Abstract
Long-term opioid therapy for non-cancer pain has increased. Caution is advised in prescribing for persons with substance use disorders, but little is known about actual health plan practices. This paper reports trends and characteristics of long-term opioid use in persons with non-cancer pain and a substance abuse history. Using health plan data (1997–2005), the study compared age–sex-standardized rates of incident, incident long-term and prevalent long-term prescription opioid use, and medication use profiles in those with and without substance use disorder histories. The CONsortium to Study Opioid Risks and Trends study included adult enrollees of two health plans, Kaiser Permanente of Northern California (KPNC) and Group Health Cooperative (GH) of Seattle, Washington. At KPNC (1999–2005), prevalence of long-term use increased from 11.6% to 17.0% for those with substance use disorder histories and from 2.6% to 3.9% for those without substance use disorder histories. Respective GH rates (1997–2005), increased from 7.6% to 18.6% and from 2.7% to 4.2%. Among persons with an opioid disorder, KPNC rates increased from 44.1% to 51.1%, and GH rates increased from 15.7% to 52.4%. Long-term opioid users with a prior substance abuse diagnosis received higher dosage levels, were more likely to use Schedule II and long-acting opioids, and were more often frequent users of sedative-hypnotic medications in addition to their opioid use. Since these patients are viewed as higher risk, the increased use of long-term opioid therapy suggests the importance of improved understanding of the benefits and risks of opioid therapy among persons with a history of substance abuse, and the need for more careful screening for substance abuse history than is the usual practice.
Keywords: Prescription opioid episodes, Substance use disorders, Chronic pain management in managed care
1. Introduction
In the last several years, opioid treatment for non-cancer pain has increased dramatically in the U.S. [8,23]. High rates of prescription drug abuse have also heightened concern about the prescribing practices and potential adverse effects [11,40]. Although drug testing of U.S. workers between 1997 and 2007 showed declining rates for most drug use, in contrast, rates of opiate use increased from 11% to 16%. However, it is not known whether this was due to prescription abuse [9].
Expert guidelines for management of non-cancer pain recommend caution in prescribing long-term opioid therapy for persons with a substance use disorder, but also recognize the importance of treating their pain [21]. However, little is known about medically prescribed use in community practice for individuals with substance abuse histories. A recent review [29] found only one study measuring rates of narcotic analgesic use for those with a substance abuse history. A recent study of long-term opioid management in primary care practices found the prevalence of substance abuse disorders to be 9.7% and opioid disorders to be 3.8% among those with daily opioid prescription use, but it did not examine them prior to the prescribed opioid use [19].
The efficacy and risks of long-term opioid use for individuals with substance abuse histories are poorly understood, because they have been excluded from almost all randomized trials of long-term opioid therapy [10,25,34,41]. In line with expert guidelines [1], physicians often have concerns about using opioids for long-term pain management for these individuals [4,32]. Some evidence shows that those using prescription drugs non-medically have higher rates of later prescription drug abuse [31], and that those with chronic pain and substance use disorders misuse medications more often than others [14,15,22,28,36].
This study examines the extent to which persons with a history of substance abuse are receiving opioids long-term. We expect to observe substantially higher rates of long-term opioid use among persons with a history of substance abuse at the beginning of the observational period, and increasing rates of use over time. We hypothesize that, among patients using opioids long-term, those with a history of substance abuse will be more likely to receive higher dosage levels, use Schedule II opioids, and use sedative-hypnotics frequently in addition to their opioid use.
2. Method
The CONsortium to Study Opioid Risks and Trends (CONSORT) was developed to improve understanding of trends in, and risks of, long-term opioid therapy for non-cancer pain in community practice [42]. Detailed methods have been published [42]; we briefly describe the study population and methods.
The two health plans studied, Kaiser Permanente Northern California (KPNC) and Group Health in Washington State (GH), provide comprehensive care to approximately 4,000,000 persons. The demographic characteristics of both plans closely resemble those of their underlying communities, and membership has been stable over time [35,38,42]. Both serve populations enrolled in Medicare and Medicaid. The study was approved by each health plan’s Institutional Review Board.
2.1. Data sources
Information on enrollment, demographics, and utilization including medications, diagnoses, and procedures is recorded in automated databases linked by a unique membership number. Information on medical encounters, including pharmacy utilization, is captured for all services provided directly by the health plans. In addition, data pertaining to medical encounters by providers and pharmacies outside the health plans are captured in billing/claims databases. Health plan pharmacy data encompass about 97% of member prescriptions [6,35,38].
2.2. Measures
2.2.1. Opioid use episodes
CONSORT data were developed using an episode approach. The beginning of an episode is defined as a dispensing for an oral or transdermal opioid with none dispensed in the prior 6 months. The last dispensing in an episode is the last opioid dispensed with no dispensing in the following 6 months. The end date of an episode is defined as the date of the last prescription plus days supply for that prescription. Total days supply is defined as the sum of days supply for each opioid dispensed during an episode [42]. We determined the indication for the opioid use episode by linking the first opioid prescription in the episode to visits to the prescribing doctor occurring during the 2 weeks prior to the episode start date. We evaluated the ICD-9 codes on the visit nearest to the episode start date to determine the nature and frequency of study subjects’ pain conditions.
2.2.2. Long-term opioid use episodes
These were defined as episodes lasting longer than 90 days that had 120+ total days supply or 10+ opioid prescriptions dispensed over the course of the episode. This was based on our previous work showing that persons who surpassed this threshold were highly likely to continue frequent use of opioids [42].
2.2.3. Medication use profiles
These were developed for prevalent and incident long-term opioid users in 2005. Prevalent users are persons in an episode of long-term opioid therapy on 1/1/2005 who also met long-term use criteria in 2005 (e.g., at least 10 opioid fills and/or 120+ days supply). Incident users are persons who initiated an episode of long-term use during 2005. The medication use profile for prevalent longterm users covers the calendar year 2005; the profile for incident long-term users covers the first 365 days of their opioid use episode. These profiles were restricted to 2005 because patient characteristics were similar for long-term opioid users across study years; we chose the most recent year.
We calculated morphine equivalents for each opioid dispensed as follows: quantity × the strength (i.e., milligrams per unit dispensed) × drug-specific conversion factors [42]. Total morphine equivalents were calculated by adding the morphine equivalents for each opioid dispensed during the episode. Average daily dose is the total morphine equivalents divided by episode duration in days. Average prescribed dose is the total morphine equivalents divided by total days supply for the episode. Average daily dose is an estimate of mean daily consumption, while average prescribed dose approximates the maximum intended daily dose [42].
Opioid medications were divided into three types: (1) Non-Schedule II; (2) short-acting Schedule II; and (3) long-acting Schedule II [42]. Longer acting opioids are recommended for chronic pain given the lower frequency of dosing needed and greater consistency in pain control. In addition, addiction risk is generally considered higher with shorter acting opioids given the more rapid onset of effects, both analgesic, and for some, euphoric. If more than one type of opioid was dispensed over an episode, the predominant type was determined by greatest total days supply.
2.3. Substance use, and opioid, dependence and abuse disorders
We used administrative databases at both health plans to identify visits with ICD-9 codes indicating a substance use disorder (drug or alcohol), or the subset with opioid use disorders. We also identified whether the diagnosis was made in Chemical Dependency or Psychiatry departments only.
We note that none of the patients were prescribed methadone for opiate disorders by the health plans; it was not covered or prescribed by them as an opioid dependence treatment. If patients were using methadone as an opioid dependence treatment, they would be accessing it through community agencies. Thus, any methadone prescriptions would be for pain rather than for opioid treatment.
Buprenorphine maintenance was not used for opioid dependence treatment in the health plans in 2005. Although it became available for use in opioid treatment in 2002–2003, it was not used for opioid dependence treatment in the health plans prior to 2006. The federal regulations required that no physician practice (with these health plans defined as a practice) could have more than 30 patients at a time. This regulation was lifted on August 15, 2005, but the use remained very limited in these and other health plans until late 2006. Buprenorphine was mainly used for detox in 2006 and wider adoption did not occur until 2007. The number of patients prescribed buprenorphine during 2007 was 667 in KPNC and was 209 in GH.
2.4. Data analysis
For each study year (1997–2005 in GH and 1999–2005 in KPNC), we compared the age- and sex-standardized rates of opioid use episodes for individuals with and without a substance use (and opioid use) disorder diagnosis. (Because complete data were not available prior to 1997 at KPNC, the rates are limited to years 1999–2005.) Specifically, we examined annual rates of any incident opioid use, incident long-term use, and prevalent long-term use. Incident episodes are episodes beginning, and prevalent episodes are those ongoing, in the calendar year of interest. For incident episodes, substance use disorder status was determined from diagnoses received in the two calendar years prior to the year the episode began. For prevalent long-term opioid use episodes, substance use disorder status was determined in the two calendar years prior to each year during which the episode was ongoing; thus if an episode was of multiple years duration, substance abuse status was determined for each year within the episode. Among incident and prevalent long-term users in 2005, we compared medication profiles for individuals with and without substance and opioid use disorders in the prior 2 years.
We limited the sample to subjects enrolled for the entire year of interest plus 182 days following the end of the year to ensure adequate time to observe long-term use after an episode began. Subjects were at least 18 years at the beginning of the year of interest and had no cancer diagnoses on or before the end of that year [42]. In addition, for each relevant year subjects were continuously enrolled in the health plan for the prior two calendar years ensuring that they were “at risk” for receiving a substance abuse diagnosis.
We directly standardized age and sex rates to the 2005 population of each plan using 10 groups (sex and age categorized as: 18–34, 35–44, 45–64, 65–74, and 75+). We estimated the percent change annualized (PCA) across the period with 95% confidence intervals for the rates using a linear regression method [18]. The linearized PCA estimates the constant annual (multiplicative) rate of change over a fixed time period.
3. Results
3.1. Sample characteristics
Clinical characteristics of the CONSORT population have been described [42]. The most common diagnoses were back pain, extremity pain and osteoarthritis [42]. Table 1 presents data on gender and age for individuals with and without prior substance use disorders, as well as for the subsets of those with and without opioid disorders, and those with and without alcohol disorders. With a few exceptions in GH (often due to small sample sizes), those in each disorder group are significantly different from those without disorders. For incident long-term use, men are over-represented among those with substance use disorders and among the subset of those with alcohol disorders in KPNC (with non-significant differences in GH). However, there are a higher proportion of women than men with prior opioid disorders. Those in the disorder groups are younger than their counterparts (e.g., mean age was 47 for those with an opioid disorder in KPNC, and was 58 for those without an opioid disorder). Characteristics of those with prevalent long-term use are similar to those with incident long-term use, with the exception that women are over-represented among those with substance abuse histories as well as with opioid disorder histories.
Table 1.
Age and gender of persons initiating incident and prevalent long-term opioid use episodes for non-cancer chronic pain in 2005 by substance use, opioid and alcohol disorders in the prior 2 years at KPNC and GH.
| KPNC | Substance use disorder history |
Opioid disorder history |
Alcohol disorder history |
|||
|---|---|---|---|---|---|---|
| SUD yes (1023) | SUD no (11,494) | OP yes (160) | OP no (12,357) | ALC yes (689) | ALC no (11,828) | |
| Incident long-term use | ||||||
| Female (%) | 47.2 | 61.5 | 55.6 | 60.4 | 42.2 | 61.4 |
| Male (%) | 52.8 | 38.5 | 44.4 | 39.6 | 57.8 | 38.6 |
| p ≤ 0.0001 | p = 0.2207 | p ≤ 0.0001 | ||||
| Mean age (SD) | 50.7 (14.0) | 58.2 (16.1) | 47.1 (10.1) | 57.8 (16.1) | 52.3 (13.2) | 57.9 (16.2) |
| p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | ||||
| GH | SUD yes (145) | SUD no (2147) | OP yes (14) | OP no (2278) | ALC yes (115) | ALC no (2177) |
| Female (%) | 55.2 | 60.8 | 57.1 | 60.5 | 52.2 | 60.9 |
| Male (%) | 44.8 | 39.2 | 42.9 | 39.5 | 47.8 | 39.1 |
| p = 0.1802 | p = 0.7985 | p = 0.0618 | ||||
| Mean age (SD) | 48.9 (16.3) | 58.0 (16.6) | 41.6 (16.7) | 57.5 (12.7) | 49.6 (15.5) | 57.8 (17.8) |
| p = 0.7328 | p = 0.2507 | p ≤ 0.0001 | ||||
| KPNC | SUD yes (3676) | SUD no (29,512) | OP yes (894) | OP no (32,294) | ALC yes (1739) | ALC no (31,449) |
| Prevalent long-term use | ||||||
| Female (%) | 55.8 | 65.5 | 60.3 | 64.5 | 44.6 | 65.5 |
| Male (%) | 44.2 | 34.5 | 39.7 | 35.4 | 55.4 | 34.5 |
| p ≤ 0.0001 | p = 0.0096 | p ≤ 0.0001 | ||||
| Mean age (SD) | 52.2 (12.9) | 59.0 (14.7) | 49.6 (11.8) | 58.5 (14.7) | 53.9 (12.4) | 58.5 (14.7) |
| p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | ||||
| GH | SUD yes (400) | SUD no (5154) | OP yes (165) | OP no (5490) | ALC yes (220) | ALC no (5334) |
| Female (%) | 54.5 | 66.2 | 59.0 | 65.5 | 48.2 | 66.1 |
| Male (%) | 45.5 | 33.8 | 41.5 | 34.5 | 51.8 | 33.9 |
| p ≤ 0.0001 | p = 0.2448 | p ≤ 0.0001 | ||||
| Mean age (SD) | 51.8 (13.5) | 58.3 (15.0) | 50.4 (14.3) | 58.0 (15.0) | 52.4 (12.3) | 58.1 (15.1) |
| p = 0.0072 | p = 0.6605 | p ≤ 0.0001 | ||||
3.2. Any incident opioid prescribing
We examined age- and sex-adjusted rates of any incident opioid prescribing from 1997 to 2005 for GH and from 1999 to 2005 for KPNC; rates for both were higher for those with prior substance use and/or opioid use disorders than others (not shown). However, while rates increased significantly for individuals without substance use disorders; there were no significant increases for those with a substance use disorder, except in GH for those with any substance use disorder (PCA 1.8%, p = 0.0004).
3.3. Incident long-term opioid episodes
Fig. 1 presents age- and sex-adjusted rates from 1997 to 2005 for GH and from 1999 to 2005 for KPNC of incident long-term opioid use by substance use disorder status in the prior 2 years. Baseline rates for incident long-term use were three to four times higher among individuals with prior substance use disorders than among those without prior substance use disorders: KPNC, 2.54% (numerator [num] = 729; denominator [den] = 28,718) versus 0.067% (num = 8187; den = 1,221,916), and GH, 2.68% (num = 84, den = 3146) versus 0.86% (num = 1638; den = 190,069), and both increased significantly over time.
Fig. 1.
Age–sex-adjusted rates (%) of incident long-term opioid use among adult non-cancer patients by drug or alcohol diagnosis and opioid diagnosis in the prior 2 years.
Rates of incident long-term use for those with prior opioid use disorders did not have significant increases, but were less stable because of sample sizes. Rates at KPNC went from 6.26% (num = 95; den = 1525) to 5.9% (num = 139; den = 2342) for those with opioid use disorders and from 7.1% (num = 8369; den = 1,249,109) to 8.4% 9 (num = 11,673; den = 1,459,152) for those without opioid use disorders. Rates at GH were 6.96% (num = 8; den = 112) for those with opioid disorders versus 8.8% (num = 1703; den = 193,103) for those without opioid disorders.
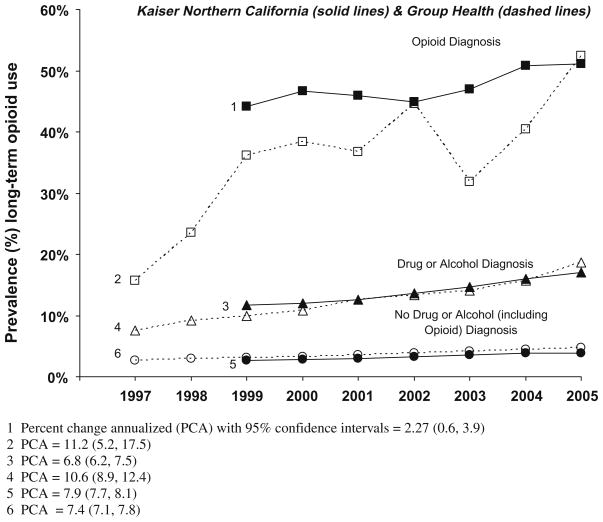
3.4. Prevalent long-term opioid episodes
Baseline rates for prevalent long-term use were higher for those with both substance use and opioid use disorders than for those without substance use and opioid use disorders, and remained higher over time in both health plans (Fig. 2). At KPNC, rates increased from 11.7% (num = 3357; den = 28,718) to 17.0% (num = 6465; den = 38,097) for those with substance use disorders compared to an increase from 2.6% (num = 31,281; den = 1,221,916) to 3.9% (num = 56,082; den = 1,423,397) for those without substance use disorders, but with similar rates of change. At GH, rates increased from 7.6% (num = 240; den = 3146) in 1997 to 18.6% (num = 727; den = 3900) in 2005 for those with prior substance use disorders, and from 2.7% (num = 5059; den = 190,069) to 4.8% (num = 9365; den = 196,942) for those without prior substance use disorders.
Fig. 2.
Age–sex-adjusted rates (%) of prevalent long-term opioid use among adult non-cancer patients by drug or alcohol diagnosis and opioid diagnosis in the prior 2 years.
For those with opioid use disorders, rates of prevalent long-term episodes at KPNC increased from 44.1% (num = 673, den = 1525) to 51.1% (num = 1197; den = 2342), while for those without opioid use disorders they increased from 2.7% (num = 33,726; den = 1,249,109) to 4.2% (num = 60,701; den = 1,459,152). Because prevalence rates of long-term opioid use were high initially among persons with an opioid use disorder history, their annualized rate of change was lower than that for those without an opioid use disorder. However, in GH, there was a larger rate of change for prevalent long-term use for those with opioid use disorders from 1997 to 2006 (15.7% in 1997 (num = 18; den = 112) to 52.4% (num = 103; den = 197) (PCA = 11.2%) than for those without opioid use disorders (2.7% (num = 5246; den = 193,103) to 5.0% (num = 9936; den = 200,645) (PCA = 7.7%).
3.5. Medication use profiles by substance use and opioid use disorder diagnoses
Table 2 presents medication use profiles for prevalent long-term opioid use in 2005 by substance use and opioid use disorder status in the prior 2 years. In both health plans, individuals with substance use disorders were on higher dose regimens, received more days supply, and were more likely to receive short- and long-acting Schedule II opioids and 180+ days of sedative-hypnotics than those without a substance use disorder. Similar patterns were again significant (p ≤ 0.0001) when comparing persons with an opioid use disorder to those without an opioid use disorder.
Table 2.
Profiles of opioid use among persons with prevalent episodes of long-term opioid use at the beginning of 2005 at KPNC and GH by substance use disorder (SUD) and opioid disorder in the prior 2 years.
| (N) | Percent of higher dose usersa | Average prescribed doseb | Average daily dosec | Total days supply | Percent with mainly Schedule IId | Percent with mainly long-acting Schedule II | Percentage with 180+ days supply sedative-hypnotics | |
|---|---|---|---|---|---|---|---|---|
| KPNC: prevalent episodes 2005 | ||||||||
| No SUDe | (29,512) | 47.1 | 50.3 | 42.3 | 277.5 | 16.2 | 11.7 | 28.4 |
| SUD | (3676) | 64.1 | 82.6 | 89.4 | 338.5 | 30.6 | 24.3 | 42.1 |
| Opioid disorder | (894) | 80.2 | 114.1 | 136.6 | 396.3 | 46.9 | 40.3 | 47.3 |
| GH: prevalent episodes 2005 | ||||||||
| No SUDe | (5155) | 43.5 | 46.0 | 41.7 | 288.4 | 44.5 | 22.8 | 27.6 |
| SUD | (400) | 63.5 | 75.9 | 85.5 | 339.5 | 60.3 | 39.8 | 39.5 |
| Opioid disorder | (65) | 83.1 | 94.0 | 109.7 | 384.5 | 67.7 | 52.3 | 53.9 |
Defined as average daily dose of 20+ mg morphine equivalents.
Average prescribed dose is the total morphine equivalents of all fills divided by total days supply of all fills, that is, the estimated average maximum allowed daily dose prescribed as opposed to the average daily dose consumed.
Average daily dose is the total morphine equivalents of all fills divided by the duration of use in days.
Schedule II = morphine sulfate; codeine sulfate; hydromorphone; meperidine; fentanyl transmucoSUl; and oxymorphone, oxycodone, morphine sulfate SR; fentanyl transdermal; levorphanol; oxycodone CR; and methadone, hydromorphone SR, oxymorphone SR.
All comparisons between no SUD and SUD, and between no SUD and opioid disorders are significant (p < 0.0001).
For example, for those with substance use disorders and prevalent long-term episodes, the average daily dose morphine equivalent supply was 89 mg morphine equivalents versus 42 mg in KPNC and 86 mg versus 42 mg in GH. Rates of predominant use of long-acting Schedule II opioids were also significantly higher in both health plans for those with substance use disorders than for those without substance use disorders (KPNC 24% versus 12%, and GH, 40% versus 23%), and in both health plans more of those with a substance use (and those with an opioid use) disorder than those without a substance use disorder were prescribed 180+ days supply of sedative-hypnotics (e.g., at KPNC, 47% with an opioid disorder versus 28% without an opioid disorder, and at GH, 54% versus 28%). It should be noted that the absolute rates for each category were higher among those with opioid use disorders than among those with substance use disorders. Profiles of incident episodes of long-term use in 2005 showed similar patterns, but the differences between individuals with and without a prior substance use (or opioid use) disorder were smaller (not shown).
For those who initiated a new long-term episode in any year and had an opioid or other substance dependence or abuse diagnosis recorded with a medical visit in the prior 2 years, we determined how many received their diagnoses from Chemical Dependency or Psychiatry departments. Of those with a substance use disorder, 39% at GH and 34% at KPNC received their diagnosis in those departments only. Of those with a prior opioid use disorder, 74% at KPNC and 54% at GH received their diagnoses solely in those departments.
4. Discussion
Data from these two health plans show that individuals with both substance use and opioid use disorders more often initiated and continued long-term opioid therapy for non-cancer pain than others. These differences were seen for both incident and prevalent episodes of long-term opioid prescribing. Rates for prevalent use were about four times higher for those with substance use disorders than for those without substance use disorders, and were seven to eight times higher for the subset with an opioid dependence or abuse diagnosis. By 2005, one in every six patients with a recent history of substance abuse treatment was receiving opioids long-term, and half of the patients with a prior opioid disorder diagnosis were receiving opioids long-term. Long-term opioid users with prior drug and alcohol diagnoses were younger than those without prior drug and alcohol diagnoses, and women were over-represented among those with prior opioid disorder diagnoses. Those with substance use disorders and the subset with opioid use disorders were much more likely to receive higher doses, more days supply, and more potent Schedule II opioids. They also had twice the rates of concurrent use of sedative-hypnotic medications. Differences in long-term use were due to differences in sustaining use more than to differences in initial prescribing.
These results add a new dimension to the literature by examining community practice patterns of prescribing opioids for individuals with substance use disorders diagnosed prior to receiving opioid prescriptions for pain. Others have found increased prevalence of prescription drug abuse in community and CD treatment populations [7,9]. Although persons with substance abuse histories were only somewhat more likely to initiate an episode of opioid use, they were much more likely to progress to long-term use.
Patients with substance use disorders commonly have co-occurring psychiatric disorders associated with pain and other physical symptoms that prompt opioid prescriptions. But those with opioid dependence are often not found in opioid treatment programs, perhaps because they lack access, or because they prefer to get treatment in a medical setting due to the inconvenience and stigma of methadone maintenance programs. Patients in methadone programs also have high rates of chronic pain [32,39]. The higher rates of opioid prescribing among opioid-dependent patients in these medical settings highlight the co-morbid nature of these disorders. These findings help in identifying this co-morbid pain and addiction population within the medical setting.
There are a number of barriers to appropriate assessment of the risks involved in long-term opioid therapy for patients with substance abuse problems. Primary care physicians frequently do not ask their patients about substance abuse histories [16]. Patients may not inform them, fearing they will not be prescribed medications, or sufficient medications, for their pain, or because of concerns about stigma and privacy in view of legal sanctions regarding use (or past use) of illicit drugs, or concerns about jeopardizing future health coverage. Some patients may also feign pain symptoms to access opioids because of non-pain-related drug addiction. Medical providers have minimal training in addiction medicine and may focus solely on managing pain complaints. Thus, it is unlikely that patients are receiving adequate treatment or monitoring for their substance use problems. This group could be the target of enhanced clinical management of these complex co-morbid conditions.
An organizational factor contributing to the higher rates of long-term opioid prescriptions for those with substance abuse histories is that over one-third of those with prior substance use disorders, and almost three-fourth of those with prior opioid use disorders, received their diagnoses in Chemical Dependency or Psychiatry departments. These diagnoses were likely not known to the prescribing physician; Federal 42CFR Part 2 privacy regulations prohibit any disclosure of chemical dependency visits or diagnoses made in that department (or in Psychiatry when CD is affiliated with Psychiatry), including disclosure in the electronic medical record used by other departments in the same health plan [2,3]. Exceptions are made only for cases where the information is needed to treat a condition posing an immediate threat to health and which requires immediate medical intervention (42 CFR. Sec. 2.51a) [17]. In that case, the medical record must document the names of the medical personnel disclosed to, the name of the individual disclosing, the date and time of the disclosure, and the nature of the emergency (42CFR. Sec. 2.51a). This process is not undertaken often or lightly.
This regulation has clear clinical and public health implications. The efficacy of opioid use for pain in patients with substance use problems is not known [10,34]. In addition, some studies show that a substance abuse history is a risk factor for future opioid misuse [30,33,37], but few rigorous studies have assessed the implications of this history for future opioid prescription use among chronic pain patients. We were not able to examine whether physicians knew their patients had substance use disorders prior to the initial prescription or during the period of ongoing prescriptions, or if they knew while prescribing higher dosages and for longer periods. We would expect that at least some physicians did not know.
Some maintain that a substance abuse history should contraindicate long-term opioid therapy or that increased prescriptions are associated with more problems [26], and that deception is difficult to detect [24]. Others have made the case that with appropriate monitoring most individuals with substance use disorders and pain can be managed [43,44]. In the absence of controlled studies of the risks and benefits of long-term opioid therapy for non-cancer pain among patients with a substance abuse history, empirical research cannot resolve this question. However, our results indicate that exposure to long-term opioid therapy is substantially greater among persons with a substance abuse history than among persons without a substance abuse history.
The extent to which higher prescription rates are due to inappropriate patient drug-seeking behavior is not known. Our findings and the policy issues surrounding non-disclosure of substance abuse diagnoses call for careful attention to assessment and screening [5], early detection, and development of more effective clinical management processes [12,13,27], as well as of efficacy studies for this population. The decline in trends for this group suggests that attention to screening and prescription practices may be increasing as concern grows about prescription drug abuse and other potential adverse effects of long-term opioid use.
Limitations include the study’s reliance on administrative data. We were not able to determine whether persons with a history of substance use disorders are more likely to need opioid therapy for chronic pain or not, including whether they have lower pain thresholds, or whether they are manipulating the system to access medication that contributes to their substance use problems. Our data only allow us to describe the change in these rates over time and the magnitude of the difference, and to estimate the proportion of individuals for whom the physicians have information on substance abuse disorders without their conducting a comprehensive screening. Since these patients are viewed as higher risk, the increased use of long-term opioid therapy suggests the importance of improved understanding of the benefits and risks of opioid therapy among persons with a history of substance abuse, and the need for more careful screening for substance abuse history than is the usual practice.
Opioid use disorders, in particular, may be over-diagnosed for those who had already been prescribed opioids, given the confusion between substance use disorders (addiction) and physiological dependence, which often occurs with regular opioid use. This confusion is less likely to occur in psychiatric and CD treatment settings. Over-diagnosis bias would more likely affect our analysis of prevalent long-term opioid prescribing, because some substance use diagnoses might have been made after opioid prescribing began. However, we found similar patterns in our analyses of incident long-term opioid therapy, where all opioid and other substance use disorder diagnoses preceded the long-term episode. In any case, it is unlikely that this potential bias explains the very large and consistent differences found. Another limitation is that individuals may be accessing medications from outside the health plans [20] or diverting them to others. KPNC and GH are integrated health plans and findings may not generalize to other types of health plans. At the same time, results are consistent with what we would expect from general population and CD treatment research. We suggest that our findings may be conservative, since these are integrated health plans and likely share records more than those with contracted CD programs, and also given that they have chronic pain programs and guidelines for care not common to all health plans or public sector care.
Acknowledgments
This research was supported by Grant #R01 DA022557 from the National Institute on Drug Abuse, National Institutes of Health. The authors thank Joe V. Selby, MD, MPH for comments on an earlier draft, and Agatha Hinman, BA, for her editorial assistance. No authors report any financial disclosures.
Footnotes
URL: http://www.dor.kaiser.org/staff/investigators/weisner.shtml (C.M. Weisner).
References
- 1.American Academy of Pain Medicine, American Pain Society. The use of opioids for the treatment of chronic pain. [accessed May 9, 2008];A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Available from: http://www.ama-assn.org/ama1/pub/upload/mm/455/opioidschronicpain.pdf.
- 2.Beckerman JZ, Pritts J, Goplerud E, Leifer JC, Borzi PC, Rosenbaum S, Anderson DR. A delicate balance: behavioral health, patient privacy, and the need to know. California HealthCare Foundation; [accessed June 9, 2008]. Available from: http://www.chcf.org/documents/chronicdisease/ADelicateBalanceBehavioralHealthAndPrivacyIB.pdf. [Google Scholar]
- 3.Beckerman JZ, Pritts J, Leifer JC, Borzi PC, Rosenbaum S. Health information privacy, patient safety, and health care quality: issues and challenges in the context of treatment for mental health and substance use. [accessed June 9, 2008];Health Care Policy Report. 16 Available from: http://ihcrp.georgetown.edu/pdfs/pritts0208.pdf.
- 4.Bhamb B, Brown D, Hariharan J, Anderson J, Balousek S, Fleming MF. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin. 2006;22:1859–65. doi: 10.1185/030079906X132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloodworth D. Opioids in the treatment of chronic pain: legal framework and therapeutic indications and limitations. Phys Med Rehabil Clin N Am. 2006;17:355–79. doi: 10.1016/j.pmr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–8. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 7.Carise D, Dugosh KL, McLellan AT, Camilleri A, Woody GE, Lynch KG. Prescription OxyContin abuse among patients entering addiction treatment. Am J Psychiatry. 2007;164:1750–6. doi: 10.1176/appi.ajp.07050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Center for substance abuse treatment. Percentage of positive employee drug tests containing marijuana and cocaine decreases; opiates and amphetamines increases over past 10 years. [accessed May 8, 2008];Cesar Fax. 17 Available from: http://www.cesar.umd.edu/cesar/cesarfax/vol17/17-13.pdf.
- 10.Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: a systematic review. J Pain Symptom Manage. 2003;26:1026–48. doi: 10.1016/j.jpainsymman.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83:S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–7. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.D’Arcy Y, McCarberg B. Pain management: patients with a substance use disorder. Nurse Pract. 2007;32:36–44. doi: 10.1097/01.NPR.0000287469.11754.a5. [DOI] [PubMed] [Google Scholar]
- 14.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–62. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Edlund MJ, Sullivan M, Steffick D, Harris KM, Wells KB. Do users of regularly prescribed opioids have higher rates of substance use problems than nonusers? Pain Med. 2007;8:647–56. doi: 10.1111/j.1526-4637.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 16.Edlund MJ, Unutzer J, Wells KB. Clinician screening and treatment of alcohol, drug, and mental problems in primary care: results from healthcare for communities. Med Care. 2004;42:1158–66. doi: 10.1097/00005650-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Electronic code of federal regulations. Title 42: public health. [accessed June 27, 2008];Part 2— confidentiality of alcohol and drug abuse patient records. Available from: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr;sid=cd0e791854053a546c17c86a675d7eac;rgn=div6;view=text;node=42%3A1.0.1.1.2.4;idno=42;cc=ecfr.
- 18.Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62:847–54. doi: 10.1111/j.1541-0420.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8:573–82. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forman RF. Innovations: alcohol & drug abuse: narcotics on the net: the availability of web sites selling controlled substances. Psychiatr Serv. 2006;57:24–6. doi: 10.1176/appi.ps.57.1.24. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283:1710–4. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 24.Jung B, Reidenberg MM. Physicians being deceived. Pain Med. 2007;8:433–7. doi: 10.1111/j.1526-4637.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 25.Katz N. Opioids: after thousands of years, still getting to know you. Clin J Pain. 2007;23:303–6. doi: 10.1097/AJP.0b013e31803cb905. [DOI] [PubMed] [Google Scholar]
- 26.Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–51. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- 27.Kuehn BM. Scientists probe ways to curb opioid abuse without hindering pain treatment. JAMA. 2007;297:1965–7. doi: 10.1001/jama.297.18.1965. [DOI] [PubMed] [Google Scholar]
- 28.Manchikanti L, Manchukonda R, Pampati V, Damron KS. Evaluation of abuse of prescription and illicit drugs in chronic pain patients receiving short-acting (hydrocodone) or long-acting (methadone) opioids. Pain Physician. 2005;8:257–61. [PubMed] [Google Scholar]
- 29.Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 30.Matzger H, Weisner C. Nonmedical use of prescription drugs among a longitudinal sample of dependent and problem drinkers. Drug Alcohol Depend. 2007;86:222–9. doi: 10.1016/j.drugalcdep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 31.McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction. 2007;102:1920–30. doi: 10.1111/j.1360-0443.2007.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17:327–33. doi: 10.1046/j.1525-1497.2002.10625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage. 2008;35:214–28. doi: 10.1016/j.jpainsymman.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Saunders KW, Davis RL, Stergachis A. Group health cooperative. In: Strom BL, editor. Pharmacoepidemiology. New York: Wiley; 2005. pp. 223–39. [Google Scholar]
- 36.Schieffer BM, Pham Q, Labus J, Baria A, Van Vort W, Davis P, Davis F, Naliboff BD. Pain medication beliefs and medication misuse in chronic pain. J Pain. 2005;6:620–9. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Sees KL, Di Marino ME, Ruediger NK, Sweeney CT, Shiffman S. Non-medical use of OxyContin tablets in the United States. J Pain Palliat Care Pharmacother. 2005;19:13–23. [PubMed] [Google Scholar]
- 38.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. New York: Wiley; 2005. pp. 241–59. [Google Scholar]
- 39.Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med. 2008;11 doi: 10.1111/j.1526-4637.2008.00420.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–93. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 41.Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207–9. doi: 10.1016/j.pain.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver M, Schnoll S. Abuse liability in opioid therapy for pain treatment in patients with an addiction history. Clin J Pain. 2002;18:S61–9. doi: 10.1097/00002508-200207001-00007. [DOI] [PubMed] [Google Scholar]
- 44.Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573–84. doi: 10.1111/j.1526-4637.2006.00254.x. [DOI] [PubMed] [Google Scholar]