Abstract
A realistic goal for cardiac cell therapy may be to attenuate left ventricular remodeling following acute myocardial infarction to prevent the development of congestive heart failure. Initial clinical trials of cell therapy have delivered cells 1 to 7 days after acute myocardial infarction. However, many patients at risk of developing congestive heart failure may not be ready for cell delivery at that time-point because of clinical instability or hospitalization at facilities without access to cell therapy. Experience with cell delivery 2 to 3 weeks after acute myocardial infarction has not to date been explored in a clinical trial. The objective of the LateTIME study is to evaluate by cardiac magnetic resonance the effect on global and regional left ventricular function, between baseline and 6 months, of a single intracoronary infusion of 150 × 106 autologous bone marrow mononuclear cells (compared with placebo) when that infusion is administered 2 to 3 weeks after moderate-to-large acute myocardial infarction. The 5 clinical sites of the Cardiovascular Cell Therapy Research Network (CCTRN) will enroll a total of 87 eligible patients in a 2:1 bone marrow mononuclear cells-to-placebo patient ratio; these 87 will have undergone successful percutaneous coronary intervention of a major coronary artery and have left ventricular ejection fractions ≤0.45 by echocardiography. When the results become available, this study should provide insight into the clinical feasibility and appropriate timing of autologous cell therapy in high-risk patients after acute myocardial infarction and percutaneous coronary intervention.
Key words: Apoptosis; bone marrow cells; bone marrow transplantation; cell therapy; colony-stimulating factors; free radicals; heart failure; infusions, intra-arterial; inflammation/prevention & control; magnetic resonance imaging; myocardial infarction/therapy; myocardial ischemia/therapy; myocardial reperfusion injury; myocytes, cardiac; prospective studies; regeneration; research design; stem cells; stem cell transplantation; time factors; ventricular function, left; ventricular remodeling
Following an acute myocardial infarction (AMI), there is replacement of myocytes by fibrotic tissue and ongoing apoptotic loss of viable cardiac myocytes in the infarct border zone as a result of reperfusion injury and ongoing ischemia from microvascular obstruction. If the infarction is large, left ventricular (LV) dysfunction may develop due to scar expansion and LV dilation, ultimately leading to the development of congestive heart failure. Congestive heart failure is the leading admission diagnosis for hospitalization in the United States and carries a 5-year mortality rate of 50%.1 Although medical therapy can improve symptoms and extend survival to a limited degree, cardiac transplantation remains the only curative procedure available.
Recent studies in animals with experimental AMI have observed significant recovery of LV function after the delivery of bone marrow–derived stem cells.2–4 Although the mechanisms of benefit are subject to ongoing investigation,5,6 the potential has led to the initiation of multiple clinical trials to test the concept that delivery of autologous bone marrow mononuclear cells (BMMNCs) into the infarct region can improve cardiac function after AMI.7–11 Several meta-analyses12–14 have demonstrated that BMMNC administration results in a small, but significant, improvement in LV function and attenuation of adverse LV remodeling.
The timing of cell administration after AMI may be of fundamental importance in determining the success of cell therapy in this patient population, given the temporal changes that occur in the myocardium in the days to weeks after AMI that can affect stem cell survival and efficacy. Initial up-regulation of chemokine production, such as stromal derived factor-1 (SDF-1), can direct cell homing and differentiation,15 while stem cell-secreted growth factors such as vascular endothelial growth factor (VEGF) and insulin growth factor-1 can improve perfusion (through angiogenesis) and reduce apoptotic cell death in the infarct border zone.16 However, the development of a vigorous inflammatory response, coupled with the release of reactive oxygen species and cytokines such as tumor necrosis factor-a in the infarct region after an AMI, can adversely affect cell survival17 and limit the efficacy of this therapeutic approach.
No clinical trial published to date has been sufficiently powered to adequately answer questions about the efficacy of late cell delivery after AMI. Recently, the National Heart, Lung and Blood Institute (NHLBI) established the Cardiovascular Cell Therapy Research Network (CCTRN) to answer important questions in cell therapy, such as the optimal timing of cell delivery after AMI. The 1st TIME trial developed by the CCTRN is currently investigating outcome differences in LV function in patients randomized to cell delivery at 3 versus 7 days after AMI.18 However, many patients eligible for cell therapy may not be suitable for randomization at these time-points, due to clinical instability or to hospitalization at facilities without access to cell therapy. To study the usefulness of cell therapy in this important subset of AMI patients, the CCTRN developed a 2nd randomized, placebo-controlled trial called LateTIME, in which autologous BMMNCs are delivered 2 to 3 weeks after AMI.
Organizational Structure and Oversight
The CCTRN was established by the NHLBI to devel-op, coordinate, and conduct multiple collaborative protocols testing the effects of stem cell therapy on cardiovascular disease. The Network builds on contemporary findings of the cell-therapy basic science community, translating this newly acquired information to the cardiac clinical setting in the Phase I/II study paradigm. The Network consists of 5 clinical research centers (Cleveland Clinic Foundation, University of Florida, Minneapolis Heart Institute/University of Minnesota, Texas Heart Institute, and Vanderbilt University), a data coordinating center (DCC) (University of Texas School of Public Health) that provides trial management and data analysis, a cell-processing quality-control center, and 5 core laboratories. Together, these Network components provide standardization of cell-therapy preparation and endpoint measurements. All clinical centers participate in the selection and design of Network protocols, which are also reviewed by an independent Protocol Review Committee and a Gene Therapy/Cell Therapy Data Safety and Monitoring Board (DSMB), under the aegis of the NHLBI. Each clinical center and the DCC has independent institutional review board (IRB) approval and oversight. By recruiting from multiple centers, the Network accelerates the speed with which its studies can be completed, increases the broad applicability of study findings, and amplifies the dissemination of its public health findings.
Objectives and Design
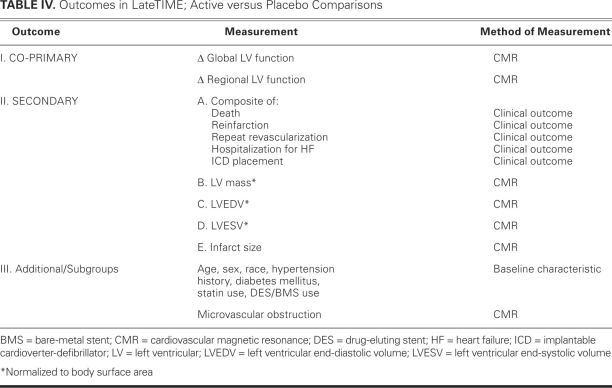
The primary objective of LateTIME, a Phase-I/II, randomized, double-blinded, placebo-controlled clinical trial, is to determine whether delayed (2–3 wk) administration of intracoronary autologous BMMNCs to patients after a moderate-to-large MI can safely produce a measurable improvement in LV global and regional function as determined by cardiovascular magnetic resonance (CMR) at 6 months, compared with baseline. Secondary objectives are to determine whether the administration of cell therapy in comparison with placebo will produce smaller end-diastolic and end-systolic volumes at 6 months, compared with baseline. Secondary objectives are to determine whether the administration of cell therapy in comparison with placebo will produce smaller end-diastolic and end-systolic volumes at 6 months. Patients will be monitored for 2 years to evaluate the effects of cell therapy on the clinical endpoints of death, reinfarction, repeat target-vessel revascularization, hospitalization for congestive heart failure, and placement of implantable cardiac defibrillators (ICDs). The effects of cell phenotype on therapeutic efficacy will be investigated through ancillary studies at 2 biorepository core facilities (the University of Minnesota and the University of Florida, Gainesville).
Patients will be randomized in a 2:1 ratio, BMMNC to placebo. The overall anticipated sample size required for LateTIME is 87 patients (58 cell therapy and 29 placebo). Hypothesis testing for the primary outcome will be carried out at the 0.05 level.
Enrollment and Study Population
The 87 patients required for LateTIME will be recruited from all sites participating in the NHLBI CCTRN and their developed satellite referral centers. Each patient will have undergone successful percutaneous revascularization of a major coronary artery after a moderate-to-large infarction with a left ventricular ejection fraction (LVEF) ≤0.45 as evaluated by echocardiography. All prospective patients will be screened by investigators or study coordinators and will be enrolled in the trial after meeting inclusion criteria (Table I) and signing the informed consent and Health Insurance Portability & Accountability Act forms.
TABLE I. Major Inclusion and Exclusion Criteria
Eligibility and Randomization
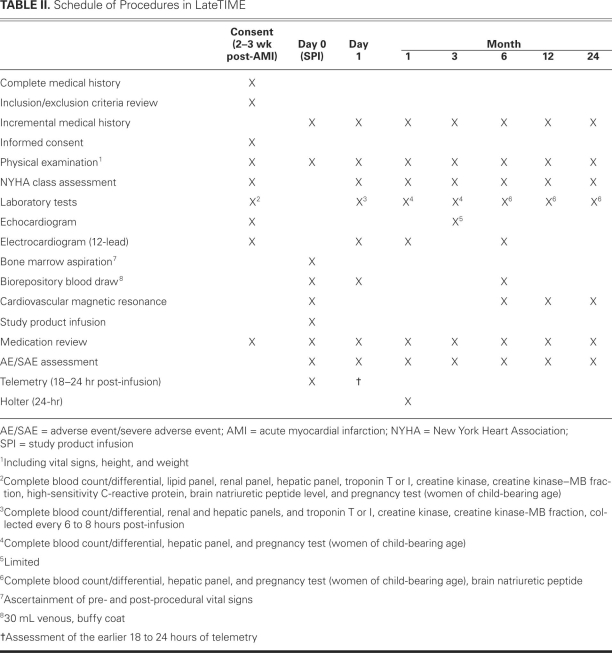
Once inclusion and exclusion criteria have been evaluated and informed consent has been obtained, eligible patients will be randomly assigned to one of the selected treatment strategies in an interactive Web-based randomization session. Patients will be randomized in a 2:1 (BMMNC-to-placebo) ratio using randomly selected block sizes of 6 or 9 and stratified by the center. The patients and research staff, including the CCTRN physicians and interventional cardiologists, will be blinded to treatment assignment. Patients will follow a prescribed schedule of procedures (Table II) and be evaluated at 6 months for efficacy of treatment. All patients will be monitored for 2 years to obtain an extensive safety profile.
TABLE II. Schedule of Procedures in LateTIME
Bone Marrow Aspiration
All patients will receive a physical examination, including evaluation of vital signs, height, weight, and New York Heart Association (NYHA) functional class. On the morning of study product infusion, patients will undergo bone marrow aspiration in accordance with standard operating procedures developed by the CCTRN. Patients will be lightly sedated or receive conscious sedation as determined by the attending physician. Approximately 80 to 90 mL of bone marrow will be aspirated from the iliac crest using standard techniques. Only one bone marrow aspiration will be attempted. As determined from preclinical experience, 150 to 200 million total nucleated cells (TNCs) can be routinely harvested with this volume of bone marrow aspirate, containing small fractions of CD34+ and CD133+ cells. The specific population of cells administered in LateTIME will be determined at the biorepository core facilities and correlated with outcomes.
Cell Preparation and Cell Product
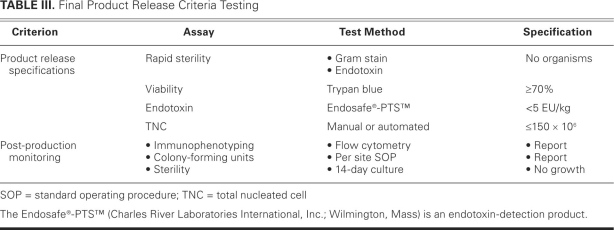
Once harvested, the cells will be transported to the institution's cell-therapy laboratory. Each site will use the Sepax® system19 (Biosafe America, Inc.; Houston, Tex) for BMMNC isolation. The Sepax system is a closed system that enables faster isolation of the cellular product and, potentially, increased safety for the patient. Each site's cell-therapy laboratory director and participating personnel have been trained with the Sepax system, thereby ensuring a more uniform cellular product. The cells will be harvested and washed 3 times in human serum albumin/saline buffer before re-suspension in 5% human serum albumin/saline. The composition of CD34+ and CD133+ cells will be determined by fluorescent-activated cell-sorting analysis. Viability of the cells will be determined by trypan blue exclusion; ≥70% viability will be required before transplantation. A 14-day sterility culture will be performed on the final product. Because this testing will not be available before product infusion, a negative Gram stain will be required before the product is released. Although cells are autologous in this protocol, the standard tests for infectious diseases will be performed. Additional testing deemed necessary by regulations or institutional policy (or both) will be performed. Final product (lot) release criteria testing results (Table III) will be available before transport of BMMNCs to the hospital for administration. The product will be labeled and tracked with adhesive labels containing the patient's study identification.
TABLE III. Final Product Release Criteria Testing
All patients randomized to the active group will receive 150 × 106 TNCs (70%–80% BMMNCs) as determined by a hematology analyzer. If the bone marrow aspiration yield is less than the target amount and the patient has already been randomized to therapy, then he or she will receive all available cells. Patients randomized to the placebo group will receive 5% albumin in normal saline, to which 100 mL of autologous blood will be added to ensure that the color and consistency of the placebo infusion matches that of the active group. If the patient consents, all cells not required for study product delivery will be provided to the CCTRN biorepository core facilities for cell characterization and functional analysis.
Infusion of Cellular Product or Placebo
The final cellular product or placebo (approximate total volume, 30 mL) will be infused less than 12 hours after bone marrow aspiration. The patients will be heparinized to an activated clotting time of >200 sec, and the infusate will be delivered via an over-the-wire percutaneous transluminal coronary angioplasty catheter (stop-flow) in 6 aliquots of approximately 5 mL each, delivered over 2 minutes of balloon inflation within the previously placed stent at the lowest possible pressure necessary to obstruct coronary blood flow (3–6 atm). Two minutes of reperfusion will occur after each cycle of cell infusion.
All patients will be monitored overnight on a telemetry unit in the hospital and seen by a physician the following morning, before discharge. Patients are advised to take aspirin for life and 75 mg of clopidogrel for 24 months, as well as the usual medications for post-AMI care. Patients with LVEFs of <0.40 will be advised to take an aldosterone antagonist unless contraindicated by a creatinine level ≥2.5 mg/dL or a serum potassium level ≥5 mEq/L.
Determination of Outcomes
A 1.5T CMR scanner will be used, with precise magnetic resonance imaging (MRI) protocols developed by the MRI core laboratory (University of Florida). Cardiovascular Angiography Analysis System/Magnetic Resonance Ventricular (CAAS/MRV) cardiac function and perfusion analysis software (PIE Medical Imaging B.V.; Maastricht, The Netherlands) will be used to measure global LV myocardial mass, volumes, and LVEF, as well as regional parameters. Regional systolic wall motion, ejection fraction, thickening, and radial displacement in the infarct and border zones will be determined. Regional ejection fraction will be calculated for the basal, midwall, and apical portions of the LV cavity. Areas of microvascular obstruction, infarct size, and degree of transmurality will be quantified by delayed gadolinium-enhanced MRI.
Wall Motion Imaging. Data on both global and segmental LV function will be obtained using a steady-state free-precession or fast-gradient echo technique. Long-axis cine images in the 2-chamber and 4-chamber projections will be acquired. In addition, a set of contiguous short-axis slices (8–10 mm thick) will be obtained from the mitral valve annulus through the apex of the LV throughout the cardiac cycle. Data will be analyzed using the CAAS/MRV software. Global measurements evaluated will include end-diastolic volume, end-systolic volume, stroke volume, ejection fraction, and LV mass. Volumetric measurements will be performed by direct planimetry on the contiguous short-axis images at both end-systole and end-diastole. Regional measurements will include wall thickening and wall motion, and will be calculated using 100 chords spaced every 3.6°, originating from the centroid of the LV. Regional data will be reported using the American Heart Association 17-segment model.20 The minimum spatial and temporal resolution requirements of the steady-state free-precession sequence are 2.5 × 2.5-mm voxels and 40 ms, respectively.
Baseline Perfusion Imaging. A 2-chamber long-axis cine image will be obtained on the basis of axial scout images, with the imaging plane spanning the center of the mitral valve coaptation point and through the apex of the LV. Based on this, a 4-chamber long-axis cine image will be obtained. Subsequently, a T1-weighted gradient-echo baseline perfusion sequence will be performed using an intravenous gadolinium chelate (for example, gadolinium-DTPA). Three short-axis slices will be obtained (positioned from the 2-chamber and 4-chamber cine images) to encompass the basal, middle, and apical thirds of the LV during a bolus administration of gadolinium chelate (0.15–0.2 mmol/kg). Imaging will be acquired for a total of 60 dynamics per slice, ensuring that the passage of contrast material through the myocardium is captured for semiquantitative analysis.
Viability Imaging. Fifteen to 20 minutes after the administration of gadolinium-chelate contrast agent, delayed-enhancement imaging (DE-MRI) will be performed with a T1-weighted inversion-recovery prepared gradient-echo sequence. The inversion delay time will be iteratively adjusted for optimal nulling of normal myocardium. Contrast-enhanced viability imaging will be performed with 2 techniques: the standard 2-dimensional technique, which acquires a single slice during each breath hold, will be performed in the short-axis projections, using the same plane prescription as the functional short-axis cine series; and a high-resolution 3-dimensional technique will be used to acquire 10 short-axis slices during a single breath hold. Regions of irreversible myocardial damage are manifested by “hyperenhancement” (bright white areas) on the images, while normal or viable tissue is “nulled” (black) on the acquired images. The presence, location, and extent of irreversibly damaged tissue will be qualitatively and quantitatively evaluated on a segmental basis. Pre- and post-therapy imaging, both cine wall motion and DE-MRI, will be carefully matched for consistency and accuracy, using internal landmarks that include the insertion sites of the right ventricular free wall and the papillary muscles.
Safety Monitoring
All CCTRN participants will be closely monitored for adverse events, and this information will be transmitted to the IRBs of all centers, the U.S. Food and Drug Administration, and the DSMB through the DCC. The DSMB will meet at least twice yearly to review the performance of the participating sites, to evaluate the accruing safety data, and to ascertain the feasibility of continuing the study. A set of stopping rules has been developed in consultation with the DSMB.
In addition, the DCC will oversee and coordinate the collection, standardization, integration, and analysis of study data from the various study components (enrolling sites and core facilities) and the preparation and distribution of the required reports to each of the safety-oversight entities. The DCC will facilitate and monitor regulatory and safety compliance at each site and core laboratory and will conduct site visits to each site and core laboratory to ensure protocol adherence and regulatory compliance, both on a regular basis and for cause.
Statistical Analyses
Assuming the independence and normalcy of the observations, we calculated the sample size using a 2-sample t-test statistic. The literature21 suggests that the absolute change (effect size, δ) achievable in global LVEF is δ=4% and that the common group SD of the difference of LVEF over time is σD = 6%. These produced a study sample size of 86, administratively rounded up to 87, with 58 participants in the active group and 29 in the control group. For a regional assessment of left ventricular function, we are assuming an absolute change in regional ejection fraction of δ=6.7% and a common group SD of σD = 9.5%, from the 2004 BOOST clinical trial report7; these assumptions produced a sample size of 77 patients. On the basis of the assumptions described above for global and regional LV function, the overall anticipated sample size required for LateTIME is 87 patients.
Exact testing for categorical variables and Student's t-testing for continuous variables will be used to evaluate the compatibility of baseline variables between treatment groups. All hypothesis testing, and all effect sizes and their 95% confidence intervals, will be evaluated using the general linear mixed model. Both unadjusted and adjusted treatment effects will be computed for primary and secondary endpoints (Table IV); adjustments will be for clinical centers, as well as for baseline covariates whose association with the dependent variable is generally accepted. In keeping with standard methodology for clinical trials, the primary and secondary comparisons will compare the randomized study groups using an intention-to-treat analysis. Logistic regression will be used to evaluate the effect of cell administration on the combined endpoint of death, reinfarction, repeat target-vessel revascularization, hospitalization for congestive heart failure, and ICD placement. No adjustments for multiple comparisons will be made.
TABLE IV. Outcomes in LateTIME; Active versus Placebo Comparisons
Discussion
To date, more than 1,000 patients with AMI have been treated with intracoronary BMMNCs throughout the world. In total, studies12–14 show that cell therapy appears safe over several years of follow-up and results in a small, but significant, improvement in LV function and remodeling. However, fundamental questions in cell therapy, such as dose, optimal cell type, and timing of administration after AMI, have not been answered. The CCTRN was established by the NHLBI to develop protocols to answer these important questions in cell therapy.
In the previously published randomized clinical trials,7–11 BMMNCs were administered between 1 and 7 days, but timing was never integrated into the randomization scheme; therefore, the effect of timing of BMMNC administration after AMI is not known. The 1st protocol developed by the CCTRN, called TIME,18 is a randomized, double-blinded, placebo-controlled trial that is evaluating the optimal time-point to deliver BMMNCs after a moderate-to-large MI. In that study, patients are randomized to cell therapy at either day 3 or day 7 after MI. This 2nd protocol developed by the CCTRN, LateTIME, will continue the investigation of the important aspect of cell-delivery timing. LateTIME is a randomized, double-blinded, placebo-controlled trial that will investigate the potential benefit of BMMNC administration 2 to 3 weeks after AMI in high-risk patients with persistent LV dysfunction. Evaluation of this time-point is important for many patients who are too ill to undergo randomization in a cell-therapy trial during the 1st few days after AMI, or who are hospitalized at centers that do not have access to cell therapy, but may present to cell-therapy centers weeks later.
The timing of cell delivery after AMI may be one of the most important criteria in determining the efficacy of cell therapy. After AMI, a confluence of changes occurs in the myocardium; these changes include the expression of growth factors and cytokines, which might promote cell survival and angiogenesis or, conversely, might encourage myocyte apoptosis and adverse LV remodeling. Expression of chemokines such as SDF-1,15 which may aid in stem cell homing, is up-regulated in the infarct zone in the 1st few days after AMI. On the other hand, a strong inflammatory reaction and release of reactive oxygen species in the infarct zone may adversely affect the survival of injected cells.17 In a pre-clinical model, Ma and colleagues22 administered 5 × 106 mesenchymal stem cells (MSCs) via the tail vein in rats at multiple time-points after AMI (12 hr, and 1, 2, 4, 8, and 16 d). They observed that the greatest number of labeled MSCs retained in the heart when measured 3 days after administration occurred in those animals that received cells 1 day after AMI. The number of retained cells declined significantly with each subsequent day of administration so that no cells were present when cell administration occurred at 8 or 16 days after AMI. These observations are consistent with recent clinical findings that homing of radiolabeled progenitor cells to the infarct zone are greatest in the 1st few days after AMI.23 These findings suggest that delayed administration of stem cells (up to several weeks after an AMI) may not be as effective as earlier administration. However, several considerations—other than the fact that the patients of LateTIME are ineligible for earlier administration—justify exploring this later time-point. These include the roles of ongoing apoptosis during the first several months after AMI,24 ongoing resolution of microvascular obstruction (which might improve intracoronary cell delivery to the infarct zone), and the abatement of a vigorous inflammatory response (which can affect stem cell survival).25 Furthermore, all previous trials in which cells have been delivered within the 1st week after an AMI have been subjected to the confounding effect of myocardial stunning26 and its variable resolution, which can dwarf the improvement in ejection fraction that is attributed to cell therapy. Because cell delivery in LateTIME will occur weeks after AMI, the effects of stunning should be minimized, so that the true effect of cell therapy can be determined.
Very few clinical studies have been performed to evaluate the efficacy of delayed delivery of stem cells after AMI. Assmus and colleagues27 compared with placebo the administration of intracoronary BMMNCs or cultured circulating blood progenitor cells (CPCs) in 75 patients at least 3 months after MI (mean, 81 ± 72 mo). They noted a small but statistically significant (2.9%) improvement in LVEF by left ventriculography in the BMMNC group (n=35), versus the CPC (–0.4%) or placebo group (–1.2%). In an expanded follow-up to that study,28 Assmus and colleagues administered 214 ± 98 × 106 BMMNCs to 121 patients at a mean of 7 years (range, 4 mo–39 yr) after AMI. They observed an increase in LVEF from 0.399 to 0.417 (P <0.001) by left ventriculography 3 months later. This was accompanied by significant reduction in N-terminal pro-ANP and -BNP levels, and those patients whose cells exhibited the highest levels of colony-forming units and migratory capacity demonstrated a survival benefit at follow-up.
The MYSTAR Trial29 randomized 60 AMI patients with moderate-to-large AMIs (LVEF, <0.45) to a combination of intramyocardial and intracoronary delivery of BMMNCs administered at an early (3–6 wk) or late (3–4 mo) time-point. Compared with baseline values, they observed a 3.5% increase in LVEF 3 months after cell therapy in both groups, as measured by single-photon-emission computed tomography. These findings indicate that the delayed administration of BMMNCs after AMI is safe and feasible and may result in clinical benefit in certain patients. The LateTIME trial will be the 1st protocol to examine the effects of cell delivery at a delayed, but focused, time-point of 2 to 3 weeks after AMI.
Conclusions
LateTIME is consistent with the goal of the CCTRN to accelerate research in the use of cell-based therapies for the management of cardiovascular diseases. LateTIME will provide data on a stage of clinical development—the subacute time-frame after AMI—that has not been much studied. This will be the 1st randomized trial in which cells are delivered 2 to 3 weeks after AMI, a time-frame bracketed by the acute and chronic phases of AMI, both of which already have growing safety profiles in regard to BMMNC delivery. When the results become available, this study should provide insight into the clinical feasibility and appropriate timing of autologous cell therapy in high-risk patients after AMI and PCI.
Footnotes
Address for reprints: Lemuel A. Moyè, MD, PhD, Department of Biostatistics, University of Texas School of Public Health, RAS Bldg., 1200 Herman Pressler, Houston, TX 77030
E-mail: Lemuel.A.Moye@uth.tmc.edu
This study is supported by the NHLBI (U01 HL087318-01) and by the Production Assistance for Cellular Therapies (PACT), N01-HB-37164. The percutaneous transluminal coronary angioplasty and guide catheters and wires were generously supplied by Boston Scientific Corporation (Natick, Mass). ClinicalTrials.gov #NCT00684021.
References
- 1.Braunwald E. The Denolin lecture. Congestive heart failure: a half century perspective. Eur Heart J 2001;22(10):825–36. [DOI] [PubMed]
- 2.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410(6829):701–5. [DOI] [PubMed]
- 3.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7(4):430–6. [DOI] [PubMed]
- 4.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001; 107(11):1395–402. [DOI] [PMC free article] [PubMed]
- 5.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004;428(6983):664–8. [DOI] [PubMed]
- 6.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004;428(6983):668–73. [DOI] [PubMed]
- 7.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004;364(9429): 141–8. [DOI] [PubMed]
- 8.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006;355(12):1199–209. [DOI] [PubMed]
- 9.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355(12):1210–21. [DOI] [PubMed]
- 10.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 2006;367(9505):113–21. [DOI] [PubMed]
- 11.Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J 2009;30(11):1313–21. [DOI] [PubMed]
- 12.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 2007;50(18):1761–7. [DOI] [PubMed]
- 13.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 2008;29(15):1807–18. [DOI] [PubMed]
- 14.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167(10):989–97. [DOI] [PubMed]
- 15.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003;362(9385):697–703. [DOI] [PubMed]
- 16.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms [published erratum appears in Circ Res 2005;97 (3):e51]. Circ Res 2004;94(5):678–85. [DOI] [PubMed]
- 17.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 2004;94 (12):1543–53. [DOI] [PubMed]
- 18.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, et al. Rationale and design for TIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction [published erratum appears in Am Heart J 2009;158 (6):1045]. Am Heart J 2009;158(3):356–63. [DOI] [PMC free article] [PubMed]
- 19.Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy 2008;10 (2):203–11. [DOI] [PubMed]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105(4):539–42. [DOI] [PubMed]
- 21.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006;113(10):1287–94. [DOI] [PubMed]
- 22.Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol 2005;100(3):217–23. [DOI] [PubMed]
- 23.Schachinger V, Aicher A, Dobert N, Rover R, Diener J, Fichtl-scherer S, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation 2008;118(14):1425–32. [DOI] [PubMed]
- 24.Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, et al. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol 2002;34(2):165–74. [DOI] [PubMed]
- 25.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 2006;13(16): 1877–93. [DOI] [PubMed]
- 26.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 2001;104(24):2981–9. [DOI] [PubMed]
- 27.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006;355(12):1222–32. [DOI] [PubMed]
- 28.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 2007;100(8):1234–41. [DOI] [PubMed]
- 29.Gyongyosi M, Lang I, Dettke M, Beran G, Graf S, Sochor H, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med 2009;6(1):70–81. [DOI] [PubMed]