Abstract
In pulmonary arterial hypertension, the blood vessels that carry blood between the heart and lungs are constricted, making it difficult for the heart to pump blood through the lungs. Prostacyclin, a prostanoid metabolized from endogenous arachidonic acid through the cyclooxygenase (COX) pathway, is a potent vasodilator that has been identified as one of the most effective drugs for the treatment of pulmonary arterial hypertension. Currently, prostacyclin and its analogues are widely used in the clinical management of pulmonary arterial hypertension patients. However, the mortality rate associated with pulmonary arterial hypertension has not been significantly reduced within the past 5 years. More powerful therapeutic approaches are needed. This article briefly reviews the current management of pulmonary arterial hypertension to identify the problems associated with present therapies; then it focuses on the emerging technology of prostacyclin synthase gene therapy and cell-based therapy using native stem cells and engineered stem cells with enhanced prostacyclin production capacity. By using the recent advances in technology and the molecular understanding of prostacyclin synthesis, researchers are prepared to make significant advances in the treatment of pulmonary arterial hypertension.
Key words: Antihypertensive agents; cell therapy; gene therapy; genetic predisposition to disease; hypertension, pulmonary/classification/drug therapy/etiology/genetics; hypertrophy, right ventricular; monocrotaline; prostacyclin; prostacyclin synthetase; pulmonary artery/physiopathology; receptors, endothelin/antagonists & inhibitors; vascular diseases/drug therapy; vasodilator agents
Pulmonary hypertension, a devastating disease with a high mortality rate, is characterized by increased pressure in the pulmonary arteries that leads to remodeling of the right ventricle (RV) and, ultimately, to right-heart failure. The most recent classification scheme divides pulmonary hypertension into 5 separate categories.1 Group 1 comprises patients with pulmonary arterial hypertension (PAH), which will be the main subject of this review. Pulmonary arterial hypertension patients have reduced levels of the potent vasodilator prostacyclin, which leads to constriction of the pulmonary vasculature. Idiopathic pulmonary hypertension, which can occur from infancy into late adulthood, has the strongest genetic component2 and the worst prognosis. Pulmonary arterial hypertension associated with other diseases has a better outcome. Despite the differences in origin, the pathophysiology of PAH at the cellular level is largely the same.3–5
This review briefly describes the current clinical methods of treating PAH in an effort to identify the problems associated with the present regimens and then focuses on promising new treatment approaches, including gene and cell therapy.
Overview of the Diagnosis and Management of Pulmonary Arterial Hypertension
The diagnosis of PAH depends on clinical suspicion in patients who have risk factors, presenting symptoms, and findings on physical examination (for example, chest radiography and electrocardiography) suggestive of PAH.6,7 In patients in whom suspicion is high, Doppler echocardiography should be performed to measure pulmonary artery pressure and RV systolic pressure, to estimate pulmonary vascular resistance, and to identify morphologic changes associated with PAH.8,9 Echocardiography is sensitive in diagnosing PAH but lacks specificity.8,9 Therefore, other diagnoses must be excluded before arriving at idiopathic PAH. Other tests to support the diagnosis of idiopathic PAH (and to exclude secondary causes of PAH) include pulmonary ventilation/perfusion scans (V/Q scans), pulmonary function tests, overnight oximetry, and serology tests for connective-tissue disorders.8–10 Right-heart catheterization is sometimes required to confirm the diagnosis and to evaluate the hemodynamics of PAH.10,11 Mean pulmonary artery pressure (mPAP) >25 mmHg and pulmonary vascular resistance (PVR) >3 Wood units are the diagnostic criteria for PAH.10,12 Patients with suspected idiopathic PAH should undergo an acute vasodilator response test with inhaled nitric oxide or with intravenously administered epoprostenol or adenosine.4,5,13 A positive vasodilator response suggests that patients may respond to oral calcium channel blocker therapy14,15; however, only about 5% of PAH patients will have a good long-term response to this therapy. For that reason, calcium channel blockers are rarely used in clinical practice at present.
The goals of current treatment regimens are to improve patients' symptoms and quality of life and to reduce death. Some patients receive anticoagulation therapy, such as warfarin, unless this is contraindicated. However, the level of support for anticoagulation therapy is low because of inconclusive data from retrospective analyses of patient registries and small prospective studies. Patients should be placed on diuretics to manage right-heart failure and on oxygen to manage hypoxemia. Patients with idiopathic PAH who have a positive vasodilator response should be placed on oral calcium channel blockers and should be monitored for sustained responsiveness and safety. First-line therapy includes oral endothelin receptor antagonists or phosphodiesterase inhibitors for lower-risk patients and intravenous prostacyclins for higher-risk patients. Prostanoids can be initiated in high-or low-risk patients, if 1st-line therapy fails. Patients not responding to medical therapy or combination medical treatment may be considered for atrial septostomy or lung transplantation.13,16–19
Prostacyclin and Prostacyclin Mimic Therapy
Prostacyclin, a member of the endogenous prostanoids family, is produced from arachidonic acid in a multistep process involving the enzymes prostacyclin synthase and cyclooxygenase (COX).20–22 In the pulmonary circulation, prostacyclin is released by endothelial cells in the pulmonary artery. Cellular and molecular techniques involving x-ray crystallography, nuclear magnetic resonance, and site-directed mutagenesis have been used to better characterize the COX and the prostacyclin synthase enzymes involved in prostacyclin synthesis.23–32 Because of this molecular understanding, an increasing amount of research is now focusing on a potentially new way to treat PAH by specifically upregulating prostacyclin in cells.
The biological functions of prostacyclin in the pulmonary circulation are mediated by a specific cell-surface receptor. Found on platelets and endothelial cells, the prostacyclin receptor belongs to the G-protein coupled receptor (GPCR) class.33 The binding of prostacyclin to the receptor triggers the activation of the G-protein and increases intracellular cAMP, which activates protein kinase A. This causes inhibition of platelet aggregation, relaxation of smooth muscle, and vasodilation of the pulmonary arteries. In this manner, prostacyclin and its analogues can counter the vasoconstrictive mediators, such as endothelin, which are active in PAH, enabling relaxation of the pulmonary arterial vasculature. However, because prostacyclin has a short half-life (only minutes) and primarily works locally, the clinical use of prostacyclin is challenging. Therefore, one of the most important avenues in PAH therapeutic research has been to look for more chemically stable prostacyclin mimics that target the receptor.
Recently, the structural and functional relationships of the prostacyclin receptor have been well characterized.34–39 Key residues involved in mediating prostacyclin binding33,34,36 and signaling27,38 have been identified. A structural model of the human prostacyclin receptor bound with the prostacyclin agonist analogue, iloprost, has been proposed.35 That information has provided an important basis for designing receptor structure-based drugs for PAH.
Intravenous or Subcutaneous Delivery. The use of prostacyclin and its analogues is arguably the most effective approach in the treatment of PAH in the United States.40 Epoprostenol, a synthetic prostacyclin, and iloprost and treprostinil, synthetic prostacyclin analogues, are currently used to treat patients with PAH. These drugs have improved exercise tolerance, breathing, hemodynamic circulation, and survival. Treatment with epoprostenol requires the use of a permanent intravenous catheter and an infusion pump, which may be associated with serious complications such as mechanical malfunction, obstruction, and infection.41 Furthermore, epoprostenol is unstable at room temperature and requires refrigeration and frequent attention during administration. Although treprostinil has pharmacodynamics similar to those of epoprostenol, it can be administered subcutaneously and intravenously. In addition, treprostinil has a long half-life and is stable at room temperature. Treatment with trepostinil improves New York Heart Association (NYHA) classification of heart failure in patients with PAH and symptoms as measured by the Borg dyspnea score.42,43 Furthermore, treprostinil has shown benefits in patients with PAH secondary to connective-tissue diseases.42 When administered intravenously, treprostinil requires double the maintenance dose of epoprostenol, making it twice as expensive.42 However, the chemical stability of treprostinil makes it a better drug to use intravenously. Transitioning from epoprostenol to treprostinil is easy and safe; however, careful follow-up is required with treprostinil because of its hemodynamic effects.43
Delivery by Inhalation. Inhaled treprostinil has been shown to benefit patients with PAH. The inhalation of treprostinil can reduce pulmonary vascular pressure without affecting systemic vascular pressure, thereby making it a safe treatment for PAH.44
Inhaled iloprost is also used to decrease pulmonary arterial resistance in a pulmonary-selective manner.45 In a retrospective study of 79 PAH patients who received iloprost therapy from 1997 through 2001 and who were monitored until 2007, iloprost did not improve long-term survival, despite being associated with immediate clinical improvements.45 However, an earlier study suggests that iloprost has an anti-remodeling effect on the pulmonary vasculature in experimental PAH.46,47 Furthermore, inhaled iloprost shows promise in identifying patients with idiopathic PAH who may respond well to calcium channel blockers.48
A recent study in rats compared the effects of inhaled nitric oxide with those of iloprost on pulmonary arterial pressure.45 Congestive heart failure (CHF) was induced in the rats by supracoronary aortic banding. Then the rats inhaled iloprost (3-min inhalations at 45-min intervals), nitric oxide (continuous), or 0.9% normal saline (continuous). Other groups were given intravenous iloprost, sodium nitroprusside, or 0.9% sodium chloride. Interestingly, no systemic or pulmonary effects were observed in the non-CHF control rats who received any of the 3 inhaled treatments. However, in rats with induced CHF that inhaled nitric oxide or iloprost, pulmonary arterial pressure was reduced without systemic hemodynamic effects. In contrast, in rats given intravenous iloprost or nitric oxide (via sodium nitroprusside), pulmonary arterial pressure and systemic vascular pressure decreased. The authors concluded that inhaled iloprost and nitric oxide are superior to intravenous infusion of iloprost and nitroprusside. Moreover, inhaled iloprost may be superior to inhaled nitric oxide, because of its selectivity.45
Prostanoid Combination Therapy
The use of prostanoids in combination with other drugs selective for the pulmonary circulation is a viable choice for PAH therapy. Iloprost in combination with tolafentrine, a dual selective phosphodiesterase 3/4 inhibitor, was used to treat chronic monocrotaline-induced PAH in rats.49 The dual regimen resulted in normalization of RV size as well as monocrotaline-induced hemodynamic changes in the pulmonary circulation. Although single therapy with tolafentrine or inhaled iloprost has been shown to reverse the remodeling process in the pulmonary vascular wall, leading to normalization of hemodynamics, the combination of the 2 drugs resulted in significantly better improvements.45,46,49
Sildenafil therapy alone has been suggested to alleviate symptoms in patients with PAH. In a study of 14 patients who were in transition from subcutaneous treprostinil to sildenafil, 4 did not tolerate treprosinil withdrawal despite the introduction of sildenafil. The rest of the patients tolerated the transition.50 In a study in France, the long-term effects of adding sildenafil to intravenous therapy with epoprostanol were studied in a 16-week, double-blind trial.51 Pulmonary arterial hypertension patients on intravenous epoprostanol therapy (n=267) were randomly assigned to receive sildenafil or placebo. The addition of sildenafil improved the 6-minute walk test, hemodynamic measurements, and quality of life but had no effect on the Borg dyspnea score. Although the combination of epoprostanol and sildenafil was clinically effective, it produced more headaches and dyspepsia than did epoprostanol alone.51
Promising results have been reported in a study of the efficacy of long-term subcutaneous treprostinil therapy alone and in combination with bosentan, an endothelin receptor antagonist used to treat moderate-to-severe PAH.52 The study group comprised 38 patients with PAH who were treated with subcutaneous treprostinil. Patients in NYHA functional class II or III who did not tolerate the side effects of prostacyclin-based therapy received supplemental oral bosentan. Patients' hemodynamic status, NYHA functional class, Borg dyspnea score, and 6-minute walk test results were assessed at 6-month intervals. All measures significantly improved in patients with treprostinil therapy alone. Additional benefit was seen in all 4 measures of clinical status in patients who received supplemental bosentan.52 Similarly, adding inhaled iloprost to bosentan therapy (STEP study) has shown promise53; however, varying results have been reported.54
Future Drug Development
Developing an oral prostanoid is important in the management of PAH because it will bypass many problems associated with intravenous and central venous catheter use. Infection, pump failure, and catheter and pump occlusion are not factors in oral delivery. Beraprost, an early-development drug, showed symptomatic benefit in patients with PAH55 but did not have a good risk-to-benefit ratio.56 The side effects overshadowed the modest clinical effects. Also, beraprost therapy provided symptomatic improvement only in the early phases of treatment.57 A longer-acting version of beraprost, TRK-100STP, is under study. In 4 patients with mild-to-moderate PAH, TRK-100STP decreased pulmonary vascular resistance and improved the results of the 6-minute walk test.57
An orally bioavailable form of treprostinil has been developed. When administered in combination with phosphodiesterase inhibitors or endothelin receptor antagonists, oral treprostinil modestly improved the 6-minute walk test, but the results were not statistically significant.45 Oral treprostinil is also being studied as a monotherapy. If trial results are positive, it could be approved for use in pulmonary arterial hypertension.
Although prostanoids have been used to dilate the pulmonary vasculature by binding to prostacyclin receptors, a different mechanism has been reported in a German study in which iloprost was used in patients with idiopathic PAH.58 Lung tissue was collected, and prostacyclin receptors (IP) and prostaglandin-E2 subtype 4 receptors (EP4) were detected by using immunoblotting. Pulmonary tissue from rats with monocrotaline-induced PAH was also used in the study to detect the receptors. The expression of IP was decreased and the expression of EP4 increased in lung samples from both the patients and the rats. The results indicate that EP4 must also be involved in signal transduction when docked with iloprost. This finding may encourage future drug development to focus on EP4-selective receptors in patients with PAH.58
The anti-inflammatory agent retinoic acid has been suggested to increase transcription of prostacyclin synthase in human endothelial cells, and retinoic acid receptors may mediate the production of prostacyclin synthase.59 The future of PAH treatment might involve developing agonists for the retinoic acid receptor or using retinoic acid to increase the expression of prostacyclin synthase. However, it is not known whether endothelial cells in PAH patients respond to retinoic acid by upregulating prostacyclin synthase (PGIS).
Gene Therapy
Gene therapy is drawing increased attention as a novel treatment approach. Recent improvements in gene transfer technology have dramatically increased gene therapy research for PAH. Genes either are transfected by using a viral vector or are delivered in another fashion into the pulmonary vasculature to produce a desired effect on the vascular wall. Adenoviruses, adeno-associated viruses, and other viral vectors have been studied for genetic incorporation of cDNA to induce the vascular endothelium to produce prostacyclin.
Viral Gene Therapy
Adenovirus. A recombinant adenovirus vector has been used to incorporate the p21 gene, which regulates cell cycle progression, into rats in a model of pulmonary hypertension. Pulmonary arterial hypertension was induced in the rats by creating a left-to-right shunt. The p21 adenovirus vector was successfully transfected into the tissue, and the overexpression of p21 inhibited the development of PAH.60
Adeno-Associated Virus. Japanese investigators used an adeno-associated viral (AAV) vector to transfect human PGIS into mice to determine the effect on PAH. Pulmonary hypertension was induced in mice by restricting them to 10% oxygen for 24 hours, and then AAV-PGIS was injected into the area of the thigh. Significant pulmonary hypertension was observed after 8 weeks. Moreover, the AAV-PGIS group showed a smaller increase in RV systolic pressures, upregulation of brain natriuretic peptide levels in the RV, and suppression of significant medial thickening of the pulmonary arteries. Furthermore, AAV-PGIS–treated mice showed a significant decrease in pulmonary arterial wall thickening and prolonged survival.61 In another Japanese study, pulmonary vascular resistance and pulmonary artery pressure were decreased in mice that received intramuscular (anterior tibial) injection of AAV-PGIS.62
Other Viruses. A murine parainfluenza virus (hemagglutinating virus of Japan[HVJ]) envelope vector system has been used for gene therapy in rats with anoxia-induced pulmonary hypertension. In rats with monocrotaline-induced PAH, PGIS was transfected within the trachea by using the HVJ-liposome method. The metabolite of prostacyclin, 6-keto-PGF1α, was significantly increased after 1 week. The authors concluded that gene transfer of PGIS increased the levels of prostacyclin, leading to significant improvement in PAH in the monocrotaline rat model.63
Nonviral Gene Therapy
Nonviral approaches have been developed for gene transfer. Naked gene transfer of PGIS has been achieved by injection into the thigh muscle in rats with monocrotaline-induced PAH. Treated rats showed improved RV pressure.64 In another study, polyplex nanomicelles were used to deliver a therapeutic plasmid with the gene for human adrenomedullin, a vasodilator peptide. Rats with monocrotaline-induced PAH were transfected with the gene via the intratracheal route, and RV pressures were significantly decreased just 3 days after transfection.65 Another approach used biocompatible micelle nanovectors for gene transfer. A polyplex micelle, PEG-b-P[Asp(DET)], has been suggested to be safe and unassociated with cytotoxicity or thrombus formation.66 Together, these studies show the potential for improvement with the use of gene-therapy approaches.
Tissue-Specific Gene Therapy
Intratracheal gene transfer has been used in PAH. The human extracellular superoxide dismutase (EC-SOD) gene has been delivered by the intratracheal route in monocrotaline-treated rats. The rationale underlying this approach is that oxidative stress might contribute to the development of PAH, and superoxide dismutase eliminates oxygen radicals involved in pulmonary vasculature injury. The overexpression of superoxide dismutase in rats directly resulted in decreased pulmonary vascular pressure as well as in decreased proliferation of the pulmonary vascular wall.67
Intratracheal gene transfer has also been used with vascular endothelial growth factor (VEGF), which has been suggested to ameliorate PAH by inducing angiogenesis in the pulmonary vasculature and preventing the degeneration of the present pulmonary vasculature.68 The intratracheal gene transfer of VEGF into rabbits with bleomycin-induced PAH resulted in significantly decreased pulmonary arterial pressure and in reduced thickening of the pulmonary vasculature.69
Genes can be delivered in tissue outside of the pulmonary vasculature to facilitate the production of enzymes such as prostacyclin synthase. The liver has been studied as an extrapulmonary site for gene transfer. By using HVJ-liposomes, researchers transfected PGIS or hepatocyte growth factor (HPF), or both PGIS and HPF, into the livers of rats with monocrotaline-induced PAH.70 Treatment with both PGIS and HPF was more beneficial than treatment with either agent alone. Medial pulmonary arterial hypertrophy and RV pressure were decreased in rats that were treated with both PGIS and HPF.70
Cell Therapy
Cell therapy is being studied for the treatment of PAH. Consequently, future treatment regimens might include the use of cell therapy to increase the production of prostacyclin. Mesenchymal stem cells (MSCs) that overexpress PGIS have been used in Japan to enhance engraftment and neovascularization in a mouse model of hind-limb ischemia. In this study, the overexpression of PGIS under hypoxic conditions led to a 40% increase in the proliferation rate of MSCs. Mice with hind-limb ischemia were injected with PGIS-overexpressing MSCs, MSCs expressing green fluorescent protein, or the vehicle alone. Doppler studies showed effective reperfusion in all mice injected with MSCs. Mice injected with MSCs that overexpressed PGIS recovered perfusion within 7 days, which is earlier than the recovery in mice that received MSCs with green fluorescent protein.71 This approach has the potential for application to the management of PAH. Mesenchymal stem cells that overexpress PGIS might have the potential to regenerate the endothelium of the pulmonary vasculature.
Combination of Cell and Gene Therapy
Endothelial nitric oxide synthase (eNOS) decreases pulmonary arterial pressure in monocrotaline-treated rats.72 The effect of eNOS has been further investigated in fibroblasts transfected with the eNOS gene. Monocrotaline-treated rats were administered fibroblasts transfected with either VEGF or eNOS or with a null plasmid as a control. Cell-based therapy with eNOS was more effective in treating PAH than was cell-based therapy with VEGF, although both were more effective than was control treatment with a null plasmid. Therapy with VEGF prevented further development of PAH, whereas eNOS was associated with regeneration of the pulmonary microvasculature.73
Endothelial progenitor cells (EPCs) are responsible for the neovascularization of ischemic tissues and for the repair of the vascular endothelial lining. Evidence suggests that EPCs may become dysfunctional after the development of hypoxia-induced PAH.74 In mouse experiments, EPCs were marked with green fluorescent protein and were exposed to chronic hypoxia. The EPCs that were recruited to the area of injury did not reverse the pulmonary hypertension, which suggests hypoxia-induced dysfunction of progenitor cells.74 Endothelial progenitor cells can also be used to repair the vascular endothelium in another interesting way. Endothelial progenitor cells from the umbilical cord have a high capacity for phagocytosis and can be readily transduced with genes that are therapeutic for PAH. Investigators studied the effects of transplanting EPCs transduced with the gene for adrenomedullin, a vasodilator peptide, in a rat model of monocrotaline-induced PAH.75 This novel type of gene therapy decreased pulmonary vascular resistance in hypertensive rats.75
The effects of endothelial progenitor-like cells (EPLCs) and eNOS-transduced EPLCs on the pulmonary vasculature have been studied in rats with monocrotaline-induced PAH. Transplantation of EPLCs improved regeneration of the pulmonary vascular endothelium and survival. Moreover, survival was further improved in rats that received eNOS-transduced cells.73
The role of prostacyclin in improving grafts for hind-limb ischemia through promoting neovascularization, as demonstrated by Nagaya and colleagues,75 suggests that prostacyclin can also improve the function of EPCs, thereby aiding in the repair of pulmonary arterial endothelium.71,76 Iloprost and erythropoietin also increase the number of EPCs in the peripheral blood of patients with critical limb ischemia. These studies illustrate the potential role of EPCs in vascular regeneration.76–78
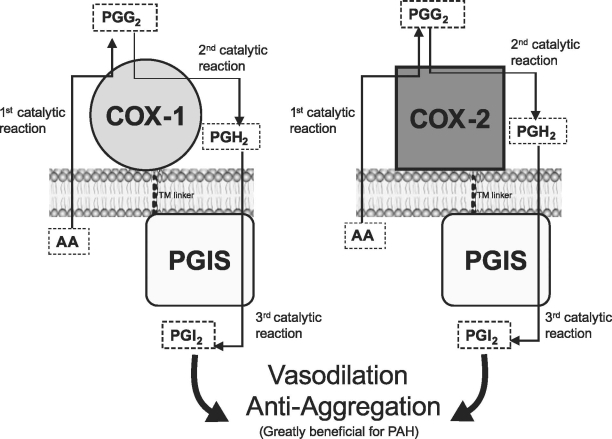
Recently, an engineered fusion (or hybrid) enzyme has been developed that links COX and PGIS together to specifically synthesize prostacyclin from arachidonic acid (Fig. 1).79–82 This fusion enzyme increases prostacyclin production in cells transfected with the fusion enzyme gene. In addition, the biologic functions of the fusion enzyme include antiplatelet aggregation, as shown in human platelet aggregation assays. The combination of the newly developed COX-PGIS fusion enzyme with gene and cell therapy may provide a more effective approach for treating PAH (Fig. 1).81–83
Fig. 1. Models of the newly created fusion (hybrid) enzymes designed to specifically increase prostacyclin production in cells. The fusion enzymes were created by linking COX-1 to PGIS (A),81 or COX-2 to PGIS (B),82 respectively, through an optimized TM linker (10 amino acid residues) without alteration of the protein topologies in the endoplasmic reticulum membrane. The direct conversion of arachidonic acid into prostacyclin by the fusion enzyme (through 3 catalytic reactions) is shown.
AA = arachidonic acid; COX = cyclooxygenase; PAH = pulmonary arterial hypertension; PGG2 = prostaglandin-G2; PGH2 = prostaglandin-H2; PGIS = prostacyclin synthase; PGI2 = prostacyclin; TM = transmembrane
Structure-Based Drug Design (Prostacyclin Receptor)
The design of new prostacyclin receptor agonist drugs is one of the most important next steps in the treatment of PAH. A novel, orally available agonist test drug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl)acetamide (NS-304), has been tested in rats with monocrotaline-induced PAH. This prodrug alleviated vascular endothelial dysfunction, pulmonary arterial wall hypertrophy, and RV hypertrophy, and it increased RV systolic pressure. In a comparison with the prostacyclin analogues beraprost and iloprost, NS-304 had an even higher affinity for the prostacyclin receptor than did the analogues. Beraprost and iloprost bind to EP4 (along with IP receptors) to dilate pulmonary arteries in patients with severe PAH who have a reduced expression of IP. Although NS-304 is not known to bind to EP4, its higher affinity for IP and its availability in oral form make it ideal for treating PAH.58,84,85 A disadvantage to using iloprost is that, like many agonists, the drug may cause desensitization of the prostacyclin receptor.86 This may be due to the co-stimulation effect (IP and EP4) of the drug.86 Research on developing more selective drugs, such as NS-304, should focus on minimizing the receptor desensitization effect caused by iloprost.
Conclusion
The loss of prostacyclin production is a key feature in patients with PAH, and prostacyclin replacement therapy is currently one of the best treatments available. Although this therapy improves physical function and survival, it has significant drawbacks and results in limited improvements in quality of life. Better orally bioavailable analogues or unrelated IP agonists are needed. Early studies of gene- and cell-based therapies have produced encouraging data in animal models. Furthermore, a clinical trial of cell-based gene therapy that uses cells overexpressing eNOS is currently ongoing. The recent production of a fusion enzyme that achieves all of the enzymatic activities necessary to produce prostacyclin from arachidonic acid is promising. Cells overexpressing this fusion gene produce prostacyclin and have been used successfully to treat animals with hind-limb ischemia. This mechanism of vasodilation can similarly be used in reducing pressures in the pulmonary arteries, which may be a viable alternative to prostacyclin therapy in PAH patients.
Acknowledgments
We thank Ahn Vu, Vanessa Cervantes, and Rebecca Bartow for excellent assistance in the preparation of this manuscript.
Footnotes
Address for reprints: Cheng-Huai Ruan, MD, Department of Internal Medicine, New York Hospital Medical Center of Queens/Weil Cornell Medical College Affiliated Hospital, 56-45 Main St., Flushing, NY 11355.
E-mail: chr9062@nyp.org
References
- 1.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54(1 Suppl):S43–54. [DOI] [PubMed]
- 2.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 2009; 119(17): 2313–22. [DOI] [PMC free article] [PubMed]
- 3.Burger CD. Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD 2009;6(2):137–44. [DOI] [PubMed]
- 4.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53(17):1573–619. [DOI] [PubMed]
- 5.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association [published erratum appears in Circulation 2009;120(2):e13]. Circulation 2009;119(16):2250–94. [DOI] [PubMed]
- 6.Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 2003;167(4):580–6. [DOI] [PubMed]
- 7.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43(12 Suppl S):5S-12S. [DOI] [PubMed]
- 8.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 1985;6(4):750–6. [DOI] [PubMed]
- 9.Hinderliter AL, Willis PW 4th, Long WA, Clarke WR, Ralph D, Caldwell EJ, et al. Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol 2003;91(8):1033–7, A9. [DOI] [PubMed]
- 10.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28(10):1250–7. [DOI] [PubMed]
- 11.Karamanoglu M, McGoon M, Frantz RP, Benza RL, Bourge RC, Barst RJ, et al. Right ventricular pressure waveform and wave reflection analysis in patients with pulmonary arterial hypertension. Chest 2007;132(1):37–43. [DOI] [PubMed]
- 12.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 2006;114(13):1417–31. [DOI] [PubMed]
- 13.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004;25(24): 2243–78. [DOI] [PubMed]
- 14.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992;327(2):76–81. [DOI] [PubMed]
- 15.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005;111(23):3105–11. [DOI] [PubMed]
- 16.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest 2007;131(6):1917–28. [DOI] [PubMed]
- 17.Bocchi EA, Bacal F, Auler Junior JO, Carmone MJ, Bellotti G, Pileggi F. Inhaled nitric oxide leading to pulmonary edema in stable severe heart failure. Am J Cardiol 1994;74(1):70–2. [DOI] [PubMed]
- 18.Morales-Blanhir J, Santos S, de Jover L, Sala E, Pare C, Roca J, et al. Clinical value of vasodilator test with inhaled nitric oxide for predicting long-term response to oral vasodilators in pulmonary hypertension. Respir Med 2004;98(3):225–34. [DOI] [PubMed]
- 19.Rich S, McLaughlin VV. The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J Am Coll Cardiol 1999;34(4):1184–7. [DOI] [PubMed]
- 20.Deng H, Wu J, So SP, Ruan KH. Identification of the residues in the helix F/G loop important to catalytic function of membrane-bound prostacyclin synthase. Biochemistry 2003; 42(19):5609–17. [DOI] [PubMed]
- 21.Dogne JM, de Leval X, Hanson J, Frederich M, Lambermont B, Ghuysen A, et al. New developments on thromboxane and prostacyclin modulators part I: thromboxane modulators. Curr Med Chem 2004;11(10):1223–41. [DOI] [PubMed]
- 22.Ruan KH. Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway. Mini Rev Med Chem 2004;4(6):639–47. [DOI] [PubMed]
- 23.Lin Y, Wu KK, Ruan KH. Characterization of the secondary structure and membrane interaction of the putative membrane anchor domains of prostaglandin I2 synthase and cytochrome P450 2C1. Arch Biochem Biophys 1998;352(1):78–84. [DOI] [PubMed]
- 24.Lin YZ, Deng H, Ruan KH. Topology of catalytic portion of prostaglandin I(2) synthase: identification by molecular modeling-guided site-specific antibodies. Arch Biochem Biophys 2000;379(2):188–97. [DOI] [PubMed]
- 25.Ren Y, Walker C, Ruan KH, Kulmacz RJ. Examination of prostaglandin H synthase-1 topology in the endoplasmic reticulum membrane. Adv Exp Med Biol 1997;400A:171–5. [DOI] [PubMed]
- 26.Ruan KH, Deng H, Wu J, So SP. The N-terminal membrane anchor domain of the membrane-bound prostacyclin synthase involved in the substrate presentation of the coupling reaction with cyclooxygenase. Arch Biochem Biophys 2005;435(2): 372–81. [DOI] [PubMed]
- 27.Ruan KH, Dogne JM. Implications of the molecular basis of prostacyclin biosynthesis and signaling in pharmaceutical designs. Curr Pharm Des 2006;12(8):925–41. [DOI] [PubMed]
- 28.Ruan KH, So SP, Zheng W, Wu J, Li D, Kung J. Solution structure and topology of the N-terminal membrane anchor domain of a microsomal cytochrome P450: prostaglandin I2 synthase. Biochem J 2002;368(Pt 3):721–8. [DOI] [PMC free article] [PubMed]
- 29.Ruan KH, Wu J, Cervantes V. Characterization of the substrate mimic bound to engineered prostacyclin synthase in solution using high-resolution NMR spectroscopy and mutagenesis: implication of the molecular mechanism in biosynthesis of prostacyclin. Biochemistry 2008;47(2):680–8. [DOI] [PubMed]
- 30.Shyue SK, Ruan KH, Wang LH, Wu KK. Prostacyclin synthase active sites. Identification by molecular modeling-guided site-directed mutagenesis. J Biol Chem 1997;272(6):3657–62. [DOI] [PubMed]
- 31.Wang LH, Matijevic-Aleksic N, Hsu PY, Ruan KH, Wu KK, Kulmacz RJ. Identification of thromboxane A2 synthase active site residues by molecular modeling-guided site-directed mutagenesis. J Biol Chem 1996;271(33):19970–5. [DOI] [PubMed]
- 32.Wu J, So SP, Ruan KH. Determination of the membrane contact residues and solution structure of the helix F/G loop of prostaglandin I2 synthase. Arch Biochem Biophys 2003;411 (1):27–35. [DOI] [PubMed]
- 33.Funk CD, Furci L, Moran N, Fitzgerald GA. Point mutation in the seventh hydrophobic domain of the human thromboxane A2 receptor allows discrimination between agonist and antagonist binding sites. Mol Pharmacol 1993;44(5):934–9. [PubMed]
- 34.Ni F, So SP, Cervantes V, Ruan KH. A profile of the residues in the second extracellular loop that are critical for ligand recognition of human prostacyclin receptor. FEBS J 2008;275 (1):128–37. [DOI] [PMC free article] [PubMed]
- 35.Ruan CH, Wu J, Ruan KH. A strategy using NMR peptide structures of thromboxane A2 receptor as templates to construct ligand-recognition pocket of prostacyclin receptor. BMC Biochem 2005;6:23. [DOI] [PMC free article] [PubMed]
- 36.Ruan KH, Wu J, So SP, Jenkins LA. Evidence of the residues involved in ligand recognition in the second extracellular loop of the prostacyclin receptor characterized by high resolution 2D NMR techniques. Arch Biochem Biophys 2003;418(1): 25–33. [DOI] [PubMed]
- 37.Zhang L, Bastepe M, Juppner H, Ruan KH. Characterization of the molecular mechanisms of the coupling between intracellular loops of prostacyclin receptor with the C-terminal domain of the Galphas protein in human coronary artery smooth muscle cells. Arch Biochem Biophys 2006;454(1):80–8. [DOI] [PubMed]
- 38.Zhang L, Huang G, Wu J, Ruan KH. A profile of the residues in the first intracellular loop critical for Gs-mediated signaling of human prostacyclin receptor characterized by an integrative approach of NMR-experiment and mutagenesis. Biochemistry 2005;44(34):11389–401. [DOI] [PubMed]
- 39.Zhang L, Wu J, Ruan KH. Solution structure of the first intracellular loop of prostacyclin receptor and implication of its interaction with the C-terminal segment of G alpha s protein. Biochemistry 2006;45(6):1734–44. [DOI] [PubMed]
- 40.Miyaji K, Matsubara H. Continuous intravenous prostacyclin therapy [in Japanese]. Nippon Rinsho 2008;66(11):2139–44. [PubMed]
- 41.Gomberg-Maitland M, Tapson VF, Benza RL, McLaughlin VV, Krichman A, Widlitz AC, Barst RJ. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med 2005;172(12): 1586–9. [DOI] [PubMed]
- 42.Oudiz RJ, Schilz RJ, Barst RJ, Galie N, Rich S, Rubin LJ, Simonneau G. Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest 2004;126(2):420–7. [DOI] [PubMed]
- 43.Skoro-Sajer N, Lang I. The role of treprostinil in the management of pulmonary hypertension. Am J Cardiovasc Drugs 2008;8(4):213–7. [DOI] [PubMed]
- 44.Voswinckel R, Reichenberger F, Gall H, Schmehl T, Gessler T, Schermuly RT, et al. Metered dose inhaler delivery of treprostinil for the treatment of pulmonary hypertension. Pulm Pharmacol Ther 2009;22(1):50–6. [DOI] [PubMed]
- 45.Yin N, Kaestle S, Yin J, Hentschel T, Pries AR, Kuppe H, Kuebler WM. Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit Care Med 2009;37(3):980–6. [DOI] [PubMed]
- 46.Schermuly RT, Yilmaz H, Ghofrani HA, Woyda K, Pullamsetti S, Schulz A, et al. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am J Respir Crit Care Med 2005;172(3):358–63. [DOI] [PubMed]
- 47.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Samidurai A, Pullamsetti S, Weissmann N, et al. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ Res 2004;94(8):1101–8. [DOI] [PubMed]
- 48.Jing ZC, Jiang X, Han ZY, Xu XQ, Wang Y, Wu Y, et al. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J 2009;33(6):1354–60. [DOI] [PubMed]
- 49.Pullamsetti S, Krick S, Yilmaz H, Ghofrani HA, Schudt C, Weissmann N, et al. Inhaled tolafentrine reverses pulmonary vascular remodeling via inhibition of smooth muscle cell migration. Respir Res 2005;6:128. [DOI] [PMC free article] [PubMed]
- 50.Keogh AM, Jabbour A, Weintraub R, Brown K, Hayward CS, Macdonald PS. Safety and efficacy of transition from subcutaneous treprostinil to oral sildenafil in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007;26(11):1079–83. [DOI] [PubMed]
- 51.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial [published errata appear in Ann Intern Med 2009;150(1):63 and Ann Intern Med 2009;151(6):435]. Ann Intern Med 2008;149(8):521–30. [DOI] [PubMed]
- 52.Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest 2008;134(1):139–45. [DOI] [PubMed]
- 53.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;174(11):1257–63. [DOI] [PubMed]
- 54.Hoeper MM, Leuchte H, Halank M, Wilkens H, Meyer FJ, Seyfarth HJ, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2006;28(4):691–4. [DOI] [PubMed]
- 55.Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002;39(9):1496–502. [DOI] [PubMed]
- 56.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost therapy for pulmonary arterial hypertension [published erratum appears in J Am Coll Cardiol 2003;42(3):591]. J Am Coll Cardiol 2003;41(12):2119–25. [DOI] [PubMed]
- 57.Ikeda D, Tsujino I, Sakaue S, Ohira H, Itoh N, Kamigaki M, et al. Pilot study of short-term effects of a novel long-acting oral beraprost in patients with pulmonary arterial hypertension. Circ J 2007;71(11):1829–31. [DOI] [PubMed]
- 58.Lai YJ, Pullamsetti SS, Dony E, Weissmann N, Butrous G, Banat GA, et al. Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am J Respir Crit Care Med 2008;178(2):188–96. [DOI] [PubMed]
- 59.Camacho M, Rodriguez C, Salazar J, Martinez-Gonzalez J, Ribalta J, Escudero JR, et al. Retinoic acid induces PGI synthase expression in human endothelial cells. J Lipid Res 2008; 49(8):1707–14. [DOI] [PubMed]
- 60.Chen SJ, Wang YB, Chen O, Zhu XB, Ma Y. Effect of p21 gene transfection mediated by replication deficient adenovirus on the pulmonary hypertensive rat model [in Chinese]. Zhonghua Er Ke Za Zhi 2008;46(2):139–42. [PubMed]
- 61.Kawakami T, Kanazawa H, Satoh T, Ieda M, Ieda Y, Kimura K, et al. AAV-PGIS gene transfer improves hypoxia-induced pulmonary hypertension in mice. Biochem Biophys Res Commun 2007;363(3):656–61. [DOI] [PubMed]
- 62.Ito T, Okada T, Mimuro J, Miyashita H, Uchibori R, Urabe M, et al. Adenoassociated virus-mediated prostacyclin synthase expression prevents pulmonary arterial hypertension in rats. Hypertension 2007;50(3):531–6. [DOI] [PubMed]
- 63.Nagaya N, Yokoyama C, Kyotani S, Shimonishi M, Morishita R, Uematsu M, et al. Gene transfer of human prostacyclin synthase ameliorates monocrotaline-induced pulmonary hypertension in rats. Circulation 2000;102(16):2005–10. [DOI] [PubMed]
- 64.Tahara N, Kai H, Niiyama H, Mori T, Sugi Y, Takayama N, et al. Repeated gene transfer of naked prostacyclin synthase plasmid into skeletal muscles attenuates monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Hum Gene Ther 2004;15(12):1270–8. [DOI] [PubMed]
- 65.Harada-Shiba M, Takamisawa I, Miyata K, Ishii T, Nishiyama N, Itaka K, et al. Intratracheal gene transfer of adrenomedullin using polyplex nanomicelles attenuates monocrotaline-induced pulmonary hypertension in rats. Mol Ther 2009;17(7): 1180–6. [DOI] [PMC free article] [PubMed]
- 66.Akagi D, Oba M, Koyama H, Nishiyama N, Fukushima S, Miyata T, et al. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 2007;14(13):1029–38. [DOI] [PubMed]
- 67.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, et al. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 2008;177(2):219–26. [DOI] [PubMed]
- 68.Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 2001;104(18):2242–8. [DOI] [PubMed]
- 69.Gong F, Tang H, Lin Y, Gu W, Wang W, Kang M. Gene transfer of vascular endothelial growth factor reduces bleomycin-induced pulmonary hypertension in immature rabbits. Pediatr Int 2005;47(3):242–7. [DOI] [PubMed]
- 70.Ono M, Sawa Y, Fukushima N, Suhara H, Nakamura T, Yokoyama C, et al. Gene transfer of hepatocyte growth factor with prostacyclin synthase in severe pulmonary hypertension of rats. Eur J Cardiothorac Surg 2004;26(6):1092–7. [DOI] [PubMed]
- 71.Ishii M, Numaguchi Y, Okumura K, Kubota R, Ma X, Murakami R, et al. Mesenchymal stem cell-based gene therapy with prostacyclin synthase enhanced neovascularization in hindlimb ischemia. Atherosclerosis 2009;206(1):109–18. [DOI] [PubMed]
- 72.Campbell AI, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature: endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol 1999;21(5): 567–75. [DOI] [PubMed]
- 73.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96(4):442–50. [DOI] [PubMed]
- 74.Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, et al. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008;26(4):1017–26. [DOI] [PubMed]
- 75.Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003;108(7):889–95. [DOI] [PubMed]
- 76.Di Stefano R, Barsotti MC, Melillo E, Iorio M, Santoni T, Armani C, et al. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost 2008;100(5):871–7. [PubMed]
- 77.Asaumi Y, Kagaya Y, Takeda M, Yamaguchi N, Tada H, Ito K, et al. Protective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload-induced left ventricular dysfunction in mice. Circulation 2007;115(15): 2022–32. [DOI] [PubMed]
- 78.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006;113(11): 1442–50. [DOI] [PubMed]
- 79.Ruan KH, inventor; Board of Regents of the University of Texas System, assignee. Hybrid protein that converts arachidonic acid into prostacyclin. United States patent article WO/2007/104000. 2007 Sep 13.
- 80.Ruan KH, Deng H, So SP. Engineering of a protein with cyclooxygenase and prostacyclin synthase activities that converts arachidonic acid to prostacyclin. Biochemistry 2006;45(47): 14003–11. [DOI] [PubMed]
- 81.Ruan KH, So SP, Cervantes V, Wu H, Wijaya C, Jentzen RR. An active triple-catalytic hybrid enzyme engineered by linking cyclo-oxygenase isoform-1 to prostacyclin synthase that can constantly biosynthesize prostacyclin, the vascular protector. FEBS J 2008;275(23):5820–9. [DOI] [PMC free article] [PubMed]
- 82.Ruan KH, So SP, Wu H, Cervantes V. Large-scale expression, purification, and characterization of an engineered prostacyclin-synthesizing enzyme with therapeutic potential. Arch Biochem Biophys 2008;480(1):41–50. [DOI] [PMC free article] [PubMed]
- 83.Yuan J, Westney OL, Ruan KH, Wang R. A new strategy, SuperEnzyme gene therapy in penile rehabilitation. J Sex Med 2009;6 Suppl 3:328–33. [DOI] [PubMed]
- 84.Kuwano K, Hashino A, Asaki T, Hamamoto T, Yamada T, Okubo K, Kuwabara K. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetamide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther 2007;322(3):1181–8. [DOI] [PubMed]
- 85.Kuwano K, Hashino A, Noda K, Kosugi K, Kuwabara K. A long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl)acetamide (NS-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}acetic acid (MRE-269), on rat pulmonary artery. J Pharmacol Exp Ther 2008;326(3):691–9. [DOI] [PubMed]
- 86.Schermuly RT, Pullamsetti SS, Breitenbach SC, Weissmann N, Ghofrani HA, Grimminger F, et al. Iloprost-induced desensitization of the prostacyclin receptor in isolated rabbit lungs. Respir Res 2007;8:4. [DOI] [PMC free article] [PubMed]