While the needs of animals vary across ecological niches and among species, they always include access to food, water, shelter and mates. The ability to anticipate these needs and to predict when necessary resources will become available optimizes the likelihood of success. In some cases this means that effort is not wasted searching for a resource that is not yet available. In other cases, effort is expended to prepare for upcoming challenges such as gathering seeds in anticipation of winter or fattening up in anticipation of migration or hibernation. The neural basis of the complex behaviors associated with anticipatory responses is now being delineated. This special topics issue of the European Journal of Neuroscience focuses on the neural basis of timing and anticipatory behaviors with special attention to regulation of food anticipatory behaviors.
What is anticipation and why the emphasis on anticipation of food?
An anticipatory system can be defined as one containing a predictive model of itself and/or its environment, which allows it to change state in accord with the model's predictions pertaining to a latter instant (Rosen, 1985). Anticipation can be construed as an agent making responses based on predictions, expectations, or beliefs about the future. Anticipation is a vital component of complex natural systems and including cognitive and non-cognitive systems. It implies the existence of an “internal model”. In the study of anticipatory behaviors of organisms, the search for this internal model requires identifying the nervous and non-nervous elements that constitute the internal components.
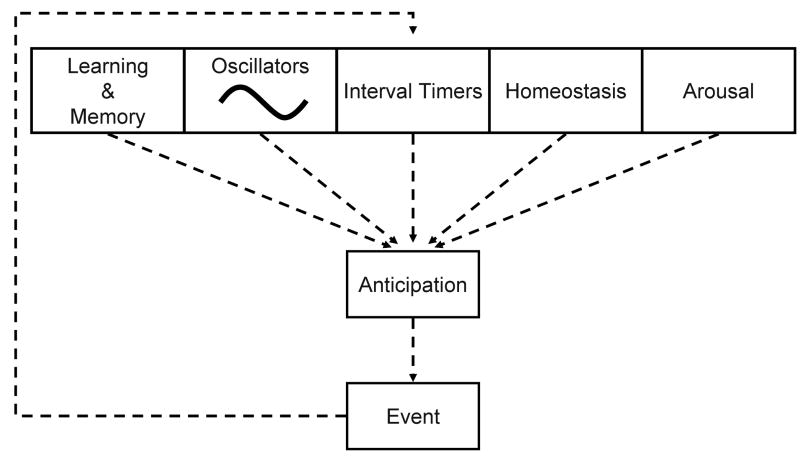
Food seeking behavior provides a convenient window into the web of hypothetical constructs, and psychological / biological phenomena associated with anticipation. Anticipation can be studied in the context of learning and memory, reward and punishment, memory and cognition, arousal and feedback associated with internal and external state changes, homeostatic processes and timing mechanisms (Figure 1). In each of these contexts, anticipation of food availability provides empirically tractable paradigms for experimental analysis.
Figure 1.
A model of how various systems regulate anticipation. Anticipation is a function of cognitive processes such as learning and memory, as well as unconscious processes such as underlying oscillators, interval timers and arousal. Homeostatic drives may be considered a special case of interval timers that help maintain physiological set-points by steadily increasing drive states as time passes and, upon reaching a threshold, trigger a response. The event anticipated can feedback to each of these processes: It can provide an event to be remembered, set the phase of an oscillator, reset an interval timer, alter a homeostatic drive and influence arousal. Furthermore, all the processes that feed into anticipation may interact.
Food anticipatory activity as a model of anticipation
The earliest studies of food anticipation derived from observations of the behavior of organisms, human and non-human, in their natural environments and pointed out that we perceive time. A century ago, August Forel (1910) related that while on vacation in the Swiss Alps, he was visited by bees that came to feed on his marmalade while he ate breakfast on the patio. The number of bees increased over a number of days until they were so numerous that he could no longer eat outdoors. On subsequent days, Forel noted that the bees continued to arrive at breakfast time even though he and his marmalade were safely indoors. He reported that the bees appeared to have a time-memory (zeitgedachtnis) for when breakfast was served. Hugo Berthold von Buttel-Reepen (1900) had earlier reported that bees exhibited a time-sense (zeitsinn) for foraging, in that they visited a buckwheat field only in the morning while the blossoms were open and secreting nectar. These studies constitute the earliest descriptions of food anticipatory activity (FAA). The earliest controlled studies were conducted by Ingeborg Beling (1929) and Oskar Whal (1932), demonstrating that bees could learn that food was available at one station in the morning and another station in the afternoon. The ability to time food availability had obvious ecological value; but what was truly important about these findings was that the bees were able to use their time sense to anticipate availability of a limited resource, and that their simple nervous system encodes this time sense.
Curt Richter (1922) conducted the earliest systematic analysis in mammals of timing behavior and its neural basis. Among his many findings was the observation that rats housed in constant illumination anticipated daily feeding with an increase in activity in the two to three hours preceding food presentation. Apart from a few phenomenological studies (e.g., Bolles & Stokes, 1965; Potter et al., 1968), interest in anticipation of scheduled feeding waned while the field focused on circadian control of rhythms in general, with the view that daily meal time was but one of many end points that could be analyzed.
In 1972, the study of circadian rhythms shifted from phenomenology to neuroscience with the discovery that the suprachiasmatic nucleus (SCN) was the neural locus of a light-entrainable circadian pacemaker. When the SCN was lesioned, animals lost circadian rhythms in locomotor activity, hormone secretion and body temperature (Moore & Eichler, 1972; Stephan & Zucker, 1972). The knowledge that circadian timing could be discretely localized in the brain prompted the revisitation of other timing phenomena, including FAA.
The discovery that FAA was not dependent on the SCN presented a substantial puzzle, first systematically studied by Fred Stephan and colleagues (1979a; b). The fact that intact animals housed in constant conditions could anticipate a scheduled meal while simultaneously expressing a freerunning locomotor rhythm of a different period argued strongly for a separate circadian clock controlling FAA (Mistlberger, 1994). The next twenty years witnessed a flurry of activity to delineate the formal properties of FAA (Mistlberger, 1994). Specifically, circadian rhythms persist for multiple cycles of ~24 hours under total food deprivation, indicating that it is controlled by a circadian clock. This property is unique to circadian oscillators, as interval timers (so called hourglasses) can only exhibit a single cycle, after which they must be reset by an external stimulus. Furthermore, FAA has many additional important properties of circadian timers, including: limits of entrainment, free running period and transient cycles following a shift in feeding time (Mistlberger, 1994).
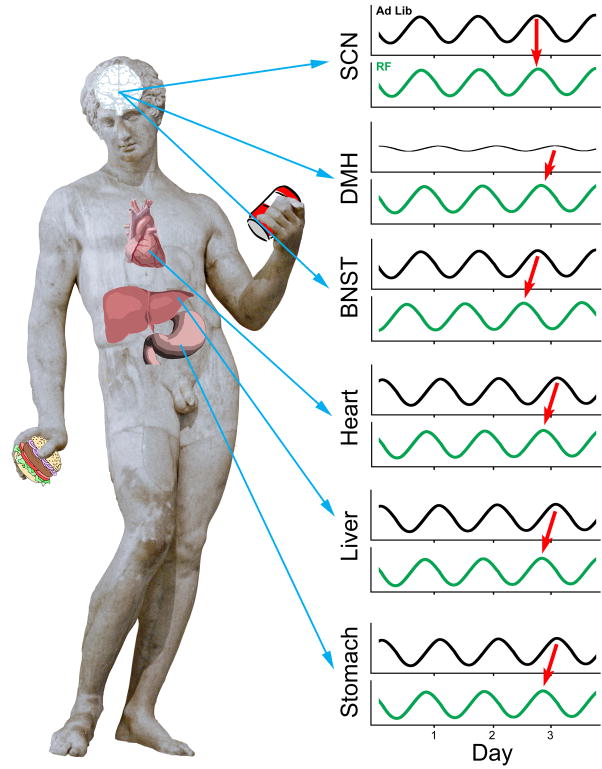
The molecular revolution and the tremendous breakthroughs in identification of genes and proteins that serve as cellular markers of time accelerated interest in the elements that are the neural basis or “internal model” of food anticipation, as it enabled a search for the loci of timing mechanisms. This new era of investigation was ushered in by two important developments: the discovery of the molecular components of the circadian clock (Reppert & Weaver, 2001); and the observation that tissues outside the brain can exhibit self-sustained oscillation (Balsalobre et al., 1998). These peripheral oscillators are driven by the same intracellular transcription translation feedback loops that give rise to oscillations in SCN cells. In general, the phases of these extra SCN oscillators are differ from that of the SCN. Under normal physiological conditions, the phases of these oscillators are set by the SCN pacemaker, but these oscillators are sensitive to many other signals as well. Importantly, feeding (or cues that are consequent to feeding), can set the phase of circadian clocks in peripheral tissues (Damiola et al., 2000; Stokkan et al., 2001; Figures 2). This finding set the stage for significantly revised views of the localization of food-entrainable circadian oscillators. A number of extra-SCN oscillators in both the brain and body are entrained by food or food-derived cues (Figure 2), notably the liver, kidney, heart, pancreas (Damiola et al., 2000; Stokkan et al., 2001), stomach (LeSauter et al., in press), dorsomedial hypothalamus (Mieda et al., 2006; Verwey et al., 2007; 2008; Verwey et al., 2009) and limbic forebrain (Verwey et al., 2007).
Figure 2.
Various tissues in the body can exhibit circadian oscillations. The suprachiasmatic nucleus (SCN) is the master light-entrainable pacemaker and under normal physiological conditions sets the phase of other oscillators in the brain and body, such as the liver and bed nucleus of the stria terminalis (BNST), each of which can have its own phase, distinct from that of the SCN. When subjected to a restricted feeding protocol, many peripheral oscillators have their phase reset, such that they acquire a particular phase angle relative to feeding time. Some tissues, such as the dorsomedial hypothalamus (DMH) only exhibit strong oscillations when the individual is on a scheduled feeding regimen.
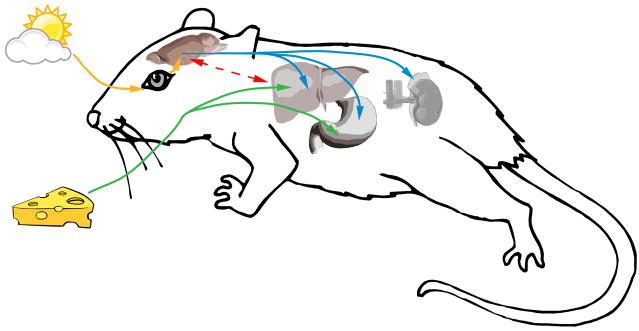
Deconstruction of the component topics embedded in the notion of food anticipatory behaviors reveals the diverse aspects that need explanation. Within the realm of circadian rhythms, interest has focused on the possibility that there exists a single brain site, a nucleus bearing food entrainable master clock that regulates food anticipatory activity (FAA), parallel to the master clock of the light-entrainable SCN (Stephan, 2002; Figure 3). Such a clock was initially referred to a food entrainable pacemaker, as it was believe that this clock was able to impose its rhythm on other systems, which is the critical property of a pacemaker. This system is now more commonly referred to as a food entrainable oscillator (FEO; Stephan, 2002) due to Jurgen Aschoff’s “admonition that pacemaker was too presumptuous and should be reserved for the SCN” (Stephan, 2002, p.286). In fact, it appears that there are many food entrainable oscillators in the brain and body, reinforcing the possibility that there may be no single FEO. Evidence is gathering for the existence of numerous oscillators entrained by food (Escobar et al., 2009; Verwey & Amir, 2009), some of which send out hormonal signals that increase activity and eating behavior prior to regularly timed meals (LeSauter et al., in press) The search for food entrainable oscillators remains a topic of intense interest (Gooley et al., 2006; Landry et al., 2006; Landry et al., 2007; Moriya et al., 2009). While there are clear oscillator components to FAA, if this behavior is viewed in a broader perspective, it is clear that some aspects introduced above, namely “…predictions, expectations, beliefs about the future” and “… learning and memory, reward and punishment, memory and cognition, arousal and feedback associated with internal and external state changes, homeostatic processes and timing mechanisms”, will not lie within an oscillator.
Figure 3.
The light entrainable pacemaker in the suprachiasmatic nucleus receives light information from the retina from the retinohypothalamic tract (yellow). The SCN sets the phase of various peripheral oscillators (blue). When individuals are placed on restricted feeding schedules, the phases of peripheral “food-entrainable oscillators” are adjusted to track feeding time (green). There may be communication between food entrainable oscillators in the brain and periphery (red). See the literature cited in the text for details.
Multifaceted control of anticipation
Anticipation is a complex behavior, and while underlying oscillators likely contribute to anticipatory responses that have a regular periodicity, there are numerous other processes that participate as well. First, the animal must be awakened if it is sleeping. The neural mechanism underlying behavioral state and state transitions have been profitably studied in both vertebrates (Jones, 2005) and invertebrates (van Staaden & Huber, 2001). Behavioral state is also subject to multiple regulatory systems, most notably homeostatic and circadian forces (Borbely, 1982). If an anticipated event occurs during the sleep phase, the appropriate neural systems must be activated to arouse the animal from its slumber.
Once activated, a timing mechanism must trigger anticipatory responses at the appropriate time. The nature of these responses can be quite varied, but include changes in both physiology and behavior. Given that bees can anticipate foraging opportunities, it is clear that anticipatory timing behaviors can be achieved by relatively simple nervous systems (cf., the mammalian brain). The role of an underlying oscillator depends on the period of the anticipated phenomenon. In terms of daily feeding, circadian FEOs may be the source of such a signal. Anticipation of non-circadian timescales have also been described (Boisvert & Sherry, 2006; Balsam et al., 2009; Crystal, 2009) and may depend on an underlying oscillator as well.
Anticipation develops gradually as the animal learns the association between an event and the time of its occurrence. In many cases, there is no reason to expect a priori that an event will be periodic (bees foraging at flowers may be an exception). When an event recurs regularly, the animal must realize that it is rhythmic, and must appreciate the period of the recurrence. Such calculations may or may not reach conscious awareness. In some cases, a circadian oscillator may serve as a continuously consulted clock (Gallistel, 1990; Mistlberger et al., 1996) that can time-stamp a memory, whereas in other cases an underlying oscillator or pacemaker may become entrained to the periodic event. The circuits underlying these forms of associative learning could be distinct for different types of periodic stimuli.
If learning is involved in an anticipatory phenomenon, then a memory engram must be stored somewhere. If the anticipation was regulated by an underlying oscillator alone, then the memory for phase could not be distinguished from the phase of the oscillator. On the other hand, the oscillator phase information may be completely separate from the memory information. An interesting question is whether FAA is context specific. Given that food sources differ both in space and time, it might be reasonable to hypothesize that that FAA may change dramatically if an animal were moved to a new place. If the event to be anticipated were complex, such as is the case with time-place learning (e.g., Biebach et al., 1989; Zhou & Crystal, 2009), it is likely that multiple systems could become involved. Anticipation that has a spatial component would likely involve, at least in part, the hippocampal formation. In this case, circadian oscillators (the SCN and/or FEOs) likely act as consulted clocks, at a minimum. Which oscillators participate, and what networks are involved in time-stamping an event may depend on the temporal event to be anticipated. It is possible that multiple oscillators, in both the brain and the periphery, can provide phase information for time place learning.
Multiple timing systems?
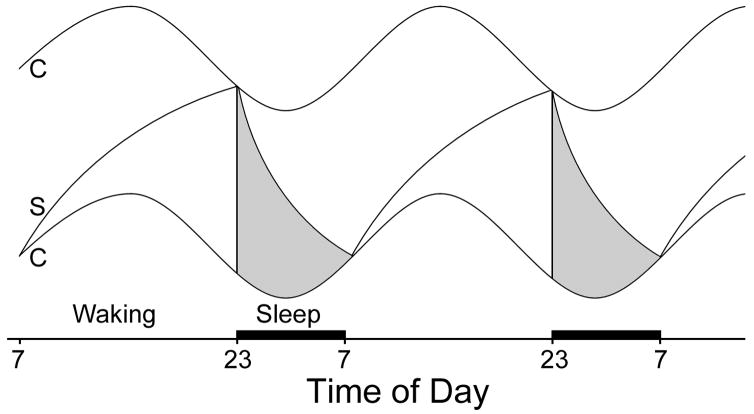
We now address the question of whether the homeostatic and circadian mechanisms underlying FAA are fundamentally different from other behaviors and physiological responses that involve joint control by these two different timing mechanisms. The joint participation of circadian and interval timing mechanisms has been discussed in the context of interacting neural and endocrine systems specifically in the study of parental care in doves, photoperiodic timekeeping, regulation of LH (and ovulation and estrus behavior) and pregnancy/pseudopregnancy (Silver & Bittman, 1984). Perhaps more familiar to circadian chronobiologists is the two-process model of sleep by Borbely (1982; Figure 4). In each of these systems, interval timers appear to be critical at one or more levels, and the final output has circadian properties. The signal duration that is measured may originate inside the animal (as in the case of estrus and LH surges) or in the outside world (as in the cases of mating induced pseudopregnancy or photoperiodism). The origin of the stimulus imposes no constraints upon the participation of circadian and interval properties in the final response.
Figure 4.
Some aspect of Borbely’s two-process model of sleep regulation may also apply to circadian/homeostatic regulation of FAA. A circadian component (C) provides thresholds for wake (lower oscillation) and sleep (upper oscillation) onset. During wake, a homeostatic sleep drive (S) increases. When the sleep drive intersect with the circadian rhythm for sleep onset, sleep is initiated, and the homeostatic drive decreases during the sleep, until it intersects with the circadian rhythm for wake onset, at which point the individual awakens, and the cycle reinitiates. Modified from (Daan et al., 1984).
Anticipation, particularly for events as critical to survival as feeding, are likely regulated by many processes with multiple interacting levels, each with numerous redundancies. In the case of ingestive behaviors, it is likely that some resources lack a daily temporal component, but could still require learning, homeostasis, and interval timers for an organism to optimally exploit these resources. There is no reason to assume that these systems would cease to operate when a resource also has a predictable temporal component. In fact, in natural settings these multiple systems would likely work in conjunction with each other. Clearly, circadian oscillators participate in anticipation of feeding (Mistlberger, 1994; Mistlberger & Marchant, 1995), but this participation does not exclude simultaneous involvement of complimentary strategies (Mistlberger, 2009).
Redundancies may operate at each level of the system. As proposed elsewhere there may be multiple FEOs that can provide phase information, possibly explaining the persistence of FAA following discrete lesions of single regions (Stephan, 2002; Davidson, 2009). Mice with mutation rendering either period1 or period2 nonfunctional (or genetic deletions of cryptochrome 1 or 2) are rhythmic, while double mutants are not (Horst et al., 1999; Zheng et al., 2001). On the other hand, the clock knockout animal sustains rhythmicity (DeBruyne et al., 2006), possibly due to the participation of another protein that dimerizes with BMAL1, such as the NPAS2 gene (DeBruyne et al., 2007). A BMAL1 deficiency alone is sufficient to eliminate circadian locomotor rhythms (Bunger et al., 2000), but FAA is sustained, though there is uncertainty as to the distinct roles of circadian oscillators vs. interval timers or homeostatic mechanisms (Challet et al., 2009; Pendergast et al., 2009; Storch & Weitz, 2009).
Interval-Circadian Systems
How might an FEO interact with homeostatic/interval-timing systems? The interval timer establishes WHETHER permissive conditions have been met. The circadian timer determines WHEN the outcome will occur. Thus the two timing processes are functionally independent, and in conjunction provide a mechanism that allows the response to occur in the appropriate sequence and time. In such systems, information appears to be accumulated by the interval timer and once threshold is reached, activation of the circadian effector system comes into play.
In this framework, the FEO will determine when the meal is expected and thus trigger FAA at the appropriate phase, while homeostatic and interval-timer mechanisms will determine if the animal is hungry enough to eat (Figure 1), reinforcing FAA during a restricted feeding schedule, and masking the FEO when animals are given ad libitum access to food following a restricted feeding protocol.
The state of the art in tackling issues related to the neural basis of timing and anticipatory behaviors, focused on FAA, is represented in the papers in this issue. The first section of this issue focuses on the current state of knowledge of the “internal state” that encodes anticipation of daily time. The first two papers explore neural basis of circadian timing and of feeding behavior. The SCN is seen as the master light-entrainable circadian pacemaker in the mammalian circadian timing system, but the mechanisms by which SCN signals are translated into circadian behaviors are only now being explored. Michael Verwey and Shimon Amir explores molecular, neuroanatomical and behavioral approaches used to identify circadian oscillators in the brain and to examine how these oscillators are synchronized with the master clock in the SCN. Their paper emphasizes the nature, modulation and function of circadian oscillators in forebrain, amygdale and BNST involved in the regulation of motivated and emotional behaviors including feeding. A classical process for exploring function is to impose loss of function. Alec Davidson reports on the contributions of ablation studies to the identification of the locus of the FEO, with a specific focus on methodological considerations and the recent implication of the dorsomedial hypothalamus. As can be seen in the review of Carolina Escobar, Cathy Cailloto, Manuel Angeles-Castellanos, Roberto Salgado Delgado, and Ruud M Buijs, peripheral oscillators can entrain to food cues, and in turn, the phase of peripheral oscillators can be set by time of eating, thereby producing signals that determine the timing of food anticipation. A recent report suggests that the display of FAA does not require any of the canonical clock genes (Storch & Weitz, 2009), though it is clear that in both the brain and the periphery, extra-SCN oscillators exhibit rhythmic expression of these genes. Etienne Challet, Jorge Mendoza, Hugues Dardente and Paul Pevet introduce a cautionary note and review the data on mutant animals and explore the experimental conditions under which FAA does and does not occur. Furthermore, they go on to examine experimental approaches to studies of the role of feeding related genes in the regulation of FAA.
The second section of this issue explores the timing of eating behavior. Early lesion studies and subsequent genetic findings identified the hypothalamus as an important regulator of food intake and energy expenditure. As discussed by Marcelo Dietrich and Tamas Horvath, molecular and genetic tools for visualizing and manipulating homeostatic systems in the brain, in combination with neuroanatomical, electrophysiological, behavioral, and pharmacological techniques have begun to elucidate the control of feeding behavior and energy expenditure. Such work enables an understanding of the link between the circadian and metabolic systems that underlie feeding. While most of the work in this issue uses experimental manipulations to control the timing of access to food, we are alerted in the studies discussed by Mario Caba and Gabriela Gonzalez-Mariscal that restricted feeding occurs in nature. The mother rabbit feeds her young for minutes once a day and the pup’s metabolism must adjust to this once-daily opportunity. The study of nursing anticipation in rabbits provides a wonderful and natural example of the development of acute and chronic mechanisms associated with the regulation of energy homeostasis.
The third section of this issue looks beyond feeding, to explore anticipation in a broader perspective. As reviewed by Ken-ichi Honma and Sato Honma it has long been appreciated that exposure to methamphetamine uncovers a robust circadian rhythm in SCN lesioned animals, which is suggested to be closely related with the food-entrainable circadian rhythm. Like FAA, methamphetamine’s influence on circadian rhythmicity does not appear to depend on the SCN or on canonical clock genes (Mohawk et al., 2009). The mechanism underlying this and many other circadian anticipatory phenomena are not well understood, in part due to the existence of redundant and/or overlapping regulatory systems that can play a role. Ralph Mistlberger discusses experimental paradigms that have been developed to tease apart the various homeostatic, interval and circadian timing aspects that participate in anticipatory phenomena. Anticipation implies not only timing, but also appropriate behavioral responses to fully exploit the event being anticipated. Ana Ribeiro, Joseph LeSauter, Christophe Dupre and Donald Pfaff point out that arousal is a critical aspect of any anticipatory behavior. While arousal and circadian cycles typically work together, they can be in conflict in cases where anticipated events occur outside the normal active phase of the organism. As Ian Webb, Ricardo Baltazar, Michael Lehman and Lique Coolen explain, anticipation of scheduled rewarding events, such as access to drugs of abuse or a mate, may broaden our understanding of the neural systems that underlie anticipation. Importantly, events other than timing of food availability can also be anticipated, and that anticipation can itself feed back to change behavior.
The final section of this issue examines cognitive aspects of anticipation. The neural mechanisms underlying the timing of non-circadian intervals are not known. Since time and time perception are fundamental to all learning, it will be important to determine these neural systems. Peter Balsam, Hugo Sanchez-Castillo, Kathleen Taylor, Heather Van Volkinburg and Ryan Ward review the cognitive and behavioral processes that underlie food anticipation at intervals of seconds and minutes that are typical of psychological studies of anticipation and timing. Their analysis points to the importance of examining multiple behavioral measures to reveal how memory, motivation and decision processes all play a role in food anticipation. Finally, Jonathon Crystal reviews the phenomena of time-place learning, whereby an organism is able to demonstrate learning in not only the spatial realm, but also the temporal dimension. This work explores the relationship of mechanisms involved in timing of short intervals (seconds to minutes) to those controlling circadian timing (hours to days).
Future Directions
A number of interesting and important questions remain to be investigated with respect to food anticipation. It is somewhat curious that animals on a restricted feeding schedule would engage in high-amplitude anticipatory activity, thereby expending a significant amount of their limited available energy. One suggestion is that this behavior is an escape response, since activity levels are exceptionally high when food is severely restricted (Yi et al., 1993). However, animals show anticipatory activity to events where escape would be counterproductive, such as anticipation of a scheduled palatable meal when standard lab chow is available ab libitum (Mistlberger & Rusak, 1987) or anticipation of a daily opportunity to mate (Landry et al., 2008). These observations are consistent with a different hypothesis namely that anticipatory activity represents a foraging drive. Specifically, it has been suggested that animals have an innate urge to engage in exploratory activity to increase the probability of encountering a desired resource (e.g., food, water, a mate), particularly those that are anticipated but absent (Mather, 1981). This fits with anticipation of opportunities to hoard seeds (Rusak et al., 1988) or foraging for nectar, activities where the food item may not actually be consumed per se. It is also possible that anticipatory activity is not be due to a single factor, but rather may result from a multitude of sources, such as responses to hunger, foraging for resources, and general autonomic arousal associated with reward.
As the neural and molecular correlates underlying FAA are uncovered, new avenues for translational research will be revealed. Already links between altered clock gene expression and obesity are being reported (Kaneko et al., 2009). Night eating syndrome, frequently associated with obesity, appears to involve circadian dysregulation between the major light-entrainable pacemaker and other peripheral oscillators (Goel et al., 2009). Anorexia nervosa, bulimia nervosa and obesity are associated with circadian abnormalities in neuroendocrine function and exhibit characteristics of resynchronization between various neuroendocrine rhythms (Ferrari et al., 1997). The extent to which the eating disorder causes these abnormalities versus the extent to which the circadian abnormality exacerbates the eating disorder remains to be determined. Anticipation may play a role in addictive behaviors as well. The same areas of the brain that are activated during anticipation and consumption of food rewards are activated in people addicted to gambling, alcohol or nicotine when they anticipate and receive the rewards associated with their respective addictions (Stice et al., 2009).
One of the problems in trying to understand the neural and network properties of FAA stems from the fact that there a multiple ways to “make a clock tick”. The complex transcriptional and translational feedback loops have multiple redundancies, so single knockout studies often don’t reveal a lack of FAA (e.g., Pitts et al., 2003; Pendergast et al., 2009). The charge in understanding the molecular underpinnings of light-entrainable rhythms was led by work in the fruit fly, which has orthologs for the mammalian clock genes. While circadian rhythms in feeding have been observed in drosophila (Xu et al., 2008), it is difficult to distinguish any FAA (Sehgal, personal communication). This likely has to do with the ecology of feeding in fruit flies, in that their food source is not temporally restricted to one part of the day. A solution to the search for exemplary simple model systems may be a return to the hive. Honey bees show circadian rhythms (Moore, 2001) and, as noted on the outset, exhibit clear and ecologically valid FAA. Orthologs for various clock genes (Toma et al., 2000; Rubin et al., 2006; Yuan et al., 2007) have been identified in the honey bee, and some of the circuitry has been determined (Bloch et al., 2003), raising the possibility that the first model of FAA may open an avenue for future investigation into the neural and molecular mechanisms of FAA.
Acknowledgments
The research from our labs is funded by NIH grants 075045 and 37919 to RS, and by an NSERC Discovery Grant to MCA.
References
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsam P, Sanchez-Castillo H, Taylor K, Van Volkinburg H, Ward RD. Timing and anticipation: Conceptual and methodological approaches. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06967.x. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beling I. Uber das Zeitgedachtnis der Bienen. Z Vergl Physiol. 1929;9:259–388. [Google Scholar]
- Biebach H, Gordijn M, Krebs JR. Time-and-place learning by garden warblers Sylvia borin. Anim Behav. 1989;37:353–360. [Google Scholar]
- Bloch G, Solomon SM, Robinson GE, Fahrbach SE. Patterns of PERIOD and pigment-dispersing hormone immunoreactivity in the brain of the European honeybee (Apis mellifera): Age- and time-related plasticity. J Comp Neurol. 2003;464:269–284. doi: 10.1002/cne.10778. [DOI] [PubMed] [Google Scholar]
- Boisvert MJ, Sherry DF. Interval timing by an invertebrate, the bumble bee Bombus impatiens. Curr Biol. 2006;16:1636–1640. doi: 10.1016/j.cub.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Stokes LW. Rat's anticipation of diurnal and a-diurnal feeding. J Comp Physiol Psychol. 1965;60:290–294. doi: 10.1037/h0022308. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, Gonzalez-Mariscal G. The rabbit pup, a natural model of nursing anticipatory activity. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06964.x. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E, Mendoza J, Dardente H, Pevet P. Neurogenetics of food anticipation. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06962.x. this issue. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Theoretical and conceptual issues in time-place discrimination. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06968.x. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol Regul Integr Comp Physiol. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ. Lesion studies targeting food-anticipatory activity. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06961.x. this issue. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M, Horvath T. Feeding signals and brain circuitry. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06963.x. this issue. [DOI] [PubMed] [Google Scholar]
- Escobar C, Cailloto C, Angeles-Castellanos M, Salgado-Delgado R, Buijs RM. Peripheral oscillators - The driving force for food anticipatory activity. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06972.x. this issue. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Magri F, Pontiggia B, Rondanelli M, Fioravanti M, Solerte SB, Severgnini S. Circadian neuroendocrine functions in disorders of eating behavior. Eat Weight Disord. 1997;2:196–202. doi: 10.1007/BF03339975. [DOI] [PubMed] [Google Scholar]
- Forel A. Das Sinnesleben der Insekten. Reinhardt; Munich: 1910. [Google Scholar]
- Gallistel CR. The Organization of Learning. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O'Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks: Methamphetamine and food restriction. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06976.x. this issue. [DOI] [PubMed] [Google Scholar]
- Horst GTJvd, Muijtjens M, Kobayashi K, Takano R, Kanno S-i, Takao M, Wit Jd, Verkerk A, Eker APM, Leenen Dv, Buijs R, Bootsma D, Hoeijmakers JHJ, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: Neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Yamada T, Tsukita S, Takahashi K, Ishigaki Y, Oka Y, Katagiri H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009;1263:58–68. doi: 10.1016/j.brainres.2008.12.071. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Mear RJ, Mistlberger R. Soc Res Biol Rhythms. Destin, Fl: 2008. Surprisingly weak effects of sex as a zeitgeber in male rats; p. 196. [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0906426106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather JG. Wheel-running activity: A new interpretation. Mammal Rev. 1981;11:41–51. [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: Dependence on meal size and nutrient content. Physiol Behav. 1987;41:219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Food anticipatory circadian rhythms: Concepts and methods. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06965.x. this issue. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, de Groot MHM, Bossert JM, Marchant EG. Discrimination of circadian phase in intact and suprachiasmatic nuclei-ablated rats. Brain Res. 1996;739:12–18. doi: 10.1016/s0006-8993(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Marchant EG. Computational and entrainment models of circadian food-anticipatory activity: Evidence from non-24-hr feeding schedules. Behav Neurosci. 1995;109:790–798. [PubMed] [Google Scholar]
- Mohawk JA, Baer ML, Menaker M. The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes. Proc Natl Acad Sci U S A. 2009;106:3519–3524. doi: 10.1073/pnas.0813366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. Honey bee circadian clocks: Behavioral control from individual workers to whole-colony rhythms. J Insect Physiol. 2001;47:843–857. [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. European Journal of Neuroscience. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R57–67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter VR, Baril EF, Watanabe M, Whittle ED. Systematic oscillations in metabolic functions in liver from rats adapted to controlled feeding schedules. Fed Proc. 1968;27:1238–1245. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, LeSauter J, Dupre C, Pfaff DW. Relationship of arousal to circadian anticipatory behavior: Ventromedial hypothalamus - One node in a hunger/arousal network. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06969.x. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. A behavioristic study of the activity of the rat. Comp Psychol Monogr. 1922;1:1–55. [Google Scholar]
- Rosen R. Anticipatory systems: Philosophical, mathematical and methodological foundations. Pergamon Press; New York: 1985. [Google Scholar]
- Rubin EB, Shemesh Y, Cohen M, Elgavish S, Robertson HM, Bloch G. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 2006;16:1352–1365. doi: 10.1101/gr.5094806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Bittman EL. Reproductive mechanisms: Interaction of circadian and interval timing. Ann N Y Acad Sci. 1984;423:488–514. doi: 10.1111/j.1749-6632.1984.tb23455.x. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: Food as a zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979a;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979b;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma DP, Bloch G, Moore D, Robinson GE. Changes in period mRNA levels in the brain and division of labor in honey bee colonies. Proc Natl Acad Sci U S A. 2000;97:6914–6919. doi: 10.1073/pnas.97.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staaden MJ, Huber R. Multidisciplinary dissection of behavioral arousal: The role of muscarinic acetylcholine stimulation in grasshopper stridulatory behavior. Proc Natl Acad Sci U S A. 2001;98:9468–9470. doi: 10.1073/pnas.181341098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M, Amir S. Food entrainable oscillators in the brain. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06960.x. this issue. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Region-specific modulation of PER2 expression in the limbic forebrain and hypothalamus by nighttime restricted feeding in rats. Neurosci Lett. 2008;440:54–58. doi: 10.1016/j.neulet.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Verwey M, Lam GY, Amir S. Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06766.x. [DOI] [PubMed] [Google Scholar]
- von Buttel-Reepen HB. Sind die Bienen Reflexmaschinen? Leipzig: 1900. [Google Scholar]
- Wahl O. Neue untersuchungen tiber das Zeitgedachtnis der Bienen. Z Vergl Physiol. 1932;16:529–589. [Google Scholar]
- Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: Is restricted food access a unique zeitgeber? Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06966.x. this issue. [DOI] [PubMed] [Google Scholar]
- Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi I, Bays ME, Stephan FK. Stress ulcers in rats: The role of food intake, body weight, and time of day. Physiol Behav. 1993;54:375–381. doi: 10.1016/0031-9384(93)90126-z. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proc Natl Acad Sci U S A. 2009;106:9525–9529. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]