Abstract
Given the high fatality rate of pancreatic cancer, an effective treatment for this devastating disease is urgently needed. We have shown that mesothelin (MSLN) expression was higher in human pancreatic cancer cells than human pancreatic ductal epithelium (HPDE) cells, and MSLN mRNA was substantially overexpressed in 18 of 21 (86%) clinical pancreatic adenocarcinoma specimens when compared with the surrounding normal tissues. However, the biological functions of MSLN in tumor progression are not clearly understood. Here we studied the effects of MSLN overexpression in pancreatic cancer cell proliferation and migration in vitro and pancreatic cancer progression in vivo. We found that forced expression of MSLN significantly increased tumor cell proliferation and migration by 90% and 300%, respectively, and increased tumor volume by 4-fold in the nude mice xenograft model when compared with the vector control cell line. Silencing of MSLN inhibited cell proliferation and migration in pancreatic cancer cells and ablated tumor progression in vivo. Vaccination with chimeric virus-like particles (VLPs) that contain human MSLN (VLP-hMSLN) substantially inhibited tumor progression in C57BL/6J mice. The increases in MSLN-specific antibodies and CTL activity, and the decrease in regulatory T cells correlated with reduced tumor progression and prolonged survival. This study revealed novel functions of MSLN and suggested a new therapeutic vaccine strategy whereby MSLN is targeted to control pancreatic cancer progression.
Keywords: Mesothelin, Pancreatic Cancer, Virus-like Particle, Therapeutic Vaccine
Introduction
Pancreatic cancer is the deadliest cancer and ranks as the fourth-leading cause of cancer death in North America. Survival statistics are poor because there are no reliable tests for early diagnosis and no effective therapies for the metastatic form (1, 2). Clearly, there is a need to understand more about the molecular mechanisms of pancreatic cancer tumorigenesis and to develop effective treatment strategies for pancreatic cancer. Global gene-expression profile studies have identified genes that are overexpressed in pancreatic cancer that might be used for the development of histologic diagnostic markers. Many genetic alterations in pancreatic cancer progression reside in oncogenes and tumor suppressor genes, such as K-ras, HER-2/neu, p16, p53, Smad4/DPC4, and BRCA2. Point mutations in the K-ras oncogene and overexpression of HER-2/neu are thought to be the earliest genetic alterations in pancreatic cancer (3-7). Many other molecules—such as mesothelin (MSLN), CEACAM-1, CEACAM-6, S100P, and Rap1GAP—are also listed as potential candidates for early detection markers in pancreatic cancer and are heavily involved in pancreatic cancer pathogenesis (8-11). However, biologic functions and molecular mechanisms that contribute to the tumor progression caused by the overexpressed genes remain largely unknown. Therefore, understanding of genes that are specifically expressed in pancreatic cancer would be critical in the development of new diagnostic and therapeutic strategies for pancreatic cancer.
The MSLN gene encodes a 69-kDa precursor protein that is proteolytically cleaved into an N-terminus secreted form and a C-terminus membrane-bound form, 40-kDa MSLN, which is a glycosylphosphatidylinositol-linked (GPI)-linked glycoprotein (12). MSLN knockout mice do not show any pathologic characteristics, so the biologic functions of MSLN are not clearly understood. Previous studies have suggested a role of MSLN in tumor adhesion and dissemination (13, 14). A recent study has shown that interaction of MSLN and mucin 16 (MUC 16) facilitates peritoneal metastasis of ovarian-cancer cells (15). Moreover, MSLN has been shown to be overexpressed in several cancer types, including mesothelioma, ovarian cancer, and pancreatic cancer (12, 16-20). Recently, high expressions of MSLN were detected in lung cancer (21), uterine serous carcinoma (22), and acute myeloid leukemia AML (23). Little or no expression of MSLN is seen in other cancer types and normal tissues (12, 17, 24). Argani et al. found that MSLN staining was positive in all 60 resected primary pancreatic adenocarcinomas, but negative or weak in adjacent normal pancreatic tissues (18). That finding has been confirmed by many other studies with microarray, serial analysis of gene expression (SAGE), U133 oligonucleotide array, and immunohistochemical staining (25-27). These latest studies indicate that MSLN may play some role in tumorigenesis.
Immunotherapy for pancreatic cancer is promising (8). Several studies have used whole-cell vaccine, peptide vaccine, and monoclonal antibody (mAb) targeting key signaling pathways, such as epidermal growth-factor receptor (EGFR), vascular endothelial growth factor (VEGF), and K-ras (28). An effective cancer vaccine must be able to elicit strong cytotoxic T-cell responses and antibody responses with little toxicity. Vaccination with virus-like particles (VLPs) has the unique property that it can induce protective antiviral immune responses without carrying the infectious and replicative capacities of the original virus. Therefore, VLPs constitute a major advantage in the design of efficient and safe vaccines. In addition, VLPs have been shown to induce maturation of dendritic cells directly to enhance innate immune responses (29). VLPs are also potent inducers of B-cell responses and are capable of breaking self-tolerance (30). In light of the overexpression of MSLN in pancreatic cancer, it is intriguing to incorporate MSLN onto VLPs to make a chimeric VLP-MSLN and to test the efficacy of VLP vaccination against pancreatic cancer. In this study, we first investigated the biologic functions of MSLN in human pancreatic cancer cells in vitro and in vivo. We then investigated the effect and mechanism of chimeric VLP-hMSLN as a potent candidate vaccine for controlling pancreatic cancer progression in an orthotopic pancreatic cancer mouse model.
Materials and Methods
Cells, chemicals, antibodies (Abs), and human tissue specimens
Human pancreatic cancer cell lines, Panc-1, MIA PaCa-2, BxPC-3, Capan-1, and mouse endothelial cell line SVEC4-10, were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr. Ming-Sound Tsao (Division of Applied Molecular Oncology, Ontario Cancer Institute, Toronto, Ontario, Canada; ref. 31). Panc02 was originally established by Corbett et al. (32), and NS47 was provided by A. Takashima (University of Texas, Dallas). All cells were cultured as previously described (33, 34). Rabbit anti-MSLN polyclonal Ab was generated from Genemed (San Jose, CA), and anti-MSLN monoclonal Ab was purchased from Abcam (Cambridge, MA). PE conjugated anti-mouse/rat Foxp3 Staining Ab was purchased from eBioscience (San Diego, CA). Other Abs and chemicals were from Sigma (St. Louis, MO). Human pancreatic adenocarcinoma specimens were collected from patients who underwent surgery according to an approved human protocol at Baylor College of Medicine (Houston, TX).
Real time RT-PCR, immunoblot and immunohistochemical analysis
The MSLN mRNA was analyzed by real time RT-PCR as described previously (33, 34). The primer sequences for human MSLN gene are: Sense, 5’CTCAACCCAGATGCGTTCTCG3’; anti-sense: 5’AGGTCCACATTGGCCTTCGT3'. Immunoblot and immunohistochemical staining were performed as previously (34) and the MSLN protein was detected with anti-MSLN Abs.
Stable cell line selection
MSLN overexpression and siRNA silencing cells were selected in MIA PaCa-2 and BxPC-3 cells, respectively, with retrovirus vectors pBabe and pSuper (Clontech, CA), following manufacturer's instructions. Briefly, full length human MSLN cDNA (NM_005823) and siRNA sequences for MSLN (5’ GAAGAATGTCAAGCTCTCA 3’) were cloned into pBabe or pSuper vectors, and the recombinant plasmid was cotransfected into 293T cells with plasmid PegPam3 and RDF. Viral supernatants were collected and transduced to the target cells. Stable cell lines were selected with adding 0.5-1 μg/mL of puromysin. At least three individual clones were selected for each stable cell line. The overexpression and silencing of MSLN in each stable cell lines were confirmed by both real-time RT-PCR and western blot.
Cell Proliferation Assay
Stable MIA PaCa-2 or BxPC-3 cells were seeded in 96-well plates (2×103 cells/well), and serum-starved (0% FBS) for 24 h. For MTS assay, cell growth was assessed on 6 days after release from starvation. Twenty μL of MTS reagent mixed with 100 μl growth medium was added to each well, and incubated in 37°C for 2 h. Absorbance was recorded at 490 nm with an EL-800 universal microplate reader (Bio-Tek Instruments, Winooski, VT).
In vitro migration assays
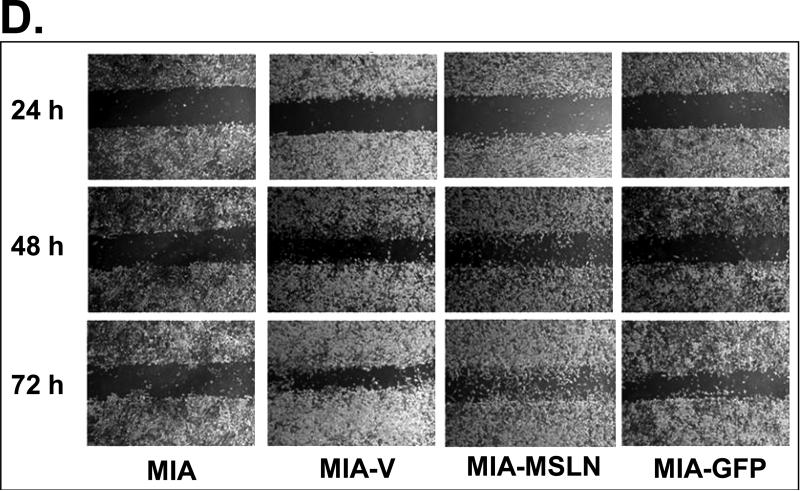
The cell migration was determined with a modified Boyden chamber assay. Briefly, cells (105cells/500μl) were added into the upper compartment of a migration chamber. After 24 h, the cells were incubated with calcein-AM (Molecular Probes, Eugene, OR) before fixation. Cells in the upper chamber were then removed, and cells that had migrated onto the lower surface of the membrane were quantified. The migration rate was presented as the ratio of the mean fluorescence reading after scraping of the cells divided by the reading before removing the top cells. A monolayer wound-healing assay was also performed. Wounds were created in confluent monolayer cells with a sterile pipette tip, and wound-healing was observed at 0, 24, 48, and 72 h within the scrape line, and representative fields for each cell line were photographed.
Subcutaneous (s.c.) and orthotopic pancreatic cancer mouse models
Subconfluent stable pancreatic cancer cells with MSLN overexpression or siRNA silencing were harvested by trypsinization, and resuspended in DMEM. 2×106 cells were inoculated either into the right flank (subcutaneous tumor model) or the pancreas (orthotopic tumor model) of 5- to 6-week-old male nude mice (NCI-Charles River) as described previously (35). For intrapancreatic injection, mice were anesthetized with 2.5% Avertin and a 0.5-1 cm incision was made in the left subcostal region. 2×106 tumor cells in a volume of 50 μL were injected into the body of the pancreas. For the subcutaneous tumor model, the tumor size was measured weekly using a digital caliper (VWR international, GA) and the tumor volume was determined with the formula: tumor volume [mm3] = (length [mm]) × (width [mm])2 × 0.52. For the orthotopic tumor model, after 4 weeks, all surviving mice were euthanized by overdose of CO2 exposure and evaluated macroscopically for the presence of orthotopic tumor and the metastases in the abdominal cavity. The orthotopic and metastatic tumor nodules were then explanted, counted and measured. For both subcutaneous and orthotopic experiments, the animals were euthanized when their tumor size reached 2 cm in diameter or the animals became moribund during the observation period, and the time of euthanization was recorded as the time of mortality.
VLP preparation and vaccination
The production and purification of VLP-hMSLN was done similarly as SHIV VLP production previously described (36). For a fixed time study, three groups of C57BL/6J mice (The Jackson Laboratory) (n=10) were implanted orthotopically with 5×105 Panc02 cells on day 0. On day 3, 50 μg of VLP-hMSLN or VLP-Gag or PBS were used to vaccinate mice with i.p. route followed by a day 7 and a day 14 boost with 100 μg of VLP-hMSLN or VLP-Gag or PBS. On days 0, 7, 14, 21, and 28, mice were bled to obtain serum. On day 28, mice were sacrificed and splenocytes were collected for cellular immune response assays. For a survival study, the three groups of mice received same treatment as above with VLPs. The survival of each mouse in each group was recorded until 9 weeks after tumor implantation.
ELISA and ELISPOT assay
All serum samples were individually collected, and titers of IgG against MSLN was determined by ELISA. Immunlon-4 HBX 96-well microtiter plates were coated with 100 μL of purified MSLN protein (Abnova Corporation, Taiwan) at 2 μg/mL. Plates were blocked with PBS containing 1% BSA, and 100-fold diluted serum was added to the wells and incubated at 4°C overnight. After four washes, the wells were treated with goat anti-mouse IgG peroxidase conjugates (Sigma-Aldrich) for 1 h at room temperature. After removal of the unbound conjugates, the substrate solution prepared in ABTS (Sigma), and hydrogen peroxide were added to the plates. ODs were read at 405 nm with an ELISA reader. IFN-γ producing MSLN-specific T cells were determined by an ELISPOT assay as previously described (37).
Cytotoxic T lymphocyte activity assay
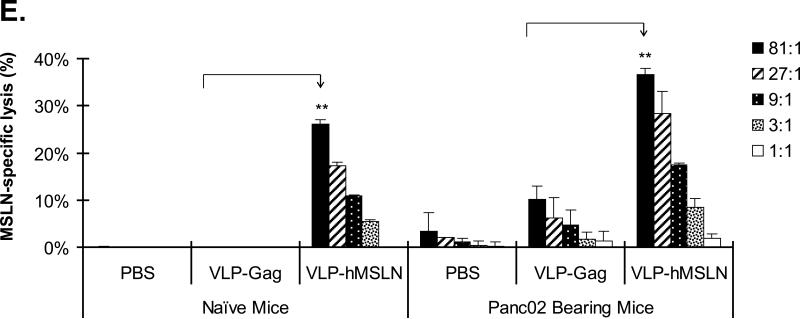
The CTL activity was measured by using the CytoTox 96® Non-Radioactive Cytotoxicity Assay according to the manufacturer's instructions (Promega, Madison, WI). Splenocytes were freshly isolated and stimulated with MSLN-specific peptide (GQKMNAQAI) at 2 μM for 5 days. These cells were used as effector cells in the CTL assay. EL-4 cells (H-2b) pulsed with MSLN-specific peptide (2 μM) for 1 h were used as target cells. EL-4 cells pulsed with no peptides were used as negative control target cells. Five different effector to target cell ratios (81:1, 27:1, 9:1, 3:1, and 1:1) were tested in the assay with 1 × 104 target cells for 4 h. The specific lysis of target cells was measured by LDH release and calculated using formula in the Cytotox 96 assay kit (Promega, Madison, WI).
Regulatory T cells assay
Freshly prepared splenocytes were collected on day 28 after tumor implantation in different vaccination groups, and were used for staining surface markers CD4, CD25, and intracellular Tregs specific marker Foxp3 followed with flow cytometry analysis. PE Anti-Mouse/Rat Foxp3 staining set were purchased from eBioscience. All experiments were repeated at least three times. For immunohistochemistry staining of Foxp3+ cells in the frozen tumor sections, PE conjugated anti-mouse/rat Foxp3 Staining Set (eBioscience, San Diego, CA) was also used.
Statistical analysis
Quantitative results are shown as means ± standard deviations. The statistical analysis was performed by Student's t test for paired data between control and treated groups or one-way ANOVA for data from multiple groups. A log-rank test was performed for comparing the survival curves between the treatment and the control group. P values < 0.05 were considered significant.
Results
MSLN is overexpressed in human pancreatic cancer cell lines and tissues
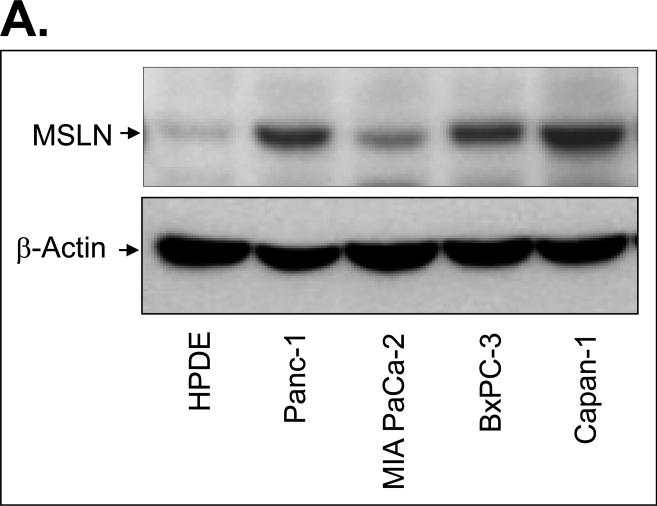
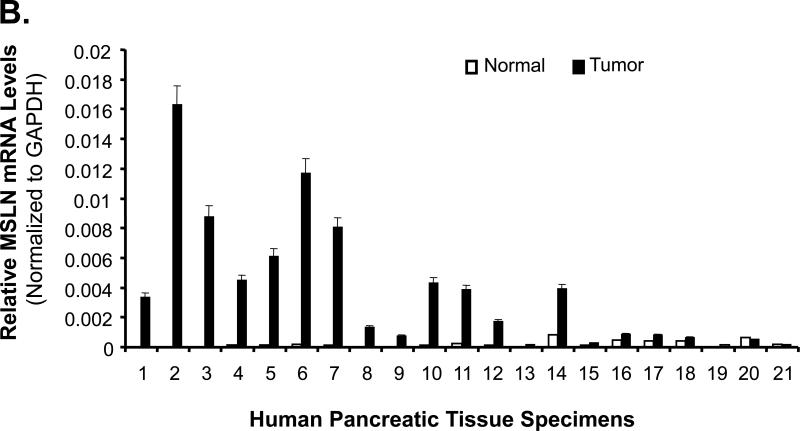
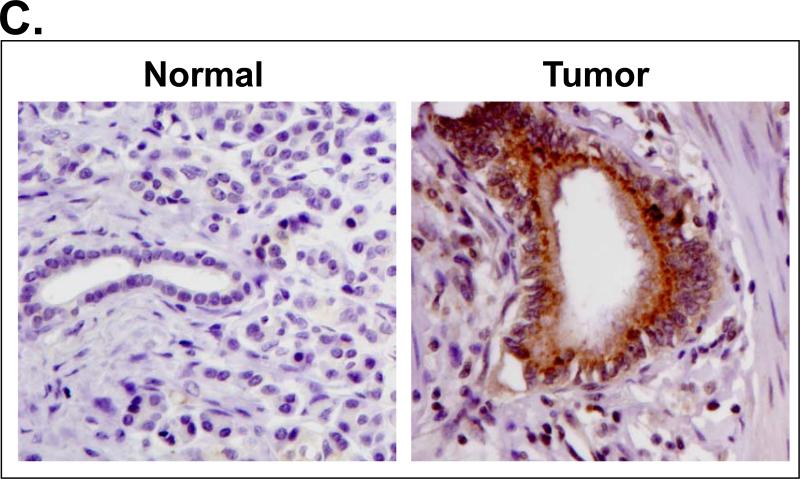
We examined MSLN expression in four human pancreatic cancer cell lines, 21 pairs of human pancreatic cancer tissues with the surrounding normal tissues, and five pancreatitis tissues. MSLN expression was differentially increased in all four cell lines tested compared with that in HPDE cells (Fig. 1A). MSLN mRNA was substantially overexpressed in 18 of 21 (86%) clinical pancreatic-adenocarcinoma samples compared with that in their surrounding normal tissues (Fig. 1B and Supplementary Table 1). In all five pancreatitis tissues, MSLN mRNA was as low as that in the surrounding normal tissues (data not shown). Overall mRNA expression in pancreatic cancer tissue was 17.5 times that in the surrounding normal tissues. The tumor samples also showed strong immunoreactivity to MSLN Ab (Fig. 1C). Thus, human pancreatic cancer is associated with increased expression of MSLN.
Fig. 1.
Expression of MSLN in pancreatic cancer cell lines and pancreatic cancer tissues. A. MSLN protein expression in pancreatic cancer cell lines and human pancreatic ductal epithelium (PHDE) cells by Western blot analysis. B. MSLN mRNA in pancreatic tissues detected by real-time RT-PCR. Twenty-one paired tumor and adjacent normal tissues were studied. Relative mRNA concentrations of MSLN were normalized to that of GAPDH and presented as 2^(Ct[GAPDH] - Ct[MSLN]). C. Immunohistochemical staining of MSLN expression in pancreatic cancer tissues.
Overexpression of MSLN increases cell proliferation and migration in pancreatic cancer cells
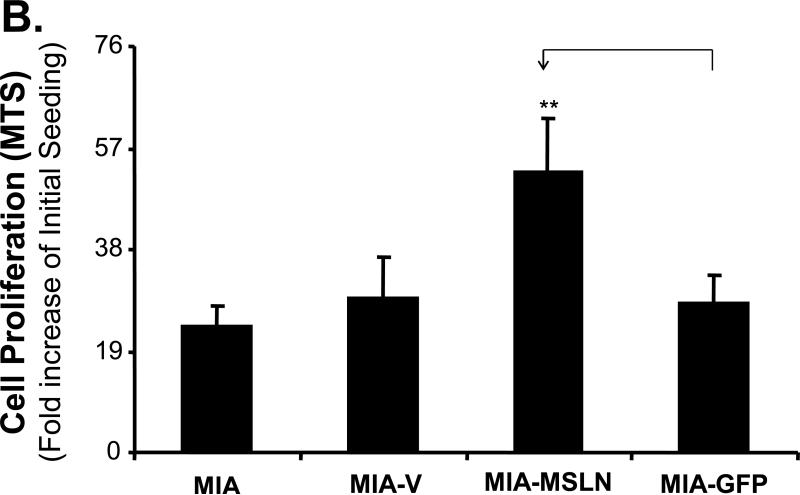
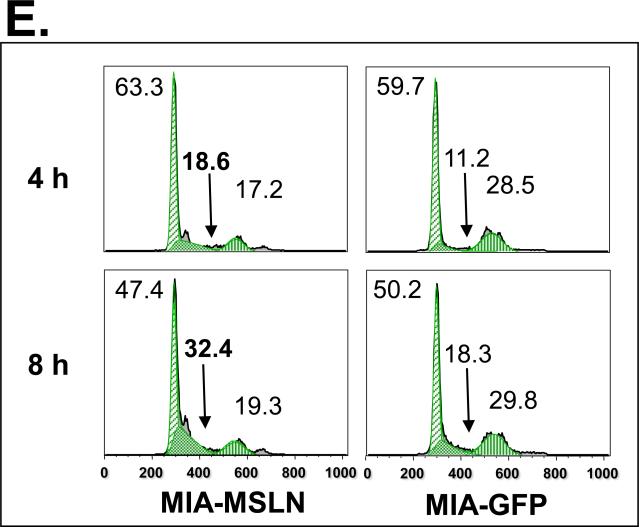
To study the potential functions of MSLN in pancreatic cancer, we established three stably overexpressed MSLN cell lines in MIA PaCa-2 cells (MIA-MSLN) because parental MIA PaCa-2 cells express MSLN less than other pancreatic cancer cell lines (Fig. 1A). Stable cells containing empty vectors (MIA-V) or nonrelated GFP gene (MIA-GFP) were also established in MIA PaCa-2 cells as controls. Overexpression of MSLN in all three MIA-MSLN cells at both mRNA and protein level was confirmed when we compared them with MIA-V and MIA-GFP controls. MSLN overexpression in a representative MIA-MSLN stable cell line is shown in Fig. 2A and Supplementary Fig. S1 (p < 0.01). MSLN overexpression in MIA-MSLN cells was associated with increases in cell proliferation and migration of 90% and 300%, respectively, compared with those in MIA-GFP cells (p < 0.01; Fig. 2B and 2C). In the monolayer wound-healing assay, MIA-MSLN cells showed much greater migration potential than the parental MIA PaCa-2, MIA-V, and MIA-GFP cells (Fig. 2D). Overexpression of MSLN is also associated with an increased S-phase cell population in a cell-cycle analysis. MIA-MSLN cells included 18.6% and 32.4% entering the S phase 4 h and 8 h, respectively, after release from serum starvation. That is 66% and 77% more than MIA-GFP cells, which had 11.2% and 18.3%, respectively, entering the S phase at those time points (Fig. 2E). Thus, overexpression of MSLN in MIA PaCa-2 cells is associated with increased cell proliferation and migration. We also constructed MSLN-overexpressing Panc-1 stable cells and found that forced expression of MSLN increased cell proliferation in those cells (data not shown).
Fig. 2.
Effects of MSLN overexpression in MIA PaCa-2 cells on cell proliferation and migration. A. MSLN expression in stable cells by Western blot analysis. B. Cell proliferation by MTS assay. Cells were seeded in 96-well plates (2 × 103 cells/well) and serum-starved for 24 h before changing to the growth medium with 2% FBS. Absorbance was recorded on day 6 after release from starvation at 490 nm. C. Cell migration by modified Boyden chamber assay. Stable cells were trypsinized and resuspended in growth medium (105 cells/500 μL) and added into the upper compartment of a migration chamber for 24 h. The cells were stained with Calcein-AM. The mean fluorescence reading after scraping of the cells at the top was divided by the reading before removal of the top cells, and the ratio was plotted. D. Cell migration by wound-healing assay. Confluent stable cells were scraped with a pipette tip and cultured in DMEM for 3 days. Pictures were taken daily. E. Cell-cycle analysis by flow cytometry. Confluent cells were serum-starved (0% FBS) for 24 h before changing to the growth medium with 2% FBS. Cells were collected at 4 h and 8 h, permeabilized, stained with propidium iodine (PI), and analyzed with flow cytometry. **p < 0.001.
Silence of MSLN expression decreases cell proliferation and migration in pancreatic-cancer cells
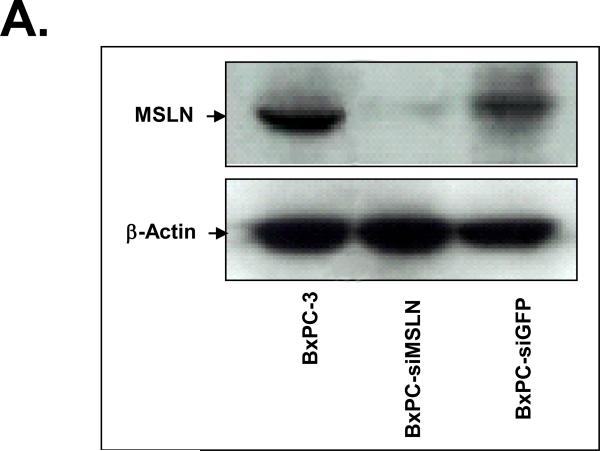
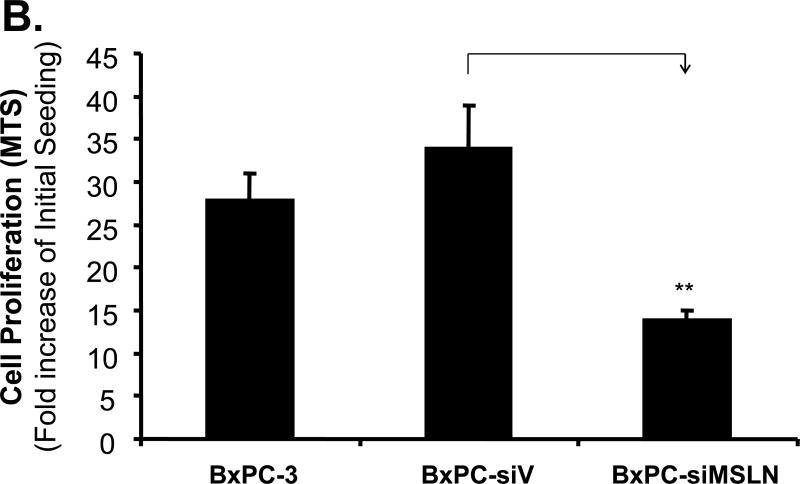
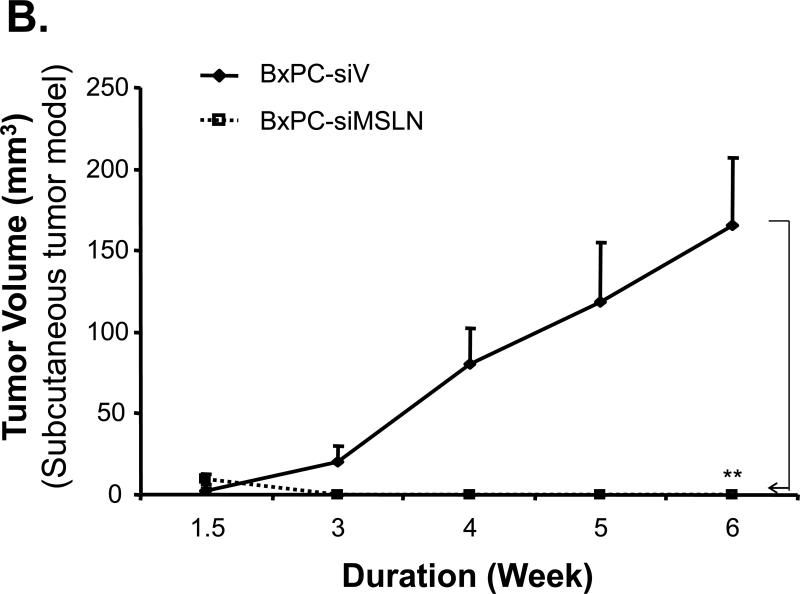
We established three stably MSLN-silenced cell lines in BxPC-3 cells (BxPC-siMSLN) because parental BxPC-3 cells express MSLN more than other pancreatic cancer cell lines (Fig. 1A). Cells containing empty vector (BxPC-siV) were included as controls. A reduction in expression of MSLN in three BxPC-siMSLN cells at the mRNA and protein levels was confirmed when we compared them with BxPC-siV cells. Silence of MSLN expression in a representative BxPC-siMSLN stable cell line is shown in Fig. 3A and Supplementary Fig. S2 (p < 0.01). The decrease in MSLN in BxPC-siMSLN cells was associated with decreases in cell proliferation and migration of 50% and 70%, respectively, compared with BxPC-siV cells (p < 0.01, Fig. 3B and 3C).
Fig. 3.
Effects of silencing of MSLN expression in BxPC-3 cells on cell proliferation and migration. A. MSLN expression in stable cells by Western blot analysis. B. Cell proliferation by MTS assay. Cells were seeded in 96-well plates (2 × 103 cells/well) and serum-starved (0% FBS). Cell growth was assessed with the MTS assay on day 6 after releasing from starvation. C. Cell migration by modified Boyden chamber assay with calcein-AM labeling. **p < 0.001.
MSLN contributes to pancreatic cancer progression in the nude mouse xenograft model
On the basis of the effects of MSLN on pancreatic cancer cell proliferation and migration in vitro, we analyzed the role of MSLN in tumor progression in vivo with an immunodeficient nude mouse model. MIA-MSLN cells showed a dramatic increase (4.3-fold) in tumor volume over MIA-V control cells in the subcutaneous tumor model (p < 0.01, Fig. 4A, Supplementary Table 2). In contrast, BxPC-siMSLN cells with reduced MSLN expression showed a significant reduction in tumor volume compared with BxPC-siV control cells (p < 0.01, Fig. 4B, Supplementary Table 2). Similarly, MIA-MSLN cells significantly increased tumor size by 6.8-fold after 4 weeks compared with MIA-V control cells in the orthotopic model (p < 0.01, Fig. 4C). Furthermore, mice given injections of MIA-MSLN cells showed jaundice (60%), local metastasis (50%), liver metastasis (60%), and abdominal ascitic fluid (60%), whereas none of the MIA-V control mice showed any of these symptoms (Supplementary Table 3). A log-rank test was performed for comparing the survival curves between the MIA-MSLN group and the MIA-V group. The log-test showed that the difference of the survival curves between the two groups are statistically significant (p<0.00001). Nude mice with MIA-MSLN cells had a substantially lower survival rate over 4 weeks than mice with MIA-V control cells (Fig. 4D). Those results indicate that MSLN is a malignant factor that significantly contributes to pancreatic cancer progression in vivo.
Fig. 4.
Effects of MSLN on pancreatic cancer growth in the xenograft nude mouse model. A. Subcutaneous tumor volume of MIA-MSLN cells. MIA-MSLN or MIA-V cells (2 × 106) were subcutaneously inoculated into nude mice (10 mice per treatment group). Tumor size was measured weekly for 4 weeks. **p < 0.01. B. Subcutaneous tumor volume of BxPC-siMSLN cells. 2 × 106 BxPC-siMSLN cells were injected into the right flank of nude mice (eight per treatment group). Tumor size was measured weekly for 6 weeks. **p < 0.01. C. Orthotopic tumor volume of MIA-MSLN cells. 2×106 MIA-MSLN or MIA-V cells were implanted into the body of pancreases in nude mice (10 in each group). The mice were euthanized at different times. Orthotopic tumor volume was measured. Tumor volume in mice given MIA-MSLN inoculation was significantly higher than that in mice given MIA-V inoculation (**p < 0.01). D. Survival rate of nude mice with orthotopically inoculated tumors. 2 × 106 MIA-MSLN or MIAV cells were implanted into the body of pancreases in nude mice (10 in each group). Death was recorded up to 4 weeks after tumor inoculation. Survival of mice with MIA-MSLN inoculations was substantially lower than that of mice with MIA-V inoculations (p<0.00001).
VLP-hMSLN constitutes an efficient therapeutic vaccine against pancreatic cancer growth in the mouse model
Because surface expression of MSLN played an important role in pancreatic cancer growth and pathogenesis, we tested the hypothesis that vaccination against MSLN could be an effective therapeutic approach to pancreatic cancer. Higher expression of mouse MSLN in Panc02 cells than in normal mouse pancreas, mouse fibroblast cells NS47, and mouse endothelial cells SVEC4-10 was confirmed with real-time RT-PCR (p < 0.01, Supplementary Fig. S3). Our previous studies have shown that VLP is a strong immunogen and vaccine candidate that elicits both cellular and humoral immune response to incorporated surface protein. Therefore, we constructed a chimeric MSLN VLP (VLP-hMSLN) in which the human MSLN protein was incorporated into the SHIV VLPs. The expression of MSLN on the VLPs was confirmed, as shown in Supplementary Fig. S4.
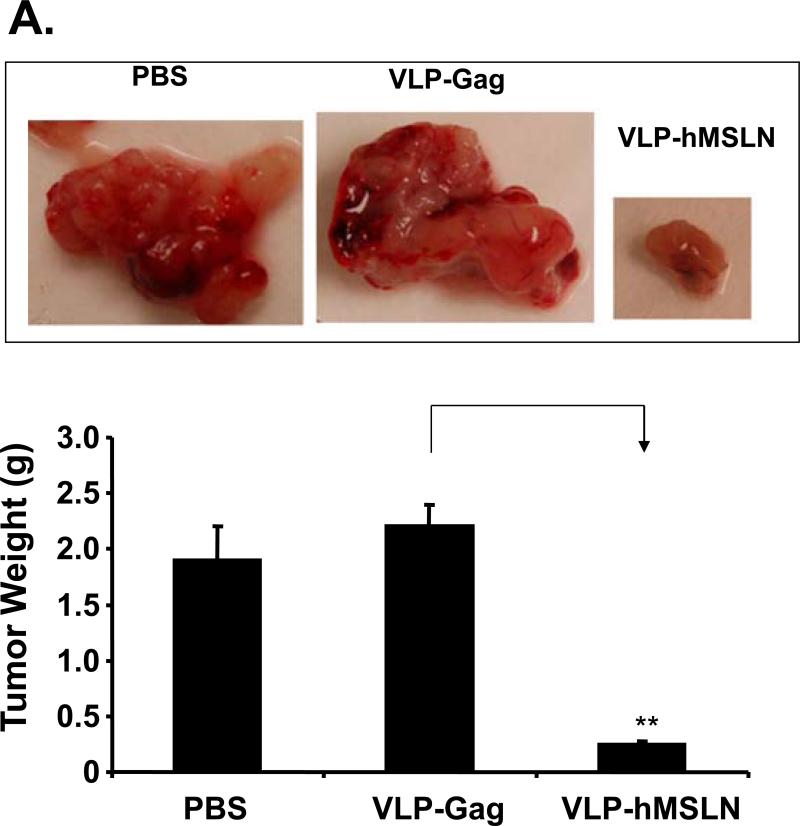
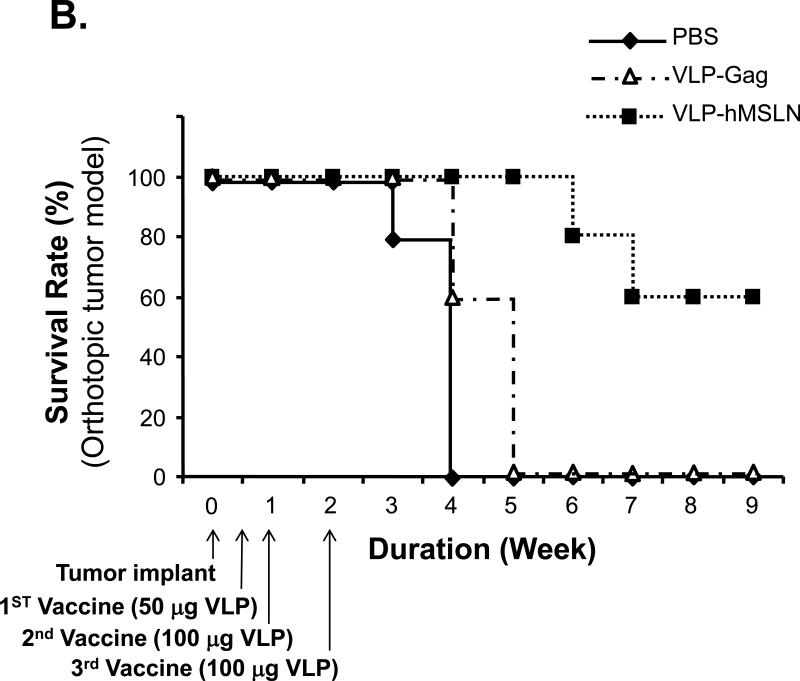
To test the therapeutic efficacy of VLP-hMSLN, we implanted Panc02 cells orthotopically in C57BL/6J wild-type mice, which were then vaccinated intraperitoneally with 50 μg of VLP-hMSLN on day 3 and 100 μg of VLP-hMSLN on days 7 and 14. We monitored tumor progression in C57BL/6J mice 4 weeks after tumor implantation. The VLP-hMSLN-vaccinated group showed significantly lower tumor progression and smaller tumor mass than VLP-Gag and PBS groups (p<0.01) (Fig. 5A). In addition, some of the VLP-hMSLN-vaccinated mice showed mild peritoneal dissemination and little abdominal ascitic fluid, whereas all the control (VLP-Gag and PBS) mice showed multiple peritoneal dissemination and severe abdominal ascitic fluid (Supplementary Table 4). Survival of the VLP-hMSLN-vaccinated mice was significantly increased: 60% of them were still alive 9 weeks after tumor implantation, but all the PBS and VLP-Gag mice died 4 and 5 weeks, respectively, after tumor implantation (Fig. 5B). The log-rank test showed that the survival of VLP-hMSLN treatment is significantly higher than VLP-Gag treatment (p=0.006).
Fig. 5.
Effects of therapeutic vaccine against MSLN on pancreatic cancer growth and pathogenesis in the orthotopic mouse model. A. Effects of VLP-hMSLN vaccination on orthotopic tumor growth in mice. 5 × 105 Panc02 cells were implanted into the body of pancreases of C57BL/6J wild-type mice. On day 3, 50 μg VLP-hMSLN, VLP-Gag, or PBS was injected intraperitoneally (10 mice in each group); this was followed on day 7 and day 14 with two 100 μg VLP-hMSLN, VLP-Gag, or PBS boosts. Tumor growth was analyzed 4 weeks after implantation. Photo images show a representative tumor size in each group at the same distance. The bar graph shows the quantitation of tumor mass in each group. **p<0.01. B. Effects of VLP-hMSLN vaccination on the survival of mice. The same groups of animals as in Fig. 5A were studied. Death was recorded up to 9 weeks after tumor inoculation. Survival of VLP-hMSLN-vaccinated mice was substantially greater than that of VLP-Gag- or PBS-treated mice (p=0.006). C. Effects of VLP-hMSLN vaccination on serum anti-MSLN antibody production. The same groups of animals as in Fig. 5A were studied. Two weeks after the final VLP boost, serum samples were individually collected, and titers of IgG against MSLN were determined by ELISA. Serum anti-MSLN antibodies in the VLP-MSLN-vaccinated mice were substantially increased compared with those in VLPGag- or PBS-treated mice. **p < 0.01 compared with the VLP-Gag group. D. Effects of VLP-hMSLN vaccination on MSLN-specific IFN-γ-producing cells by ELISPOT assay. The same groups of animals as in Fig. 5A were studied. Two weeks after the final VLP boost, mouse spleen cells were collected and stimulated with recombinant vaccinia virus expressing MSLN in vitro. Spleen IFN-γ-producing T cells in the VLP-MSLN-vaccinated mice were substantially increased compared with those in VLP-Gag- or PBS-treated mice. **p < 0.01 compared with the VLP-Gag group. E. Increased MSLN-specific CTL activity elicited by VLP-hMSLN immunization. Three groups of naïve mice and three groups of Panc02 implanted mice were immunized with PBS, VLP-Gag, or VLP-hMSLN at days 3, 7, and 14. At 28 days post immunization, splenocytes isolated from PBS, VLP-Gag, or VLP-hMSLN immunized groups were stimulated with an MSLN-specific peptide at 2 μM for five days to generate effector cells. EL-4 cells pulsed with MSLN-specific peptide (2 μM) for 1 h were used as target cells. Different effector-to-target cell ratios (81:1, 27:1, 9:1, 3:1, and 1:1) were mixed for 4 h. The specific lysis of target cells was measured by LDH release and calculated using formula in the Cytotox 96 assay kit (Promega, Madison, WI). Data are presented as mean ±standard deviation. The data represent one of five independent experiments with different mice. **p<0.01 .
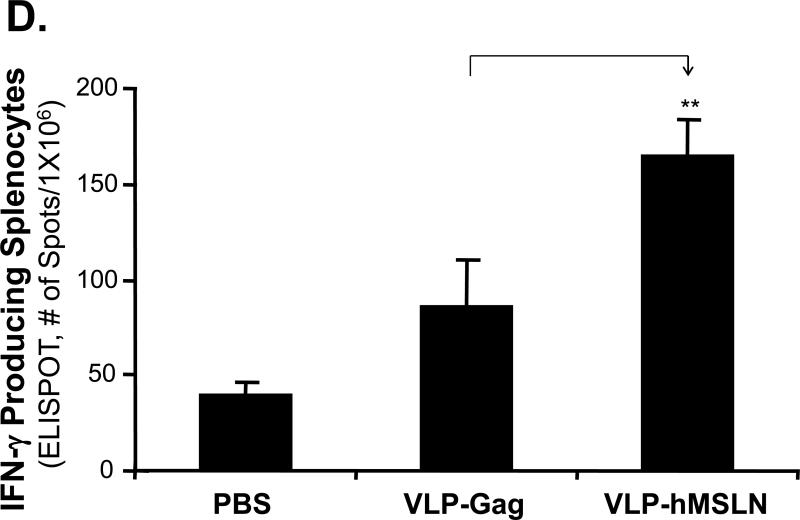
To determine hMSLN-specific immune responses elicited by VLP-hMSLN vaccination, we examined serum hMSLN-specific antibody production and splenocyte hMSLN-specific cellular immune responses. As shown in Fig. 5C, higher concentrations of specific antihuman MSLN antibodies were detected in the serum of VLP-hMSLN-vaccinated mice than in control mice (10 in each group, p < 0.01). To examine hMSLN-specific T cells, we collected spleens from mice sacrificed 4 weeks after tumor implantation. Splenocytes were activated by recombinant vaccinia virus that expresses hMSLN or vaccinia-virus vector controls, and IFN-γ production T cells was determined with the ELISPOT assay. More IFN-γ-producing hMSLN-specific T cells were detected in VLP-hMSLN-vaccinated mice (10 in each group, p < 0.01, Fig. 5D) than in other control mice that received PBS or VLP-Gag. Thus, increased hMSLN-specific antibody production and number of T cells correlated well with inhibition of tumor growth and increase in survival in animals vaccinated with VLP-hMSLN.
To determine and compare the hMSLN-specific immune responses elicited by VLP-hMSLN immunization in naïve mice and Panc02 implanted mice, three groups of naïve mice and three groups of Panc02 implanted mice were immunized with PBS, VLP-Gag, or VLP-hMSLN. At 28 days post immunization, hMSLN-specific CTL activity were analyzed and compared with the two groups. As shown in Fig. 5E, VLP-hMSLN immunization in naïve mice can stimulate strong CTL responses against MSLN, VLP-hMSLN immunization in Panc02 tumor cell bearing mice can stimulate even higher CTL responses but with high baseline MSLN-specific CTL activity in PBS and VLP-Gag immunized animals. These data indicate that although Panc02 itself can induce weak MSLN-specific immune responses, VLP-hMSLN immunization can stimulate much stronger specific CTL responses which contribute to controlling pancreatic cancer progression.
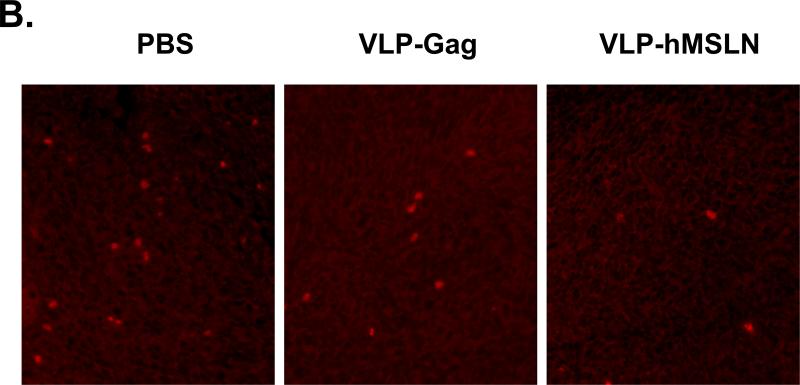
To study the immune response in the VLP-hMSLN-vaccinated mice further, we examined the populations of regulatory T cells (Tregs) in these mice and in the PBS and VLP-Gag control mice with flow cytometry analysis. CD4+Foxp3+ Tregs were decreased to 5.7% in VLP-hMSLN-vaccinated mice compared with 10.4% in PBS mice and 8.4% in VLP-Gag mice, representing a reduction of CD4+Foxp3+ Tregs by 45% and 32%, respectively. In addition, CD4+CD25+ Tregs were also decreased in VLP-hMSLN-vaccinated mice to 3.3% compared with 8.4% in PBS mice and 5.4% in VLP-Gag mice, or 61% and 39% lower, respectively (Fig. 6A). To determine whether Treg is affected by the different treatment in Panc02 tumors, immunohistochemistry staining of Foxp3+ cells in the tumor tissues was performed. Shown in Fig. 6B, the amount of Treg in Panc02 tumor tissue were slightly reduced in VLP-Gag and dramatically reduced in VLP-hMSLN immunized mice tumor tissue compared with that in PBS group. Our data indicate that VLP-hMSLN-induced immune responses may be involved in the reduction in inhibitory Tregs and thereby enhance the immune response against tumor progression.
Fig. 6.
Reduced numbers of Tregs in VLP-hMSLN-vaccinated mice. About 5 × 105 Panc02 cells were implanted into the body of pancreases of C57BL/6J wild-type mice. On day 3, 50 μg VLP-hMSLN, VLPGag, or PBS was injected intraperitoneally (10 mice in each group); this was followed on day 7 and day 14 with two 100-μg VLP-hMSLN, VLP-Gag, or PBS boosts. Two weeks after the final VLP boost, mouse splenocytes were collected and stained for CD4, CD25, and Foxp3 expression by flow cytometry (A.); or mouse Panc02 tumor sections were stained with Foxp3 Ab to visualize number of Treg cells (red cells) in tumors with PBS, VLP-Gag, or VLP-hMSLN immunization (B.). The number of Tregs was lower in VLP-hMSLN vaccinated mice. All data shown are representatives of three separate experiments.
Discussion
We have shown that MSLN was overexpressed in human pancreatic cancer cells and clinical specimens. Increased MSLN is associated with increased cell proliferation and migration of pancreatic cancer cells in vitro and contributes to tumor progression in the nude mouse xenograft model. Silencing of MSLN expression significantly decreased cell proliferation and migration in pancreatic cancer cells and inhibited tumor growth in vivo. Vaccination with chimeric VLP-hMSLN significantly inhibited tumor progression in C57BL/6J mice. The increases in MSLN-specific antibodies and T-cell responses and decrease in regulatory T cells correlated with reduced tumor progression and prolonged survival.
MSLN overexpression was reported to be present in all of 60 resected primary pancreatic adenocarcinomas but not in the adjacent normal pancreas or benign pancreatic ducts (18, 19). Significantly more (p < 0.05) metastatic pancreatic adenocarcinomas were found to be positive for MSLN (64%) than bile duct adenomas (0%) and hepatic hamartomas (0%) (38). We found that MSLN was significantly increased in all pancreatic cancer cell lines tested compared with HPDE cells and was substantially overexpressed in 86% of clinical pancreatic adenocarcinoma specimens compared with surrounding normal tissues and pancreatitis tissues. Those results confirmed that MSLN can serve as a marker of pancreatic adenocarcinoma. Therefore, detection of MSLN could be valuable in diagnosing pancreatic adenocarcinoma, particularly in small biopsy or cytopathology samples (39). It might be possible to use soluble MSLN-related proteins as biomarkers of pancreatic carcinoma with previously used markers, such as CA19-9 and CEA, to increase the sensitivity of diagnosis (39).
MSLN is a surface glycoprotein that is attached to the cell membrane by a glycosyl phosphatidylinositol (GPI) anchor and is postulated to function in cell adhesion (12). However, the details of the biologic functions of MSLN are not clear. Methylation-specific PCR revealed that the MSLN gene displayed a high prevalence of hypomethylation in pancreatic cancer cell lines and in primary pancreatic carcinomas (40). The identification of novel CTL epitopes of MSLN that could efficiently activate human T cells to lyse human tumors supports the hypothesis that MSLN is a potential target for immune-based therapy for pancreatic cancer (41).
In the study reported here, we chose MIA PaCa-2 cells, which expressed relatively little endogenous MSLN, and overexpressed MSLN by selecting several stable cell lines. We found that MSLN overexpression in MIA PaCa-2 cells is associated with increased cell proliferation, migration, and S-phase cell population. In contrast, in BxPC-3, a pancreatic cancer cell line that expressed more endogenous MSLN, reduction in expression of MSLN by siRNA stable silencing resulted in decreased cell proliferation and migration. In the cell cycle study as shown in Fig. 2E, we observed a second peak in proximity of the G0/G1 peak, which we believe are cells in mitosis that going into S phase but not an aneuploid peak because of the following possible reasons: it might be that these cells have different levels of mesothelin expression and hence some cells showed faster cell cycle progression than the rest of the cells. We further found that when we blocked the cell proliferation by using STAT3siRNA, we do not see the peak or see a very low and shifted peak almost merging with the dominant G0/G1 peak (unpublished data). Those results indicate that MSLN is an important factor in pancreatic cancer growth and a potential target for pancreatic cancer treatment. The significant reduction in pancreatic cancer growth by MSLN siRNA indicated the importance of siRNA blockage and opened a door for siRNA pancreatic cancer therapy that targets MSLN.
Toxicity and lack of tumor specificity are the most obvious limitations of conventional approaches, such as chemotherapy and radiation therapy in cancer treatment. The therapeutic-vaccine approach, which would exploit a naturally occurring defense system, embodies an ideal nontoxic treatment capable of evoking tumor-specific immune responses. Vaccination immunotherapy is being tested for many types of cancers, but no current vaccination regimen has been demonstrated in randomized controlled trials. Identification of novel CTL epitopes of MSLN that could efficiently activate human T cells to lyse human tumors would shed light on the hypothesis that MSLN is a potential vaccine candidate for immune-based therapy for pancreatic cancer (41). We have shown that vaccination with VLP-hMSLN significantly inhibited pancreatic cancer progression in C57BL/6J mice by increasing concentrations of hMSLN-specific antibodies, increasing CD8+ T cell responses, and decreasing Tregs. We found that high baseline of MSLN-specific Ab and CTL responses were observed in Panc02 bearing mice model. It led us to further study whether Panc02 tumor cells implantation by itself can stimulate immune response against human mesothelin. As shown in Fig 5, although Panc02 itself can induce weak MSLN-specific immune responses, VLP-hMSLN immunization can stimulate much stronger MSLN-specific CTL responses which attribute to controlling pancreatic cancer progression.
Because of their size and structure, VLPs appear to be actively taken up by antigen-presenting cells; this leads to antigen delivery to cell compartments that are inaccessible to soluble protein antigens. Some VLPs reportedly include papilloma virus-like particles (PPV-VLP), and SHIV VLPs have intrinsic adjuvanticity, which avoids the requirement for adjuvants to stimulate strong T-helper and CTL responses (36, 37, 42). Our study has extended the VLP-based vaccine to pancreatic cancer and showed a great potential of VLP-hMSLN as a therapeutic pancreatic cancer vaccine because of its excellent immunogenic property, safety, and feasibility for multiple vaccinations. The i.p. route of immunization was chosen initially to prove the efficacy of hMSLN-VLP immunization in the mouse pancreatic cancer orthotopic model. Since i.p. route approach limits the clinical applicability of this finding, we plan to further compare the immunotherapeutic efficacy among different routes of immunization such as subcutaneously, intradermally, or intramuscularly which are more practical for the clinical use.
We found from our in vitro data that MIA-MSLN cells had higher cell migration rate than MIA-V cells. This correlates well with the in vivo migration and in vivo metastasis in our MIA PaCa-2 xenograft mouse pancreatic cancer model. We did find a nice correlation of higher metastasis in MIA-MSLN than in MIA-V group. In our orthotopic pancreatic cancer wild type mouse model, Panc02 cells grow aggressively in a short period of time to reach the tumor burden big enough for sacrifice, metastasis is hard to establish in this model. We observed that Panc02 cells predominantly grow in local implant area and we did not observe any distal metastasis in Panc02 orthotopic pancreatic cancer model. Therefore, it was inconclusive whether VLP vaccine could affect metastasis of Panc02 cells. In our future study, we plan to use a highly metastatic mouse pancreatic cancer cell line, Panc H7, to investigate if the VLP vaccine affects metastasis.
One reason for the ineffectiveness of tumor immune response is the acquisition of tolerance of tumor antigen during the development of the immune system as though it is self-antigen. The breaking of immune tolerance of autologous tumor antigen should be a useful approach for a cancer vaccine. We have shown here suppression of tumor progression by the xenogeneic homolog VLP-hMSLN vaccination in the mouse model. Previous studies have shown successful immunotherapy for tumors in a cross-reaction between the xenogeneic homologs and self-molecules in a mouse model (43). In a recent study, mice vaccinated with Ad-hEp-CAM (epithelial cell adhesion molecule) or Ad-rhEp-CAM were protected from a tumor challenge with MC38-hEp-CAM cells; this showed that the immune response induced against hEp-CAM with both vaccines is sufficient to produce a biologic antitumor effect (44). VLPs have the capacity to break self-tolerance to produce antibody responses against self-proteins expressed on them (45). Therefore, human MSLN is used in this study to evaluate the vaccine efficacy against mouse mesothelin. Human mesothelin sequence (NM_005823, 622 amino acid residues) and mouse mesothelin (BC023753, 625 amino acid residues) are 58% homologous in amino acid sequence. Our study proves the effectiveness of using xenogeneic antigen as a candidate tumor vaccine.
Tregs protect the host from autoimmune disease by suppressing self-reactive immune cells. Therefore, Tregs may also block antitumor immune responses (46). Recent reports have shown that the prevalence of Tregs is increased in several solid tumors, particularly in the tumor environment. Those Tregs are usually CD4+CD25+Foxp3+ (in some circumstances, CD4+CD25-Foxp3+). One study in a mouse pancreatic cancer model suggests that the tumor actively promotes the accrual of Tregs through several mechanisms involving both activation of naturally occurring Tregs and conversion of non-Tregs to Tregs (46). However, little is known about the molecular mechanisms responsible for increases in Tregs in cancer. For the first time, we have found that vaccination with VLP-hMSLN could significantly decrease the population of Tregs in both systemic immune organ (mouse spleen) and local tumor site. CD4+Foxp3+ Tregs and CD4+CD25+ Tregs were significantly decreased in VLP-hMSLN vaccinated mice compared with PBS- and VLP-Gag-treated groups, respectively. Therefore, VLP-hMSLN-induced decrease in the Tregs population could be the important mechanism of enhanced immune responses and inhibition of pancreatic cancer growth in VLP-hMSLN-vaccinated mice.
In conclusion, VLP-based vaccination immunotherapy for pancreatic cancer shows exciting hope of not only attacking the tumor but also dealing with important regulatory mechanisms that may have adversely influenced clinical efficacy. This novel strategy has potential clinical applications in treatment for human pancreatic cancer and other cancers with high expression of MSLN.
Supplementary Material
Acknowledgement
This work was supported in part by NIH grants DE15543, AT003094, Dan L Duncan Cancer Center pilot grant (Q. Yao), and American Cancer Society Grant #IRG-93-034-09 (M. Li). We thank Drs. Craig D. Logsdon, James L. Abbruzzese, Mary K. Estes, and David M. Spencer for critical review and stimulating discussions; Dr. Ira Pastan (Laboratory of Molecular Biology, National Cancer Institute (NCI), NIH, Bethesda, MD) for providing human mesothelin construct; and Fei Li, Dr. Hao Wang, Dr. Hong Chai, and Dr. Qihui Zhai for their technical assistance.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 3.Day JD, Digiuseppe JA, Yeo C, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27:119–24. doi: 10.1016/s0046-8177(96)90364-0. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–54. [PMC free article] [PubMed] [Google Scholar]
- 5.DiGiuseppe JA, Hruban RH, Offerhaus GJ, et al. Detection of K-ras mutations in mucinous pancreatic duct hyperplasia from a patient with a family history of pancreatic carcinoma. Am J Pathol. 1994;144:889–95. [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm PD, Hruban RH, Ramsoekh TB, et al. The potential diagnostic use of K-ras codon 12 and p53 alterations in brush cytology from the pancreatic head region. J Pathol. 1998;186:247–53. doi: 10.1002/(SICI)1096-9896(1998110)186:3<247::AID-PATH179>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.van Heek T, Rader AE, Offerhaus GJ, et al. K-ras, p53, and DPC4 (MAD4) alterations in fine-needle aspirates of the pancreas: a molecular panel correlates with and supplements cytologic diagnosis. Am J Clin Pathol. 2002;117:755–65. doi: 10.1309/5RQ0-JCQU-5XF2-51LQ. [DOI] [PubMed] [Google Scholar]
- 8.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer. 2005;5:459–67. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Chenwei L, Mahmood R, et al. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006;66:898–906. doi: 10.1158/0008-5472.CAN-05-3025. [DOI] [PubMed] [Google Scholar]
- 10.Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. A novel role for carcinoembryonic antigen-related cell adhesion molecule 6 as a determinant of gemcitabine chemoresistance in pancreatic adenocarcinoma cells. Cancer Res. 2004;64:3987–93. doi: 10.1158/0008-5472.CAN-04-0424. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–64. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 12.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–8. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 14.Bast RC, Jr., Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 15.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frierson HF, Jr., Moskaluk CA, Powell SM, et al. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003;34:605–9. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 17.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–7. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 18.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 19.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 20.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 21.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–5. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 22.Dainty LA, Risinger JI, Morrison C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol. 2007 doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 23.Steinbach D, Onda M, Voigt A, et al. Mesothelin, a possible target for immunotherapy, is expressed in primary AML cells. Eur J Haematol. 2007;79:281–6. doi: 10.1111/j.1600-0609.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52:181–6. [PubMed] [Google Scholar]
- 25.Watanabe H, Okada G, Ohtsubo K, et al. Expression of mesothelin mRNA in pure pancreatic juice from patients with pancreatic carcinoma, intraductal papillary mucinous neoplasm of the pancreas, and chronic pancreatitis. Pancreas. 2005;30:349–54. doi: 10.1097/01.mpa.0000160281.56828.76. [DOI] [PubMed] [Google Scholar]
- 26.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–22. [PubMed] [Google Scholar]
- 27.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–12. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 28.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Li M, Chen C, Yao Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine. 2004;23:139–47. doi: 10.1016/j.vaccine.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Ruedl C, Schwarz K, Jegerlehner A, Storni T, Manolova V, Bachmann MF. Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies. J Virol. 2005;79:717–24. doi: 10.1128/JVI.79.2.717-724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–26. [PubMed] [Google Scholar]
- 33.Li M, Yang H, Chai H, et al. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101:2341–50. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Zhai Q, Bharadwaj U, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–94. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zhang Y, Liu Z, Bharadwa U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc Natl Acad Sci U S A. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Q, Zhang R, Guo L, Li M, Chen C. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J Immunol. 2004;173:1951–8. doi: 10.4049/jimmunol.173.3.1951. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313:502–13. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- 38.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–9. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W, Sokoll LJ, Bruzek DJ, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev. 1998;7:109–12. [PubMed] [Google Scholar]
- 40.Sato N, Maitra A, Fukushima N, et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res. 2003;63:4158–66. [PubMed] [Google Scholar]
- 41.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–51. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Expert Rev Vaccines. 2002;1:101–9. doi: 10.1586/14760584.1.1.101. [DOI] [PubMed] [Google Scholar]
- 43.Wei YQ, Wang QR, Zhao X, et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6:1160–6. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 44.Elia L, Mennuni C, Storto M, et al. Genetic vaccines against Ep-CAM break tolerance to self in a limited subset of subjects: Initial identification of predictive biomarkers. Eur J Immunol. 2006;36:1337–49. doi: 10.1002/eji.200535514. [DOI] [PubMed] [Google Scholar]
- 45.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169:6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 46.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–68. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.