Abstract
Mesothelin (MSLN) is a cell-surface glycoprotein that is overexpressed in human pancreatic cancer. Although its value as a tumor marker for diagnosis and prognosis and as a preferred target of immunointervention has been evaluated, there is little information on the growth advantage of MSLN on tumor cells. In this study, we examined the effect of MSLN on pancreatic cancer cell proliferation, cell cycle progression, expression of cell cycle regulatory proteins, and signal-transduction pathways in two pancreatic cancer cell lines, MIA-MSLN (overexpressing MSLN in MIA PaCa-2 cells) and BxPC-siMSLN (silencing MSLN in BxPC-3 cells). Increased cyclin E and cyclin-dependent kinase 2 (CDK2) expression found in MIA-MSLN cells correlated with significantly increased cell proliferation and faster cell cycle progression compared with control cells. BxPC-siMSLN cells showed slower proliferation and slower entry into the S phase than control cells. Signal transducer and activator of transcription protein 3 (Stat3) was constitutively activated in MIA-MSLN cells, but not in control cells. Inhibition of Stat3 activation in MIA-MSLN cells by the JAK-selective inhibitor tyrphostin AG490 was followed by a marked decrease in proliferation of the cells. siRNA against Stat3 significantly reduced the MIA-MSLN cell cycle progression with a concomitant decrease in cyclin E expression. Our data indicate that overexpression of MSLN in pancreatic cancer cells leads to constitutive activation of the transcription factor Stat3, which results in enhanced expression of cyclin E and cyclin E/CDK2 complex formation as well as increased G1-S transition.
Keywords: Pancreatic cancer, Mesothelin, Cell Proliferation, Cyclin E, Stat3
Introduction
Mesothelin (MSLN) is a differentiation antigen that is present on normal mesothelial cells of the pleura, peritoneum, and pericardium (1). Accumulating evidence has shown that MSLN is overexpressed in various cancers, including ovarian cancer, pancreatic adenocarcinoma, mesothelioma, lung adenocarcinoma, and acute myeloid leukemia (2–5). The human MSLN gene encodes a 71-kDa precursor protein that is cleaved by furin-like proteinases to produce an N-terminal 31-kDa soluble fragment megakaryocyte potentiating factor (MPF) and a C-terminal 40-kDa membrane-bound fragment, MSLN (5). MSLN is reportedly involved in cell adhesion and plays a role in attachment of ovarian cancer cells onto peritoneal mesothelial cells (6); however, not much is known about its role in pancreatic cancer pathogenesis.
We have shown that MSLN-overexpressing stable MIA PaCa-2 cells (MIA-MSLN) led to development of much larger tumors than the vector control cells in subcutaneous and orthotopic mouse models of pancreatic cancer (7). Our in vitro data also showed that MIA-MSLN cells proliferated faster than control cells; this explains their induction of larger tumors. It has been reported that MSLN may play a role in the generation, and hence the proliferation, of corneal limbic epithelial cells (8), and that there is an increased proliferation rate of MSLN-high virgin mammary gland epithelial cells in response to carcinogenic stimuli, in contrast to age-matched parous mammary control cells that lack MSLN expression (9). In a tumor model in C57BL/6 mice with multiple oncogene-transformed peritoneal cells, Cheng et al showed that continuous isolation and passage of early-stage tumor cells (WF-0) from the ascites fluid of the mice resulted in an aggressive tumor cell line named WF-3 that expressed high levels of MSLN and had increased proliferation and migration rates (10). Although these studies indicate the pro-proliferative effect of MSLN, direct evidence and the detailed mechanism of MSLN involvement in cell proliferation remain unclear.
Progression of eukaryotic cells through the cell cycle is regulated by the sequential formation, activation, and inactivation of a series of cyclin/cyclin-dependent kinase (CDK) complexes and negative regulation through cyclin-dependent kinase inhibitors (CKIs) (11–13). Cyclin D/CDK4/6 complexes phosphorylate the retinoblastoma (Rb) gene products, and this releases the E2F transcription factors. E2Fs then stimulate the transcription of mRNAs that encode proteins required for the cell to progress further through the cycle. The next complex, cyclin E/CDK2, further phosphorylates Rb family proteins, and the cell begins to synthesize DNA (S phase). The cyclin A/CDK2 kinase complex is formed once the cell enters the S phase. Finally, the cyclin B/CDC2 complex phosphorylates proteins involved in chromosomal condensation and the progression of the cell through mitosis (11, 12). Two classes of CKIs have been identified. The first, represented by pl6INK4a and p15INK4b (including p19 and p18), primarily regulates CDK4 and CDK6 (14–16). The second, characterized by p21cip1 (including p27KIPl and p57KP2), regulates the activities of the CDK2 and CDK4/6 complexes (11, 12, 17). Aberrations in the cyclin/CDKs and G1/S checkpoint function are associated with many cancers, including pancreatic cancer (18).
Stats are transcription factors activated by a wide array of cytokines and growth factors (19). Stat3 is activated by phosphorylation primarily at Tyr705 by a wide array of tyrosine kinases, including receptor tyrosine kinase like EGFR (20) and ErbB2 (21). Stat3 is also indirectly activated by receptor-associated kinases like JAK2 (21), as well as non-receptor-associated tyrosine kinase src (22). Phosphorylation of Stat3 leads to its dimerization and translocation to the nucleus, where it binds to the specific DNA response element in target gene promoters and enables gene transcription (23). Constitutive activation of Stat3 is associated with a number of human epithelial cancers in which it modulates the expression of target genes that are involved in various physiological functions (24), including apoptosis (Survivin, Bcl-xL, and HSP27), cell-cycle regulation (Cyclin D1, c-fos, and c-myc), and angiogenesis (VEGF). Approximately 30% of pancreatic cancers have activated Stat3 (24). Conversely, inactivation of Stat3 leads to an inhibition of cell proliferation in pancreatic cancer (25–29).
In this study, we examined the direct role of MSLN in pancreatic cancer cell proliferation and cell cycle progression. We examined the relevance of Stat3 in these processes by overexpressing and silencing MSLN in pancreatic cancer cell lines MIA PaCa-2 and BxPC-3, respectively. This study demonstrates that overexpressing MSLN induces Stat3 activation and leads to up-regulation of S-phase promoting cyclin E. The enhanced cyclin E/CDK2 complex is responsible for faster progression through the cell cycle. Blocking Stat3 by using specific siRNA abrogated the growth-promoting effect of MSLN on the pancreatic cancer cells by blocking cyclin E expression.
Results
Overexpression of MSLN enhances proliferation of pancreatic cancer MIA PaCa-2 cells
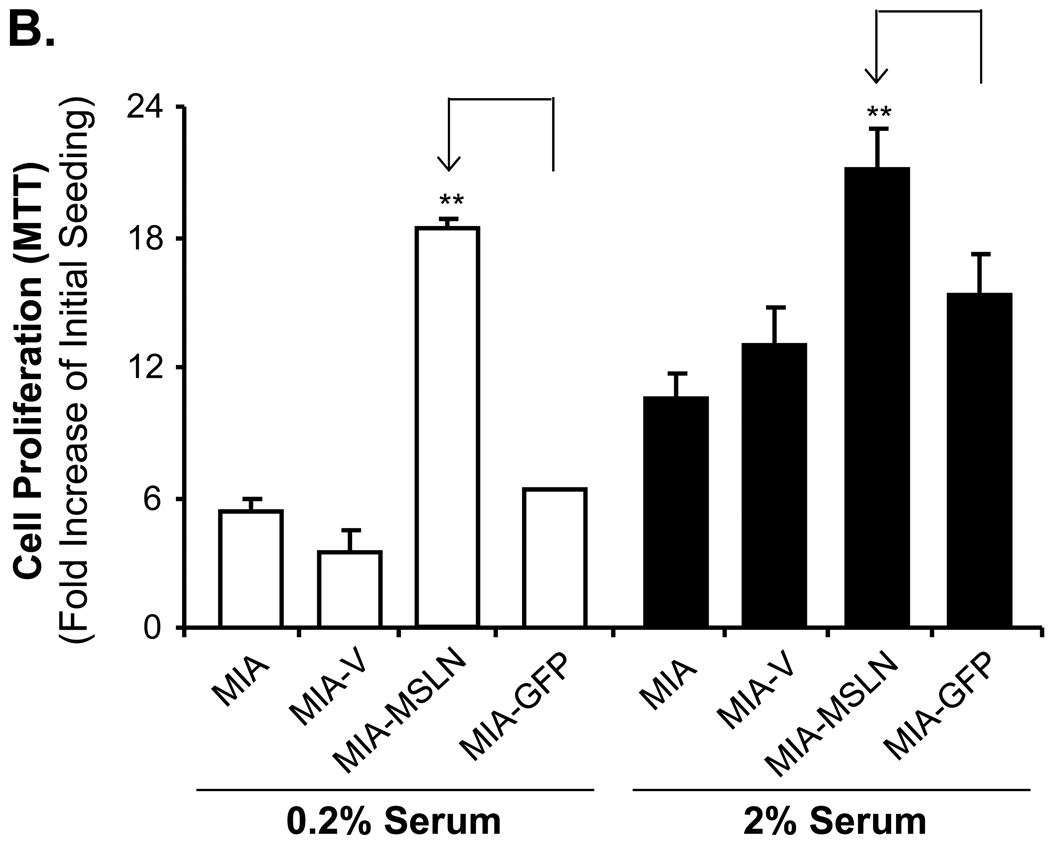
To elucidate the role of MSLN overexpression in pancreatic cancer cell proliferation, we used the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, comparing the cell growth rate among the MSLN-overexpressing MIA PaCa-2 stable cell line (MIA-MSLN), the empty vector MIA PaCa-2 stable cell line (MIA-V), and the unrelated GFP gene-overexpressing MIA PaCa-2 stable cell line (MIA-GFP). The MTT assay showed that MIA-MSLN cells proliferated almost 2.9 times faster than the MIA-V cells at day 3 (p < 0.001, Fig. 1A), and almost 2.3 times faster at day 6 (p < 0.001, Fig. 1A). To determine the serum dependence of MSLN-induced cell proliferation, we cultured cells in either 2% or 0.2% serum-containing media and compared cell-proliferation rates. Results depicted in Fig. 1B showed that the MIA-MSLN cells proliferated at almost the same rate at both serum concentrations, whereas the control cells proliferated at a much lower rate in 0.2% serum (p < 0.001, after 3 days of proliferation in growth medium). These results indicate that the effect of MSLN on cell proliferation is probably independent of serum concentration. To confirm the role of MSLN in cell proliferation, we performed the above assay with another stably MSLN-overexpressing pancreatic cancer cell line, Panc-1 (Panc1-MSLN). The similarity of the results provides further evidence for the role of MSLN in inducing cell proliferation (Fig. 1C).
Figure 1.
Overexpression of MSLN promotes pancreatic cancer cell proliferation and cell cycle progression. A. Cell proliferation of MIA-PaCa-2 cells according to MTT assay. MIA-MSLN and control cells were seeded in 96-well plates (2×103 cells/well), serum-starved (0% FBS) for 24 h before changing to 2% FBS growth medium, and cultured for 6 d. Viability was measured with MTT. Relative increase in viability was measured by dividing viability at some time by viability of same cell at day 0 (day of addition of growth medium after initial serum starvation) and is plotted along Y axis. Data plotted show mean of triplicate wells. B. Serum dependence of proliferation. After initial 24 h of serum starvation, cells were treated with 0.2% and 2% serum growth medium; viability was measured with MTT after 3 d. C. Cell proliferation in Panc-1 cells (MTT assay). Panc1-MSLN and control cells were serum-starved (0% FBS) for 24 h before changing to 2% FBS growth medium, and cultured for 6 d. D. Cell cycle analysis. After initial 24 h of serum starvation and then release by 2% serum growth medium for 4 and 8 h, cells were collected, fixed, PI-stained, and analyzed for cell cycle phase distribution (percentage of cells) with FACS. E. Plating efficiency. MIA-V and MIA-MSLN cells (600) were plated in 150-mm dishes, allowed adhering for 48 h, and starved for 24 h. Cells were then allowed to form colonies in complete medium for 15 d, which were then stained with MTT. Percentage plating efficiency was determined as (number of colonies formed/cells seeded) × 100. Columns, mean of replicates; bars, SD; ** p < 0.001 compared with controls according to t test.
To elucidate the detailed effects of MSLN-induced cell proliferation, we examined and compared the cell cycle progression of MIA-MSLN, MIA-V, and MIA-GFP cells by using fluorescence-activated cell sorting (FACS) analysis. As depicted in Fig. 1D, 50% and 61% of the MIA-MSLN cells entered the S phase at 4 h and 8 h, respectively, after release to 2% serum-containing growth medium from 24 h of serum starvation. Those proportions were significantly higher than the 20% and 28% of MIA-V cells or the 14% and 28% of MIA-GFP cells entering the S phase at 4 h and 8 h, respectively. Thus, overexpression of MSLN is associated with increased cell proliferation and faster progression into the S phase.
We used a plating efficiency assay to determine any difference in clonogenic capacity between MIA-MSLN cells and MIA-V cells,. As shown in Fig. 1E, the MIA-MSLN cells exhibited greater plating efficiency (54%) than the MIA-V cells (~ 27%; p < 0.05). This result further suggests the enhanced cell proliferation ability and survival efficiency of MIA-MSLN cells.
MSLN overexpression leads to increased expression of S-phase cyclins and the association with their binding partners in pancreatic cancer cells
To delineate the mechanism of MSLN-induced, faster progression of pancreatic cancer cells into the S phase, we examined the protein expression of various cell cycle-related molecules from the asynchronous cultures of MIA-MSLN cells and other control cells. MIA-MSLN cells had significantly higher expression of the S-phase-initiating cyclin E and the S-phase-promoting cyclin A (Fig. 2A). CDK2, which interacts with those cyclins at the initiation and progression of the S phase, respectively, was also increased in the MIA-MSLN cells. There was no difference in the expression of the cyclin D1, although the expression of CDK4, one of the cyclin-dependent kinases interacting with cyclin D for the termination of G0/G1 arrest and entering into the S phase, was slightly increased in the MIA-MSLN cells (Fig. 2A). We also found that p21 was up-regulated in MIA-MSLN cells. The entry of eukaryotic cells into mitosis is regulated by CDC2 kinase (CDK1) activation, a process controlled at several steps, including cyclin binding and phosphorylation of CDC2 at Thr161 (30). However, the critical regulatory step in activating CDC2 during progression into mitosis appears to be dephosphorylation of CDC2 at Tyr15 and Thr14 (31). Consequently, the magnitude of phosphorylation at Tyr15 is highest in cells in the S phase. MIA-MSLN cells with higher S-phase populations had increased phosphorylation at Tyr15 of CDC2 (Fig. 2A), although the expression of CDC2 in these cells was similar to that of the control cells. Thus, changes in expression of cell cycle related molecules, especially up-regulation of cyclin E and CDK2 in the MIA-MSLN cells, may be responsible for the increased cell proliferation and faster S-phase progression.
Figure 2.
S-phase cyclin E and its binding partner CDK2 are up-regulated in MIA-MSLN cells. A. Sub-confluent cells were used to prepare lysates, and 60 µg of protein were subjected to SDS-PAGE and Western blot. Various cell-cycle-related proteins were detected with antibodies mentioned in methods.B. Control cells and MIA-MSLN cells were serum-starved (0% FBS) for 24 h, changed to 2% FBS medium, and collected at indicated times, and whole cell proteins were subjected to SDS PAGE, Western blotting, and probing for cyclin E, CDK2, and β actin. C. 400 µg of MIA-V and MIA-MSLN proteins was used to immunoprecipitate CDK2 by using immobilized protein G-conjugated-anti-CDK2 monoclonal antibody, and the immune-complex precipitate was washed and subjected to SDS-PAGE under denaturing conditions, gel-transferred to nitrocellulose membrane, and probed for cyclin E and CDK2.
In normal cells, there is a cyclic pattern of expression of the cyclins in progression through the cycle, and this cyclic pattern is often lost in cancer cells. To determine whether MSLN overexpression leads to a loss of the cyclic pattern, we starved MIA-MSLN and control MIA-V cells for 24 h in serum-free medium, released them to 2% serum-containing medium, and determined cyclin E and CDK2 expression at different times after release. As shown in Fig. 2B, even at the G0 synchronized state there was an appreciable expression of cyclin E in MIA-MSLN cells, and it remained high at each of the times tested, although there was an increased induction at later time points. In the control MIA-V cells, there was a clear cyclic pattern of cyclin E expression after release from G0/G1 arrest. Most importantly, the expression of cyclin E at time zero was negligible, and it was induced only upon stimulation by 2% serum-containing medium. CDK2 induction in the MIA-MSLN cells was also more rapid and persistent (Fig. 2B). Thus, the aberrant cyclin E and CDK2 expression pattern may explain the faster S-phase entry and progression in the MSLN-overexpressed cells.
To find out whether cyclin E overexpressed in the MIA-MSLN cells was functionally active, we determined cyclin E and CDK2 complex formation by using a co-immunoprecipitation assay. When whole proteins from the cells were immunoprecipitated with CDK2 antibody and blotted with cyclin E antibody, we found increased cyclin E in the MIA-MSLN cells, suggesting CDK2 interacts with cyclin E in these cells (Fig. 2C). MIA-MSLN cells thus have an increased level of cyclin E/CDK2 complexes, which might be responsible for the increased G1/S transition in these cells.
Constitutively active Stat3 in MIA-MSLN cells is responsible for enhanced cell proliferation
Constitutive activation of the transcription factor Stat3 has been implicated in the pathogenesis of many cancers, including pancreatic cancer (24–29). However, the mechanism of Stat3 activation and precisely what leads to it are largely unknown. We found that MSLN overexpression leads to aberrant activation of Stat3 (Fig. 3A) in MIA-MSLN cells, which had significantly higher levels of activated pStat3 (Tyr705) than MIA-V and MIA-GFP cells. We further assessed the nuclear translocation of Stat3 in the different cells and found that MIA-MSLN cells had a substantial amount of Stat3 transcription factor in the nucleus, whereas the control cells had negligible amounts in their nuclei (Fig. 3B). These data indicate that MSLN overexpression may be responsible for constitutive Stat3 activation in MIA-MSLN cells.
Figure 3.
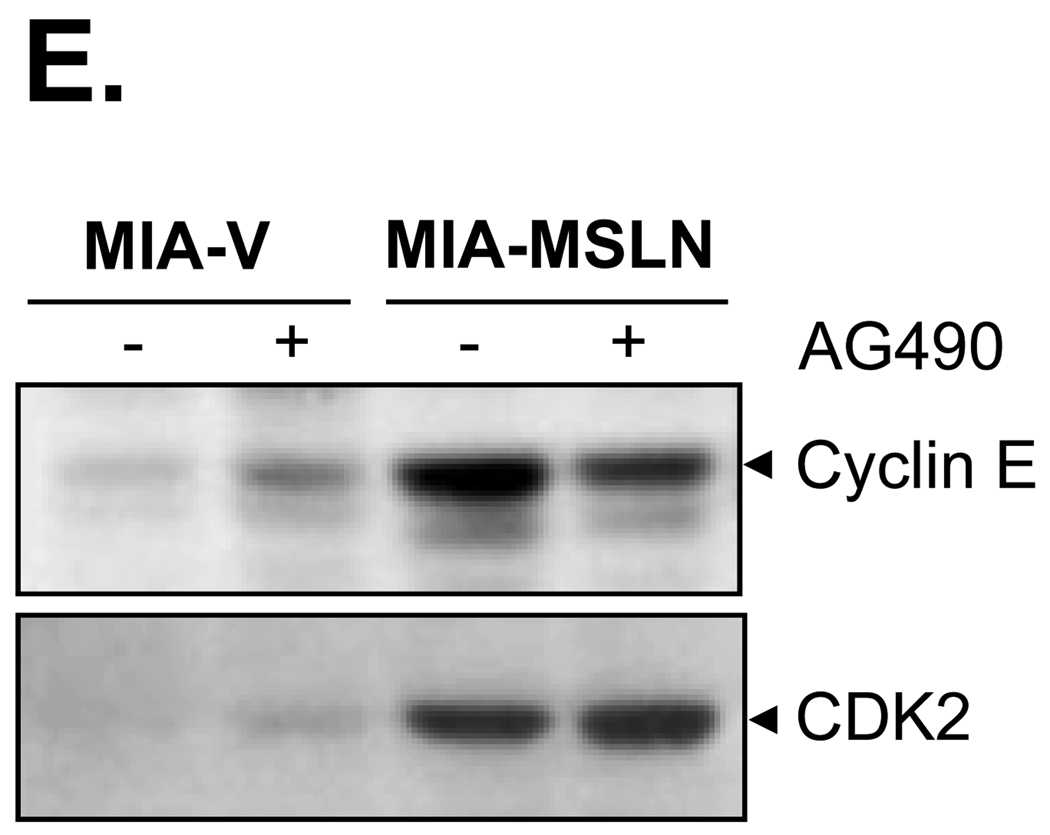
Blocking of Stat3 activation in MSLN-overexpressed MIA PaCa-2 cells led to decrease in proliferation and cell cycle progression. A. Activation of Stat3 in MIA-MSLN cells. 60 µg of total proteins from control and MIA-MSLN cells was subjected to immunoblot analysis with antibodies against phosphorylated form of Stat3 (pStat3Tyr705) and total Stat3. B. Nuclear translocation of Stat3 in MIA-MSLN cells. Nuclear protein was isolated from MIA-MSLN and control cells and subjected to SDS-PAGE and Western blot and probed for total Stat3 protein and nuclear envelope marker Lamin A as loading control. C. Blocking of Stat3 physophorylation with JAK-selective inhibitor Tyrphostin AG490. Total lysates from cells treated with Tyrphostin AG490 for 0, 4, 8, or 12 h were used to immunoblot for relative amount of pStat3Tyr705 and Stat3. D. Cell proliferation according to MTT assay. For cell-proliferation assay, control and MIA-MSLN cells were serum-starved for 24 h, treated with DMSO/Tyrphostin AG490 (50 µM) for 24 h in 2% serum medium, and washed. Proliferation was continued for 6 d, and cell viability was assayed with MTT. Cell proliferation of MIA-MSLN cells was significantly reduced by pretreatment with Tyrphostin AG490 (*p<0.05, ***p < 0.001). E. Effect of AG490 treatment on expression of cyclin E and CDK2. Whole proteins from MIA-V and MIA-MSLN cells, untreated or treated with AG490 for 48 h, were used to detect cyclin E and CDK2 with Western blot.
To determine whether the activated Stat3 is responsible for MSLN-induced cell proliferation, we blocked Stat3 activation with JAK-selective inhibitor AG490, a widely used inhibitor for Stat3 phosphorylation. As shown in Fig. 3C, the phosphorylation and activation of Stat3 in MIA-MSLN cells were substantially blocked by the inhibitor treatment within 12 h. AG490 treatment substantially abrogated the enhanced cell proliferation (p < 0.001 at day 4 and day 6; p < 0.05 at day 2; see Fig. 3D) in MIA-MSLN cells but had little or no effect on MIA-V and MIA-GFP cells. Furthermore, slightly reduced cyclin E expression was found in AG490-treated MIA-MSLN cells after 48 h, but no significant change in CDK2 levels was observed (Fig. 3E). Thus, the results indicate the Stat3 pathway may be involved in MSLN-induced pancreatic cancer cell proliferation through alterations in cyclin E expression.
Stat3 siRNA abrogates increased cell proliferation in MIA-MSLN cells
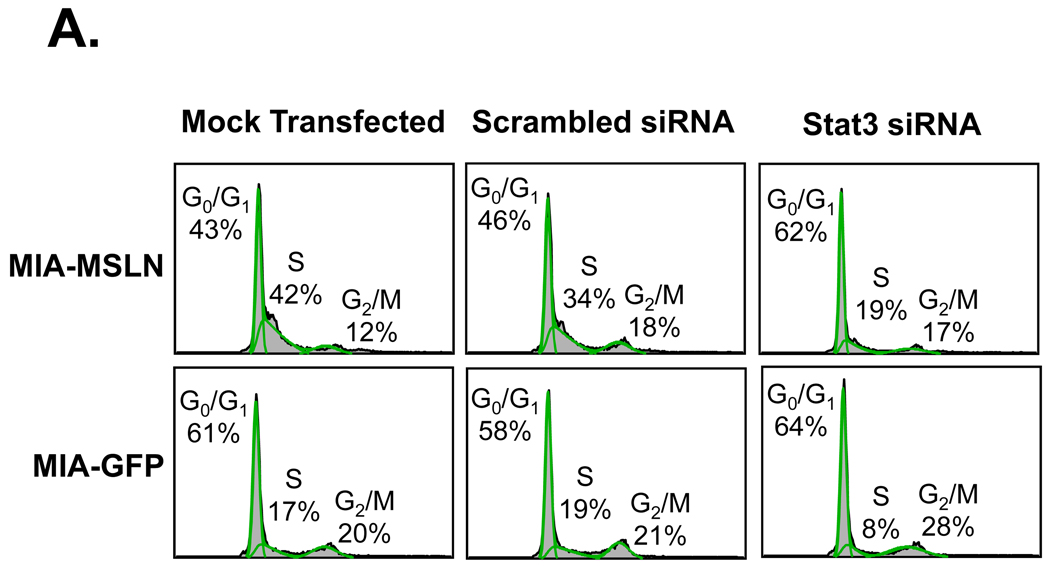
After finding that blocking Stat3 activation with pharmacologic inhibitor AG490 decreased the growth potential of MIA-MSLN cells, we wanted to confirm the involvement of Stat3 in MSLN-mediated cell proliferation. We used Stat3-specific siRNA to knock down the expression of Stat3 in the MIA-MSLN cells and the MIA-GFP control cells. We analyzed the cell cycle by using mock-transfected, nonspecific scrambled siRNA-transfected, and Stat3 siRNA-transfected MIA-MSLN cells and MIA-GFP cells. Approximately 42% and 34% of the mock-transfected and scrambled siRNA-transfected MIA-MSLN cells, respectively, entered the S phase after 8 h of release by growth medium following initial serum starvation (Fig. 4A). Those rates were significantly higher than the 17% and 19% of the similarly transfected GFP control cells entering S phase. The Stat3 siRNA-transfected MIA-MSLN cells had only 19% of cells in the S phase. These findings demonstrate that the increase in cell cycle progression of the MIA-MSLN cells may be caused by the increased Stat3 activation in these cells.
Figure 4.
Stat3 siRNA treatment decreases normally increased cell cycle progression of MIA-MSLN cells. A. MIA-MSLN or MIA-GFP cells were transfected with either nonspecific scrambled siRNA oligonucleotide or Stat3-specific RNA pool. Cells treated with only transfection reagent was mock transfection control. For cell cycle analysis, 24 h after transfection, cells were serum-starved for 24 h, released with 2% serum medium, collected after 8 h, and processed for cell cycle analysis. B. Stat3 silencing decreased cyclin E expression in MIA-MSLN cells. Whole proteins from cells collected 48 h after transfection with Stat3-specific siRNA pool or scrambled siRNA control or transfection reagent control were used for Western blot with Stat3, cyclin E, and β actin antibodies.
We had postulated that higher expression of cyclin E in the MIA-MSLN cells was responsible for the increase in cell cycle progression. We examined the fate of cyclin E as a result of Stat3 silencing in MIA-MSLN and MIA-GFP control cells. After silencing of Stat3, there was a decrease in cyclin E expression in MIA-MSLN cells but not in MIA-GFP cells (Fig. 4B). Taken together, these results further show that Stat3 activation could be responsible for the up-regulation of cyclin E expression in MIA-MSLN cells.
Silencing MSLN expression decreases cell proliferation in pancreatic cancer cells
To further elucidate the role of MSLN in pancreatic cancer cell proliferation, we compared the cell-proliferation properties of three cell lines: BxPC-3, a pancreatic cancer cell line expressing high MSLN; a stable MSLN-silenced cell line in BxPC-3 cells (BxPC-siMSLN); and a control cell line containing empty vector (BxPC-siV). MTT assay showed that the cell proliferation capacity of BxPC-siMSLN cells decreased by 60% compared with that of BxPC-siV cells (p < 0.01; Fig. 5A), further indicating the possible involvement of MSLN in enhancement of cell proliferation.
Figure 5.
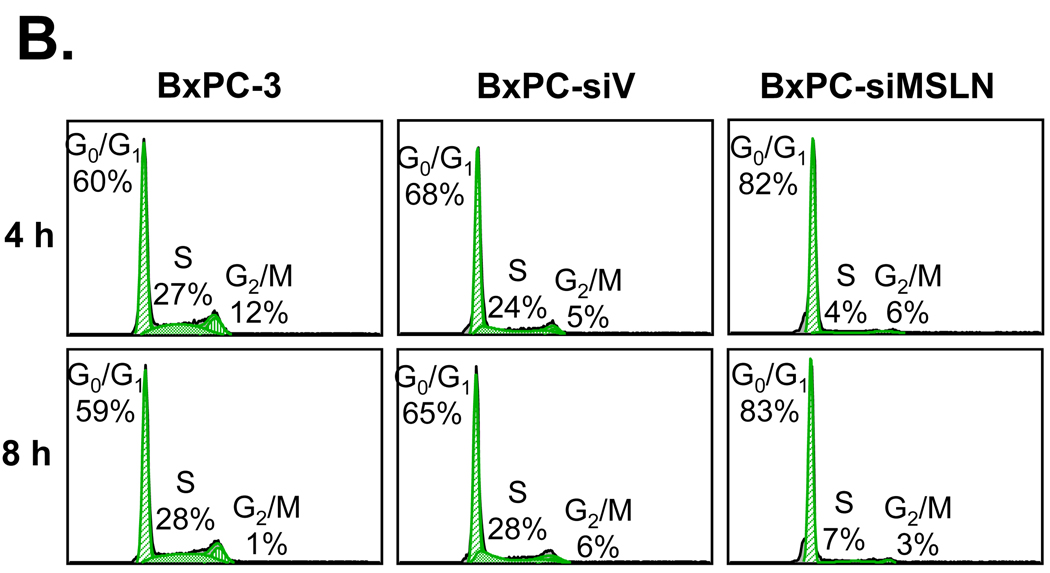
Silencing MSLN expression decreases pancreatic cancer cell proliferation and cell cycle progression. A. Cell proliferation according to MTT assay. MSLN siRNA-silenced BxPC-3 stable cell line (BxPC-siMSLN) and control cells BxPC-3 parental cells (BxPC-3) and empty siRNA-vector-integrated stable cell line (BxPC-siV) were seeded in 96-well plates (2×103 cells/well) and serum-starved (0% FBS) for 24 h before being changed to growth medium with 2% FBS and cultured for 6 d. Cell growth was assessed at 2, 4, and 6 d after growth-medium addition with MTT assay. Cell proliferation of BxPC-siMSLN cells was significantly reduced compared with parental BxPC-3 and BxPC-siV cells (***p < 0.001). B. After initial serum starvation for 24 h, BxPC-siMSLN cells and controls were treated with 2% serum medium; cells were collected after 4 and 8 h, fixed, PI-stained, and analyzed for cell cycle phase distribution (percentage of cells) with FACS. C. Cyclin A and CDK2 expression was decreased in BxPC-siMSLN cells. Whole proteins from sub-confluent BxPC-siMSLN and control cells were subjected to SDS-PAGE and immunoblot for cell-cycle-related proteins.
To study the effect of MSLN silencing on BxPC-3 cell proliferation, we examined cell cycle progression in BxPC-3 cells, BxPC-siMSLN cells, and BxPC-siV cells with FACS analysis. As shown in Fig. 5B, 4% and 7% of BxPC-siMSLN cells entered the S phase at 4 h and 8 h, respectively, after being released from serum starvation. This marked a significant decrease compared with the S phase populations of BxPC-siV cells in the same periods (24% at 4h and 28% at 8h). Thus, silencing of MSLN in BxPC-3 cells is associated with decreased cell proliferation and slower progression into the S phase, suggesting the possible involvement of MSLN in pancreatic cancer cell proliferation.
Complementing the finding that MIA-MSLN cells had higher expression of CDK2, the silencing of MSLN in the BxPC-siMSLN cells reduced the expression of CDK2 and cyclin A compared with control cells (Fig. 5C). MSLN overexpression in pancreatic cancer may induce proliferation in these cells through the up-regulation of the S-phase-promoting cyclins E and A, and their binding partner CDK2.
Discussion
The important role of MSLN in several cancers has gained more and more attention in recent years, but its exact function in cancer pathogenesis has not been explored in depth. In the current study, we found that MSLN overexpression in pancreatic cancer cell MIA PaCa-2 increases cell proliferation through faster cell cycle progression. Overexpression of MSLN up-regulates S-phase-promoting cyclin E and its partner kinase CDK2. The increase in cyclin E expression is mediated by the increased activation of the transcription factor Stat3, as using specific siRNA against Stat3 reduced cyclin E expression in those cells. Blocking MSLN expression in the MSLN-high cell line BxPC-3 inhibited the proliferation and cell cycle progression of these cells with concomitant decreases in cyclin A and CDK2.
Cancer is primarily a disease of uncontrolled proliferation. MSLN is selected to be up-regulated in pancreatic cancer (3) and plays a role advantageous to the tumor cells. The question is whether it leads to increased proliferative capacity of the pancreatic cancer cells. We have shown previously, in both subcutaneous and orthotopic models, injection of MSLN-overexpressing tumor cells led to the formation of bigger tumors than that of control cell injection (7) and suggested a role for MSLN in proliferation. However, little additional information on the pro-proliferative properties of MSLN is available except for some indirect evidence. In a carcinogen-induced rat mammary-carcinoma model, up-regulated MSLN in the mammary glands was observed concomitantly with increased cell proliferation (8); moreover, reported high expression of MSLN in the stem-cell-rich corneal epithelial cells suggested roles of MSLN in cell proliferation, migration, and wound healing (9). In this study, we have found a difference in the proliferation rate between MIA-V and MIA-MSLN cells at lower serum concentrations of 0.2 and 2%, which is indicative of a probable role of MSLN in growth factor-independent survival. Furthermore, we found that the MIA-MSLN cells had the ability to resist anoikis (data not shown). The exceptionally low number of cells in plating efficiency experiments provides the cells with a condition devoid of adherence to neighboring cells, and tests the survival and the proliferative capacity of individual clones. Several previous reports have suggested the clonogenic assay should be commonly used in oncological research to test the proliferative capacity of cancer cells after radiation and/or treatment with anticancer agents (32, 33). The average colony-forming ability of the MIA-MSLN cells with an initial ultra-low seeding of cells was greater than the control cells, may indicate that MSLN affects both survival and proliferative capacity of pancreatic cancer cells under stringent conditions. Our findings are consistent with previous reports (34), and indicate that better plating efficiency of the cells depends on both survival and ability to proliferate for eventual colony formation.
Cyclin E is increasingly evident in pancreatic cancer pathogenesis, particularly in the later stages, as is the association of high cyclin E expression with a poor prognosis (35). Our data clearly demonstrate that cyclin E expression was increased in MSLN overexpression cell lines. Maitra et al. showed that MSLN and cyclin E were both relatively late up-regulated genes in the multi-step progression model of pancreatic cancer pathogenesis (36), suggesting a pro-proliferative role of MSLN in later stages of pancreatic cancer pathogenesis. In addition, CDK2, the binding partner of cyclin E involved in G1/S transition, was found to be up-regulated in the MSLN-overexpressing cells. It was reported that CDK2 inhibitors efficiently blocked the proliferation of human pancreatic cancer cells regardless of their mutations in K-ras, p53 or p16 genes (37), cementing the importance of these kinases in pancreatic cancer cell proliferation. That MSLN overexpression could up-regulate CDK2 expression points toward another crucial role in pancreatic cancer pathogenesis. It remains an intriguing question why CDK2 is up-regulated in the MSLN overexpressing cells. The answer may involve gene amplification, as happens in a subset of human colorectal-cancer tissues (38), or may be under the control of other transcription factors simultaneously activated by MSLN overexpression. The association of cyclin E and CDK2 complex may indicate the critical function in cell cycle progression. We showed here that increased cyclin E/CDK2 complex correlated with the MSLN-overexpressed cell line.
In pancreatic cancer, Stat3 is stated to have a pivotal role in oncogenic transformation (26, 27), cell survival and proliferation (26, 28), and resistance to apoptosis (25), and has been found to be aberrantly activated in a subset of pancreatic tumor tissues and cell lines (28). Blockade of activated Stat3 by ectopic expression of a dominant-negative Stat3 or by JAK-selective inhibitor AG490 significantly inhibited the growth of pancreatic cancer cell lines (28). We have shown in this study that not only activated Stat3, but also total Stat3 are elevated in MIA-MSLN cells compared with the control cells. Many reports demonstrated increased total Stat3 (with or without phosphorylation) expression in various cancers (24), particularly pancreatic cancer, in the nucleus (39). In fact, Yang et al (40) showed the over-expression of unphosphorylated forms of Stat3 can induce many well-know oncoproteins such as MRAS and MET by a novel mechanism. Thus, MSLN may likely exert its effects through an increase in total Stat3. In addition, the Stat3 promoter has a binding site for Stat3 dimers; the total amount of Stat3 protein may increase when Stat3 is activated (41). Thus, it is not entirely unexpected to observe an increased Stat3 expression in Stat3-active MIA-MSLN cells.
There is no precise information on what leads to Stat3 activation, although reports have linked ErbB2 tyrosine kinase activity to Stat3 activity and shown that functional inhibition of Stat3 signaling by expression of a dominant-negative Stat3 mutant reduced the growth of human pancreatic cancer cells (27). Our results indicate that overexpression of MSLN could be one of the important factors leading to Stat3 activation. How a GPI-anchored glycoprotein mesothelin leads to Stat3 activation remains to be explored. Based on our preliminary data about the relationship between MSLN expression and Stat3 activation, we hypothesize that high expression of MSLN may directly interact with some unknown adaptor molecules on the cell membrane and induc unique signal transduction pathways which activate Stat3. Therefore, MSLN-activated Stat3 may be a critical mechanism of pancreatic cancer pathogenesis. Various mechanisms have been proposed for constitutive Stat3 activation in tumors (24), including the autocrine activation of IL-6/gp130/JAK2/stat3 pathway (42, 43), autocrine ErbB2/stat3 pathway (27), TGF-α/EGFR/stat3 pathway (20), and mutant-EGFR/stat3 pathway (44). To test our hypothesis, we are applying various strategies including the use of specific pathway inhibitors, the study of MSLN-interacting proteins, and activation of various growth factor receptors in the MIA-MSLN cells.
Major cell-cycle-related genes under transcriptional control by Stat3 are cyclin D1, Bcl-xL, and Mcl-1, and down-regulation of cyclin D3 and cyclin E in pancreatic cancer cells by AG1478 and AG879 through the blocking of Stat3 activation has been reported (27). Sinibaldi et al. suggested that v-src-mediated transformation of mouse fibroblasts involved Stat3 activation that led to cyclin D1 and p21 up-regulation with eventual cyclin E up-regulation (45). Our study shows direct evidence that Stat3 is essential for cyclin E up-regulation. Although blocking Stat3 expression with Stat3 siRNA reduced the expression of cyclin E in the MIA-MSLN cells, CDK2 was unaffected by Stat3-siRNA or AG490. These observations are similar to those in previous studies that showed AG490 was able to reduce cyclin E expression in hepatocellular carcinoma cells (46) by down-regulating activated Stat3.
While we demonstrated that Stat3 siRNA decreased the proportion of MIA-MSLN cells in the S-phase, we also found that Stat3 siRNA can slightly decrease the number of S phase cells in the MIA-GFP control cells. Stat3 is a very important general transcription factor controlling a number of genes regulating various aspects of cell growth, differentiation and apoptosis. These include Mcl-1, Bcl-xL and survivin, all of which suppress apoptosis; c-myc98 and cyclin D1, which mediate cell proliferation; matrix metalloproteinase-9, which mediates cellular invasion; and vascular endothelial growth factor (VEGF), which mediates angiogenesis (24). In pancreatic cancer cells, Stat3 has been reported to support proliferation and viability (28), and growth factor-independent survival through autocrine ERBb2 signaling (27). Therefore, knocking down the expression of such a ubiquitous factor using siRNA is bound to negatively affect cell growth. In addition, taking into account the results of Yang et al (40), if non-phosphorylated stat3 is also playing a major role in pancreatic cancer cell survival/proliferation, abrogating stat3 must be deleterious for the cell. On the other hand, addition of AG490 had no effect on cell proliferation in MIA-GFP cells. Since AG490 is a JAK-selective inhibitor which blocks stat3 activation (phosphorylation), it should theoretically only have an effect on Stat3-activated cells such as MIA-MSLN cells, but not on the control cells such as MIA-GFP cells. In addition, from the actual experimental method point of view, the data for the AG490 treatment was derived after treating the cells with AG490 for 24 hrs, removing it and washing the cells, and then continuing for 2, 4, and 6 days to observe the viability.The siRNA blocking assay, on the other hand, was performed when all the cells were treated with the continued presence of the inhibitor in the medium, which may have a relatively long lasting effect on all the cells.
We noticed that both Stat3 siRNA-treated MIA-MSLN and MIA-GFP cells had substantially low levels of Stat3 proteins, showing a potent silencing effect. However, Stat3 siRNA-treated MIA-MSLN cells had a relatively low level of cyclin E compared with Stat3 siRNA-treated MIA-GFP cells. The exact reasons for different cyclin E levels are not clear, but may suggest that MIA-MSLN cells could be more sensitive to Stat3 siRNA treatment because Stat3 is activated in these cells.
The pro-proliferative effect of MSLN observed in MIA-MSLN cells was further obtained in the BxPC-siMSLN cells by blocking MSLN. We showed that blocking of MSLN dramatically reduces cyclin A and CDK2 expression when compared with both sets of control cells (BxPC-3 and BxPC-V cells), and this correlates well with decreased cell proliferation and cell cycle progression. We observed that BxPC-V cells had relatively high levels of cyclin A and CDK2 compared BxPC parental cells. This observation may reflect the potential effects of the vector viral infection and persistent puromycin selective pressure on expression levels of these genes. This also provides the general rationale for the use of vector-infected cells as controls for any gene delivery experiments. Indeed, MSLN siRNA-delivered stable cells (BxPC-siRNA) showed much lower levels of cyclin A and CDK2 proteins, which could be an implication for MSLN-specific functions.
In summary, we have shown that MSLN up-regulation induces the activation of Stat3, which leads to increased expression of cyclin E and makes pancreatic cancer cells proliferate faster. Knowing the pathways and mechanism of MSLN-induced tumor-cell survival and proliferation will help in formulating new combinatorial multidrug regimens in which specific inhibitors could be used with anti-MSLN immunotherapy.
Materials and Methods
Cell culture, chemicals and antibodies
Human pancreatic cancer cell lines MIA PaCa-2, BxPC-3 and Panc-1 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Puromycin, Tyrphostin AG490, and anti-β-actin antibody were purchased from Sigma (St. Louis, MO). Rabbit anti-MSLN polyclonal antibody was custom-generated by Genemed (San Jose, CA). Antibodies against the phosphorylated (Tyr 705) and total form of Stat3 and antibodies against cyclin D1, CDK4, p21 and p15, goat anti-rabbit IgG (H&L) antibody conjugated to horseradish peroxidase (HRP) and goat anti-mouse IgG (H&L) antibody-HRP were obtained from Cell Signaling Technology Laboratories Inc (Beverly, MA). Antibodies to cyclin A, cyclin E, CDK1 and CDK2 were procured from Santa Cruz Biotechnology (Santa Cruz, CA).
Stable cell line selection
MSLN overexpressing and siRNA silencing stable cell lines were selected in MIA PaCa-2, BxPC-3 and Panc-1 cells using retroviral vectors pBabe and pSuper (Clontech, CA), respectively, following manufacturer’s instructions. Full length human MSLN cDNA (kindly provided by Dr. Ira Pastan, NCI, Bethesda, Maryland) was cloned into pBabe vector for overexpression. The MSLN-gene-specific siRNA sequence (5’GAAGAATGTCAAGCTCTCA3’) separated by a 9-nucleotide non-complementary spacer (TCTCTTGAA) from the reverse complement of the same 19-nucleotide sequence was cloned in the pSuper vector. The recombinant plasmids were co-transfected into 293T cells with plasmid PegPam3 (containing the gag-pol) and plasmid RDF (containing the RD114 envelope). Viral supernatants were collected after 48 h and used to transduce the target cells, MIA PaCa-2, BxPC-3 and Panc-1 cells. Stable cell lines were selected by adding puromycin (0.5–1 µg/ml) into the medium, and cultured for at least 7 days before confirming the expression level of MSLN by real time PCR and western blot.
Cell proliferation measurement by cell viability assay (MTT)
The effect of MSLN on cell proliferation was determined by measuring cell viability using the MTT assay. Briefly, 2000 cells were plated and serum-starved for 24 h. A day 0 reading (viability corresponding to basal number of cells plated) was then measured by MTT. Media with 0.2% or 2% serum was added to each well and incubated for 6 days. At each time point indicated, cell viability was measured using MTT. Briefly, 1 mg/ml of MTT in medium with 2% serum was added to each well and incubated for 2 h at 37°C. An extraction buffer (20% SDS, 50% dimethylformamide) was added, and the cells were incubated overnight at 37°C. The optical density was measured at 590 nm using a 96-well multi-scanner (EL-800 universal microplate reader, BioTek Inc, VT). The proliferating capacity of the cell was measured by dividing the viability at a certain time point by the viability at day 0.
Cell cycle analysis
Cells were starved for 24 h in serum-free medium, released with media containing 2% serum, and collected at various time points after release. Cells were harvested and processed using the CycleTEST™ PLUS DNA Reagent Kit from Beckton Dickinson according to the manufacturer’s instructions. Briefly, cells were first washed thrice in a buffer containing sodium citrate, sucrose, and dimethyl sulfoxide (DMSO) provided for the collection of cell suspensions. Before analysis, cells were incubated sequentially for ten minutes each in solution A (for the enzymatic digestion of cell membranes and cytoskeletons), solution B (to inhibit the trypsin activity and to digest the RNA) and solution C (for the stoichiometric binding of PI to the DNA at a final concentration of 125 µg/ml). Flow cytometry (FACS) analysis was carried out to examine the cell cycle distribution by a FACSCalibur (Becton Dickinson, San Jose, CA, USA). Data was further analyzed using the software FLOWJOW Ver. 6.1.1 (Tree Star, San Carlos, CA).
Plating efficiency
Plating efficiency was measured by a slight modification of a previously published procedure (47). Briefly, 600 cells each of MIA-V and MIA-MSLN were plated in duplicate in 150 mm dishes, so that the single cells were separated from one another and allowed to adhere for 48 h. Cells were then starved for 24 h, and subsequently grown in complete medium for 15 days. The colonies growing from single cell were stained with MTT and photographed. The colonies were counted manually from the photographs and the mean number of colonies/well was plotted. The percent survival/plating efficiency was determined using the following equation: Plating efficiency/Clonogenic survival = (colonies formed/cells seeded) × 100. The Student’s t-test was applied to compare the two groups.
Immunoblot analysis
Cells were lysed with 100 µl of lysis buffer (Cell Signaling Technology, Beverly, MA) with phosphotase inhibitor cocktails I and II (Sigma) and protease inhibitors Aprotinin and Leupeptin (Sigma). The lysates (60 µg total protein) were then resolved by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The levels of different proteins were detected using specific primary antibodies and were visualized using appropriate secondary antibodies conjugated to HRP followed by ECL detection system (Amersham Biosciences, UK). The nuclear and cytoplasmic extracts were prepared using the N-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL) and used for studying nuclear translocation of Stat3.
Immunoprecipitation using CDK2 antibodies and subsequent immunoblot
MIA-V and MIA-MSLN cells were lysed with IP lysis buffer (50 mM Tris [pH 8.0], 10 mM EDTA, 150 mM NaCl, 0.5% NP-40) with phosphotase inhibitor and protease inhibitors on ice for 60 min. CDK2 monoclonal antibody (Santa Cruz) was conjugated to immobilized protein G using the SeizeX Mammalian Immunoprecipitation kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions. An equal amount of total protein (400 µg) from each sample was incubated with immobilized protein G-conjugated anti-CDK2 antibody overnight at 4°C. Immunocomplexes were washed with IP wash buffer (50 mM Tris [pH 8.0], 10 mM EDTA, 100 mM NaCl, 0.5% NP-40) plus protease inhibitors, boiled with reducing sample loading buffer, and subjected to SDS-PAGE, western blotting and probing for CDK2 and cyclin E using specific antibodies as mentioned above.
Treatment with AG490
MIA-MSLN and MIA-V control cells were seeded in 6-well plates. After they reached 50% confluence, the medium was replaced with the same growth medium containing 50 µM of Tyrphostin AG490 (Calbiochem La Jolla, CA) and the cells were collected after 4, 8 and 12 h. Whole cell proteins were prepared according to procedures already described and used in a western blot to detect the phosphorylated (Tyr705) and total Stat3 levels. For the cell proliferation assay, the cells, previously starved for 24 hours, were treated with either DMSO or Tyrphostin AG490 (50 µM) for 24 h in the medium with 2% serum for 2, 4 and 6 days. An MTT test was performed as described above.
Stat3 siRNA transfection
The MIA-MSLN or MIA-GFP cells were transfected with either a non-specific scrambled siRNA oligonucleotide or a Stat3-specific RNA pool (SMARTpool™ Stat3, Upstate cell signaling solutions, New York, NY) at a final concentration of 100 nM, using Lipofectamine 2000 (Invitrogen, CA, USA). Mock transfection controls received only the transfection reagent. Cells were collected 48 h after transfection for whole cell protein extraction for western blot. For cell cycle analysis, 24 h after transfection cells were serum-starved for 24 h and then released using 2% serum-containing medium. The cells were then collected after 8 h and processed for cell cycle analysis according to procedures described above.
Acknowledgments
We thank Christian Marin-Muller for editing this manuscript.
This work was supported in part by National Institutes of Health (NIH) Research Grants DE15543, AT003094, and Dan L Duncan Cancer Center pilot grant (Q. Yao).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors do not have potential conflicts of interest.
References
- 1.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 2.Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52:181–186. [PubMed] [Google Scholar]
- 3.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 4.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 5.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Bharadwaj U, Zhang R, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi W, Ulanovsky H, Li Y, et al. Serial analysis of gene expression (SAGE) in the rat limbal and central corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3801–3810. doi: 10.1167/iovs.06-0216. [DOI] [PubMed] [Google Scholar]
- 9.Uehara N, Unami A, Kiyozuka Y, et al. Parous mammary glands exhibit distinct alterations in gene expression and proliferation responsiveness to carcinogenic stimuli in Lewis rats. Oncol Rep. 2006;15:903–911. [PubMed] [Google Scholar]
- 10.Cheng WF, Hung CF, Chai CY, et al. Generation and characterization of an ascitogenic mesothelin-expressing tumor model. Cancer. 2007;110:420–431. doi: 10.1002/cncr.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doree M, Galas S. The cyclin-dependent protein kinases and the control of cell division. Faseb J. 1994;8:1114–1121. doi: 10.1096/fasebj.8.14.7958616. [DOI] [PubMed] [Google Scholar]
- 12.Pines J. Cyclins, CDKs and cancer. Semin Cancer Biol. 1995;6:63–72. doi: 10.1006/scbi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 13.Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 14.Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall M, Bates S, Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995;11:1581–1588. [PubMed] [Google Scholar]
- 16.Roberts JM, Koff A, Polyak K, et al. Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harb Symp Quant Biol. 1994;59:31–38. doi: 10.1101/sqb.1994.059.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez I, Dynlacht BD. Transcriptional control of the cell cycle. Curr Opin Cell Biol. 1996;8:318–324. doi: 10.1016/s0955-0674(96)80004-4. [DOI] [PubMed] [Google Scholar]
- 18.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 20.Grandis JR, Drenning SD, Chakraborty A, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Z, Schaefer TS. ErbB-2 activates Stat3 alpha in a Src- and JAK2-dependent manner. J Biol Chem. 2002;277:38486–38493. doi: 10.1074/jbc.M112438200. [DOI] [PubMed] [Google Scholar]
- 22.Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680–45687. doi: 10.1074/jbc.M204255200. [DOI] [PubMed] [Google Scholar]
- 23.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Sethi G, Ahn KS, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 25.Greten FR, Weber CK, Greten TF, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–2063. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 26.Scholz A, Heinze S, Detjen KM, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 27.DeArmond D, Brattain MG, Jessup JM, et al. Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene. 2003;22:7781–7795. doi: 10.1038/sj.onc.1206966. [DOI] [PubMed] [Google Scholar]
- 28.Toyonaga T, Nakano K, Nagano M, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107–116. doi: 10.1016/s0304-3835(03)00482-8. [DOI] [PubMed] [Google Scholar]
- 29.Wei D, Le X, Zheng L. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 30.Atherton-Fessler S, Liu F, Gabrielli B, et al. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norbury C, Nurse P. Cyclins and cell cycle control. Curr Biol. 1991;1:23–24. doi: 10.1016/0960-9822(91)90116-e. [DOI] [PubMed] [Google Scholar]
- 32.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 33.Scholz CC, Berger DP, Winterhalter BR, Henss H, Fiebig HH. Correlation of drug response in patients and in the clonogenic assay with solid human tumour xenografts. Eur J Cancer. 1990;26:901–905. doi: 10.1016/0277-5379(90)90196-z. [DOI] [PubMed] [Google Scholar]
- 34.Vogler M, Giagkousiklidis S, Genze F. Inhibition of clonogenic tumor growth: a novel function of Smac contributing to its antitumor activity. Oncogene. 2005;24:7190–7202. doi: 10.1038/sj.onc.1208876. [DOI] [PubMed] [Google Scholar]
- 35.Skalicky DA, Kench JG, Segara D, et al. Cyclin E expression and outcome in pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1941–1947. doi: 10.1158/1055-9965.EPI-06-0319. [DOI] [PubMed] [Google Scholar]
- 36.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 37.Iseki H, Ko TC, Xue XY, Seapan A, Townsend CM., Jr A novel strategy for inhibiting growth of human pancreatic cancer cells by blocking cyclin-dependent kinase activity. J Gastrointest Surg. 1998;2:36–43. doi: 10.1016/s1091-255x(98)80101-7. [DOI] [PubMed] [Google Scholar]
- 38.Kitahara K, Yasui W, Kuniyasu H, et al. Concurrent amplification of cyclin E and CDK2 genes in colorectal carcinomas. Int J Cancer. 1995;62:25–28. doi: 10.1002/ijc.2910620107. [DOI] [PubMed] [Google Scholar]
- 39.Doucas H, Mann CD, Sutton CD, et al. Expression of nuclear Notch3 in pancreatic adenocarcinomas is associated with adverse clinical features, and correlates with the expression of STAT3 and phosphorylated Akt. J Surg Oncol. 2008;97:63–68. doi: 10.1002/jso.20894. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Chatterjee-Kishore M, Staugaitis SM, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 41.Narimatsu M, Maeda H, Itoh S. Tissue-specific autoregulation of the stat3 gene and its role in interleukin-6-induced survival signals in T cells. Mol Cell Biol. 2001;21:6615–6625. doi: 10.1128/MCB.21.19.6615-6625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh HH, Lai WW, Chen HH, Liu HSSu WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–4309. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 43.Lang SA, Moser C, Gaumann A, et al. Targeting heat shock protein 90 in pancreatic cancer impairs insulin-like growth factor-I receptor signaling, disrupts an interleukin-6/signal-transducer and activator of transcription 3/hypoxia-inducible factor-1alpha autocrine loop, and reduces orthotopic tumor growth. Clin Cancer Res. 2007;13:6459–6468. doi: 10.1158/1078-0432.CCR-07-1104. [DOI] [PubMed] [Google Scholar]
- 44.Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinibaldi D, Wharton W, Turkson J, et al. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 46.Fuke H, Shiraki K, Sugimoto K, et al. Jak inhibitor induces S phase cell-cycle arrest and augments TRAIL-induced apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2007;363:738–744. doi: 10.1016/j.bbrc.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 47.Lewis A, Du J, Liu J, et al. Metastatic progression of pancreatic cancer: changes in antioxidant enzymes and cell growth. Clin Exp Metastasis. 2005;22:523–532. doi: 10.1007/s10585-005-4919-7. [DOI] [PubMed] [Google Scholar]