Abstract
Concanavalin A (Con A)–induced injury is an established natural killer T (NKT) cell–mediated model of inflammation that has been used in studies of immune liver disease. Extracellular nucleotides, such as adenosine triphosphate, are released by Con A–stimulated cells and bind to specific purinergic type 2 receptors to modulate immune activation responses. Levels of extracellular nucleotides are in turn closely regulated by ectonucleotidases, such as CD39/NTPDase1. Effects of extracellular nucleotides and CD39 on NKT cell activation and upon hepatic inflammation have been largely unexplored to date. Here, we show that NKT cells express both CD39 and CD73/ecto-5’-nucleotidase and can therefore generate adenosine from extracellular nucleotides, whereas natural killer cells do not express CD73. In vivo, mice null for CD39 are protected from Con A–induced liver injury and show substantively lower serum levels of interleukin-4 and interferon-γ when compared with matched wild-type mice. Numbers of hepatic NKT cells are significantly decreased in CD39 null mice after Con A administration. Hepatic NKT cells express most P2X and P2Y receptors; exceptions include P2X3 and P2Y11. Heightened levels of apoptosis of CD39 null NKT cells in vivo and in vitro appear to be driven by unimpeded activation of the P2X7 receptor.
Conclusion
CD39 and CD73 are novel phenotypic markers of NKT cells. Deletion of CD39 modulates nucleotide-mediated cytokine production by, and limits apoptosis of, hepatic NKT cells providing protection against Con A–induced hepatitis. This study illustrates a further role for purinergic signaling in NKT-mediated mechanisms that result in liver immune injury.
Concanavalin A (Con A) administration results in murine liver injury that is thought to be mediated by T cells, natural killer T (NKT) cells, and antigen-presenting cells such as dendritic cells.1,2 Hepatic NKT cells are highly enriched in rodent liver3 and are required for Con A–induced hepatitis.4–6 CD1d-deficient mice that lack all NKT cells appear resistant to Con A–induced hepatitis.1,4–6 Similarly, sufficient numbers of NKT cells are required to propagate hepatic injury.7,8 Importantly, the activation of NKT cells is associated with a degree of apoptosis that occurs rapidly after induction by Con A or the NKT cell–specific ligand α-galactosylceramide (αGalCer).4,9,10 NKT cell–linked injury is associated with the secretion of the cytokines interleukin 4 (IL-4), interferon-γ (IFN-γ), and tumor necrosis factor-α.5,6,11,12
In addition to cytokines, extracellular nucleotides also accumulate at inflammatory sites. These latter mediators modulate immune reactions and are operative through the activation of specific P2Y and P2X receptors that are expressed on many cell types.13–17 Lymphocytes release adenosine triphosphate (ATP) and accumulate a halo of pericellular nucleotides upon stimulation with polyclonal stimuli such as anti-CD318,19 or mitogenic lectins such as Con A.20 Activation of these P2 surface receptors regulates lymphocyte and leukocyte functions such as cytokine secretion and/or migration.21,22 As a pertinent example, lymphoid cell activation and proliferation in response to Con A is closely associated with the activation of P2 receptors.23,24
Interestingly, mice deficient in the ATP receptor P2X7 are resistant to Con A–induced hepatitis.25 However, in such mice null for P2X7, the NKT cells exhibit a dimorphic phenotype in which responses appear to be dictated by the prior state of activation. Activation of P2X7 receptors on naïve cells induces inhibitory signals, whereas in primed cells the activation responses appear to be facilitated further. It remains unclear whether putative alterations in levels of extracellular nucleotides that develop during hepatic injury might impact the inflammatory response in a NKT cell–dependent manner.
In a closely related manner, adenosine, an end product of nucleotide hydrolysis, has potent anti-inflammatory and immune suppressive properties. These effects are executed by activation of certain P1 receptors. Anti-inflammatory outcomes have been demonstrated to involve the adenosine A2A receptor in various liver injury models, including Con A–induced hepatitis.26
Levels of extracellular nucleotides and the generation of adenosine are tightly regulated by cell surface ectoenzymes known as ectonucleotidases. Within the vasculature, CD39 is crucial for the hydrolysis of extracellular nucleotides such as ATP and adenosine diphosphate (ADP) to the respective monophosphates, and, in concert with 5′ ectonucleotidase or CD73, these generate adenosine. 27
To test whether the major ectonucleotidase CD39 is expressed by NKT cells and alters the outcome of Con A–induced hepatitis, we have derived and tested mice deficient in CD39.28 We describe a novel phenotype of hepatic NKT cells in that these cells express both ectonucleotidases that operate in tandem to regulate membrane P2 receptors. We also note how disordered metabolism of extracellular nucleotides following on genetic deletion of CD39 protects against immune liver injury in a murine model of Con A–induced hepatitis. We provide evidence that the mechanism involves heightened apoptosis of hepatic NKT cells in mutant mice resulting in hepatoprotection.
Materials and Methods
Animals and Animal Models
Animals were housed in accordance with the guidelines from the American Association for Laboratory Animal Care. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees approved all research protocols. We studied wild-type C57BL/6 mice (Taconic, Germantown, NY) and CD39-null mice backcrossed with the same derivation of C57BL/6 for over six generations. Mice deficient in both CD1d genes were prepared as described. 29 CD1d knockout mice were further backcrossed for a total of 12 generations to the C57BL/6 background. Mice had free access to a standard mouse chow. Intravenous injections of Con A or αGalCer were performed into the left saphenous vein in animals of 8 to 10 weeks of age under anesthesia using xylacin (10 mg/mL) and ketamine (80 mg/kg). At least three animals from each group were sacrificed at each time point analyzed. At the time of sacrifice, mice were anesthetized, blood was taken from the inferior vena cava, and liver lobes were removed and further processed.
For adoptive transfer NKT cells (NK1.1.+, CD3+.) were sorted and then injected into the left liver lobe of CD1d-null mice using a 29-gauge needle.4,30
Reagents and Antibodies
The following reagents and antibodies were used: rabbit anti-mouse CD39 polyclonal antibody,31 fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), anti-mouse NK1.1 (allophycocyanin [APC]), CD3 (FITC), CD4 (Pacific blue), CD73 (phycoerythrin), IFN-γ (phycoerythrin, FITC) (eBioscience, San Diego, CA), and anti-mouse NK1.1 (phycoerythrin), annexin V (BD Biosciences, Franklin Lakes, NJ). Invariant NKT-reactive αGalCer-loaded CD1d tetramer was provided by the NIH tetramer facility. αGalCer (Kirin Brewery) was dissolved in dimethyl sulfoxide.
Purification of Liver Mononuclear Cells
Livers were perfused through the portal vein with cold collagenase type IV (Worthington, Lakewood, NJ) 0.2% in phosphate-buffered saline. Livers were excised and incubated for 30 minutes at 37°C and then passed through a 100 µm nylon mesh. If cells were stained for annexin V or intracellular IFN-γ livers were solely minced and passed through a 200G stainless steel mesh without collagenase perfusion. The filtrate was centrifuged at 50g for 1 minute and the supernatant was collected. The nonparenchymal cell supernatant fraction was washed once. Cells were suspended in a 40% percoll (GE Healthcare) solution and overlaid on a 70% percoll solution. After centrifugation with 2500 rpm for 20 minutes, the interphase was collected. For cell sorting with electromagnetic beads, the manufacturer’s protocol (Miltenyi Biotec Inc. Auburn, CA) was followed. Liver mononuclear cells (MNCs) were labeled with CD3 FITC and NK1.1 phycoerythrin and double-positive cells were sorted with FACSaria.
Assessment of NTPDase Activity/Thin Layer Chromatography
The pattern of nucleotide hydrolysis was determined via thin layer chromatography (TLC) using [2,8-3H]ATP (PerkinElmer, Boston, MA) as substrate as described.32 In brief, the lymphocytes (1 × 105 cells) were incubated with 20 µM [3H]ATP in a starting volume of 120 µL RPMI-1640 medium supplemented with 5 mM β-glycerophosphate. Aliquots of the mixture were periodically applied onto Alugram SIL G/UV254 TLC sheets (Nacherey-Nagel, Duren, Germany) and [3H]ATP and its radiolabeled derivates were separated using an appropriate solvent mixture and quantified via scintillation β-counting. For further analyses of sorted NKT cells, 2 mCi/mL [14C]ADP (GE Healthcare) was added to cell suspensions; aliquots were removed and analyzed for the presence of [14C]ADP hydrolysis products by TLC (three different cell culture preparations). Adenosine uptake and deamination was blocked with dipyridamole 10 µmol/L.
Measurement of Liver Injury
Alanine aminotransferase (ALT) levels were measured on a Cobas Mira (GMI Inc., Ramsey, MN) analyzer with an ALT reagent (JAS Diagnostics, Miami, FL)
Stimulation of Cytokine Secretion In Vitro
Liver MNCs were extracted and incubated at a density of 5 × 105 per well in a 96-well plate. Cells were stimulated with αGalCer at a concentration of 100 ng/mL or Con A at a concentration of 3 µg/mL for up to 48 hours. Supernatants (100 µL) were taken after 24 and 48 hours. For activation of NKT by dendritic cells, splenic dendritic cells were isolated using PanDC MACS beads. Isolated dendritic cells were loaded with αGalCer (100 ng/mL) for 2 hours and subsequently incubated with liver MNCs for 24 hours.
Cytokine Measurement by ELISA
Commercially available ELISA kits were used for determination of IL-4 and IFN-γ (eBioscience, San Diego, CA). Serum levels of circulating cytokines were determined according to the manufacturer’s instructions.
Expression of P2 Receptors by NKT cells (Reverse-Transcription Polymerase Chain Reaction)
Total RNA was extracted from 106 sorted NKT using Trizol (Invitrogen, Carlsbad, CA) and chloroform and precipitated with isopropanol. 0.5 to 1 µg of RNA was reverse-transcribed to complementary DNA using the Reverse Transcription Kit (Applied Biosystems, Foster City, CA). One microliter of the reverse-transcription (RT) product was added to the reaction mixture containing 1 × polymerase chain reaction (PCR) buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl), 1.5 mM MgCl2, 0.2 mM dNTPs, 2.5 U Taq polymerase, and the following receptor-specific primers: P2Y1 (607 bp), 5′-ttatgtcagcgtgctggtgt-3′ (forward), 5′-cgtgtctccattctgcttga-3′ (reverse); P2Y2 (518 bp), 5′-gaggacttcaagtacgtgct-3′ (forward), 5′-acggagctgtaagccacaaa-3′ (reverse); P2Y4 (745 bp), 5′-aacaactgcttcctccct-3′ (forward), 5′-aagtcctagaggtaggtg-3′ (reverse); P2Y6 (580 bp), 5′-cctgatgtatgcctgttcac-3′ (forward), 5′-cacagccaagtaggctgtct-3′ (reverse); P2Y11 (rat), 5′-tgtggcccatactggtggttgag-3′ (forward), 5′-gaagaaggggtgcacgatgccca-3′ (reverse); P2Y12 (768 bp), 5′-atatgcctggtgtcaacacc-3′ (forward), 5′-ggaatccgtgcaaagtggaa-3′ (reverse); P2Y13 (902 bp), 5′-tgcagggcttcaacaagtct-3′ (forward), 5′-cctttccccatctcacacat-3′ (reverse); P2Y14 (726 bp), 5′-ggaacaccctgatcacaaag-3′ (forward), 5′-tgaccttccgtctgactctt-3′ (reverse); P2X1 (682 bp), 5′-tcattgccagaggctttc-3′ (forward), 5′-gtggagggttgtatgtgt-3′ (reverse); P2X2 (738 bp), 5′-caaagcctatgggattcg-3′ (forward), 5′-cctatgaggagttctgtt-3′ (reverse); P2X3 (806 bp), 5′-gtgaaaagctggaccattgg-3′ (forward), 5′-gctgccattctccatcttgt-3′ (reverse); P2X4 (641 bp), 5′-tacgtcattgggtgggtgtt-3′ (forward), 5′-cttgatctggatacccatga-3′ (reverse); P2X5 (753 bp), 5′-aggacattgacacttccctg-3′ (forward), 5′-catcaggtcacggaactcta-3′ (reverse); P2X6 (953 bp), 5′-gtggtagtctacgtgatagg-3′ (forward), 5′-gcctctctatccacatac- ag-3′ (reverse); P2X7, variants 1–3 (659 bp), 5′-cttgccaactatgaacgg-3′ (forward), 5′-cttggcctttgccaactt-3′ (reverse); P2X7, variant 4 (719 bp), 5′-tcactggaggaactggaagt-3′ (forward), 5′-ttgcatggattggggagctt-3′ (reverse).
After PCR amplification, the products were detected by agarose gel electrophoresis. A total of 15 µL of each PCR product was subjected to electrophoresis on a 1.5% agarose gel, which was then stained with ethidium bromide and photographed.
For real-time PCR experiments, the SYBR Green assay was used for detecting products from the isolated complementary DNA samples. PCR reactions for each sample were performed in duplicate. The level of target gene expression was normalized against glyceraldehyde 3-phosphate dehydrogenase expression in each sample.
Statistical Analyses
Results are expressed as the median and range and mean ± standard deviation. For statistical analyses, the Student t test, Mann-Whitney U test, or two-way analysis of variance was used. Significance was defined as P < 0.05.
Results
NKT Cells Express CD39 and CD73
First, we characterized the phenotype of the major population of hepatic NKT cells with regard to the expression of ectonucleotidases. Staining for CD39 was assessed via flow cytometry. All NK1.1-positive liver MNCs (both NK1.1hi CD3-negative NK and NK1.1low CD3low NKT cells) were positive for CD39 (Fig. 1A). Also, all of the major invariant NKT cells (CD3low subset positive for αGalCer-loaded CD1d tetramer) were positive for CD39 (Fig. 1B). Mice null for CD39 are shown as controls (Fig. 1A,B). Invariant NKT cells were mostly positive for CD4 (83.2%) and the remainder (15.6%) was CD4−, CD8− (double negative). Hepatic and splenic subsets of NK1.1+CD3− NK cells, NK1.1+CD3+ NKT cells, and NK1.1−CD3+ conventional T cells were also stained for CD73/ecto-5′-ectonucleotidase (Fig. 2). NK cells were mostly CD73/ecto-5′-ectonucleotidase–negative, whereas NKT and T cells were CD73-positive. Double expression of CD39 and CD73 provides the entire ectonucleotidase cascade, potentially facilitating the generation of adenosine from extracellular nucleotides by NKT cells.
Fig. 1.
NKT cell populations in wild-type and CD39-null mice. (A) Characterization of isolated liver MNCs from wild-type mice and CD39-null mice. Basal levels of hepatic NK1.1+CD3+, NK1.1+CD3−, and αGalCer-loaded CD1d tetramer+ cells are shown. (B) All hepatic NK1.1+ and αGalCer-loaded CD1d tetramer+ cells expressed CD39. Data are representative of four independent experiments with similar results.
Fig. 2.
CD73 expression is confined to NKT and T cells. Expression patterns of CD73/ecto-5′-ectonucleotidase on NK1.1+CD3− (NK), NK1.1+CD3+ (NKT), and NK1.1−CD3+ (T cell) subsets of liver MNCs and splenocytes are shown in two sections. (A) NKT and T cells in liver MNCs express CD73; NK cells do not. (B) Splenocyte populations were examined and, as in the liver, show that the majority of NK cells do not express CD73. Data are representative of three independent experiments with similar results.
NKT Cells Efficiently Hydrolyze ATP to Generate Adenosine
The pattern of nucleotide hydrolysis by hepatic MNCs was then evaluated via TLC with [3H]ATP, ADP and other tracer nucleotide substrates (Fig. 3). Liver MNCs from wild-type mice (n = 4) efficiently hydrolyzed ATP through the stepwise reactions ATP → ADP → adenosine monophosphate (AMP) → adenosine, whereas cells from CD39-null mice (n = 4) displayed lower nucleotide-hydrolyzing capacity (Fig. 3A). Statistical analysis confirmed significant decreases in the rates of [3H]ATP hydrolysis (by 51.0 ± 6.6%) and [3H]ADP hydrolysis (by 75 ± 7.2%) in MNCs from CD39-null mice when compared with wild-type animals (P < 0.05). Likewise, sorted hepatic NKT cells also efficiently hydrolyzed ADP with respective generation of AMP and adenosine (Fig. 3B). Deletion of CD39 significantly attenuated generation of AMP and consequently of adenosine formation by liver MNCs and NKT cells (Fig. 3A,B). Collectively, these data clearly demonstrate that both ATP and ADP can be efficiently and sequentially inactivated by wild-type mice, whereas cells from CD39-null mice show markedly attenuated hydrolysis of nucleotides and production of adenosine.
Fig. 3.
Biochemical analysis of NTPDase activity of hepatic MNCs and sorted NKT cells from wild-type and CD39-null mice. (A) Time course of [3H]ATP hydrolysis and formation of its dephosphorylated 3H-metabolites by liver MNCs from wild-type (left panel) and CD39-null (right panel) mice (n = 4 each). The ordinate shows relative contents of [3H]-nucleotides/adenosine expressed as percentage of initial concentrations. (B) Analysis of the products of [14C]ADP hydrolysis by purified hepatic NKT cells from wild-type and CD39-null mice (n = 6). Extracellular nucleotides are efficiently hydrolyzed to AMP and adenosine by wild-type cells, whereas CD39-null cells showed delayed hydrolysis of nucleotides and limited production of adenosine.
Mice Null for CD39 Are Protected from Con A–Mediated Liver Injury
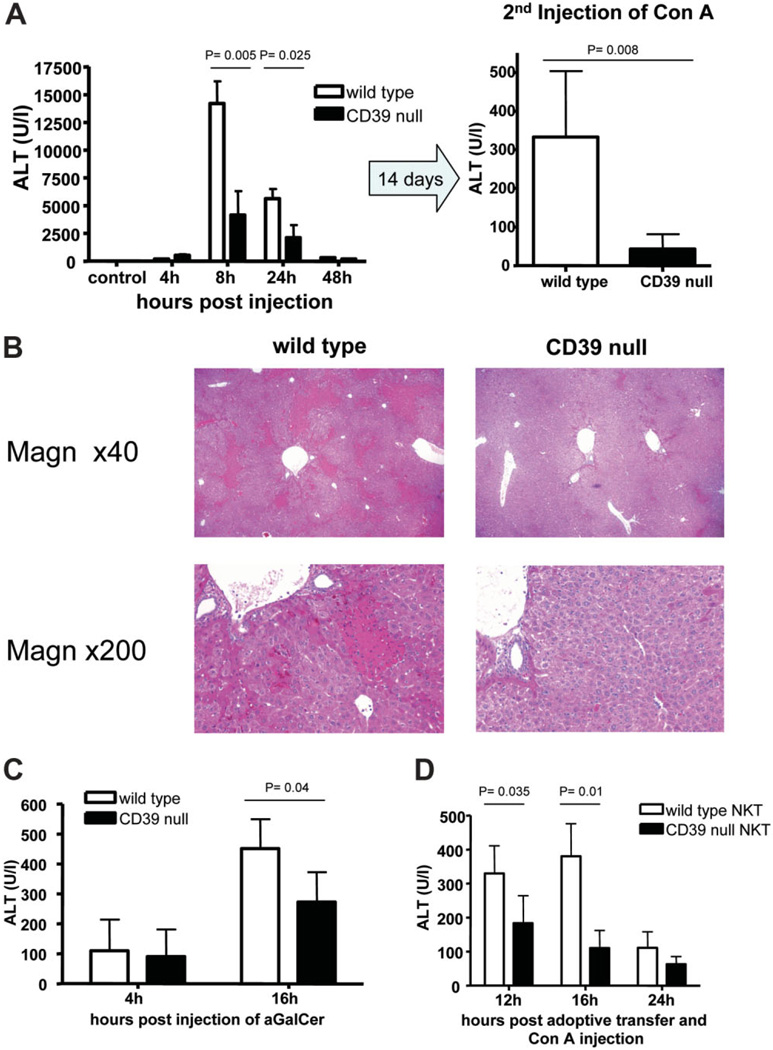
Levels of ALT were significantly lower in CD39-null mice after injection of 15 mg/kg Con A (Fig. 4A). Tolerance induction has been observed as a response to regulatory T cell (Treg) activation after a second administration of Con A.33 Deletion of CD39 on Treg inhibits immune suppressive properties of these cells. In order to test any influence of Treg functions on tolerance induction after Con A administration, we performed a second injection with 20 mg/kg Con A 14 days after Con A injury. CD39-null mice were again significantly protected against repeat Con A administration compared with wild–type mice. The area of necrotic tissue was clearly increased in wild-type mice compared with CD39-null mice after initial injection of Con A (Fig. 4B). In order to activate NKT specifically, an αGalCer hepatotoxicity model was tested. CD39-null mice were also significantly protected against αGalCer induced hepatitis (Fig. 4C).
Fig. 4.
Immune hepatitis model and CD39. (A). CD39-null mice were significantly protected from Con A–induced hepatitis (15 mg/kg) at various time points (n = 5 per time point) after injection. Minimal injury was observed in CD39-null mice after a second injection of 20 mg/kg Con A (n = 5). (B) Representative histological sections 16 hours after initial injection of Con A are shown. Magnifications are ×40 and ×200. (C) Liver injury was assessed after injection of 100 ng αGalCer per mouse at various time points (n = 4 per time point). CD39-null mice showed significantly reduced injury as assessed by ALT levels. (D) Adoptive transfer of wild-type and CD39-null NKT cells was performed in CD1d-null mice with concurrent injection of 15 mg/kg Con A (n = 4). NKT null for CD39 failed to induce significant liver injury 12 and 16 hours after injection. Data are presented as the mean ± standard deviation.
Next, adoptive transfer of purified NKT cells (NK1.1+, CD3−) was performed into the livers of CD1d-null mice with concurrent Con A (15 mg/kg) administration. Recipients of CD39-null NKT cells were significantly protected 12 and 24 hours after transfer when compared with recipients of wild-type NKT cells (Fig. 4D).
CD39 Expression by Hepatic NKT Cells Affects Cytokine Secretion In Vivo and In Vitro
Con A administration was associated with significantly decreased plasma levels of IL-4 3 hours after injection (Fig. 5A). Levels of IFN-γ were significantly lower 12 and 20 hours after injection. After incubation of liver MNCs with αGalCer for 24 hours, secretion of IL-4 and IFN-γ was absent in cells null for CD39 (Fig. 5B). As a control, secretion of IFN-γ after incubation with Con A is shown. Unlike after stimulation with αGalCer, both wild-type and mutant liver MNCs were capable of secreting IFN-γ in response to Con A, though significantly less so in CD39-null cells.
Fig. 5.
NKT cell–associated cytokine levels in vivo and in vitro. (A) Serum levels of IL-4 and IFN-γ determined after Con A injection were significantly decreased in CD39-null mice. (B) Secretion of IL-4 and IFN-γ by NKT cells in response to αGalCer. Liver MNCs were stimulated with αGalCer or Con A for 24 hours in vitro. The secretion of IL-4 and IFN-γ was significantly less from CD39-null cells. (C) Secretion of IL-4 and IFN-γ by NKT cells in response to αGalCer-primed dendritic cells. Sorted splenic dendritic cells were loaded with αGalCer for 2 hours and subsequently incubated with liver MNCs (as designated on horizontal axis) in vitro for 24 hours. The secretion of IL-4 was significantly less in MNCs null for CD39, whereas in the presence of dendritic cells the secretion of IFN-γ was increased over wild-type controls (*P < 0.05). The differential expression of CD39 on splenic dendritic cells in coculture experiments did not alter the cytokine secretion. Data are given as the mean ± standard deviation of at least four animals per group (A) and/or representative of three independent experiments (B,C).
Sorted splenic dendritic cells were loaded with αGalCer for 2 hours and then incubated with liver MNCs (Fig. 5C). Cytokine secretion in the supernatant fluid was determined and showed decreased levels of IL-4 and increased levels of IFN-γ after incubation with liver MNCs null for CD39 when compared with wild-type. There were no additional or differential effects mediated by co-cultures of mutant versus wild-type dendritic cells, suggesting that CD39 expression on the NKT cells mediates this somewhat dimorphic effect.
Increased Levels of Apoptosis in NKT Cells Null for CD39 in Response to Con A In Vivo
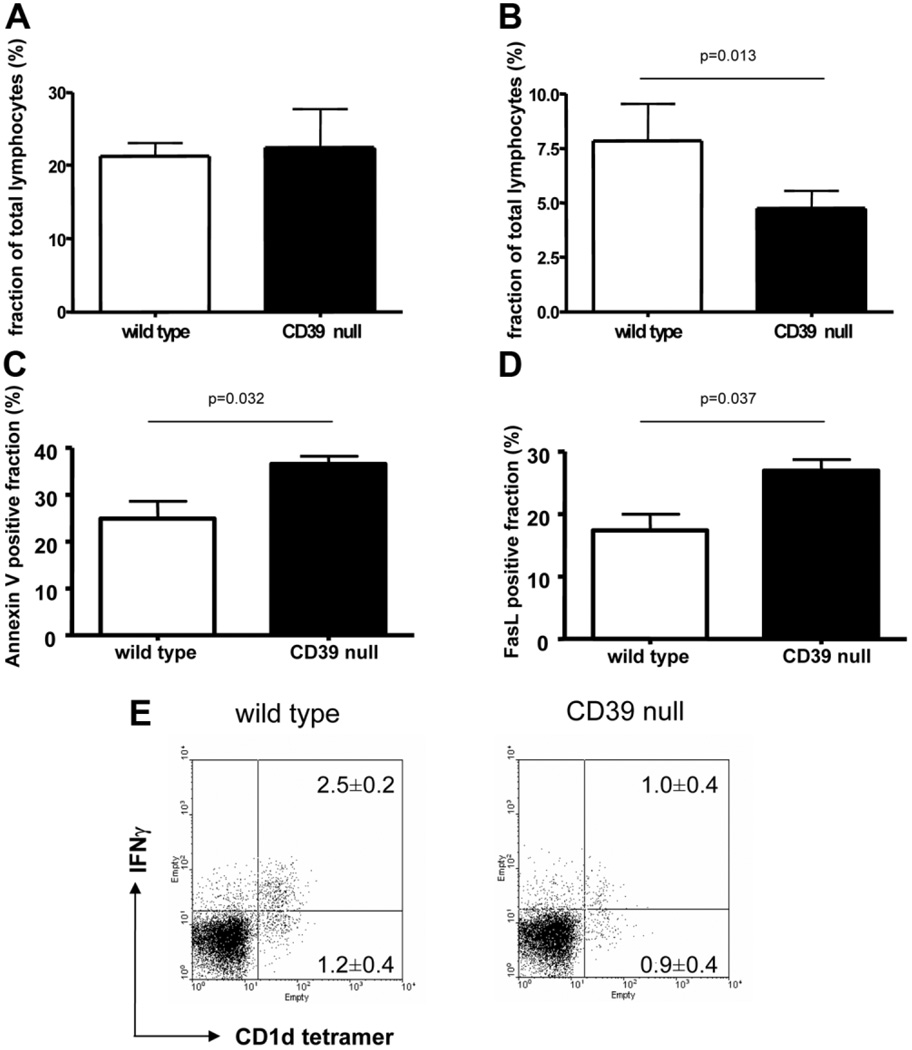
Absolute basal numbers of liver NKT cells were not affected by CD39 deletion (1.2 ± 0.3 × 105 NKT cells/g liver tissue in untreated wild-type mice versus 1.2 ± 0.5 × 105/g liver tissue in CD39-null mice; P value not significant). Overall, levels of NKT cells in the liver 60 minutes after injection with Con A were lower in mutant mice compared with wild-type mice (Fig. 6A,B). Absolute numbers of NKT cells at this time point was decreased to 0.43 ± 0.09 × 105/g liver tissue in wild-type mice and more substantially to 0.27 ± 0.04 × 105/g liver tissue in CD39-null mice (P = 0.02). Annexin V–positive (Fig. 6C) and FasL-positive (Fig. 6D) NKT cells were increased in hepatic NKT cells null for CD39 compared with wild-type mice after Con A administration. Liver MNCs showed decreased relative cell counts of IFN-γ–positive cells within the NKT cell fractions (Fig. 6E) in CD39-null mice.
Fig. 6.
Apoptosis of NKT cells in Con A–induced liver injury. (A) Under basal conditions the fraction of hepatic invariant NKT cells is not different between wild-type mice and CD39-null mice. (B) Sixty minutes after injection of Con A, the NKT cell count was significantly decreased in CD39-null livers (n = 4). (C,D) Fractions of (C) Annexin V–positive and (D) FasL-positive NKT cells were increased in mice null for CD39 compared with wild-type mice. Data are representative of at least three experiments with similar results. (E) Expression of fractions of intracellular IFN-γ–positive and IFN-γ–negative NKT cells (αGalCer-loaded CD1d tetramer+ cells) of liver MNCs after Con A–induced hepatitis were assessed via fluorescence-activated cell sorting. The number of NKT cells and the fraction of IFN-γ–positive NKT cells was decreased in CD39-null mice compared with wild-type cells (P = 0.005). Data are given as the mean ± standard deviation of four independent experiments.
NKT Cells Express P2 Receptors for Extracellular Nucleotides
The expression of purinergic receptors on NKT cells as assessed via RT-PCR revealed the presence of the P2Y receptors P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, P2Y13, and P2Y14 and of the P2X receptors P2X1, P2X2, P2X4, P2X5, P2X6, and P2X7 (Fig. 7A,B). Analysis of subsets of NKT subsets (CD4+, CD8− and CD4−, CD8− double negative) from the liver, neither the spleen nor the thymus revealed relevant changes in P2 receptor expression (Fig. 7B).
Fig. 7.
NKT cells express multiple P2 receptors. (A) Analysis of the messenger RNA expression of P2Y and P2X receptors on hepatic NKT cells (NK1.1+, CD3+). RT-PCR revealed that all of the known P2 receptors, with the exception of P2Y11 and P2X3, were found on quiescent NKT cells. (B) The expression of P2 receptors was assessed using real-time RT-PCR. The CD4+ and CD4− subsets of isolated hepatic, splenic, and thymic NKT (CD3+, NK1.1+) cells were analyzed separately.
Induction of Apoptosis by P2X7
Apoptosis triggered via the activation of P2 receptors is a well-defined mechanism.34,35 After stimulation with various doses of ATP, NKT cells rapidly became annexin V–positive in a dose-dependent manner (Fig. 8A). Significantly higher levels of annexin V–positive cells were observed at lower concentrations of ATP in CD39-null cells. With increasing concentrations of ATP, we observed marked decreases in the total number of CD39-null NKT cells (Fig. 8B). T cells (NK1.1−, CD3+) and NK cells (NK1.1+, CD3−) were also studied. With higher ATP concentrations, T cells but not NK cells show increased staining for Annexin V. Preincubation of liver MNCs with oxidized ATP, an antagonist of P2X7 receptor,36,37 results in decreased apoptosis of CD39-null NKT cells (Fig. 8C).
Fig. 8.
Induction of NKT apoptosis by extracellular ATP in vitro. Isolated liver MNCs from wild-type and CD39-null mice were incubated with various levels of ATP, and the extent of apoptosis was then determined. (A) The fraction of Annexin V–positive NKT cells is given as green histograms for wild-type cells and CD39-null cells. Extracellular ATP resulted in significantly increased expression of Annexin V on hepatic NKT cells null for CD39 compared with wild-type NKT cells (analysis of variance, P = 0.02). (B) The fraction of NKT versus the total number of MNCs reveals a dose-dependent decrease of NKT cells with increasing doses of ATP (analysis of variance, P = 0.015). (C) Administration of oxidized ATP (a P2X7 antagonist) was associated with a relative decrease of Annexin V–positive NKT cells (*P < 0.05 wild-type versus CD39-null). (A,C) Representative results of three experiments. (B) Mean and standard error of the mean from five experiments.
Discussion
In the present study, we show that CD39 and CD73 are novel phenotypic markers of NKT cells (Figs. 1 and 2); that CD39 and CD73 exhibit a biochemical signature for these cells (Fig. 3); and that genetic deletion of CD39 has salutary effects in the small animal model of Con A–induced hepatitis (Fig. 4). We propose that this protective mechanism involves loss of the specific biochemical activity of CD39 typically expressed by wild-type NKT cells and that it can be modeled by adoptive transfer of mutant and wild-type NKT cells to immunodeficient mice that lack these cells (Fig. 4D). Deletion of ectonucleotidase expression results in specific perturbations in pericellular levels of nucleotides that impact cytokine secretion (Fig. 5) and also seem to promote targeted NKT cellular apoptosis after P2X7 activation (Figs. 6–8). This increased loss of pathogenetic NKT cells as a consequence of CD39 deletion results in decreased hepatic inflammatory injury after Con A exposure.
Both CD39 and CD73 were also shown to be both highly expressed and specific to NKT cells, facilitating adenosine generation by these cells. CD39 expression on quiescent cells of the immune system has been shown to occur in Treg, B cells, and dendritic cells.38 Conversely, expression of CD73 on lymphoid populations is limited to subsets of CD4 cells and can be confirmed to be absent on NK cells.39 The pattern of coexpression of both ectoenzymes is especially interesting as it distinguishes hepatic NKT cells not only from NK cells, but also from CD39-negative CD4 effector cells. The presence of CD39 in addition to CD73 provides surface phenotype markers to define NKT cells within the liver MNC compartment.
Curiously, we have also shown recently that the coexpression of these two enzymes is present on another important regulatory cell type: the CD4+, CD25+, FoxP3+ Treg cells derived from spleen, thymus, and blood MNCs.15 In these Tregs, the suppressive function is clearly linked to the presence of adenosine. The functional consequences of CD39 and CD73 expression on NKT cells might therefore be comparable to that of Treg and hence could account for the immunosuppressive properties of these cells, some models of which involve innate immune reactions. As in the case of Treg,15 generated adenosine has potent anti-inflammatory properties on hepatic NKT-like cells, as elegantly shown in a model of ischemia/reperfusion injury in which adenosine clearly afforded protection via the NKT cell adenosine-2A receptor. 15,40
Our data showing that CD39 deletion is associated with decreased injury in Con A–induced hepatitis might initially appear counterintuitive. It is known that Con A is proinflammatory and causes ATP release from cells to drive immune responses.20 Hence, we might have predicted that loss of CD39 would result in unfettered inflammation and more marked immune responses. This is clearly not the case in this model of liver injury. Importantly, these recent findings in this NKT cell–dependent model are comparable, although only in part, to the marked inhibition of hapten-mediated type IV hypersensitivity responses witnessed previously with CD39 deletion. The antiapoptotic effects of CD39 were also seen in these prior studies where heightened levels of mutant CD39-null dendritic cell apoptosis were noted, in response to extracellular nucleotides.19
We propose that the observed altered outcomes of liver injury seen in CD39 deletion in models that test cellular immunity (as in Con A hepatitis) are dependent upon heightened nucleotide-driven P2X7-mediated selective apoptosis of proinflammatory cells, specifically the NKT cell population,19,27 as shown in our adoptive transfer experiments (Fig. 4D). This scenario would represent an alternative mechanism to that noted in innate responses (such as reperfusion injury) where CD39 expression and/or pharmacological adenosine supplementation has major beneficial effects.27,40
Our biochemical studies have further shown CD39 to be the dominant ectonucleotidase on NKT cells. Furthermore, CD39 is required for efficient production of AMP and ultimately adenosine. Other ectonucleotidases such as ecto-pyrophosphatase and alkaline phosphatases accounted for no more than 25% of hydrolysis of extracellular ATP (Fig. 3).
In Con A–induced hepatitis, we have demonstrated that extracellular nucleotides modulate secretion of cytokines by NKT cells. Interestingly, cytokine responses of IL-4 (a hallmark of NKT cells) and later IFN-γ were markedly decreased in CD39-null mice in vivo. Lack of CD39 in vitro likewise resulted in significantly decreased levels of αGalCer-mediated release of IL-4 levels and of IFN-γ from liver MNCs (Fig. 5B).
In contrast, where αGalCer is used to prime high numbers of splenic dendritic cells, which then activate NKT cells, we observed that CD39 deletion boosted IFN-γ secretion (Fig. 5C). Dendritic cells are known to interact with effector T cells and NK cells to boost other cellular sources of IL-4 and IFN-γ.41 Our own finding is also in agreement with published findings showing that the secretion of IFN-γ by effector T cells can be decreased after incubation with soluble apyrase, whereas secretion of IL-4 is not affected.22
In the experiments using nonfractionated primary liver MNCs, there are interactions between different cell types. However, the presence of CD39 in antigen-presenting cells did not differentially affect the patterns of cytokine expression in our model. Possibly the presence of high levels of splenic dendritic cells presenting αGalCer (irrespective of CD39 expression) was sufficient to impact NKT cell functions and boost both IL-4 and IFN-γ (Fig. 5C). This was somewhat surprising given our prior studies showing clearly that function of Langerhans cells can be modulated by extracellular nucleotides.14,19,42 It is feasible that effects of CD39 on costimulatory signals might be of lesser relevance in NKT cellular systems using αGalCer and other such high affinity ligands.
In essence, we propose that the triggering of increased NKT cellular apoptosis in the setting of CD39 deletion is the dominant feature in this model. Indeed, we observe lower circulating levels of IL-4 and IFN-γ in CD39-null mice after Con A administration and after αGalCer administration. Depletion of NKT cells has been likewise associated with decreased liver injury in various studies. 4,30,43 Similarly, prevention of apoptosis of NKT cells promotes liver failure,44 and blockade of IL-4 abolishes liver injury in response to Con A.44
The analysis of the phenotype of hepatic NKT cells with regard to P2 receptors show that nearly all known P2 receptors are expressed; exceptions include P2X3 and P2Y11. Therefore, multiple receptors were available for modulating purinergic responses. For example, activation of P2X7 leads to induction of apoptosis after activation of NKT cells by Con A.25 Interestingly, mice deficient in P2X7 are resistant to Con A–induced hepatitis.25 These prior studies show that NKT cells exhibit a dimorphic phenotype where responses appear to be dictated by the prior state of activation. We may speculate that low-level activation of P2X7, as limited by CD39-removing agonist, might be required for Con A–mediated liver injury. In contrast, excessive levels of P2X7 activation in the setting of CD39 deletion may result in NKT cell apoptosis and terminate the injurious process. Unlike other lymphocytes, NKT cells undergo apoptosis much more rapidly and after exposure to much lower doses of ATP; Treg also respond to stimulation with P2X7, despite potential resistance to T cell receptor– dependent induction of apoptosis. 45,46
In conclusion, we have shown that genetic deletion of CD39 protects against Con A–induced hepatitis in mutant mice by increasing NKT cell death and modulating cytokine secretion in vivo. This study has potential clinical implications in that the data indicate novel involvement of purinergic signaling in this model of immune liver injury. Pharmacological modulation of extracellular nucleotides by ectonucleotidases might provide new avenues of investigation for the treatment of acute or chronic liver inflammation.
Acknowledgments
Supported by National Institutes of Health Grants HL57307, HL63972, and HL076540 (to S. C. R.) and Swiss National Research Foundation Grants PASMA-115700 and PBBEB-112764 (to G. B.). M. E. and M. N. supported by DK 066917.
Abbreviations
- αGalCer
α-galactosylceramide
- ADP
adenosine diphosphate
- ALT
alanine aminotransferase
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- Con A
concanavalin A
- FITC
fluorescein isothiocyanate
- IFN-γ
interferon-γ
- IL-4
interleukin-4
- MNC
mononuclear cell
- NKT
natural killer T
- PCR
polymerase chain reaction
- RT
reverse-transcription
- RT-PCR
reverse-transcription polymerase chain reaction
- TLC
thin layer chromatography
- Treg
regulatory T cell
Footnotes
This work was presented in part at Digestive Diseases Week, Washington, DC, May 17–22, 2007.
Potential conflict of interest: Nothing to report.
References
- 1.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, et al. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671–1683. doi: 10.1016/S0002-9440(10)64804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. HEPATOLOGY. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyabe S, Seki S, Iiai T, Takeda K, Shirai K, Watanabe H, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 6.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito T, Okumura A, Watanabe H, Asano M, Ishida-Okawara A, Sakagami J, et al. Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J Immunol. 2004;173:579–585. doi: 10.4049/jimmunol.173.1.579. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Dong Z, Wei H, Ding C, Sun R, Tian Z. Selective elimination of hepatic natural killer T cells with concanavalin A improves liver regeneration in mice. Liver Int. 2006;26:339–345. doi: 10.1111/j.1478-3231.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 9.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, et al. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Leite-de-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol. 2000;165:4367–4371. doi: 10.4049/jimmunol.165.8.4367. [DOI] [PubMed] [Google Scholar]
- 11.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 12.Kunstle G, Hentze H, Germann PG, Tiegs G, Meergans T, Wendel A. Concanavalin A hepatotoxicity in mice: tumor necrosis factor-mediated organ failure independent of caspase-3-like protease activation. HEPATOLOGY. 1999;30:1241–1251. doi: 10.1002/hep.510300517. [DOI] [PubMed] [Google Scholar]
- 13.Denlinger LC, Angelini G, Schell K, Green DN, Guadarrama AG, Prabhu U, et al. Detection of human P2X7 nucleotide receptor polymorphisms by a novel monocyte pore assay predictive of alterations in lipopolysaccharide-induced cytokine production. J Immunol. 2005;174:4424–4431. doi: 10.4049/jimmunol.174.7.4424. [DOI] [PubMed] [Google Scholar]
- 14.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 15.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marteau F, Communi D, Boeynaems JM, Suarez Gonzalez N. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J Leukoc Biol. 2004;76:796–803. doi: 10.1189/jlb.0104032. [DOI] [PubMed] [Google Scholar]
- 17.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Yegutkin GG, Mikhailov A, Samburski SS, Jalkanen S. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17:3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 20.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 22.Langston HP, Ke Y, Gewirtz AT, Dombrowski KE, Kapp JA. Secretion of IL-2 and IFN-gamma, but not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol. 2003;170:2962–2970. doi: 10.4049/jimmunol.170.6.2962. [DOI] [PubMed] [Google Scholar]
- 23.Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DosReis GA, Nobrega AF, de Carvalho RP. Purinergic modulation of T-lymphocyte activation: differential susceptibility of distinct activation steps and correlation with intracellular 3′,5′-cyclic adenosine monophosphate accumulation. Cell Immunol. 1986;101:213–231. doi: 10.1016/0008-8749(86)90199-1. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–2160. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 26.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 27.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 28.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 29.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Sun R, Wei H, Gao B, Tian Z. Interleukin-15 prevents concanavalin A-induced liver injury in mice via NKT cell-dependent mechanism. HEPATOLOGY. 2006;43:1211–1219. doi: 10.1002/hep.21174. [DOI] [PubMed] [Google Scholar]
- 31.Sevigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- 32.Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 33.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. HEPATOLOGY. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 35.Goepfert C, Imai M, Brouard S, Csizmadia E, Kaczmarek E, Robson SC. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 36.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 38.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 39.Christensen LD, Andersen V. Natural killer cells lack ecto-5′-nucleotidase. Nat Immun. 1992;11:1–6. [PubMed] [Google Scholar]
- 40.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 43.Matsuoka S, Tsurui H, Abe M, Terashima K, Nakamura K, Hamano Y, et al. A monoclonal antibody to the alpha2 domain of murine major histocompatibility complex class I that specifically kills activated lymphocytes and blocks liver damage in the concanavalin A hepatitis model. J Exp Med. 2003;198:497–503. doi: 10.1084/jem.20021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajuebor MN, Hogaboam CM, Le T, Swain MG. C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol. 2003;170:5252–5259. doi: 10.4049/jimmunol.170.10.5252. [DOI] [PubMed] [Google Scholar]
- 45.Taylor SR, Alexander DR, Cooper JC, Higgins CF, Elliott JI. Regulatory T cells are resistant to apoptosis via TCR but not P2X7. J Immunol. 2007;178:3474–3482. doi: 10.4049/jimmunol.178.6.3474. [DOI] [PubMed] [Google Scholar]
- 46.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]