Abstract
We used an experimental rat model to compare the therapeutic efficacy of teicoplanin, linezolid, and quinupristin/dalfopristin with that of vancomycin as standard therapy for infective endocarditis.
Aortic endocarditis was induced in rats by insertion of a polyethylene catheter into the left ventricle, followed by intravenous inoculation of 106 colony-forming units of methicillin-resistant Staphylococcus aureus 24 hours later. Forty-eight hours after bacterial challenge, intravenous antibiotic therapies were initiated. There were 6 groups of 8 rats each: uninfected control; infected, untreated control; vancomycin-treated (40 mg/kg twice daily); teicoplanin-treated (20 mg/kg twice daily after a loading dose of 40 mg/kg); linezolid-treated (75 mg/kg 3 times daily for 1 day, then 75 mg/kg twice daily); and quinupristin/dalfopristin-treated (30 mg/kg twice daily and an additional 10 mg/kg dalfopristin infusion over 6 to 12 hr daily). At the end of therapy, the aortic valve vegetations in the drug-treated rats were evaluated microbiologically.
Compared with the infected, untreated group, all drug-treated groups had significantly reduced bacterial titers in the vegetations. Vancomycin, teicoplanin, and quinupristin/dalfopristin all effectively reduced the quantitative bacterial cultures of aortic valve vegetations. In addition, there was no significant difference in the comparative efficacy of teicoplanin, linezolid, and quinupristin/dalfopristin. Vancomycin significantly reduced bacterial counts in comparison with linezolid, which was nonetheless also effective.
Our experimental model showed that each of the investigated antimicrobial agents was effective in the treatment of infective endocarditis.
Key words: Anti-bacterial agents/pharmacology/therapeutic use; disease models, animal; drug resistance, microbial; endocarditis, bacterial/microbiology/drug therapy; linezolid; methicillin resistance; microbial sensitivity tests; rodents; staphylococcal infections/epidemiology; Staphylococcus aureus/drug effects; teicoplanin; vancomycin
Infective endocarditis is a severe disease with high morbidity and mortality rates. Staphylococcus aureus is the most frequent cause of endocarditis. Endocarditis from methicillin-resistant S. aureus (MRSA) is associated with higher mortality rates and lower bacteriologic eradication than is endocarditis from methicillin-sensitive strains. The glycopeptide antibiotic vancomycin is the standard therapy for MRSA endocarditis; however, the reported rate of treatment failure is high. In addition, staphylococci have become less susceptible to vancomycin.1–4 Teicoplanin is a glycopeptide antibiotic that is tolerated better than vancomycin, and its half-life is longer.5 Although available for years in Europe, teicoplanin is not standard therapy for endocarditis and is not available at all in the United States.1
The prevalence of MRSA is increasing worldwide. Most strains are now resistant to fluoroquinolones, macrolides, tetracycline, and aminoglycosides.6,7 There are few options for treating MRSA endocarditis and similar infections. Linezolid and quinupristin/dalfopristin (Q/D) are other agents that are active against gram-positive cocci, including MRSA. However, regarding the efficacy of these drugs in the treatment of endocarditis, data are limited and randomized controlled trials are warranted.8
Using an experimental rodent endocarditis model, we evaluated the therapeutic efficacy of teicoplanin, linezolid, and Q/D in comparison with vancomycin in the treatment of MRSA endocarditis.
Materials and Methods
This study was approved by the Local Animal Research Ethics Committee. The experimental animals were kept at our institution's Animal Research Laboratory under veterinary supervision during the study. All received humane care in compliance with the Principles of Laboratory Animal Care, formulated by the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences.9
Organisms and Susceptibility Testing
The strain of MRSA used in this study was isolated from a blood culture from a patient who had endocarditis. Identification of the clinical isolate was specified by gram-staining, catalase reaction, tube coagulation test, and API® staph test (bioMèrieux SA; Craponne, France). Methicillin sensitivity was evaluated by means of an oxacillin disk diffusion test.10 The antimicrobial susceptibility of the strain was determined by use of the microbroth dilution method, in accordance with outlined procedures and specifications.11,12 The lowest antibiotic concentration at which observable growth was inhibited was accepted as the minimum inhibition concentration. According to the microbroth dilution method, the clinical isolate was susceptible to vancomycin, teicoplanin, linezolid, and Q/D. In the strain of S. aureus that was used in this study, the minimal inhibitory concentrations were 1 μg/mL for vancomycin and teicoplanin, 2 μg/L for linezolid, and 0.5 μg/L for Q/D.
Drugs
Vancomycin (Abbott France, S.A.; Rungis Cedex, France), teicoplanin (Aventis Pharma S.p.A.; Lainate, Italy), linezolid (Pfizer AS; Lysaker, Norway), and Q/D (VLG Chem; Vienneuve-la-Garenne, France) were diluted in accordance with their manufacturers' recommendations. All drug solutions were freshly made on the day of assay.
In Vivo Rat Model
To investigate antibiotic treatment regimens, we used an established experimental model of aortic valve endocarditis.13,14 Rats were anesthetized with a 2:1 mixture of ketamine hydrochloride (100 mg/mL) and xylazine hydrochloride (20 mg/mL) at a dose of 0.75 mL/kg intramuscularly. Aortic endocarditis was induced in the rats by inserting a polyethylene catheter into the left ventricle, followed by intravenous (IV) inoculation of 106 colony-forming units (CFU/mL) of MRSA 24 hours later. Forty-eight hours after bacterial challenge, therapy with each antimicrobial agent was initiated, for 3 days. The catheters were left in place during the entire experiment. After the rats were humanely killed, infective endocarditis was confirmed when vegetations across the aortic valve were seen or were determined from the bacteriologic culture results. Experiments were limited to 3 days, for ethical reasons. Catheter placement was proper in every animal.
We randomly divided 48 adult male Wistar rats (weight, 300–350 g) into 6 groups of 8: uninoculated control rats (group 1) were neither infected nor treated; infected, untreated control rats (group 2) received no drug treatment; vancomycin-treated rats (group 3) were given a 40 mg/kg IV dose twice daily; teicoplanin-treated rats (group 4) were given a 20 mg/kg IV dose twice daily after a loading dose of 40 mg/kg; linezolid-treated rats (group 5) were given a 75 mg/kg IV dose 3 times daily for 1 day, then twice daily; and Q/D-treated rats (group 6) were given a 30 mg/kg IV dose twice daily and an additional 10 mg/kg dalfopristin infusion over 6 to 12 hr daily due to its short half-life in rats.
The untreated control animals (groups 1 and 2) were killed at treatment onset (48 hr after inoculation) in order to measure the frequency and severity of valve infection at the initiation of therapy. The drug-treated rats were killed 12 hours after being given the last dose of antibiotic.
Evaluation of Infection
The aortic valve vegetations were removed, weighed, homogenized in 1 mL of sterile phosphate-buffered saline solution, and serially diluted. The serial diluted homogenates were inoculated (0.01 mL) onto blood agar and incubated at 37 °C. After 48 hours, colonies of S. aureus growing on the agar plates were counted, and the results were determined as CFU/g tissue. The number of bacteria was converted to log10 CFU/g of tissue.
Statistical Analysis
Quantitative culture results were presented as arithmetic mean ± SD of log10 CFU/g of vegetation. Differences among the groups were investigated using 1-way analysis of variance. Multiple comparisons between the groups were performed using a post hoc test (the Tukey Honest Significant Difference test). Differences of P <0.05 were considered statistically significant. Data were analyzed by SPSS statistical software for Windows, version 11.0 (SPSS Inc.; Chicago, Ill). The data conformed to each test by which they were analyzed.
Results
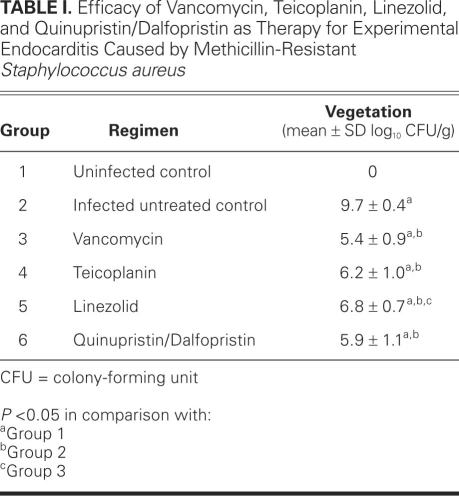
Table I shows the bacterial counts in each group. Each of the 4 antimicrobial agents significantly reduced mean bacterial titers in aortic valve vegetations in comparison with the titers in group 2 (all P <0.05). No significant differences were determined in the mean bacterial titers in vegetations among the vancomycin-, teicoplanin-, and Q/D-treated groups (all P >0.05). In addition, no significant difference was found in the linezolid-treated group in comparison with the teicoplanin- and Q/D-treated groups (both P >0.05). In contrast, vancomycin significantly reduced bacterial counts compared with linezolid (P <0.05). Although less effective than vancomycin, linezolid nevertheless significantly reduced the amount of growing microorganisms in the aortic valve vegetations in comparison with the amounts found in the group 2 animals.
TABLE I. Efficacy of Vancomycin, Teicoplanin, Linezolid, and Quinupristin/Dalfopristin as Therapy for Experimental Endocarditis Caused by Methicillin-Resistant Staphylococcus aureus
No rats in group 1 and all rats in group 2 showed anatomic or microbiologic evidence of endocarditis. Rats in the drug-treated groups also showed macroscopic evidence of infection, but to varying and diminished degrees in comparison with the animals in group 2. We observed no clinical evidence of drug-related adverse effects, such as anorexia, vomiting, diarrhea, or behavioral alterations in any of the treated rats.
Discussion
Linezolid,15 Q/D,16 vancomycin,17 and teicoplanin18,19 were previously shown to be effective in the treatment of MRSA endocarditis; however, no comparison among them had been reported. We performed our experimental study in accordance with models described previously,13,14 and with drug doses that were used in earlier studies.16–18
Although glycopeptide antibiotics remain the standard therapy for serious systemic infections from resistant S. aureus strains,20 several authors have reported the emergence of strains that are less susceptible to these antibiotics, which indicates an increased need for new therapeutic options.21,22 In addition, some patients cannot tolerate vancomycin because of side effects, such as renal failure and hearing loss.23,24 Previous investigators found that linezolid and Q/D provided effective antimicrobial activity against a wide variety of gram-positive cocci, including MRSA.25–27 We found the same, except that linezolid's effect was significantly less than that of vancomycin.
Endocarditis is a life-threatening infection, and bactericidal antibiotics are the preferred therapy. Despite recent medical advances, the mortality rate in staphylococcal endocarditis remains high.1 One reason behind the failure of antibacterial therapy is inadequate penetration of the drug within the infection site.28 However, a relatively good concentration of vancomycin—the standard antimicrobial therapy in MRSA endocarditis—has been found in heart tissue.29 In addition, the effective penetration of vancomycin into vegetations has been shown in an experimental endocarditis model.30 Conversely, the penetration of teicoplanin into the heart has been found to be adequate,31 but the drug was concentrated only at the periphery of the vegetation.32 Quinu-pristin has been found to be homogeneously distributed within infected vegetations, but dalfopristin intensified only at the periphery,33 which may affect the clinical bactericidal effectiveness of the compound in combination.
Whereas vancomycin, teicoplanin, and Q/D are bactericidal, linezolid exerts bacteriostatic activity against staphylococci and kills them slowly.34 For that reason, it is not recommended as a 1st-line agent to treat staphylococcal endocarditis. However, because the bioavailability of oral linezolid is very high, it can be administered orally when the patient's medical condition becomes stable after IV therapy.25
Studies of linezolid therapy have produced controversial results,35,36 but the findings warrant further investigation. In the treatment of MRSA endocarditis, Chiang and Climo17 showed (similarly to our study) that vancomycin alone was more effective than was linezolid alone, and they found in addition that vancomycin alone was more effective than was the combination of linezolid and vancomycin.
Quinupristin/dalfopristin, a semisynthetic injectable streptogramin, is a combination of 2 synergistic antibiotic components that are derived from pristinamycin37: a type B streptogramin (quinupristin) and a type A streptogramin (dalfopristin), in a 30:70 weight-to-weight ratio. Separately, both drugs are bacteriostatic; in combination, they exert bactericidal activity. Quinupristin/dalfopristin and vancomycin were similarly active in the treatment of experimental endocarditis that was caused by S. aureus strains with various susceptibilities.33,38 Although Q/D was very effective therapy for infective endocarditis in our experimental model, this drug does not yet have U.S. Food and Drug Administration approval as therapy for MRSA endocarditis.8
Limitations of the Study
Our study has several limitations. The aim of this study did not include the evaluation of combination therapies, which will heavily influence consensus regarding the treatment of endocarditis. We did not determine serum antibiotic concentrations in the rats; rather, we administered the antibiotics at doses similar to validated human doses and investigated efficacy by evaluating the presence and amount of the staphylococcal strains in the infected areas. The biochemical reactions in our animal model are not directly comparable with biochemical reactions that would be expected in human beings, which would surely affect the final outcome of the applied drug. Further preclinical and clinical comparisons of vancomycin with teicoplanin, linezolid, and Q/D are needed to determine the role of these last 3 drugs in the treatment of MRSA endocarditis.
Footnotes
Address for reprints: Mustafa Sacar, MD, Pamukkale Universitesi Tip Fakultesi Hastanesi, Kinikli, 20070 Denizli, Turkey
E-mail: mustafasacar@hotmail.com
References
- 1.Fowler VG Jr, Scheld WM, Bayer AS. Endocarditis and intravascular infections. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. p. 975–1022.
- 2.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169(5):463–73. [DOI] [PMC free article] [PubMed]
- 3.Staphylococcus aureus resistant to vancomycin–United States, 2002. MMWR Morb Mortal Wkly Rep 2002;51(26):565–7. [PubMed]
- 4.Hill EE, Peetermans WE, Vanderschueren S, Claus P, Herregods MC, Herijgers P. Methicillin-resistant versus methicillin-sensitive Staphylococcus aureus infective endocarditis. Eur J Clin Microbiol Infect Dis 2008;27(6):445–50. [DOI] [PubMed]
- 5.Campoli-Richards DM, Brogden RN, Faulds D. Teicoplanin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential [published erratum appears in Drugs 1991;41(5):716]. Drugs 1990;40(3):449–86. [DOI] [PubMed]
- 6.Schindler B. Worldwide spread of resistant Staphylococcus [in German]. Med Monatsschr Pharm 2007;30(4):155–6. [PubMed]
- 7.Santos Sanches I, Mato R, de Lencastre H, Tomasz A; CEM/NET Collaborators and the International Collaborators. Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb Drug Resist 2000;6(3): 199–211. [DOI] [PubMed]
- 8.Drees M, Boucher H. New agents for Staphylococcus aureus endocarditis. Curr Opin Infect Dis 2006;19(6):544–50. [DOI] [PubMed]
- 9.Institute of Laboratory Animal Resources National Research Council: Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; 1996.
- 10.Kloos WE, Barnerman TL. Staphylococcus and micrococcus. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 7th ed. Washington (DC): ASM Press; 1999. p. 264–77.
- 11.Wikler MA, Low DE, Cockerill FR, Sheehan DJ, Craig WA, Tenover FC, Dudley MN. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 7th ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2006. p. M7-A7.
- 12.Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swenson JM. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. Wayne (PA): National Committee for Clinical Laboratory Standards; 1997. p. M26-A.
- 13.Perlman BB, Freedman LR. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med 1971;44(2):206–13. [PMC free article] [PubMed]
- 14.Durack DT, Beeson PB. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol 1972;53 (1):44–9. [PMC free article] [PubMed]
- 15.Dailey CF, Pagano PJ, Buchanan LV, Paquette JA, Haas JV, Gibson JK. Efficacy of linezolid plus rifampin in an experimental model of methicillin-susceptible Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 2003;47(8): 2655–8. [DOI] [PMC free article] [PubMed]
- 16.Entenza JM, Drugeon H, Glauser MP, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or-resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob Agents Chemother 1995;39(7):1419–24. [DOI] [PMC free article] [PubMed]
- 17.Chiang FY, Climo M. Efficacy of linezolid alone or in combination with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2003;47(9):3002–4. [DOI] [PMC free article] [PubMed]
- 18.Pavie J, Lefort A, Ploy MC, Massias L, Chau F, Garry L, et al. Influence of reduced susceptibility to glycopeptides on activities of vancomycin and teicoplanin against Staphylococcus aureus in experimental endocarditis. Antimicrob Agents Chemother 2003;47(6):2018–21. [DOI] [PMC free article] [PubMed]
- 19.Wilson AP, Gaya H. Treatment of endocarditis with teicoplanin: a retrospective analysis of 104 cases. J Antimicrob Chemother 1996;38(3):507–21. [DOI] [PubMed]
- 20.Rubinstein E, Carbon C. Staphylococcal endocarditis—recommendations for therapy. Clin Microbiol Infect 1998;4 Suppl 3:S27-S33. [PubMed]
- 21.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997;350(9092):1670–3. [DOI] [PubMed]
- 22.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, et al. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 1999;340(7): 493–501. [DOI] [PubMed]
- 23.Fekety R. Vancomycin, teicoplanin, and the streptogramins: quinupristin and dalfopristin. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 382–92.
- 24.Traber PG, Levine DP. Vancomycin ototoxicity in patient with normal renal function. Ann Intern Med 1981;95(4):458–60. [DOI] [PubMed]
- 25.Stevens DL, Dotter B, Madaras-Kelly K. A review of linezolid: the first oxazolidinone antibiotic. Expert Rev Anti Infect Ther 2004;2(1):51–9. [DOI] [PubMed]
- 26.Fass RJ. In vitro activity of RP 59500, a semisynthetic injectable pristinamycin, against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother 1991;35(3):553–9. [DOI] [PMC free article] [PubMed]
- 27.Leclercq R, Nantas L, Soussy CJ, Duval J. Activity of RP 59500, a new parenteral semisynthetic streptogramin, against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J Antimicrob Chemother 1992;30 Suppl A:67–75. [DOI] [PubMed]
- 28.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin Infect Dis 2004;38 (6):864–70. [DOI] [PubMed]
- 29.Torres JR, Sanders CV, Lewis AC. Vancomycin concentration in human tissues–preliminary report. J Antimicrob Chemother 1979;5(4):475–7. [DOI] [PubMed]
- 30.Nicolau DP, Freeman CD, Nightingale CH, Coe CJ, Quintiliani R. Minocycline versus vancomycin for treatment of experimental endocarditis caused by oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1994;38(7): 1515–8. [DOI] [PMC free article] [PubMed]
- 31.Martin C, Bourget P, Alaya M, Sertin A, Atlani C, Ennabli K, Said R. Teicoplanin in cardiac surgery: intraoperative pharmacokinetics and concentrations in cardiac and mediastinal tissues. Antimicrob Agents Chemother 1997;41(5):1150–5. [DOI] [PMC free article] [PubMed]
- 32.Cremieux AC, Maziere B, Vallois JM, Ottaviani M, Azancot A, Raffoul H, et al. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis 1989;159(5):938–44. [DOI] [PubMed]
- 33.Fantin B, Leclercq R, Ottaviani M, Vallois JM, Maziere B, Duval J, et al. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother 1994;38(3):432–7. [DOI] [PMC free article] [PubMed]
- 34.Jacqueline C, Batard E, Perez L, Boutoille D, Hamel A, Caillon J, et al. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob Agents Chemother 2002;46(12):3706–11. [DOI] [PMC free article] [PubMed]
- 35.Bassetti M, Di Biagio A, Del Bono V, Cenderello G, Bassetti D. Successful treatment of methicillin-resistant Staphylococcus aureus endocarditis with linezolid. Int J Antimicrob Agents 2004;24(1):83–4. [DOI] [PubMed]
- 36.Corne P, Marchandin H, Macia JC, Jonquet O. Treatment failure of methicillin-resistant Staphylococcus aureus endocarditis with linezolid. Scand J Infect Dis 2005;37(11–12):946–9. [DOI] [PubMed]
- 37.Bouanchaud DH. In-vitro and in-vivo synergic activity and fractional inhibitory concentration (FIC) of the components of a semisynthetic streptogramin, RP 59500. J Antimicrob Chemother 1992;30 Suppl A:95–9. [DOI] [PubMed]
- 38.Zarrouk V, Bozdogan B, Leclercq R, Garry L, Carbon C, Fantin B. Influence of resistance to streptogramin A type antibiotics on the activity of quinupristin-dalfopristin in vitro and in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother 2000;44(5):1168–73. [DOI] [PMC free article] [PubMed]