Abstract
In an observational study, we examined the effect of statins on low-density-lipoprotein (LDL) subfractions.
Using density-gradient ultracentrifugation, we measured small, dense LDL density in 612 patients (mean age, 61.7 ± 12.6 yr), some with and some without coronary artery disease, who were placed in a statin-treated group (n=172) or a control group (n=440) and subdivided on the basis of coronary artery disease status.
Total cholesterol, LDL cholesterol, apolipoprotein B, and the LDL cholesterol/apolipoprotein B ratio were significantly lower in the statin group. However, the proportion of small, dense LDL was higher in the statin group (42.9% ± 9.5% vs 41.3% ± 8.5%; P=0.046) and the proportion of large, buoyant LDL was lower (23.6% ± 7.5% vs 25.4% ± 7.9%; P=0.011). In the statin group, persons without coronary artery disease had higher proportions of small, dense LDL, and persons with coronary artery disease tended to have higher proportions of small, dense LDL.
Our study suggests that statin therapy—whether or not recipients have coronary artery disease—does not decrease the proportion of small, dense LDL among total LDL particles, but in fact increases it, while predictably reducing total LDL cholesterol, absolute amounts of small, dense LDL, and absolute amounts of large, buoyant LDL. If and when our observation proves to be reproducible in subsequent large-scale studies, it should provide new insights into small, dense LDL and its actual role in atherogenesis or the progression of atherosclerosis.
Key words: Antilipemic agents/therapeutic use; arteriosclerosis/blood; biological markers/blood; cholesterol, LDL/analysis/blood/classification/drug effects; coronary artery disease/prevention & control/therapy; hyperlipidemias/prevention & control; lipids; lipoproteins, LDL/blood/chemistry; particle size
The predominance of small, dense low-density lipoprotein (LDL) has been designated as an emerging cardiovascular risk factor by the National Cholesterol Education Program Adult Treatment Panel III.1 Griffin and colleagues2 showed that the predominance of small, dense LDL is associated with an increased risk of coronary artery disease (CAD). However, another study showed that LDL particle size is rarely a significant and independent predictor of CAD risk.3 Therefore, it is a matter of debate whether the apparent increase in atherogenic potential of small, dense LDL is a consequence of the broader pathophysiology of which these particles are a part—for example, higher triglyceride (TG) levels, lower high-density-lipoprotein cholesterol levels, increased LDL particle number, obesity, insulin resistance, diabetes mellitus, and metabolic syndrome.4 Statins are potent inhibitors of hydroxy-methyl-glutaryl-coenzyme A reductase (the rate-limiting enzyme in hepatic cholesterol synthesis) and are the main drugs of choice in the treatment of elevated plasma LDL-cholesterol (LDL-C) concentrations. Several large clinical studies have shown that lipid-lowering therapy is effective in the primary and secondary prevention of cardiovascular disease.5,6 One mechanism that prevents cardiovascular disease when statins are used is the reduction of total LDL particle concentration, including small, dense LDL and large, buoyant LDL.7 Statins potentially lower all LDL subclasses (large, medium, and small particles); therefore, the net effect of statins on LDL particle size is often null or, at most, only moderate.8 Although statins clearly decrease total LDL particle concentration, it is unclear whether statins can affect the proportion of small, dense LDL.
In addition, there is still controversy regarding whether statins decrease the small, dense LDL subfraction and increase the LDL peak particle size.9–11 High-carbohydrate diets in Korea possibly contribute to higher TG levels and to the formation of small LDL particles.12 However, there have been no reports regarding the effect of statins on the proportion of small, dense LDL in the Korean population. Therefore, we examined the effect of statins on LDL subfractions and on the proportion of small, dense LDL in this population.
Study Population and Methods
The study was conducted at the Cardiovascular Center of Korea University's Guro Hospital in Seoul, Korea. We selected the study population from patients who visited the Cardiovascular Center because of chest symptoms or for preoperative evaluation. Exclusion criteria were as follows: acute coronary syndrome or prior myocardial infarction, cardiomyopathy, more than mild valvular disease, atrial fibrillation, congestive heart failure (left ventricular ejection fraction, <0.40), previous history of stent implantation or coronary artery bypass grafting, cerebrovascular disease, or renal insufficiency (creatinine level, >2 mg/dL).
The study population comprised 612 patients who did and did not have CAD. The mean age of the 279 men and 333 women was 60.3 ± 12.7 yr. Seventy-seven persons had diabetes mellitus (12.6%), 273 had hypertension (44.6%), and 125 were current smokers (20.4%). We performed coronary angiography in 321 patients whose chest symptoms suggested angina pectoris and in patients whose preoperative evaluations suggested CAD consequent to demonstrable ischemia on noninvasive testing. All patients who underwent coronary angiography gave written informed consent. Our study was approved by the local ethics committee.
We divided the study participants into 2 groups on the basis of statin therapy. The statin-treated group included 172 individuals who had been prescribed statins for at least 6 weeks before we sampled their blood for lipid profiling and the measurement of small, dense LDL density. These patients had taken atorvastatin, simvastatin, lovastatin, pravastatin, fluvastatin, pitavastatin, or rosuvastatin. The mean duration of statin therapy was 11.03 ± 5.32 wk. The control group (n=440) had no history of statin use or the use of other medications that affect lipoprotein size and density (such as fibrates or niacin). No one in either group had a total cholesterol (TC) level more than 350 mg/dL or less than 100 mg/dL, or a TG level more than 400 mg/dL or less than 30 mg/dL.
We then subdivided each group on the basis of CAD status, determined the proportions of small, dense LDL, and examined other lipid profiles. Coronary artery disease was defined as significant coronary stenosis (>50% by quantitative coronary angiography) on a coronary angiogram. In the statin-treated group, 119 persons had CAD and 53 did not; in the control group, 130 had CAD and 310 did not. We compared the change in LDL subfraction in the statin-treated group to that of the control group. We also compared the CAD risk of each group in accordance with average score on the Framingham risk assessment13 and the prevalence of metabolic syndrome. Metabolic syndrome was defined in accordance with the National Cholesterol Education Program Adult Treatment Panel III.1
Biochemical Measurements of Lipid Profiles, Insulin, and Glucose
Commercially available assay kits were used for biochemical measurements. Concentrations of TC, high-density-lipoprotein cholesterol, TG, and LDL-C were measured with use of an enzymatic assay (Roche Diagnostics GmbH; Mannheim, Germany). Serum apolipoprotein (apo) B and apo A-1 levels were determined by use of an immunoturbidometric assay (Roche Diagnostics). Fasting plasma insulin levels were measured by use of a double-antibody radioimmunoassay (Beckman Coulter, Inc; Brea, Calif). Glucose concentrations were measured with use of an enzymatic assay kit. The homeostasis model assessment–insulin resistance (HOMA–IR) value was calculated by the following formula: (fasting plasma insulin [μIU/mL] × fasting plasma glucose [mmol/L])/22.5.14
Low-Density-Lipoprotein Subfractions and Particle Sizes
We measured LDL peak particle diameter by means of gradient gel electrophoresis, and small, dense LDL density by means of density-gradient ultracentrifugation. To evaluate LDL particle size and the relative proportions of LDL I, LDL II, and LDL III, we used the method described by Griffin and colleagues15 and isolated LDL from serum by ultracentrifugation, at a density of 1.019 to 1.063 g/mL. Electrophoresis was performed using an LPE-4003 Pore Gradient Lipoprotein Electrophoresis System (C.B.S. Scientific Company, Inc.; Del Mar, Calif) with commercially available, non-denatured 2%–16% polyacrylamide gels.16 The buffer system within the gel apparatus was tris(hydroxymethyl)aminomethane base (90 mM), boric acid (80 mM), and EDTA (2.5 mM; pH, 8.3). Before electrophoresis, the gels were pre-equilibrated at 70 V for 20 min. Electrophoresis was conducted at 20 V for 20 min, at 70 V for 30 min, and at 120 V for 24 hr. The gels were then fixed for 30 min in sulfosalicylic acid (10% w/v) and stained with Coomassie Blue R-250 (0.1% w/v) for 1 hr. The gels were de-stained in 7.5% acetic acid for 24 hr and then were standardized against the following markers: polystyrene latex beads (36 nm), thyroglobulin (17 nm), apoferritin (12.2 nm), and catalase (10.4 nm). The gels were scanned with use of a Molecular Imager GS-800™ Calibrated Densitometer (Bio-Rad Laboratories, Inc.; Hercules, Calif).
The LDL particle size was reported as LDL peak particle diameter and LDL mean particle diameter. The LDL peak particle diameter was reported as the size of the major LDL fraction. The LDL mean particle diameter was calculated to yield the mean diameter across the entire LDL profile. To achieve this, the peak area under the curve (volume) was calculated. For each portion, the particle size was calculated using the known reference sizes of LDL I, LDL II, and LDL III. Then, the frequency for each particle was calculated (size × volume). Finally, the sum of the frequencies divided by the sum of the volumes yielded the mean particle diameter. Subjects were classified into 2 groups on the basis of distinct LDL subclass patterns. Pattern A was defined as an LDL subclass pattern with the major gradient gel peak at a particle diameter of >25.5 nm and the presence of a minor peak of smaller LDL particles. Pattern B had the major peak at a particle diameter of ≤25.5 nm, with skewing of the curve toward larger particle diameters.15
Because a single molecule of apo B is found in every LDL particle, serum apo B concentrations equate to the LDL particle concentration.1,7 Therefore, we regarded the product of the serum apo B multiplied by the percentage of small, dense LDL as the absolute amount of small, dense LDL; and we regarded the product of the serum apo B multiplied by the percentage of large, buoyant LDL as the absolute amount of large, buoyant LDL.
Statistical Analysis
Statistical analysis was performed with use of SPSS software version 10.0 (SPSS Inc.; Chicago, Ill). Continuous variables were expressed as mean ± SD, and categorical variables were reported as number and percentage. Because all of the LDL variables were normally distributed, the 2-tailed Student t test was used to analyze the LDL data. The Mann-Whitney U test was used to analyze the TG variables, which were not normally distributed. The χ2 test was used to analyze categorical variables. The Pearson correlation was used to analyze the association between the proportion of small, dense LDL and LDL-C. A P value less than 0.05 was considered statistically significant. The data conformed to each test by which they were analyzed.
Results
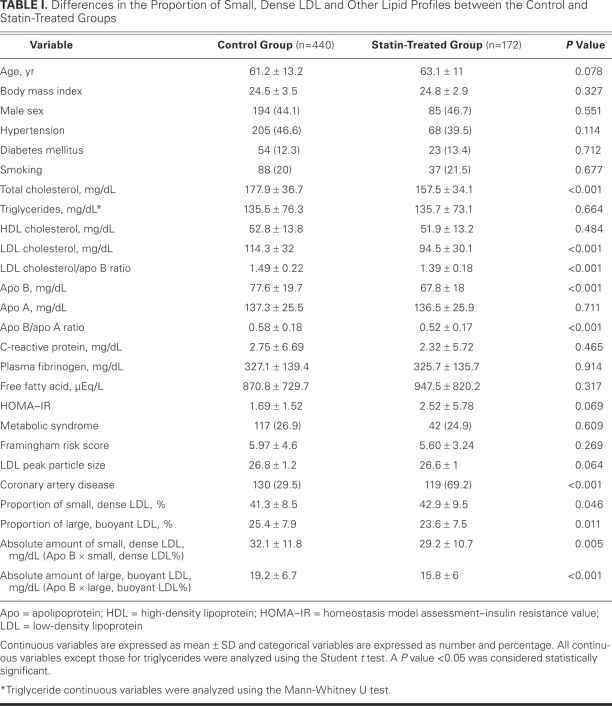
Table I shows the differences between the groups in the proportion of small, dense LDL and other lipid profiles between the statin-treated and control groups. Coronary artery disease was more common in the statin group than in the control group. The TC, LDL-C, and apo B levels, and the LDL-C/apo B ratio and apo B/apo A ratio, were lower in the statin group. No significant differences in TG, HOMA–IR, LDL peak particle size, or C-reactive protein (CRP) were found between the groups. The absolute amounts of small, dense LDL and large, buoyant LDL were significantly lower in the statin group. However, in comparison with the control group, the proportion of small, dense LDL was significantly higher in the statin group and the proportion of large, buoyant LDL was lower. Neither the number of study participants with metabolic syndrome nor the average score on the Framingham risk assessment was significantly different between the 2 groups.
TABLE I. Differences in the Proportion of Small, Dense LDL and Other Lipid Profiles between the Control and Statin-Treated Groups
Study Participants without Coronary Artery Disease
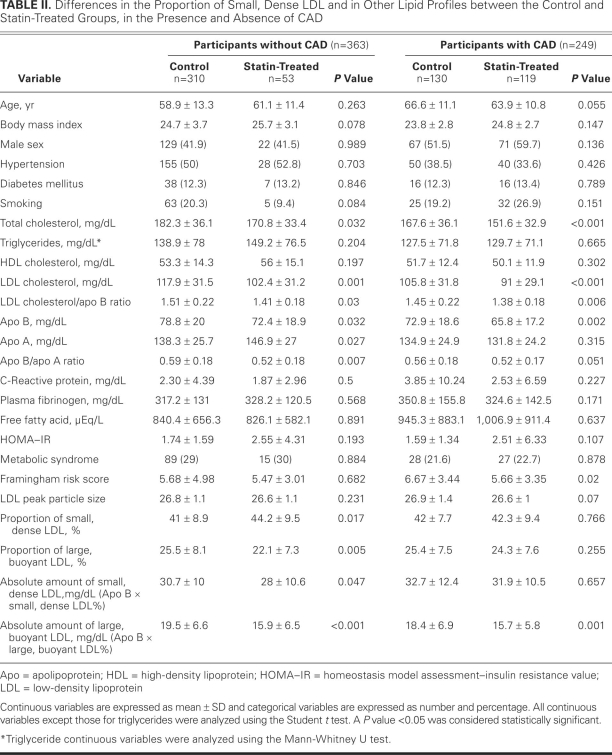
In participants who did not have CAD, the TC, LDL-C, and apo B levels and the LDL-C/apo B and apo B/apo A ratios were significantly lower in the statin-treated group than in the control group (Table II). No significant differences in TG, HOMA–IR, LDL peak particle size, or CRP were found between the groups. The absolute amounts of small, dense LDL and of large, buoyant LDL were significantly lower in the statin group than in the control group. However, the proportion of small, dense LDL was significantly higher in the statin group, and the proportion of large, buoyant LDL was lower. Neither the number of study participants with metabolic syndrome nor the average score on the Framingham risk assessment was significantly different between the 2 groups.
TABLE II. Differences in the Proportion of Small, Dense LDL and in Other Lipid Profiles between the Control and Statin-Treated Groups, in the Presence and Absence of CAD
Study Participants with Coronary Artery Disease
In participants who had CAD, the TC, LDL-C, and apo B levels and the LDL-C/apo B ratio were significantly lower in the statin-treated group than in the control group (Table II). There were no significant differences in TG, HOMA–IR, LDL peak particle size, or CRP between the 2 groups. The absolute amount of small, dense LDL had a tendency to be lower in the statin group (although not to the level of statistical significance), and the absolute amount of large, buoyant LDL was significantly lower in the statin group. However, in comparison with the control group, the proportion of small, dense LDL had a tendency to be higher in the statin group and the proportion of large, buoyant LDL had a tendency to be lower. In contrast with participants in the statin group who did not have CAD, there was no statistically significant difference (NS); however, in comparison with the control group, the proportion of small, dense LDL had a tendency to be higher in the statin group (NS), and the proportion of large, buoyant LDL showed a tendency to be lower (NS). The average score on the Framingham risk assessment was significantly lower in the statin group than in the control group. The number of patients with metabolic syndrome was not significantly different between the 2 groups.
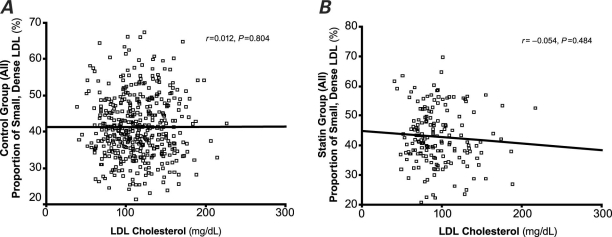
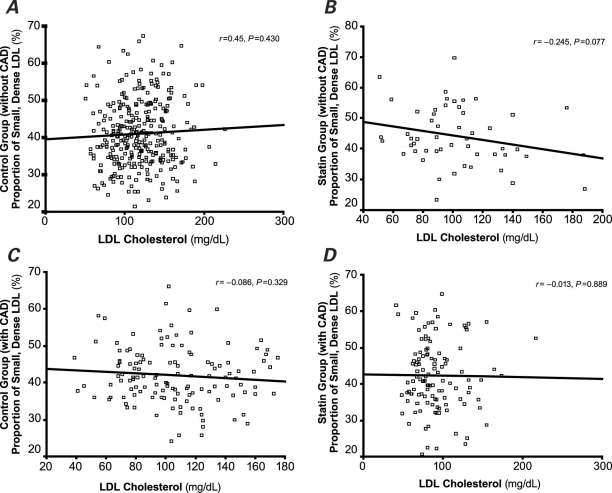
Figures 1 and 2 show that the LDL-C concentration did not correlate with the proportion of small, dense LDL, regardless of statin treatment.
Fig. 1 Correlation between the proportion of small, dense low-density-lipoprotein (LDL) and LDL cholesterol in all study participants in A) the control group and B) the statin-treated group. The LDL-cholesterol concentration did not correlate with the proportion of small, dense LDL, regardless of statin treatment. P < 0.05 was considered statistically significant.
Fig. 2 Correlation between the proportion of small, dense low-density-lipoprotein (LDL) and LDL cholesterol in participants without coronary artery disease (CAD) in A) the control group and B) the statin-treated group; and correlation between the proportion of small, dense LDL and LDL cholesterol in persons with coronary artery disease in C) the control group and D) the statin-treated group. The LDL-cholesterol concentration did not correlate with the proportion of small, dense LDL, regardless of statin treatment. P < 0.05 was considered statistically significant.
Discussion
In this study, we found that statin therapy might be associated with a higher proportion of small, dense LDL and a lower proportion of large, buoyant LDL, although statin therapy was clearly associated with a decrease in the concentrations of TC, LDL-C, and apo B; in the apo B/apo A ratio; and in the absoluteamounts of small, dense LDL and large, buoyantLDL.
Many factors influence the LDL subfractions. Previous studies have shown that CRP, plasma fibrinogen, HOMA–IR, body mass index, and metabolic syndrome can affect the proportion of small, dense LDL.18–22 In our study, the proportion of small, dense LDL was higher and there were more patients with CAD in our statin group than in our control group (Table II). Because the presence of CAD can be associated with the proportion of small, dense LDL, we analyzed the effect of statins on small, dense LDL subfractions in persons without CAD. Unexpectedly, in that analysis, the proportion of small, dense LDL was also significantly higher in patients who were treated with statins (Table II). Moreover, there were no differences in CRP, plasma fibrinogen, HOMA–IR, body mass index, or metabolic syndrome between the statin and control groups (Tables I and II). Therefore, we concluded that the increase of small, dense LDL proportion was influenced by statins but not by the other variables.
The exact mechanism by which statin therapy is associated with an increase in the proportion of small, dense LDL is unclear. One possible mechanism is that up-regulation of LDL receptor activity by statins decreases large, buoyant LDL more than small, dense LDL, because statins increase LDL receptor activity and because large, buoyant LDL is a better ligand for the LDL receptor than is small, dense LDL.23 More than 90% of apo B is found on LDL particles, and therefore patients with small, dense LDL (which is relatively low in cholesterol) would be expected to have a low LDL-C/apo B ratio, as has been described previously.17,24 The present study showed that statin therapy was associated with a greater decrease in apo B than in LDL-C, although in the group treated with statins, the levels of LDL-C and apo B were significantly lower than those in the control group. This result suggests that statin therapy is associated with a decrease in total LDL-particle concentration but with a higher proportion of small, dense LDL. In addition, these results support a possibility that statin therapy increases the proportion of small, dense LDL, although controversy still exists as to whether or not statins decrease the small, dense LDL subfraction and increase the LDL peak particle size.9–11 The present study brings up a fundamental question regarding the actual role of small, dense LDL in atherosclerosis.
In the present study, the effect of statins on the LDL subfraction was weaker in patients with CAD than in those without CAD, which might be related to the following: first, as shown in Table I, the Framingham risk score and LDL peak particle size were higher in the control group than in the statin group. These 2 variables can influence the LDL subfraction,8,22 and, therefore, the effect of statins on the LDL subfraction could be changed by these variables. Second, because patients with CAD were fewer in number than were persons without CAD, there were no statistically significant differences in the effect of statins on the LDL subfraction in patients who had CAD. Had more participants been enrolled, we would have expected to obtain similar results.
The present study showed that LDL-C was not correlated with the proportion of small, dense LDL (Figs. 1 and 2). These results are consistent with those of a previous study.25 Because the predominance of small, dense LDL has been accepted as a bona fide cardiovascular risk factor,1 this result suggests that we should not estimate the risk of CAD from the LDL-C level alone.
As mentioned in the exclusion criteria, all persons with known CAD were excluded before the study began. However, in our study, the statin-treated group included more CAD patients than did the untreated group. A misleading inference would be that persons who are treated with statins develop more CAD. In our opinion, because the mean duration of the therapy in the statin-treated group was relatively short (11.03 ± 5.32 wk), statin therapy would not have affected the severity of CAD.
Limitations of the Study
Our study had some limitations. First, we did not investigate other factors that are known to influence the generation of small, dense LDL, such as hepatic lipase activity and cholesterol ester transfer protein (CETP) activity. The generation of small, dense LDL is associated with elevations in plasma TG levels, in hepatic lipase activity, and in CETP activity.26 Triglyceride-enriched LDL is a good substrate for hepatic lipase. This LDL particle, through the action of hepatic lipase, loses the core TG and surface phospholipids and, in the process, is converted to a small, dense LDL.27 Since LDL, including rich TG, cannot be absorbed well by the LDL receptor, this LDL is converted to small, dense LDL. Because we did not check the CETP activity in our study, we could not evaluate the relationship between the proportion of small, dense LDL and CETP activity affected by statins. Second, we did not directly check the concentrations of absolute LDL particles, of small, dense LDL, or of large, buoyant LDL. Had the absolute amounts of small, dense LDL and large, buoyant LDL concentrations been measured directly, more precise results could have been obtained. Third, since CAD status was defined in accordance with angiographic findings alone, it is possible that we overlooked patients with CAD who did not undergo catheterization. Fourth, we did not compare the effect of each statin on the small, dense LDL subfraction. Previous studies have shown that the effects of statins on small, dense LDL and LDL peak particle size vary according to the type of statin.9–11 However, when we divided subjects in accordance with the types of statins, each group was too small to analyze. Fifth, fewer patients were in our statin group than in our control group. This study was observational, and we could not divide the population into equal numbers. However, we expect that a randomized, well-controlled study would produce more exact results concerning the effect of statins on small, dense LDL.
Conclusion
Whether or not individuals have been diagnosed with existing CAD, our study suggests that statin therapy does not decrease the proportion of small, dense LDL among total LDL particles, but in fact increases it, while expectedly reducing total LDL-C, absolute amounts of small, dense LDL, and absolute amounts of large, buoyant LDL. If and when our observation proves to be reproducible in large-scale studies, such studies should provide new insights into small, dense LDL and its actual role in atherogenesis or the progression of atherosclerosis.
Footnotes
Address for reprints: Hong Seog Seo, MD, Cardiovascular Center, Korea University, Guro Hospital, 97 Guro Dong, Guro Gu, Seoul 152–703, ROK
E-mail: wmagpie@yahoo.co.kr
This work was supported by Korea University grant #R0800841.
References
- 1.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285(19): 2486–97. [DOI] [PubMed]
- 2.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis 1994;106(2):241–53. [DOI] [PubMed]
- 3.Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low-density lipoprotein size, pravastatin treatment, and coronary events. JAMA 2001;286(12):1468–74. [DOI] [PubMed]
- 4.Sacks FM, Campos H. Clinical review 163: Cardiovascular endocrinology: low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab 2003;88 (10):4525–32. [DOI] [PubMed]
- 5.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383–9. [PubMed]
- 6.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335(14):1001–9. [DOI] [PubMed]
- 7.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO Jr. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol 2007;50(18):1735–41. [DOI] [PubMed]
- 8.Berneis K, Rizzo M. LDL size: does it matter? Swiss Med Wkly 2004;134(49–50):720–4. [DOI] [PubMed]
- 9.Franceschini G, Cassinotti M, Vecchio G, Gianfranceschi G, Pazzucconi F, Murakami T, et al. Pravastatin effectively lowers LDL cholesterol in familial combined hyperlipidemia without changing LDL subclass pattern. Arterioscler Thromb 1994;14(10):1569–75. [DOI] [PubMed]
- 10.Gaw A, Packard CJ, Murray EF, Lindsay GM, Griffin BA, Caslake MJ, et al. Effects of simvastatin on apoB metabolism and LDL subfraction distribution. Arterioscler Thromb 1993;13(2):170–89. [DOI] [PubMed]
- 11.Superko HR, Krauss RM, DiRicco C. Effect of fluvastatin on low-density lipoprotein peak particle diameter. Am J Cardiol 1997;80(1):78–81. [DOI] [PubMed]
- 12.Anuurad E, Shiwaku K, Enkhmaa B, Nogi A, Kitajima K, Yamasaki M, Yamane Y. Ethnic differences in the formation of small LDL particles in Asians: a comparison of Koreans, Japanese and Mongolians. Eur J Clin Invest 2004;34(11):738–46. [DOI] [PubMed]
- 13.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837–47. [DOI] [PubMed]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. [DOI] [PubMed]
- 15.Griffin BA, Caslake MJ, Yip B, Tait GW, Packard CJ, Shepherd J. Rapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugation. Atherosclerosis 1990;83(1):59–67. [DOI] [PubMed]
- 16.Hulthe J, Wiklund O, Olsson G, Fagerberg B, Bokemark L, Nivall S, Wikstrand J. Computerized measurement of LDL particle size in human serum. Reproducibility studies and evaluation of LDL particle size in relation to metabolic variables and the occurrence of atherosclerosis. Scand J Clin Lab Invest 1999;59(8):649–61. [DOI] [PubMed]
- 17.Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res 1988;29(11):1461–73. [PubMed]
- 18.St-Pierre AC, Bergeron J, Pirro M, Cantin B, Dagenais GR, Despres JP, Lamarche B. Effect of plasma C-reactive protein levels in modulating the risk of coronary heart disease associated with small, dense, low-density lipoproteins in men (The Quebec Cardiovascular Study). Am J Cardiol 2003;91(5): 555–8. [DOI] [PubMed]
- 19.Maki KC, Davidson MH, Marx P, Cyrowski MS, Maki A. Association between elevated plasma fibrinogen and the small, dense low-density lipoprotein phenotype among postmenopausal women. Am J Cardiol 2000;85(4):451–6. [DOI] [PubMed]
- 20.Slyper AH, Zvereva S, Schectman G, Hoffmann RG, Mueller RA, Walker JA. Insulin resistance is not a major determinant of low-density lipoprotein particle size. Metabolism 1997;46 (11):1275–80. [DOI] [PubMed]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement [published errata appear in Circulation 2005;112(17):e297, e298]. Circulation 2005;112(17):2735–52. [DOI] [PubMed]
- 22.Vekic J, Topic A, Zeljkovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. LDL and HDL subclasses and their relationship with Framingham risk score in middle-aged Serbian population. Clin Biochem 2007;40(5–6):310–6. [DOI] [PubMed]
- 23.Nigon F, Lesnik P, Rouis M, Chapman MJ. Discrete subspecies of human low density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J Lipid Res 1991;32(11):1741–53. [PubMed]
- 24.Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, et al. LDL particle size distribution. Results from the Framingham Offspring Study. Arterioscler Thromb 1992;12(12):1410–9. [DOI] [PubMed]
- 25.Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study. Arterioscler Thromb Vasc Biol 2000;20(9):2140–7. [DOI] [PubMed]
- 26.Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol 2000; 74 Suppl 1:S17–22. [DOI] [PubMed]
- 27.Caslake MJ, Packard CJ. Phenotypes, genotypes and response to statin therapy. Curr Opin Lipidol 2004;15(4):387–92. [DOI] [PubMed]