictly controlled so that any observable effects could be clearly linked to puberty not age. All participants were 12 and 13-years old. There was no age differences between pre/early pubertal (M = 13 yrs, 1 mos, SD = 0.63) and mid/late pubertal adolescents (M = 13 yrs, 3 mos, SD = 0.43); t(92) = 1.4, p = .16.
Procedures
Participants were selected so that roughly half of each sex would be pre/early pubertal and half mid/late pubertal. Selection was accomplished by screening the participants via parent report over the phone at the time of recruitment. Questions from the Pubertal Development Scale (PDS), parent report (Petersen, Crockett, Richards, & Boxer, 1988) were used to establish a tentative initial classification. Participants scoring 2.5 or above during phone screening were recruited as mid/late pubertal while those scoring below 2.5 were recruited as pre/early pubertal participants. Girls who had not reached menarche were classified as pre/early pubertal, irrespective of their score on the PDS. Occasionally experimenters were aware of the first tentative classification from parent report, but not of the final classification, which was established after participants finished the procedure. Potential participants that had a history of psychiatric or physical illnesses were screened out at the time of recruitment.
Participants arrived at the laboratory with at least one parent. After consent and assent were obtained participants sat on a chair at a distance of 135 cm from a 21-in computer monitor positioned directly in front of them. Following electrode placement, physiological responses were recorded using a PC computer running ERP-W 32 software (New Boundaries Technologies Inc.), which was also used to deliver the emotionally valenced pictures and noise probes. Before starting the experiment, both the parent and the experimenter left the room but could see the participant through closed circuit camera. Placement of the electrodes and viewing of the pictures took 45 minutes. The electrodes were removed and the participant completed ratings of the pictures for emotional valence and arousal (Lang, 1980). Pubertal status was verified at the time of testing by having the participants complete the Pubertal Development Scale. The PDS correlates with Tanners Stages on physical exam and predicts basal hormones related to pubertal development (Brooks-Gunn, Warren, Rosso, & Gargiulo, 1987; Shirtcliff, Heiligenstein, Hoornstra, Squires, & Pollak, 2007). To score the PDS, we included only questions that reflected the main axes of puberty: growth (item 1), adrenal (items 2, 3), and gonadal (males: items 4 and 5, females: item 6). Placement in pre/early pubertal and mid/late pubertal groups was based on the participant-reported data. The result was a 4 point scale composite measure with a median score of 2.5 that was used to split participants into pre/early pubertal and mid/late pubertal groups.
Experimental Stimuli and Measures
Participants viewed 75 pictures from the International Affective Picture System (Lang & Bradley, 1999) and heard brief loud burst of white noise while viewing the pictures. The acoustic probe was a bilateral 40 ms probe (M intensity= 99.84 dB, SD = 1.09) with nearly instantaneous rise time; acoustic probes were presented within an average of 3579 ms, SD = 1027 after picture onset. To diminish probe predictability, 9 no-probe picture trials were interspersed with probed trials and 6 probes were delivered during no-picture presentation intervals. The probes were presented binaurally through Sony insert earphones. The mean inter-probe interval was 11019 milliseconds, SD = 2601.8; the mean inter-trial interval was 5881 ms, SD = 687. Pictures were presented for 6 seconds in 5 different counterbalanced orders with appropriate stimulus substitutions made for female and males. Within and between orders, the position of the pictures was counterbalanced such that all valence, content, and intensity conditions were represented equally across orders at each serial position. No more than two picture of the same valence occurred consecutively. Additionally, within any stimulus order pictures of the same content never appeared consecutively.
Picture contents were gender balanced on dimensions of normatively rated valence. Five pictures were included in each of the following rationally established content categories: (1) Pleasant Valence: Romantic (couples in romantic postures and activities), Food (appetizing depictions of individual food items or buffets), Adventure (thrilling scenes, such as high-speed skiing and cliff-diving), Nurturing/Attachment (scenes of young children, animals, and families), Attractive People (attractive members of the opposite sex). (2) Neutral Valence: Buildings (scenes of large edifices), Landscape: (low arousal nature scenes), Objects (household utensils and appliances), Humans (depictions of humans with neutral facial expressions and poses; 2 sets of 5 pictures of this content category were presented to compensate for the large number of humans depicted in both pleasant and aversive pictures). (3) Aversive Valence: Threat (depictions of attackers with pointed guns or other weapons, aimed at the viewer), Disgust (depictions of violations of social and hygiene norms), Disgusting Animals (cockroaches and other vermin); Victim (depiction of attacks), and Dangerous Animals (snakes, dogs, and sharks in attack postures aimed at the viewer).
Manipulation Check
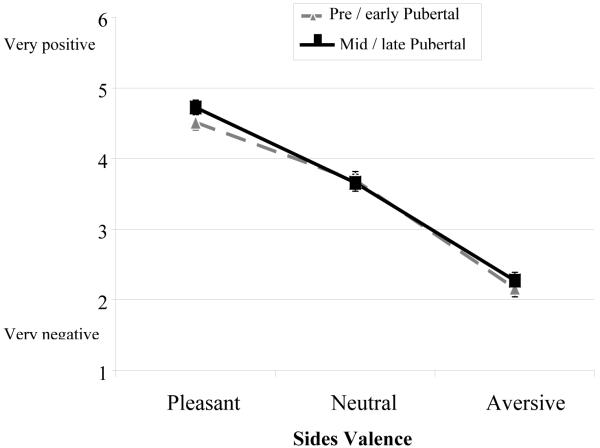
As a manipulation check, participants rated each image for both valence and arousal in a 1-6 scale employing a variation of the Self-Assessment Manikin (SAM) system (Bradley & Lang, 1994). For valence ratings, there was the expected main effect of picture Valence, F(1.71,127) = 264, p < .001, η2p = .781, in which pleasant pictures were rated as more pleasant than aversive pictures, linear Valence F(1,75) = 385, p < .001, η2p = .837, and neutral pictures were rated intermediately between pleasant and aversive. No other main effects or interactions of interest were significant; in particular, the Valence x Puberty interaction was not significant, F(1.71,127) = 0.78, p = .444, η2p = .010 (see Figure 1).
Figure 1.
Valence Ratings for Pre/early and Mid/late Pubertal Adolescents
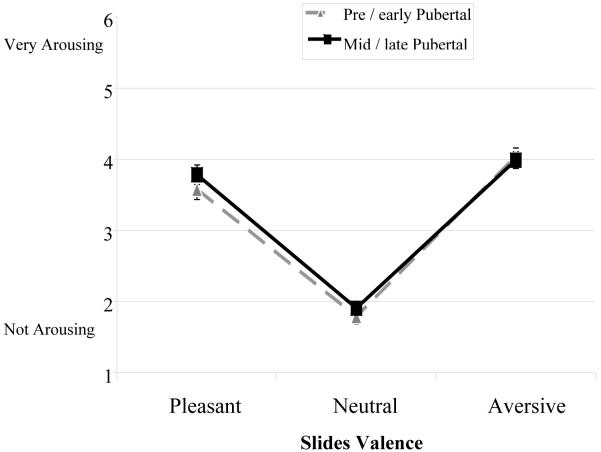
For arousal ratings, there was the expected main effect of picture Valence, F(2.00,150) = 342, p < .001, η2p = .822, in which pleasant and aversive pictures were rated as more arousing than neutral pictures, quadratic contrast F(1,75) = 683, p < .001, η2p = .902 (linear contrast F(1,75) = 14.3, p < .001, η2p = .162). As before, the Valence x Puberty interaction was not significant for arousal ratings, F(2.00,150) = 1.05, p = .355, η2p = .014 (see Figure 2). The results of the manipulation check indicated that regarding valence and arousal dimensions, mid/late pubertal and pre/early pubertal participants judged the stimuli comparably.
Figure 2.
Arousal Ratings for Pre/early and Mid/late Pubertal Adolescents
Physiological Measures
Both eye-blink startle and PA reflexes were measured by recording electromyographic (EMG) activity employing disk electrodes (Ag-AgCl) using a Grass bioamplifier. The ground electrode was placed in the forehead (all impedance levels less than 10 kΩ). Rectification, integration, and (in the case of PA reflexes) averaging of trials were done employing ERP-W 32 software; the sampling rate was 1000 Hz. The time constant for integrating the startle blink data was 20 ms. Because ERP-W 32 did not allow rectifying the PA channels without integrating the signal, PA reflexes were integrated with a time constant of 1 ms to most closely approximate the raw rectified data. Baseline was calculated as the average rectified EMG activity during the 100 ms before the onset of the noise probe. Baseline-corrected EMG activity was used in the analyses. The initial picture trial was excluded from all analyses, as it acted as a habituation stimulus (Benning, Patrick, & Iacono, 2005). For both startle blink and PA reflexes, trials were inspected and coded visually. Startle blinks were inspected and analyzed on a trial-by-trial basis, whereas PA reflexes were inspected and analyzed after signal averaging of all trials within each content category. Startle blink trials or averaged waveforms for each PA content category with excessively noisy background EMG activity were excluded from averaging procedures.
Sensor placement for the eye-blink startle reflex was done as described by Blumenthal et al. (2005). Startle EMG activity was filtered with a bandpass of 30-300 Hz and amplified 20,000x. Peak startle EMG activity was selected employing ERP-W software within a window of 20-175 ms after probe onset. The PA reflex was collected and measured as described by Sollers and Hackley (1997). In this study, raw EMG signals for each ear were recorded for 103 ms before noise onset until 400 ms after probe onset. PA data were filtered on-line with a bandpass of 10-1000 Hz and amplified 20,000x. Average PA waveforms per picture content were then exported to a spread sheet and maximum EMG peak activity was selected within a window of 8-30 ms after noise probe onset.
Trait Measures
To obtain measures of defensive and appetitive motivation, participants completed several trait measures: the easy reading version of the Multidimensional Personality Questionnaire, brief form (MPQ, Patrick, Curtin, & Tellegen, 2002), the Sensation Seeking Scale for Children (SSSC, Russo, 1991), and the Behavior Assessment System for Children, 2nd Edition (BASC-2, Reynolds & Kamphaus, 1998). These instruments were used to derive measures of anxiety/stress reaction as an index of defensive motivation and sensation/thrill seeking as an index of appetitive motivation. For anxiety/stress reaction the MPQ Stress Reaction scale and the Anxiety scale from the BASC-2 were chosen because they are conceptually linked to brain systems underlying defensive-withdrawal behaviors (Tellegen, 1985). Two subscales of the SSSC (Thrill and Adventure Seeking, and Social Disinhibition) and the Sensation Seeking scale from the BASC-2 were chosen because they are conceptually and empirically linked to traits of sensation seeking in physical and social domains (Russo, Stokes, Benjamin, Christ, McBurnett et al., 1993). These five scales were subjected to principal component analysis with varimax rotation which yielded, as expected, two factors explaining 78.7% of the variance. An Anxiety/Stress Reaction factor (eigenvalue = 1.78) was marked by high loadings of BASC anxiety (.93) and MPQ stress reaction (.95); and a Sensation/Thrill Seeking factor (eigenvalue = 2.22) marked by high loadings of BASC sensation seeking (.86), SSSC thrill and adventure seeking (.85), and SSSC social dishinibition (.84). The individual scales were then standardized and the resulting z-scores were added to yield two summary composite measures: Anxiety/Stress Reaction and Sensation/Thrill Seeking.
Missing Data
All physiological data for three participants were lost due to equipment failure, and PA reflex data were lost for one participant due to sensor failure. Insufficient responses across all conditions, defined as overall mean reflex magnitude < 2 μv, resulted in elimination of 9 participants for startle and 5 for the PA reflex. Participants with missing psychophysiological data were not different on trait measures from participants with usable psychophysiological data, t(90)= 1.22, p < .22. Psychophysiological data could assumed to be missing at random [Little’s MCAR test, χ2 (1568) = 109, p = 1.00]. However, imputation of psychophysiological data was not advisable because these were our main outcome variables. Thus a total of 82 participants had usable startle blink data (20 pre/early pubertal girls, 28 pre/early pubertal boys, 18 mid/late pubertal girls, 16 mid/late pubertal boys) and 85 participants had usable PA reflex data (19 pre/early pubertal girls, 28 pre/early pubertal boys, 21 mid/late pubertal girls, 17 mid/late pubertal boys). Two participants had missing data on the BASC-2 (one of them also with missing the MPQ data) due to an error of collating the questionnaires. However, these participants had complete SSSC data. There were no differences in psychophysiology between participants with and without complete questionnaire data, t(89) = 1.54, p = .13; and data were missing at random as indicated by Little’s MCAR test, χ2(148) = 0.00, p = 1.00. Therefore, scores in the missing scales that comprised the composite behavioral measures for these two participants were imputed employing expectation-maximization procedures.
Data Analysis Plan
Startle and PA reflex data were analyzed using a mixed analysis of variance (ANOVA) on baseline corrected (pre-peak baseline EMG activity) peak responses. For the startle blink, the design was 3 (Picture Valence) x 2 (Pubertal Status) x 2 (Gender). For the PA reflex, the design also included a factor for ear (Left/Right). Significant interactions were decomposed using simple effects, and picture Valence effects were parsed a priori into difference (for startle blink) or Helmert (for PA reflex) orthogonal contrasts. We chose different contrasts for startle and PA reflexes so that we could describe the specific patterns of emotion modulation that were meaningful for each reflex. Partial eta squared (η2p) was calculated as a measure of effect size. Effects in the ANOVAs were evaluated at an α level of .05, with the Greenhouse-Geisser correction applied to the degrees of freedom in the omnibus ANOVAs to correct for non-sphericity; follow-up t-tests were evaluated at an α level of .01 (owing to Bonferroni correction for the number of contents within each valence). Because the PA reflex is a relatively new measure, to better understand the stimuli evoking PA responses, we examined PA reflex modulation to each of the pleasant and aversive contents, comparing these responses against the average of the neutral valence pictures using t-tests. All analyses were performed on raw data, square root-transformed data (to normalize the distribution of the psychophysiological data), and within-subjects z-scored data (to ensure that all participants contributed equally to the overall findings). However, as the results were comparable for both raw and transformed data, only the analyses of the raw data are presented to ease interpretation of the findings.
The final set of analyses examined the two trait indices, Anxiety/Stress Reaction and Sensation/Thrill Seeking. To examine sex and pubertal status effects on these measures, ANOVAs were computed. We then computed Pearson correlations to examine relations between behavioral and psychophysiological data. These correlations were computed within each pubertal group. For startle, correlations were computed with both aversive potentiation (aversive minus neutral) and pleasant inhibition (pleasant minus neutral) of the startle response. For the PA reflex, correlations were computed with pleasant potentiation (pleasant minus neutral). To further examine the role of puberty in moderating behavior-psychophysiology associations, moderation analyses were computed using regression with puberty by behavior measure interaction terms.
Results
Startle Blink Reflex
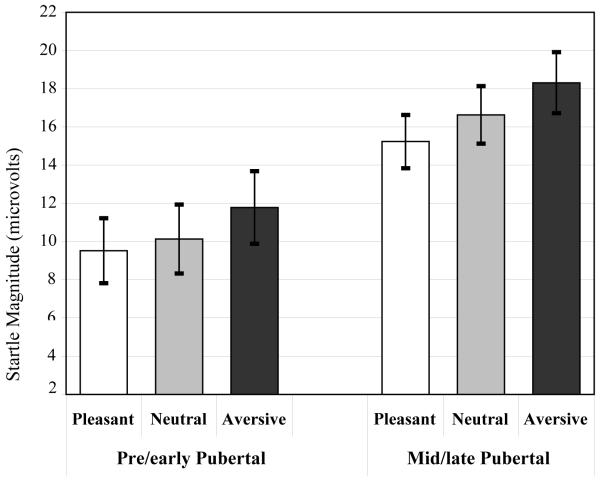
As shown in Figure 3, there was a main effect of picture Valence on startle blink magnitude, F(1.82,140) = 27.7, p < .001, η2p = .264. The magnitude of startle was larger during aversive than during other pictures, F(1,78) = 37.8, p < .001, η2p = .326, and startle blink magnitudes during neutral pictures were also greater than those during pleasant pictures, F(1,78) = 9.77, p = .002, η2p = .111, reflecting startle inhibition during pleasant picture contents. There was a main effect of puberty with mid/late pubertal children showing significantly greater overall startle blink magnitudes than pre/early pubertal children, F(1,78) = 6.26, p = .014, η2p = .074. There was no Valence x Puberty interaction, F(1.83,143) = 0.68, p = .495, η2p = .009, nor were any other main effects or interactions significant in the model, Fs < 1.7, ps > .19, η2ps < .025. Further examination indicated that approximately equal numbers of pre/early pubertal and mid/late pubertal children exhibited numerically greater startle blink reflexes (startle potentiation) during aversive pictures compared to those during neutral pictures (pre/early pubertal = 73%, mid/late pubertal = 70%).
Figure 3.
Effect of Puberty on Startle Reflex Magnitude
Postauricular Reflex
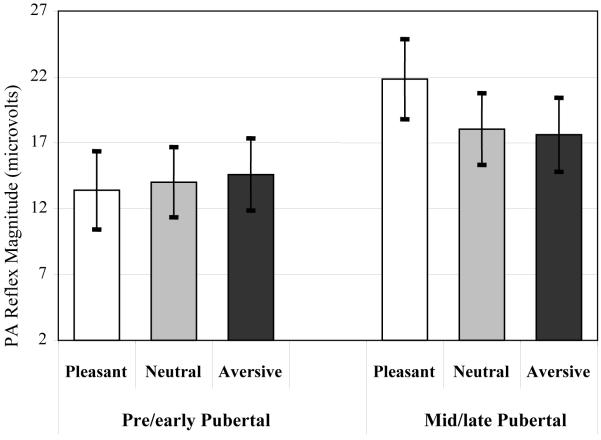
As depicted in Figure 4, there was a significant Valence x Puberty interaction for PA reflex magnitude, F(1.77,143) = 5.47, p = .007, η2p = .063. Tests of simple effects showed that there was a significant effect of picture Valence in mid/late pubertal children, F(1.64,59.1) = 17.9, p < .001, η2p = .332. Follow-up contrasts showed that mid/late pubertal children had significantly greater PA reflexes during pleasant pictures than during all other pictures, F(1,36) = 24.3, p < .001, η2p = .403, and PA reflexes during neutral pictures were not different from those during aversive pictures, F(1,36) = 0.35, p = .560, η2p = .010. In contrast, there was no significant Valence effect in pre/early pubertal children, F(1.67,74.6) = 0.03, p = .970, η2p = .001. Thus, mid/late pubertal children exhibited the expected appetitive modulation of PA reflex modulation, whereas pre/early pubertal children failed to show emotional modulation of the PA reflex. Consistent with these results, 82% of the mid/late pubertal but only 49% of the pre/early pubertal children exhibited numerically greater PA reflexes during pleasant pictures compared to those during neutral pictures. Additionally, there was a trend for PA reflex magnitudes to be larger in mid/late pubertal participants than pre/early pubertal participants, F(1,81) = 2.94, p = .090, η2p = .035.
Figure 4.
Effect of Puberty on PA Reflex Emotional Modulation
To examine which picture contents produced the greatest PA reflex modulation, follow-up t-tests for content categories were conducted using data only from mid/late pubertal children. PA reflexes during nurturant pictures (M = 25.7 μV, SE = 5.08), scenes of food (M = 23.5 μV, SE = 3.76), romantic pictures of couples (M = 23.1 μV, SE = 3.52), and scenes involving attractive opposite-sex individuals (M = 22.2 μV, SE = 3.44) were all significantly greater than those during neutral pictures (M = 18.7 μV, SE = 3.11), t(37)s > 2.83, ps < .008. PA reflexes during adventure and thrill seeking situations (M = 19.5 μV, SE = 3.35) were not significantly different from those during neutral pictures, t(37) = 1.27, p = .213. No PA reflex magnitudes during aversive contents were significantly different from those during neutral pictures, |t(37)s| < 1.5, ps> .16.
Trait Measures: Puberty, Gender and Associations with Psychophysiology
There were no effects of puberty, F(1,90) = 1.29, p = .259, or gender, F(1,90) = 1.47, p = .239, on Anxiety/Stress Reaction. However, mid/late pubertal adolescents had significantly higher Sensation/Thrill Seeking scores than pre/early pubertal adolescents, F(1,90) = 4.33, p = .040, η2p = .046. There was also a significant effect of gender with boys reporting more total sensation seeking than girls, F(1,90) = 15.5, p < .001, η2p = .147.
To test our hypothesis of puberty as a moderator we computed regression equations for both Anxiety/Stress and Sensation/Thrill Seeking personality traits with both startle potentiation during aversive pictures and startle inhibition during pleasant pictures. In preliminary analyses both trait measures were associated with startle potentiation; therefore, the regression model involved puberty by trait terms for both trait summary measures. However, only Anxiety/Stress reaction was associated with startle inhibition; therefore, only one interaction term was entered into the prediction of startle inhibition. The results shown in Table 1 confirm a moderating role of puberty in associations between behavioral traits and startle modulation. To further clarify the meaning of the significant interaction terms in our moderation analyses, we computed correlations between behavioral traits, startle and PA reflex modulation within puberty groups. As shown in the Table 2, for mid/late pubertal participants startle potentiation was positively correlated with Anxiety/Stress Reaction and negatively correlated with Thrill/Sensation Seeking, while startle inhibition was negatively correlated with Anxiety/Stress Reaction. PA reflex potentiation during pleasant pictures was negatively correlated with Anxiety/Stress Reaction but unrelated to Sensation/Thrill Seeking. In contrast, there were no significant correlations between startle modulation and trait measures for pre/early pubertal children, even though as previously reported, pre/early pubertal children exhibited startle modulation during pleasant and aversive pictures. In combination, the ANOVA and correlational findings suggested that, as predicted, puberty moderates the association between startle modulation and behavioral traits.
Table 1.
Associations of Startle Potentiation and Inhibition with Behavioral Measures: Moderation by Pubertal Status
| Outcome: Startle Potentiation during Aversive Pictures | ||||
|---|---|---|---|---|
| Predictors | B | SE B | β | Significance |
| Puberty | .601 | .695 | .087 | ns |
| Anxiety/Stress Reaction | .061 | .260 | .034 | ns |
| Sensation/Thrill Seeking | −.223 | .165 | −.164 | ns |
| Puberty*Anxiety/Stress Reaction | .781 | .369 | .300 | .037 |
| Puberty*Sensation/Thrill Seeking | −.540 | .293 | −.224 | .069 |
|
| ||||
| Outcome: Startle Inhibition during Pleasant Pictures | ||||
|
| ||||
| Puberty | .441 | .548 | .082 | ns |
| Anxiety/Stress Reaction | −.128 | .205 | −.090 | ns |
| Puberty*Anxiety/Stress Reaction | −.725 | .291 | −.358 | .015 |
Dependent variable startle potentiation to aversive pictures, R= .487, R2=.238, R2 change=.238, p<.001
Dependent variable startle inhibition to pleasant pictures, R= .442, R2=.195, R2 change=.195, p<.001
Table 2.
Pearson Correlation Coefficients for Startle Reflex Modulation with Behavioral Traits in Pre/early and Mid/late Pubertal Adolescents: Anxiety/Stress Reaction and Sensation/Thrill Seeking
| Pubertal Status |
Trait Measures |
Startle Reflex N=82 |
PA Reflex N=85 |
|
|---|---|---|---|---|
| Potentiation During Aversive |
Inhibition During Pleasant |
Potentiation During Pleasant |
||
| Pre/early | Anxiety/Stress Reaction | .054 | −.101 | .093 |
| Sensation/Thrill Seeking | −.231 | .053 | .039 | |
| Mid/late | Anxiety/Stress Reaction | .426** | −.546** | −.332* |
| Sensation/Thrill Seeking | −.420* | .134 | −.026 | |
= p<.01
=p<.05
Our initial hypothesis that the PA reflex would index sensation and thrill seeking was not confirmed. Therefore, to better understand trait correlates of PA potentiation, we explored the psychological correlates of this measure among the mid/late pubertal adolescents by examining additional subscales of the questionnaires we employed. Overall, PA reflex potentiation during pleasant pictures was associated with measures of well-being (i.e., MPQ Well-being, r = .40, p = .013, BASC-2 Personal Adjustment, r = .40, p = .013) and interpersonal affiliation (i.e., BASC-2 relationships with parents, r = .39, p = .017, BASC-2 interpersonal relations, r = .36, p = .027). Additionally, this measure was negatively correlated with MPQ Negative Emotionality = −.58, p< .001.
Discussion
Our findings regarding the effects of puberty on the psychophysiology of fear and positive emotions are particularly striking given our unique design. Some theoretical models would predict little to no developmental effects arising from a design where participants were so close in age and varied only in puberty. It was precisely this age restricted design which allowed us to scrutinize puberty-specific maturational changes.
We predicted that measures of anxiety/stress reaction would increase with puberty, and that startle potentiation during aversive pictures would be greater for mid/late pubertal than pre/early pubertal children, but neither of these predictions were confirmed. Instead, we obtained a significant increase in overall startle magnitude with puberty. Additionally, only among mid/late pubertal participants was startle modulation associated with measures of anxiety/stress reaction and sensation/thrill-seeking. We predicted that puberty would be associated with increased sensation/thrill seeking and this was observed. In addition, our prediction that puberty would increase the potentiation of the PA reflex to pleasant pictures was confirmed. In fact, we found that only among mid/late pubertal children was the PA reflex modulated by picture valence. However, while we hypothesized that PA reflex potentiation during pleasant pictures would be associated with greater sensation and risk taking, this hypothesis was not confirmed. Post-hoc analyses revealed that among mid/late pubertal adolescents, PA reflex potentiation during pleasant pictures was associated with higher scores on measures of social well-being and positive emotionality. We now consider each of our findings and their implications.
Startle Reflex and Puberty
Puberty appears to increase the overall magnitude of the startle reflex, though both mid/late pubertal and pre/early pubertal participants showed similar patterns of fear-potentiated startle and there were no differences in self-reported anxiety. This intriguing finding can be interpreted in several ways, given the neural circuitry of the startle response and the neurodevelopmental changes associated with the onset of puberty. The magnitude of the auditory startle response can be parsed in two substantive ways, general reactivity and affective modulation, which are mediated by different neural pathways. A primary pathway which, after activation of auditory sensors in the cochlea, involves a first synapse in the cochlear nucleus and a second one in the brainstem specifically regions of the nucleus reticularis pontis caudalis (Wu, Suzuki, & Siegel, 1988). These impulses are then transmitted to the spinal cord resulting in motor output such as blinking and/or full body startle (Lee, Lopez, Meloni, & Davis, 1996). If this primary pathway is damaged, the startle response is completely eliminated (Davis, Gendelman, Tischler, & Gendelman, 1982). However, at the level of the nucleus reticularis pontis caudalis, the primary pathway receives modulatory input from the amygdala, which supports processing of aversive stimuli and defensive behavior. Lesions of this secondary pathway eliminate fear-potentiated startle leaving intact both startle reactivity and the capacity to startle (Davis, Falls, Campeau, & Kim, 1993). Therefore it is possible that the overall enhancement of startle taking place with onset of puberty is mediated by changes at the level of the primary reflex pathway but not at the level of the amygdala input.
Potential candidates are neurotransmitters and structures which control arousability, alertness, and wakefulness such as the nucleus reticularis pontis caudalis; and adolescent development is accompanied by dramatic changes in the timing and the amounts of sleep and wakefulness. These changes, including the temporal delay of sleep wakefulness cycles, (Carskadon, Acebo, & Jenni, 2004) and changes in sleep activity patterns, have been linked specifically to the pubertal transition (Carskadon, Harvey, Duke, Anders, Litt et al., 1980). Nevertheless, it is important to underscore the complexity of the factors that might link puberty to sleep arousal cycles and startle reactivity as they are likely to be both puberty-specific and puberty-independent factors.
Puberty related changes in the catecholaminergic system might account for increased startle amplitude. Substances such as yohimbine which activate norepinephrine (NE) systems exacerbate startle reactivity (Morgan, Grillon, Southwick, Nagy, Davis et al., 1995; 1993), while decreasing NE by clonidine dampens the startle response (Kumari, Cotter, Corr, Gray, & Checkley, 1996). Research suggests that the “triggers” of puberty involve both weakening of inhibitory tone and amplified excitatory tone such as increased NE activity, (Genazzani, Bernardi, Monteleone, Luisi, & Luisi, 2000; Spear, 2000). In non-human primates NE increased significantly from the pre- and early pubertal to the mid pubertal stage, acting as a key trigger of puberty (Gore & Terasawa, 1991). Therefore an initial “hyperactive” phase of central catecholamines seems to contribute to the onset of puberty. There are evidences of increased NE output and heightened sympathetic reactivity with increased pubertal development in humans (Teinturier, 2002; Weise, Eisenhofer, & Merke, 2002). Thus increased excitatory output of catecholamines associated with gonadarche might enhance startle reactivity in pubertal adolescents who are transitioning toward sexual maturity.
While attractive, there are some inconsistencies with this hypothesis. First, a general increase in NE activity also would be expected to heighten NE transmissions in the amygdala (Fendt, Koch, & Schnitzler, 1994). Thus this hypothesis is difficult to reconcile with our evidence that fear-potentiated startle was not greater among the mid/late pubertal participants. This inconsistency points to other puberty-associated mechanisms, perhaps in addition to increased catecholaminergic activity, being involved in the overall increase in startle amplitude we observed. Clearly further work would require longitudinal studies of the psychophysiology of appetitive and defensive motivations. Interestingly, Avenevoli and collegues (2003) recently found greater fear-potentiated startle to a conditioned threat stimulus linked to the onset puberty in a longitudinal and cross-sectional study. It will also be important to determine whether these effects are replicable.
Postauricular Reflex and Puberty
Regarding the PA reflex, we found that mid/late pubertal adolescents showed appetitive potentiation of the PA reflex, while their same age pre/early pubertal peers showed no modulation of this reflex. Puberty appears to have divergent effects in PA and startle reflexes, in terms of general reactivity and affective modulation. The overall magnitude of the PA reflex was not significantly different across pubertal groups, but its magnitude was significantly modulated by emotion only in the mid/late pubertal participants. While these findings, and the ones discussed below, were statistically significant, a cautionary note is warranted. We found more inter-subject variability in PA reflex magnitude than in the startle response. As a result, the observed power for detecting magnitude differences was relatively low (observed power = .396). Thus we need to be cautious about concluding that the PA reflex is unaffected by picture valence until puberty. Nevertheless, the present results clearly indicate that despite generally low power, among mid/late pubertal adolescents a clear PA reflex potentiation to pleasantly-valenced pictures was noted. It is curious that for our adolescent participants the magnitude of the PA reflex was larger than that typically observed for adult samples. Developmental studies across the lifespan could elucidate whether PA reflex magnitudes decline during the course of normal development, or whether the overall magnitude of this reflex reaches an asymptote at some as-yet unstudied point in the lifespan.
Little is known about the neurobiology of the PA reflex to guide interpretation of why PA reflex potentation might be less readily detected among pre/early pubertal than mid/late pubertal adolescents. Davis (1965) theorized that the PA reflex is an analogue of Preyer’s reflex, an orienting response to alerting sounds that is frequently studied in guinea pigs (cf Douek & Clarke, 1976). The PA reflex appears to originate from the cochlea (Yoshie & Okudaira, 1969), and the facial nerve may be the efferent nerve to the postauricular muscle that generates the PA reflex (Bochenek & Bochenek, 1976). Nevertheless, the circuitry of the PA reflex between the cochlea and the facial nerve, including its emotional modulation, remain unstudied. However, in our mid/late pubertal participants, PA reflexes were significantly greater during appetitive stimuli (food, sexuality and bonding/attachment) that reliably activate the neurobiology of reward and primary reinforcers. Our findings and other accounts of PA reflex potentiation (Benning, 2006; Hess et al., 2007) suggest that the PA reflex might receive input from reward processing structures. Thus puberty’s effect in appetitive potentiation of the PA reflex may be linked to characteristic changes in reward directed behavior and reward neurobiology that take place during adolescence. For example during adolescence there are increased basal PFC dopamine levels (Andersen, Thompson, Rutstein, Hostetter, & Teicher, 2000). Additionally, there is evidence of exaggerated accumbens activity relative to prefrontal activity in adolescents, compared with children and adults (Galvan, 2006). Scrutiny from an affective neuroscience framework would be necessary in order to disintangle specific neural systems of both appetitive and defensive motivation that are impacted by pubertal maturation.
Although the PA reflex was clearly potentiated by appetitive stimuli among the mid/late pubertal adolescents, our hypothesis that it would be associated with thrilling, high sensation stimuli was not confirmed. Among the picture contents, those depicting rewarding social events modulated the PA reflex, while those depicting high sensation, thrilling activities (e.g.,white water rafting, rollercoaster rides) had no impact on this reflex. Notably, among adults, sensation/thrill seeking pictures also did not evoke significant potentiation of the PA reflex (Benning, 2006). We are also cautious about generalizing beyond the education and income group we studied. While puberty may play a role in the emergence of PA potentiation to appetitive stimuli, it is also likely that experience with various thrilling and risky situations will impact the developing adolescent’s understanding of the risks and rewards inherent in different high stimulation conditions. With age or among different socioeconomic groups, adolescents may have more experience with high sensation activities or may view these situations as more unambiguously positive than the participants in our sample. Finally, although the PA reflex was not modulated by adventure/thrilling picture content, it emerged as an indicator of positive emotions, adaptive functioning and rewarding affiliation. These findings suggest that in mid/late pubertal adolescents, PA reflex potentiation may serve as a sensitive reflexive measure of individual differences in trait positive emotionality and well being.
Personality/Behavior Traits and Psychophysiology
As we hypothesized, puberty moderates how personality and behavioral traits are associated with emotional modulation of psychophysiology. This moderating effect was not surprising for the PA reflex, as pre/early pubertal adolescents did not modulate the PA reflex with picture valence. It was more striking for the startle response, as there were no puberty effects on emotion modulation of the startle reflex.
Individual differences in trait anxiety and traits such sensation seeking have been studied as predictors of startle potentiation in both children and adults (Lissek & Powers, 2003). Additionally, the association between biological measures with certain behavior patterns emerges only after puberty (Granger, Shirtcliff, Zahn-Waxler, Usher, Klimes-Dougan et al., 2003; Twitchell et al., 2000). But to our knowledge, this is the first instance where appetitive and defensive psychophysiology relations to behavioral characteristics are moderated by puberty in participants so close in age. Steinberg et al.(2006) noted that the adolescent transition marks a relative consolidation of patterns in behavior and psychopathology. In our study the onset of puberty signaled enhanced coherence between the psychobiology of emotional processing and specific behavioral traits. This can be interpreted as a crystallization of traits and symptoms taking place during adolescence, conceivably due to puberty specific processes. A related idea is that with increased psychosocial challenges the “emotional stakes” rise during the transition through puberty, and individual differences in reactivity to danger become more pronounced. Pubertal adolescents who are stress reactive and anxious would lean toward increased vigilance in threatening contexts, whereas those prone to seek thrills and new sensations will tend to lower vigilance and stress reactivity in similar contexts. In a sense, it is as if puberty enhances individual predispositions in fast emotional processing mechanisms of both threats and rewards.
We found that mid/late pubertal participants had a significantly greater propensity to seek new sensations and thrills compared to their pre/early pubertal peers. Thus, we replicated Martin and colleagues’ (2002) findings regarding the particular role of puberty in the rise of risky behavior in a clinical sample of adolescents. However, higher sensation seeking in typical mid/late pubertal adolescents may also be linked to the increased importance of peer and romantic relationships as well as the excitement of trying more adult-like roles. We had expected that increased sensation/risk seeking during adolescence would relate to reward systems (heightened appetitive motivation as measured by the PA reflex). Instead we found that increased sensation seeking was expressed through the defensive motivational system as indexed by its negative relation to startle potentiation. This is congruent with the triadic model of motivated behavior in adolescence (Ernst et al., 2006), which posits that during this period reward and novelty seeking in the face of potential harm is due to: first - a strong reward system (nucleus accumbens), second - a weak harm-avoidant system (amygdala), and/or third - an inefficient supervisory system (medial/ventral PFC). However, our findings suggest an amendment to the second point by demonstrating that in fact there is a very strong harm-avoidant system during adolescence linked to puberty specific increases in overall startle reactivity. If puberty is associated with an increase in the harm-avoidance system, then alternative explanations are needed to explain the increase in risk-taking behavior noted at this stage of development. Likely candidates include the third component in Ernst et al.’s (2006) model, an immature regulatory executive system which is insufficiently developed to harness the arousal levels and intense emotions experienced during adolescence.
Limitations
There are a number of limitations of the present study. First, while we were able to differentiate pubertal effects from age by studying only very young adolescents, this necessarily precluded an examination of the role of later stages of pubertal maturation in affecting fear-potentiated startle and PA potentiation to appetitive stimuli. Second, our participants represented only a circumscribed group of adolescents, those from the dominant racial group living in middle- to upper-middle class homes with parents sufficiently interested in child development to register their children at birth to take part in such research. We cannot be sure that our findings will generalize beyond similar groups of young adolescents. Finally, although the behavioral correlates we obtained with the PA reflex were consistent with the picture content that evoked PA reflex potentiation, these were not the correlates we predicted. Thus, these post-hoc findings need to be replicated.
Implications for Psychopathology and Intervention
The results of the present study have implications for our understanding of the role of puberty in heightened risk for psychopathology during adolescence. Heightened startle amplitude with puberty might be expected to increase the physiological repercussions of anxiety-provoking events, allowing greater sensitivity to threat stimuli among adolescents at risk for affective disorders. Indeed, individuals with higher baseline startle are more likely to evidence higher startle potentiation to aversive stimuli (Grillon & Baas, 2002). Pubertal moderation of the association between psychological traits and the psychophysiology of defensive and appetitive motivation may increase the odds that individuals at risk for both externalizing and internalizing psychopathology will evidence clinically significant symptoms with the onset of puberty. Additionally, if pubertal processes mark a crystallization of predispositions in physiological reactivity and behavior, intervention efforts for those individuals at risk should take place before the onset of puberty. However, the clearest implication for research on psychopathology comes from evidence in this study and in the others in this special section that puberty plays a role in re-organizing physiological systems associated with defensive and appetitive motivation. These data, coming from low-risk samples of youth, should provide strong motivation for continued examination of the role of puberty in altering the neurobiological substrates underlying the various forms of psychopathology that exhibit increases in prevalence during adolescence.
Acknowledgments
Preparation of this manuscript was supported by a National Institute of Mental Health pre-doctoral fellowship (T32 MH15755) to the first author, the Adolescent Development, Affect-regulation, & the Pubertal Transition (ADAPT) Network, and a National Institute of Mental Health Senior Scientist Award (K05 MH66208) to the third author. We are grateful to the staff and faculty of the Center for Neurobehavioral Development for support and technical expertise. Thanks to Bonny Donzella, Christopher Patrick, Terry Blumenthal, Birdie Shirtcliff, and Greg Siegle for their scholarly advice and sense of humor.
References
- Adams GR, Berzonsky MD, editors. Blackwell handbook of adolescence. Blackwell Publishing; Malden, MA: 2003. [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Merikangas KR, Stolar M, Grillon C. Pubertal development, startle modulation, and changes in affective expression; Society for Research in Child Development Biennal Meeting; Tampa, Fl. 2003. [Google Scholar]
- Barb CR, Hausman GJ, Czaja K. Leptin: A metabolic signal affecting central regulation of reproduction in the pig. Domestic Animal Endocrinology. 2005;29(1):186–192. doi: 10.1016/j.domaniend.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Benning S. Embedding the postauricular reflex in a psychophysiological nomological network of emotion: Modulation by pictures, sounds, and dysphoria. University of Minnesota; Minneapolis, MN: 2006. [Google Scholar]
- Benning S, Patrick C, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41(3):426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42(6):753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzin F, Fortinguerra CR. Emg study of the anterior, superior and posterior auricular muscles in man. Annals of Anatomy. 1993;175(2):195–197. doi: 10.1016/s0940-9602(11)80182-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bochenek W, Bochenek Z. Postauricular (12 msec latency) responses to acoustic stimuli in patients with peripheral, facial nerve palsy. Acta oto-laryngologica. 1976;81(3-4):264–269. doi: 10.3109/00016487609119961. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Fred RA, Toth SL. A developmental psychopathology perspective on depression in children and adolescents. In: Reinolds WM, Johnston HF, editors. Handbook of depression in children and adolescents. Plenum Press; New York: 1994. [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: Vulnerabilities and opportunities. New York Academy of Sciences; New York, NY: 2004. Regulation of sleep and arousal: Comments on part vii; pp. 292–293. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. Vol. 1021. New York Academy of Sciences; New York, NY: 2004. [DOI] [PubMed] [Google Scholar]
- Daitzman RJ, Zuckerman M, Sammelwitz P, Ganjam V. Sensation seeking and gonadal hormones. Journal of Biosocial Science. 1978;10(4):401–408. doi: 10.1017/s0021932000011895. [DOI] [PubMed] [Google Scholar]
- David H. The young deaf child: Identification and management. Proceedings of a conference held in toronto, canada on 8-9 october, 1964. Acta oto-laryngologica. 1965;(Suppl 206):205. [PubMed] [Google Scholar]
- Davis M. The potentiated startle response as a measure of conditioned fear and its relevance to the neurobiology of anxiety. In: Simon P, Soubrie P, editors. Selected models of anxiety, depression and psychosis animal models of psychiatric disorders. Vol. 1. S. Karger AG; Basel, Switzerland: 1988. pp. 61–89. [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioural Brain Research. 1993;58(1-2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: Lesion and stimulation studies. Journal of Neuroscience. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek E, Clarke GP. A single average crossed acoustic response. The Journal of Laryngology and Otology. 1976;90(11):1027–1032. doi: 10.1017/s0022215100083079. [DOI] [PubMed] [Google Scholar]
- Eccles JS. The development of children ages 6 to 14. The Future of Children. 1999;9(2):30–44. [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler H-U. Amygdaloid noradrenaline is involved in the sensitization of the acoustic startle response in rats. Pharmacology, Biochemistry & Behavior. 1994;48(2):307–314. doi: 10.1016/0091-3057(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter M. Psychobiology of maternal behavior in nonhuman mammals. In: Bornstein M, editor. Handbook of parenting. Erlbaum; Hillside, NJ: 1995. pp. 59–86. [Google Scholar]
- Galvan A. Neural substrates and development of reward-related behavior. Weill Medical Coll, Cornell U; US: 2006. [Google Scholar]
- Ge X, Kim IJ, Brody GH, Conger RD, Simons RL, Gibbons FX, et al. It’s about timing and change: Pubertal transition effects on symptoms of major depression among african american youths. Developmental Psychology. 2003;39(3):430–439. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Bernardi F, Monteleone P, Luisi S, Luisi M. Neuropeptides, neurotransmitters, neurosteroids, and the onset of puberty. Annals of the New York Academy of Sciences. 2000;900:1–9. doi: 10.1111/j.1749-6632.2000.tb06210.x. [DOI] [PubMed] [Google Scholar]
- Gore AC, Terasawa E. A role for norepinephrine in the control of puberty in the female rhesus monkey, macaca mulatta. Endocrinology. 1991;129(6):3009–3017. doi: 10.1210/endo-129-6-3009. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Greist JH. The diagnosis of social phobia. The Journal of Clinical Psychiatry. 1995;56(Suppl 5):5–12. [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33(8-9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP. Comments on the use of the startle reflex in psychopharmacological challenges: Impact of baseline startle on measurement of fear-potentiated startle. Psychopharmacology. 2002;164(2):236–238. doi: 10.1007/s00213-002-1164-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44(10):990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grunbaum JA, Kann L, Kinchen SA, Williams B, Ross JG, Lowry R, et al. Youth risk behavior surveillance. MMWR Surveill Summ. 2002;51(4):1–62. [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Huerta R, Brizuela-Gamino OL. Interaction of pubertal status, mood and self-esteem in adolescent girls. The Journal of Reproductive Medicine. 2002;47(3):217–225. [PubMed] [Google Scholar]
- Klorman R, Cicchetti D, Thatcher JE, Ison JR. Acoustic startle in maltreated children. Journal of Abnormal Child Psychology. 2003;31(4):359–370. doi: 10.1023/a:1023835417070. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter P, Corr PJ, Gray JA, Checkley SA. Effect of clonidine on the human acoustic startle reflex. Psychopharmacology. 1996;123(4):353–360. doi: 10.1007/BF02246646. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, H JJ, A WT, editors. Technology in mental health care delivery systems. Ablex: Ablex; NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ. The emotion probe. Studies of motivation and attention. The American Psychologist. 1995;50(5):372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, editors. International affective picture system (iaps): Instruction manual and affective ratings. University of Florida, The Center for Research in Psychophysiology; Gainesville, FL: 1999. tech. Rep. No. A-4. [Google Scholar]
- Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Development. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neuroscience & Biobehavioral Reviews. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle pathway: Obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. Journal of Neuroscience. 1996;16(11):3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS. Sensation seeking and startle modulation by physically threatening images. Biological Psychology. 2003;63(2):179–197. doi: 10.1016/s0301-0511(03)00053-x. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith J, et al. Sensation seeking, puberty and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Corticosteroids and hippocampal plasticity. Annals of the New York Academy of Sciences. 1994;746:134–144. doi: 10.1111/j.1749-6632.1994.tb39223.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg W, Cuthbert BN, Lang PJ. Emotional reactions in children: Verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38(2):222–231. [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Nagy LM, Davis M, Krystal JH, et al. Yohimbine facilitated acoustic startle in combat veterans with post-traumatic stress disorder. Psychopharmacology. 1995;117(4):466–471. doi: 10.1007/BF02246220. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110(3):342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychological Assessment. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB, O’Beirne GA. A correlation method for detecting the sound-evoked post-auricular muscle response (pamr) Hearing Research. 1999;138(1-2):147–162. doi: 10.1016/s0378-5955(99)00161-6. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children; behavioral assessment of children. American Guidance Service; Circle Pines: MN: 1998. [Google Scholar]
- Romeo RD. The pubertal maturation of male sexual behavior: The role of steroid hormones, their receptors, and pheromones. Michigan State U; US: 2001. [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Roth M. The differentiation of self-consciousness in adolescence. Zeitschrift fur Differentielle und Diagnostische Psychologie. 1999;20(2):116–125. [Google Scholar]
- Russo MF. A sensation seeking scale for children: Further refinement and psychometric development. U Georgia; US: 1991. [Google Scholar]
- Russo MF, Stokes GS, Benjamin BL, Christ MAG, McBurnett K, Loeber R, et al. A sensation seeking scale for children: Further refinement and psychometric development. Journal of Psychopathology and Behavioral Assessment. 1993;15(2):69–86. [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, et al. Emotion-based decision-making in healthy subjects: Short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dorshorst JJ, Nguyen J, Pollak SD. Pubertal maturation and age influence adolescent’s perception of the impact of recent negative life events; Paper presented at the 2006 Biennial Meeting of the Society for Child Development; 2006. [Google Scholar]
- Shirtcliff EA, Heiligenstein M, Hoornstra L, Squires K, Pollak SD. Raging hormones? Stages of pubertal development largely capture underlying hormonal processes in early adolescence; Paper presented at the Society for Research in Child Development 2007 Biennal Meeting; 2007. [Google Scholar]
- Sollers JJ, Hackley SA. Effects of foreperiod duration on reflexive and voluntary responses to intense noise bursts. Psychophysiology. 1997;34:518–526. doi: 10.1111/j.1469-8986.1997.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturationa and parent-adolescent distance: An evolutionary perspective. In: Adams GR, Montemayor R, P GT, editors. Advances in adolescent behavior and development. Sage Publications; Newbury Park, CA: 1989. pp. 71–97. [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Developmental neuroscience. 2nd ed. Vol. 2. John Wiley & Sons, Inc.; Hoboken, NJ: 2006. pp. 710–741. [Google Scholar]
- Teinturier C. Neuroendocrine mechanisms of puberty onset. Gynecologie, Obstetrique & Fertilite. 2002;30(10):809–813. doi: 10.1016/s1297-9589(02)00450-2. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: H TA, D MJ, editors. Anxiety and the anxiety disorders. Erlbaum; Hillsdale, NJ: 1985. [Google Scholar]
- Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA. Serotonergic function, behavioral disinhibition, and negative affect in children of alcoholics: The moderating effects of puberty. Alcoholism, Clinical and Experimental Research. 2000;24(7):972–979. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Weise M, Eisenhofer G, Merke DP. Pubertal and gender-related changes in the sympathoadrenal system in healthy children. Clinical Endocrinology and Metabolism. 2002;87(11):5038–5043. doi: 10.1210/jc.2002-020590. [DOI] [PubMed] [Google Scholar]
- Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain Research. 1988;457(2):399–406. doi: 10.1016/0006-8993(88)90716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie N, Okudaira T. Myogenic evoked potential responses to clicks in man. Acta oto-laryngologica. 1969;252:89–103. doi: 10.3109/00016486909120515. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. The Journal of Comparative Neurology. 2005;493(1):51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]