Abstract
The extent (density and diameter) of the native (preexisting) collateral circulation in healthy tissues and the capacity of collaterals to enlarge/remodel in obstructive arterial disease are important determinants of ischemic injury. Evidence suggests that these parameters vary widely from yet-to-be-identified genetic and environmental factors. Recently, a locus on chromosome 7 was linked to less recovery of perfusion after femoral artery ligation in BALB/c and A/J versus C57BL/6 mouse strains. Moreover, evidence suggested that BALB/c and A/J share an allele(s) at this locus that is different from C57BL/6 mice. Here we tested the hypothesis that differences in collateral extent and/or remodeling underlie these findings. Compared with C57BL/6, BALB/c and A/J strains have fewer native collaterals in hindlimb (also confirmed in brain)—associated with greater reduction in perfusion immediately after femoral ligation, slower recovery of perfusion, greater hindlimb use impairment, and worse ischemia. However, A/J also differed from BALB/c in a number of these parameters, including having more robust collateral remodeling. Analysis of A/J → C57BL/6 chromosome substitution strains confirmed that a difference in an allele(s) on chromosome 7 conferred most, but not all, of the magnitude of the differences in collateral function. Additional studies of C57BL/6 × BALB/c F1 mice demonstrated that alleles of the C57BL/6 strain exert dominance for collateral traits. Finally, negative results were obtained from studies examining a previously identified candidate gene potentially responsible for these differences—Bcl2-associated athanogene-3. These findings emphasize the major contribution of genetic background to variation in the collateral circulation and its capacity to lessen ischemia in obstructive disease.
Keywords: collateral density, collateral remodeling, genetic variability, hindlimb ischemia
native collaterals are preexisting arteriole-to-arteriole anastomoses that interconnect adjacent arterial trees and limit ischemic tissue injury that occurs with sudden (e.g., atherothrombotic) or progressive (e.g., atherosclerotic) arterial obstructive diseases. The amount of protection is dependent on collateral density and diameter (i.e., collateral extent), as well as the capacity of these vessels to enlarge (remodel) during chronic obstruction (the latter termed “arteriogenesis”); all three parameters are known to vary widely within and among species (1, 10, 13, 19, 20, 22). Consistent with this, evidence suggests that conductance of the collateral circulation also differs greatly among healthy humans (16). The sources of these differences are unknown. Presumably they arise from as-yet unidentified variation in genetic and/or environmental factors that affect the molecular programs responsible for formation of these vessels, which occurs in the embryo (2), and remodeling of them in ischemic disease.
Evidence for involvement of variation in genetic background began with studies showing that recovery of hindlimb perfusion after femoral artery ligation (FAL) was significantly lower in BALB/c compared with C57BL/6 mice (8, 21). However, the underlying mechanisms were not evident because native collateral extent and/or collateral remodeling were not quantified. Subsequent studies found evidence for reduced collateral density and impaired remodeling in the BALB/c strain (1, 10). Recently, expression of two genes, Vegfa and Clic4, were identified as possible contributors (1, 4, 5). BALB/c mice express less VEGF-A during ischemia, and targeted increase in expression of either protein causes gene dose-dependent increase in native collateral formation plus, in the case of VEGF-A, increase in collateral remodeling in models of obstructive disease (1, 4, 5).
Recently, Dokun et al. (7) mapped an identical quantitative trait locus (QTL) on chromosome 7 for two related traits—recovery of leg perfusion and tissue necrosis score, both measured 21 days after FAL—in a BALB/c backcross of a C57BL/6 × BALB/c F1 population, with the C57BL/6 strain harboring a dominant protective allele(s) for recovery from hindlimb ischemia. They then narrowed down the confidence interval based on evidence for the assumption that BALB/c and A/J strains share a common haplotype block(s) at the locus that is different from C57BL/6 mice, yielding a list of candidate genes potentially responsible for the differential recovery/necrosis. However, the major physiological mechanisms potentially underlying the QTL, e.g., differences in native collateral number and diameter and/or collateral remodeling, were not examined. Therefore, we hypothesized that underlying genetic differences in hindlimb collateral function exist among the above three inbred strains to account for these previous results. We quantified collateral extent, remodeling, and other collateral-dependent outcomes in the three strains, in a C57BL/6 × BALB/c F1 population to test for inheritance pattern (e.g., dominance) and in strains in which individual chromosomes of the C57BL/6 strain have been replaced with the corresponding chromosome from the A/J strain to test for genomic location of alleles contributing to differences in collateral extent and remodeling.
MATERIALS AND METHODS
Animals.
C57BL/6, BALB/cBy (BALB/c), A/J, C57BL/6J-Chr7A/J/NaJ [chromosome substitution strain (CSS)7] and C57BL/6J-Chr17A/J/NaJ (CSS17) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred for one or two generations to acquire sufficient numbers of 10- to 12-wk-old mice. Heterozygotes for Bcl2-associated athanogene-3 (Bag3) were generously provided by Dr. Shinichi Takayama (Boston Biomedical Research Institute, Boston, MA) (11). BALB/c and C57BL/6 strains were reciprocally crossed to yield F1 progeny, designated as CXB-F1. Animals were maintained under standard conditions and diet, randomized for procedures, and analyzed in a blinded manner. Procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina.
Hindlimb ischemia.
FAL was performed as previously described (1, 3). Briefly, mice were anesthetized with 1.25% isoflurane-O2, and the hindlimbs were depilated. Rectal temperature was maintained at 37.0 ± 0.5°C. The right femoral artery was exposed through a 2-mm incision and ligated with two 7-0 ligatures placed distal to the origin of the lateral caudal femoral and superficial epigastric arteries (the latter was also ligated) and proximal to the genu artery. The artery was transected between the sutures and separated 1–2 mm. The wound was irrigated with sterile saline and closed, and cefazolin (50 mg/kg im), furazolidone (topical), and pentazocine (10 mg/kg im) were administered. We minimized trauma in the adductor region of interest (ROI) region (as we have done in previous hindlimb studies) by the following: the skin incision is 2 mm in length and outside of the ROI (see Fig. 1B; the incision is evident above the ROI), retraction is not used, the tissue underlying the incision is minimally disturbed with no. 5 dumonts and stereomicroscopy, and the wound is closed with a single 5.0 ligature.
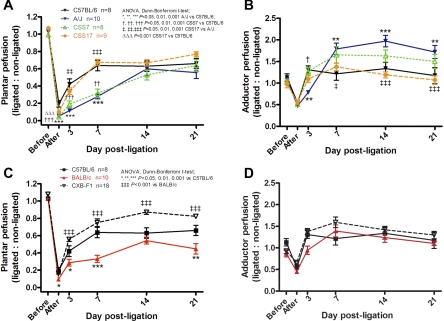
Fig. 1.
Perfusion patterns after femoral artery ligation (FAL) differ among C57BL/6, BALB/c, and A/J strains. Doppler perfusion images and regions of interest for quantification (white dotted outlines) of the plantar foot (index of overall hindlimb perfusion; A and C) and adductor thigh (index of collateral perfusion; B and D) are shown. Plantar flow immediately after FAL and recovery during the first week were lower in BALB/c and A/J than in C57BL/6 (A). Thereafter, recovery of perfusion was greater in A/J, presumably from greater collateral remodeling as suggested by the greater adductor perfusion (D). Lower plantar perfusion immediately after ligation and less recovery thereafter suggest lower native collateral conductance (due to fewer and/or smaller collaterals) and less collateral remodeling, respectively. Values are means ± SE.
Laser Doppler perfusion imaging.
As detailed previously (1, 3), under 1.125% isoflurane-O2 anesthesia and 37 ± 0.5°C, noninvasive perfusion imaging of the plantar foot (index of the overall leg perfusion) and adductor thigh (index of perfusion of superficial collaterals in the thigh) regions (1, 3) was performed before, immediately after, and at 3, 7, 14, and 21 days after FAL. ROIs were drawn to anatomic landmarks (3).
Muscle function and ischemia.
At 3, 7, 14, and 21 days after FAL, animals were evaluated for right hindlimb use with a “clinical use score”: 0 = normal toe and plantar flexion, 1 = no toe but plantar flexion, 2 = no toe or plantar flexion, and 3 = dragging foot. Mice were also scored for appearance (index of ischemia): 0 = normal, 1 = cyanosis or loss of nail(s), 2 = partial or complete loss of digit(s), and 3 = dry necrosis beyond digits into front part of foot.
Postmortem arterial microangiography.
As detailed previously (1), 21 days after right FAL animals were cannulated via the descending aorta, heparinized, and perfusion cleared at 100 mmHg with phosphate-buffered saline (PBS, pH 7.4) containing adenosine (10 mg/ml) and papaverine (4 mg/ml), followed by fixation with 4% paraformaldehyde (PFA). The left femoral artery was acutely ligated. An X-ray opaque Microfil casting agent (MV-122, FlowTech, Carver, MA) with a viscosity adjusted to restrict capillary transit (8:1 latex to diluent) was injected into the cannulated abdominal aorta and allowed to cure. After overnight fixation in 4% PFA, the skin was removed and arteriograms were obtained. Films were digitized, and a Rentrop-like line analysis was performed by counting vessels (primarily collaterals) crossing the middle of the posterior thigh as described previously (1).
Histology and morphometry.
Hindquarters blocks from the midpoint of the medial adductor/abductor of the ligated and sham-ligated limbs, taken from the above dilated and fixed legs, were embedded in paraffin. Tissue sections (5 μm thick) were stained with modified cyano-Masson's elastin stain. Diameters of collaterals at their approximate midpoints in the anterior and posterior gracilis muscles were determined at baseline and 21 days after FAL.
Cerebral collateral circulation.
Animals were cannulated via the descending abdominal aorta, heparinized, perfusion cleared, and maximally dilated (as above). The dorsal calvarium and adherent dura mater were removed to expose the pial circulation. A second catheter was placed retrogradely into the thoracic aorta, and a polyurethane solution with a viscosity sufficient to minimize capillary transit (1:1 resin to methylethyl ketone; PU4ii, Vasqtec, Zurich, Switzerland) was infused with the aid of a stereomicroscope. PFA (4%) was applied topically, and the polyurethane was allowed to cure. After postfixation overnight in 4% PFA, the pial circulation was imaged (Leica MZ16FA, Leica Microsystems, Bannockburn, IL). Collaterals connecting the middle cerebral artery (MCA) and anterior cerebral artery (ACA) trees of both hemispheres were counted, and lumen diameters were determined at their midpoints (ImageJ, National Institutes of Health).
Statistics.
Four preplanned comparisons were subjected to ANOVA followed by Dunn-Bonferroni corrected t-tests for parametric data: 1) C57BL/6, BALB/c, A/J; 2) C57BL/6, A/J, CSS7; 3) C57BL/6, A/J, CSS17; and 4) C57BL/6, BALB/c, CXB-F1. Dependent variables are denoted on the x-axis and as the groups listed in the keys of Figs. 1, 4, 5, and 7, A and B, and as the groups labeled on the y-axis in Figs. 2, 3, 6, and 7, C and D. Paired t-test for within-animal comparisons and unpaired t-tests for group comparisons were also performed as indicated in the figures. Data are reported as means ± SE. Use and appearance scores were tested with the Kruskal-Wallis nonparametric equivalent of ANOVA, followed by the nonparametric Dunn multiple comparisons test; by definition, no mean or variance is defined for these categorical data.
Fig. 4.
Perfusion patterns after FAL differ among CSS7, CSS17, and CXB-F1 relative to C57BL/6 and A/J strains. CSS7 and CSS17 are strains in which Chr 7 or 17 from the A/J strain has been substituted in place of the same chromosome in the C57BL/6 genome. A and B: CSS7 data suggest that a locus(loci) on Chr 7 has a major effect on the difference in perfusion between A/J and C57BL/6. C and D: CXB-F1 data show a trend toward overdominance of the C57BL/6 genome for recovery of perfusion. See Fig. 2 for additional analysis. Values are means ± SE.
Fig. 5.
Use impairment and ischemic appearance scores follow the patterns A/J > BALB/c > C57BL/6 and A/J > CSS7 > CSS17, respectively. Together with data in Figs. 1–4, these data show that tissue injury after FAL is more closely linked to baseline native collateral number than to subsequent collateral remodeling. These data also agree with the other metrics measured in Figs. 1–4 and the conclusions stated in their legends. y-Axis labels for A and B also apply to other panels; x-axis labels for B, D, and F also apply to A, C, and E, respectively. n sizes per strain: C57BL/6 (8), CSS7 (8), BALB/c (10) A/J (10), CSS17 (9), CXB-F1 (18).
Fig. 7.
Hindlimb perfusion after FAL (A and B) and native pial collateral number (C) and diameter (D) are not affected by deletion of 1 or both alleles of Bag3. This suggests that a functional variant in the Bag3 gene, which is located close to the peak of the quantitative trait locus (QTL) previously identified on Chr 7 for recovery from hindlimb ischemia in a CXB-N2 population (7), is unlikely to underlie the differences in native collateral extent or collateral remodeling and perfusion recovery among C57BL/6 and BALB/c strains. Values are means ± SE.
Fig. 2.
Plantar and adductor perfusion values immediately after and 21 days after FAL (taken from Figs. 1 and 4 to aid comparisons). A and C: data from the first 3 bars in each panel, taken together, suggest that the rank order for native collateral number and/or diameter in hindlimb is C57BL/6 > BALB/c > A/J. Data for CSS7 and CSS17 suggest that a locus(loci) on chromosome (Chr) 7 has a major effect on the difference in native collateral number and/or diameter between A/J and C57BL/6, with Chr 17 also contributing. B and D: given that plantar and adductor perfusion on day 21 (relative to perfusion immediately after FAL) together provide an index of the amount of collateral remodeling, CSS7 and CSS17 data suggest that loci on Chr 7 and another chromosome(s) are responsible for greater remodeling of A/J than C57BL/6 (see text). A–D: CXB-F1 data show that the C57BL/6 genome is dominant for the measured traits. Values are means ± SE.
Fig. 3.
Hindlimb collateral number before ligation (“baseline”) and collateral remodeling after FAL vary among strains. A: X-ray arteriogram for determination of collateral number (vessels intersecting red dotted lines). D: native collaterals in the posterior gracilis muscle stained with cyano-Masson's elastin for determination of collateral diameter. B, C, E, and F: baseline (native) and % increase in collateral number and diameter 21 days after FAL for the 6 strains confirm the conclusions suggested by the perfusion data given in Figs. 1, 2, and 4. That is, native collateral number in hindlimb skeletal muscle follows the pattern C57BL/6 > BALB/c > A/J, collateral remodeling follows the pattern A/J > C57BL/6 > BALB/c, a locus(loci) on Chr 7 has a major effect on baseline differences in collateral number between A/J and C57BL/6 with Chr 17 also contributing, loci on Chr 7 and an additional chromosome(s) influence differences in remodeling between A/J and C57BL/6, and the C57BL/6 genome is dominant in the CXB-F1 population for the traits shown in B, C, E, and F. Values are means ± SE.
Fig. 6.
Native pial collateral number, diameter, and size of the cerebral artery trees vary among mouse strains. A: branches of the middle cerebral artery (MCA) and anterior cerebral artery (ACA) trees supply distinct territories of the cortex, as revealed by polyurethane-filled arteriograms obtained after clearance, dilation, and fixation of the cerebral vasculature. B and C: the rank order for collateral number between the MCA and ACA trees, i.e., C57BL/6 > A/J > BALB/c, differs from that in the hindlimb (Fig. 3). In general agreement with Figs. 1–5 in hindlimb, CSS7 and CSS17 data suggest that a locus(loci) on Chr 7 has a major effect on the difference in native collateral number and diameter between A/J and C57BL/6, with Chr 17 also contributing to the diameter difference. D: % of cerebral cortex supplied by MCA, ACA, and posterior cerebral artery (PCA) trees. The MCA tree is larger in A/J than in C57BL/6 and BALB/c. In contrast to collateral metrics in hindlimb and brain (Figs. 1–5 and A–C), loci on Chr 7 and 17 do not contribute to the difference in tree territories between A/J and C57BL/6. B–D: CXB-F1 data show that the C57BL/6 genome is dominant for pial collateral number (consistent with hindlimb collateral number), while the BALB/c genome is dominant for pial collateral diameter. Values are means ± SE.
RESULTS
Native collateral extent and collateral remodeling in skeletal muscle are reduced in BALB/c, A/J, and CSS7 compared with C57BL/6 mice.
Candidate genes were identified within a QTL on chromosome 7 for differences in recovery from hindlimb ischemia and necrosis score after FAL, based on evidence that BALB/c and A/J mice share a haplotype(s) at the locus that is different from C57BL/6, together with analysis of CXB-F1 and CSS7 mice (7). The CSS7 strain is one of a set of “chromosome substitution strains” of C57BL/6 mice in which a single chromosome has been substituted for the corresponding chromosome from the A/J strain (17). Whether differences in native collateral number and diameter and/or collateral remodeling underlie the above mentioned QTL and whether these traits are similar in BALB/c and A/J have not been examined.
To determine variation in these traits among C57BL/6, BALB/c, and A/J mice, we measured a number of parameters, including recovery of hindlimb perfusion over 21 days after FAL with a high-resolution infrared laser Doppler imager (1, 3) (Fig. 1). Plantar perfusion [index of leg perfusion (3)] dropped more immediately after FAL in A/J than in BALB/c, and both strains declined more than C57BL/6 (Fig. 1C and Fig. 2A). The level of perfusion after acute FAL depends primarily on native collateral number and diameter in the adductor/abductor thigh (1, 10). Thus these data suggest that collateral number and/or diameter differ among the three strains in the hindlimb. They also indicate that BALB/c and A/J, although more similar to each other than to C57BL/6 with respect to collateral extent, are nevertheless significantly different. This finding, which is confirmed by findings described below, complicates assumptions about sharing of haplotypes involved in specifying collateral function.
To examine collateral number directly, we determined the number of angiographically detectable arteries at the midpoint of the adductor/abductor thigh after acute FAL. We have recently shown (6) that the number detected with our angiographic method [optical line resolution ∼25 μm (1)] is the same as the number of collaterals >20 μm in diameter that are directly identified in the optically cleared abductor/adductor thigh during layered microdissection. In agreement with the plantar perfusion data, A/J have fewer collaterals within this diameter range than BALB/c, and both have fewer than C57BL/6 (Fig. 3, A and B). Histomorphometry indicated no significant difference in native (baseline) diameter of the single collateral found in each of the anterior and posterior gracilis muscles (Fig. 3, D and E). We (1–6) and other investigators are not able to determine diameter of the entire population of collaterals in the adductor/abductor thigh of the mouse because of variability in their location and thus identification of them histologically, excepting the gracilis collaterals. This is why we also studied collaterals in the pial circulation (see below)—where all of the collaterals supplying the dorsal cerebral cortex can be imaged with high fidelity for determination of number and diameter for the entire population of collaterals.
In addition to native collateral number and diameter, which are determinants of collateral conductance immediately after FAL, outward remodeling of collateral lumen diameter (i.e., arteriogenesis) governs the increase in collateral conductance and thus recovery of perfusion that occurs over several weeks (20). Therefore, we measured hindlimb perfusion at several time points after FAL. Relative to C57BL/6, both BALB/c and A/J strains had less recovery of perfusion during the first week after ligation (Fig. 1C). However, by the end of the second week, perfusion was comparable among the strains, although BALB/c ultimately attained less recovery by day 21 (relative to C57BL/6) (Fig. 1C and Fig. 2B).
These data (plus other data below) agree closely with data obtained previously in C57BL/6 and BALB/c mice (1, 3). We used new mice of both strains in the present study both as a check on methodological consistency and to permit comparison of the other groups in this study to contemporary C57BL/6 and BALB/c mice. Plantar values agree closely with microsphere determinations of overall hindlimb perfusion (3). In these previous (1, 3) and the present studies we used the same moderate FAL model, consisting of ligation distal to the lateral caudal femoral artery (LCFA) and proximal to the popliteal/saphenous bifurcation, transection and separation in between, and ligation of the superior epigastric artery. Many investigators use a more severe ligation model, with ligation at positions above the LCFA as high as the common iliac artery, resulting in significant necrosis, autoamputation, and less recovery of flow.
We also measured perfusion in the upper 2 mm [penetration depth of our laser Doppler (1, 3)] of the adductor region where the gracilis collaterals and a portion of the other collaterals of the thigh reside. Perfusion declined immediately after FAL in the ROI (Fig. 1, B and D and Fig. 4, B and D), in agreement with our previous studies (e.g., Refs. 1, 2, 4–6). Perfusion was acquired during a 5-min scan interval begun ∼1–2 min after FAL (2). It is possible that edema or vasoconstriction in the ROI, secondary to trauma caused by the ligation surgery, could contribute to this decline, although the surgery was performed delicately and outside of the ROI (see materials and methods). The decline is not likely from vasoconstriction in the vasculature below the ligation, since myogenic and metabolic changes therein are well known to favor dilation, nor can the decline be attributed to any increase in arterial pressure caused by FAL that was not buffered by baroreflexes, since this would favor an increase in flow. Of note, the latter mechanisms would also similarly impact plantar perfusion values. Also, increased shear stress in the collateral network favors collateral dilation. Thus, although we cannot completely rule out contributions of edema and constriction in the ROI, the most likely mechanism for the drop in flow immediately after FAL is as follows. The value obtained for perfusion in the adductor ROI before ligation is an average dominated by capillary flow, together with flow in the distal branches of opposing arteriolar and venular trees originating outside of the ROI. Collaterals interconnecting these arterial trees in the midzone of the adductor have little or no net flow before ligation (2). Immediately after ligation 1) flow in the trees within the lateral portion of the ROI declines to whatever values are provided by the conductance of the native collateral circulation; 2) in the collaterals themselves, flow abruptly increases by ∼50-fold (2); and 3) flow increases in the arterioles in the medial portion of the ROI that supply the adductor collaterals (6). The overall ROI value immediately after FAL represents the average of flow in these three compartments. That this value is well above the contemporary value in the plantar foot [which agrees with overall leg flow as determined by microspheres (3)] reflects the contribution of collateral flow. Thus the adductor values provide an index of collateral flow, which is borne out by their subsequent increase that occurs as collateral remodeling progresses (Fig. 1D and Fig. 4, B and D).
Immediately after ligation, perfusion in the adductor region was comparable among C57BL/6, BALB/c, and A/J mice (Fig. 1D and Fig. 2C), in agreement with similar diameter of the gracilis collaterals (discussed below). However, adductor perfusion 3 days later was lower in A/J and trended (i.e., not statistically significant at the P < 0.05 level) similarly in BALB/c, compared with C57BL/6 (Fig. 1D). Thereafter, perfusion was comparable between C57BL/6 and BALB/c but significantly greater in A/J (Fig. 1D and Fig. 2D). These data are consistent with baseline collateral number among the strains (Fig. 3B) and with the detection of more vessels at day 21 in A/J (Fig. 3C). Together, they suggest that a larger fraction of the native collaterals of A/J mice have diameters too small for detection by angiography (<20 μm) that then undergo greater remodeling after FAL to reach detectable diameters by 21 days. The assumption of a smaller average diameter of the population of native collaterals in skeletal muscle of A/J mice is supported by data in the cerebral pial collateral circulation (discussed below).
The above parameters were also measured, with the same experimental design, in CSS7 and CXB-F1 mice to test the hypothesis that genetic differences in collateral function underlie the ischemic recovery locus mapped to chromosome 7 by Dokun et al. (7) and to test for mode of inheritance of the locus. Since no QTL was identified on chromosome 17 (7), we also studied CSS17 mice to validate the importance of the chromosome 7 genome for collateral function. Substitution of chromosome 7 of A/J into the C57BL/6 background (i.e., CSS7 mice) “transferred” most of the characteristics of plantar and adductor perfusion (Fig. 2A and Fig. 4, A and B) and baseline collateral number (Fig. 3B) of the A/J strain to the C57BL/6 strain. This confirms that chromosome 7 harbors a locus with a major effect on collateral number, with the C57BL/6 allele(s) promoting greater collateral number (confirmed below for cerebral collaterals). Although transfer of part of the enhanced remodeling of A/J is evident in the overall perfusion data (Fig. 2A and Fig. 4, A and B), these data are only supported by trends that do not reach statistical significance in the perfusion (Fig. 2, B and D) and angiography (Fig. 3C) data obtained on day 21. In contrast, the CSS17 strain differed little from C57BL/6, although an allele(s) on chromosome 17 of the A/J strain negatively affects native collateral number and thus flow immediately after FAL (Fig. 2A, Fig. 3B, and Fig. 4A). Comparison of the CXB-F1 mice with their parental strains showed that C57BL/6 alleles are dominant for perfusion values (Fig. 2 and Fig. 4, C and D), baseline (native) collateral number and diameter (Fig. 3, B and E), and collateral remodeling (Fig. 3, C and F). Recovery of plantar perfusion in the CXB-F1 mice trended higher than in C57BL/6 (Fig. 2B and Fig. 4C), along with a similar trend in remodeling (Fig. 3F)—effects that, if significant (e.g., in a larger sample size), may arise from overdominance (hybrid vigor) (23). Comparison of the above metrics (and others below) in F1 litters birthed and reared by either a C57BL/6 or a BALB/c dam revealed no differences, indicating that the Y chromosome or mitochondrial genome does not contribute to the phenotypic differences (data not shown). No sex differences were detected in the above strains or in the F1 population, in agreement with Dokun et al. (7) for recovery of perfusion and necrosis score.
Perfusion to the distal hindlimb immediately after FAL and its subsequent recovery are important determinants of hindlimb function and level of ischemia. We thus scored hindlimb use and ischemic appearance over the 21 days after FAL. The A/J strain showed greater use impairment and more ischemia than C57BL/6 mice, which trended better than BALB/c (Fig. 5, A and B). Values for both scores were consistent with the above measurements of native collateral extent and collateral remodeling. Consistent with chromosome 7 transferring most of the characteristics of perfusion (CSS7 data in Fig. 2 and Fig. 4, A and B), baseline collateral number and diameter (Fig. 3, B and E), and collateral remodeling (Fig. 3, C and F) of the A/J strain to the C57BL/6 strain, use and appearance scores were also similar for A/J and CSS7 (Fig. 5, C and D). On the other hand, CSS17 mice were more similar to C57BL/6 (Fig. 5, C and D) despite fewer hindlimb collaterals (Fig. 3B). This suggests that although a locus(loci) on chromosome 17 contributes to differences in collateral number, it does not affect collateral remodeling. Since C57BL/6 and CXB-F1 had similar use and appearance scores after FAL (Fig. 5, E and F), this finding suggests that the C57BL/6 genome is dominant for the metrics measured.
Native collateral extent in cerebral circulation is reduced in BALB/c, A/J, and CSS7 compared with C57BL/6 mice.
Considering BALB/c, A/J, and CSS7 together, their greater reduction in plantar perfusion immediately after ligation (Fig. 1C, Fig. 2A, and Fig. 4A), fewer collaterals in the hindlimb circulation detected by angiography at baseline (Fig. 3B), and greater hindlimb use impairment and ischemia scores (Fig. 5, A–D) suggest that deficits in perfusion in BALB/c, A/J, and CSS7 (relative to C57BL/6) are due in part to a smaller population of native (preexisting) collaterals in their hindlimbs. To examine whether this extends to another bed where collateral number and diameter can be determined with high resolution for the entire collateral population (1, 4, 5, 26), we imaged the collateral circulation that interconnects the MCA and ACA trees in the pial circulation (Fig. 6A). Also, our previous studies (1, 4–6) show that differences in collateral number and diameter in the pial circulation among inbred mouse strains or strains with targeted alterations in specific genes predict similar qualitative differences in hindlimb [and intestine (1)].
As in hindlimb, BALB/c and A/J had fewer collaterals than C57BL/6 (Fig. 6B); however, BALB/c had fewer than A/J, which is opposite to that observed in hindlimb (compare with Fig. 3B). In agreement with hindlimb data, analysis of CSS7 mice showed that the collateral number phenotype of A/J was “transferred” to the C57BL/6 background by substitution of chromosome 7 of A/J (Fig. 6B). Collateral number in CSS17 was not different from C57BL/6 (Fig. 6B), and analysis of CXB-F1 mice agreed with hindlimb data indicating that C57BL/6 alleles are dominant for collateral number (Fig. 6B).
We also measured pial collateral diameter. Diameter followed the rank order C57BL/6 > BALB/c > A/J (Fig. 6C). The smaller diameter of A/J collaterals largely followed A/J chromosome 7 (CSS7 mice), but substitution of A/J chromosome 17 into the C57BL/6 genome (CSS17 mice) also conferred some of the smaller diameter phenotype of A/J (Fig. 6C). Pial collateral diameter in CXB-F1 mice was similar to that in the BALB/c strain, suggesting that the BALB/c genome is dominant for pial collateral diameter (Fig. 6C). These diameter data for cerebral collaterals are in contrast to diameter of the collaterals in the gracilis muscles, which did not differ among the mouse strains (Fig. 3E); however, average values for pial collateral diameter are derived from measurement of the entire population, whereas it was not possible to determine the average diameter of the population of collaterals in the adductor/abductor thigh, because unambiguous identification of collaterals in histological cross sections of muscles below the gracilis muscles is not possible.
In fact, the data shown in Figs. 1–5 in hindlimb largely parallel those in Fig. 6 in brain for native collateral number and diameter. For example, comparing C57BL/6, BALB/c, and A/J, the data in Fig. 1, C and D, Fig. 2A, Fig. 3B, and Fig. 5, A and B, taken together, are consistent with A/J having a larger number of smaller-diameter (i.e., <20-μm diameter, thus not detectable by angiography) native collaterals in hindlimb compared with BALB/c, and with both of these strains having fewer smaller-diameter native collaterals than C57BL/6, which is what they evidence in the pial circulation where the entire population can be measured.
To determine the relationship between pial collateral density and cerebral artery tree size, we measured the percentage of cortical area (territory) supplied by the cerebral artery trees. Tree sizes were comparable between C57BL/6 and BALB/c mice, whereas the MCA tree was larger in the A/J strain (Fig. 6D). Tree territories also differed between BALB/c and A/J. Territories in CSS7 and CSS17 mice show that, unlike native collateral extent and remodeling (plus perfusion and use/appearance scores that follow these metrics), which are largely conferred by the chromosome 7 genome, substitution of either chromosome 7 or 17 does not transfer the A/J phenotype for cerebral artery tree size into the C57BL/6 background. Since a previous study showed no relationship between variation in cerebral artery tree size and collateral number or diameter among 15 inbred mouse strains, including the 3 examined in the present study (26), the data in Fig. 6D thus serve as a “negative control” for the specificity of the CSS strains as used here to examine the influence of chromosomes 7 and 17 on collateral metrics.
Recovery of hindlimb perfusion and native pial collateral number and diameter are not altered in Bag3-deficient mice.
Among the candidate genes identified by Dokun et al. (7), Bag3 is located on the peak of the chromosome 7 QTL within an apparent haplotype shared by BALB/c and A/J but not by C57BL/6. Moreover, Bag3 expression is increased in ischemia (14), and, in addition to several suggested functions (see discussion), acts as a negative regulator of apoptosis (15, 18). Thus Bag3 could be a determinant of formation/maintenance of native collaterals and/or of collateral remodeling after FAL. If correct, a polymorphism in Bag3 leading to altered expression could underlie the QTL obtained by Dokun et al. (7) for hindlimb ischemia, which our above findings suggest is largely due to differences in native collateral extent and remodeling. That is, a deficiency in Bag3 expression could result in altered native collateral extent and/or recovery of perfusion after FAL. Since Bag3−/− mice do not survive past postnatal day 25 because of failure of striated muscle development (e.g., diaphragm and heart) (11), we examined hindlimb ischemia in adult heterozygotes, which have no obvious phenotype (11). Hindlimb perfusion after FAL in Bag3+/− mice was comparable to that in wild-type littermates (Fig. 7, A and B). In addition, postnatal day 7 pups with deletion of one or both Bag3 alleles exhibited pial collateral numbers and diameters similar to wild type (Fig. 7, C and D). These data do not support the hypothesis that a variant in Bag3 leading to differences in expression in C57BL/6 versus BALB/c and A/J underlies the differences in collateral function in these strains identified in the present study or the QTL for recovery of hindlimb perfusion reported previously (7).
DISCUSSION
Recent studies reporting significant variation in native collateral extent among healthy individuals and in collateral remodeling in obstructive disease (1, 10, 19, 20, 22) have generated significant interest in identifying the underlying genetic factors. However, to date none has been identified. Collateral variation is striking among strains of mice, with C57BL/6 and BALB/c exhibiting the greatest difference in native collateral conductance among 15 inbred strains (26). BALB/c mice also evidence less collateral remodeling (1, 10). When combined with their reduced collateral extent, this likely underlies the impaired recovery of blood flow and more severe ischemic tissue injury after arterial obstruction in the BALB/c strain (1, 8, 10, 21, 26).
In an interesting recent study, Dokun et al. (7) reported an identical QTL on chromosome 7 for recovery of flow [logarithm of odds (LOD) 3.71, P < 0.05] and tissue necrosis score (LOD 7.96, P < 0.001) after FAL in a C57BL/6 × BALB/c mapping population. The underlying physiological basis for this finding has not been determined. Several candidate genes, including Bag3, were identified based on evidence suggesting that A/J and BALB/c mice share a common haplotype(s) at the locus that is different from C57BL/6 mice (7). The main goals of the present study were to determine whether hindlimb collateral extent and remodeling are indeed comparable in BALB/c and A/J mice and to test the hypothesis that variations in these traits are important physiological mechanisms underlying the reported QTL.
Our findings show that collateral extent in the hindlimb is less in BALB/c and A/J mice compared with C57BL/6 mice. It is also less in brain, which was studied to see whether the difference extends to another tissue. These findings provide the physiological mechanism underlying the QTL identified by Dokun et al. (7). We also find that although BALB/c and A/J mice share similarities in collateral extent, they also exhibit differences, e.g., A/J mice show greater collateral remodeling than BALB/c (and C57BL/6) mice. These findings are important in identifying candidate genes based on assumptions of haplotype sharing between BALB/c and A/J (7). Analysis of the CSS7 chromosome substitution strain was done to test whether chromosome 7 carries genetic information conferring differences in collateral extent between C57BL/6 and A/J mice, since it does for necrosis score and recovery of perfusion after FAL (7). Our results show that chromosome 7 confers part, but not all, of the difference in collateral extent and remodeling and that chromosome 17 also contributes. To test whether alleles governing these traits in the hindlimb circulation are dominant in C57BL/6 or BALB/c, we evaluated these parameters in an F1 population and found that C57BL/6 alleles are dominant. These latter two findings, which are consistent with our other data as well as studies in the literature (7, 12, 25), help to focus future investigations. Finally, because Bag3 has been proposed as a candidate gene (7) and has been implicated in pathways that could underlie differences in necrosis score and recovery of perfusion versus collateral formation and remodeling—parameters measured in the previous (7) and present studies, respectively—we examined mice heterozygous and homozygous-null for this gene. However, we found no evidence causally implicating Bag3. This result favors examining other high-priority candidate genes within the QTL locus (7) in future studies.
Our results show that, although collateral traits in BALB/c and A/J are more similar to each other than to C57BL/6, they nevertheless exhibit significant differences. The hindlimb circulation of A/J compared with BALB/c appears to have more native collaterals but with smaller diameters (see results). This was confirmed directly in another tissue, the cerebral circulation; previous studies in mouse strains have demonstrated that genetically determined differences in native collateral density and diameter in this tissue correctly predict similar differences in the hindlimb and other tissues (1, 4, 5). Consistent with lower conductance in the native hindlimb collateral circulation of A/J mice, this strain suffered more severe ischemia and use impairment after FAL compared with BALB/c. Dokun et al.(7) also observed greater necrosis in A/J than in BALB/c. These data indicate that ischemic injury and impaired use are more closely linked to differences in native collateral extent than to collateral remodeling, which in skeletal muscle takes several days to become appreciable.
Compared with BALB/c, A/J mice exhibited greater collateral remodeling, as evidenced by higher adductor perfusion, more collaterals detected angiographically by day 21, and better recovery of flow by day 21. Consistent with this, plantar flow [an index of overall leg flow (3, 10, 20, 21)] by day 21 was similar to C57BL/6. Greater collateral remodeling in A/J was also observed in the cerebral circulation after MCA ligation (26). In that study, greater remodeling in A/J and certain other strains was attributed, in part, to smaller native diameters; collateral remodeling correlated with native collateral diameter among 15 inbred strains (r2 = 0.55, P < 0.0001) (26). This finding is predicted by the well-known inverse relationship between diameter and shear stress and the fact that shear stress is known to initiate collateral remodeling (13, 19, 20, 22). However, flow-induced positive remodeling of the carotid artery also follows the rank order A/J > C57BL/6 > BALB/c (9), despite similar baseline diameters (Zhang H and Faber JE, unpublished results), indicating that additional factors besides hemodynamics influence genetic variation in remodeling. Differences in collateral extent, remodeling, and associated traits (e.g., perfusion recovery and use/injury scores after artery ligation) between BALB/c and A/J, reported here, complicate their use in haplotype mapping to narrow QTL confidence intervals and candidate genes for collateral traits.
Analysis of CSS17 mice showed that chromosome 17 transferred several small (compared to chromosome 7, i.e., the CSS7 strain) but significant differences to the C57BL/6 background. These effects are consistent with our previous studies (5) showing that differences in expression of the Vegfa gene, which resides on chromosome 17, impact native collateral density and diameter, and collateral remodeling after FAL, and VEGF expression is significantly lower in BALB/c compared with C57BL/6 (1, 2). However, there are thousands of genes on chromosome 17, several of which could harbor polymorphisms affecting collateral function. That no QTL was identified on chromosome 17 in previous studies (7, 12) may reflect a smaller contribution of such a locus(loci) than the chromosome 7 locus, as evidenced by our present data comparing CSS7 versus CSS17, plus the fact that variation in the traits measured previously [necrosis, recovery of perfusion, cerebral infarct volume (7, 12)] likely reflect genetic variation in additional mechanisms besides the dominant impact of native collateral extent and remodeling (e.g., cellular sensitivity to ischemia, apoptosis, cell metabolism, hemostasis). Moreover, it is not surprising that loci on other chromosomes impact native collateral extent and collateral remodeling, since the chromosome 7 QTL accounts for much (50–60%) but not all of the heritability of variation in tissue injury after artery ligation between C57BL/6 and BALB/c (7, 12). The use of the CSS strains, as used in our study, is widely regarded as an important and valid approach (e.g., Refs. 7, 12, 17, 23).
In contrast to the present findings, Dokun and coworkers (7) reported that recovery of hindlimb perfusion and necrosis score were not different between BALB/c and A/J (although both were different from C57BL/6). They also reported no difference in perfusion immediately after FAL in the three strains. In addition to differences in resolution of the Doppler systems used and the ROI quantified, different models of FAL were examined. In the study of Dokun et al., the femoral artery was ligated at the inguinal canal, which is much more proximal than the ligation in the present study between the LCFA and popliteal-saphenous bifurcation. More proximal ligation results in greater ischemia, tissue atrophy, and necrosis, plus removal of a greater fraction of the thigh collaterals from serving in that capacity. Indeed, partial or complete necrosis and loss of the thigh were observed in a significant fraction of BALB/c and especially A/J (7); we also observed that A/J sustained the greatest ischemia and use impairment, although this was limited to dry necrosis extending into the metatarsal area by day 21 in 20% of the mice. Thus many mechanisms including apoptosis, tissue metabolism, inflammation, angiogenesis, and skeletal muscle degeneration and regeneration likely differ between the two studies. Polymorphisms in genes affecting these mechanisms could obscure detection of QTL linked to variation in collateral extent and remodeling.
Despite these differences, our findings with CSS7 mice agree in part with data obtained by Dokun et al. (7). They showed that in CSS7 the A/J phenotype of reduced recovery of blood flow on day 21 was fully transferred into the C57BL/6 background. We found that CSS7 fully transferred plantar flow immediately after FAL but only partially transferred the recovery and the use scores. Likewise, native (baseline) collateral number and diameter in hindlimb and brain, and hindlimb collateral remodeling after FAL, were not (or trended to not be) completely transferred, suggesting that a locus(loci) on another chromosome(s) contributes to differences in these traits between C57BL/6 and A/J. Indeed, CSS17 also transferred a portion of the A/J phenotype for perfusion immediately after FAL, native collateral number in hindlimb, and pial collateral diameter. VEGF-A has been shown to be a significant positive regulator of native collateral formation (i.e., number and diameter) in the embryo and thus the adult (5). Moreover, BALB/c mice express less VEGF-A than C57BL/6 mice (1). We have also shown (4) that expression of chloride intracellular channel-4 (CLIC4) is a positive regulator of native collateral formation in the embryo and thus density and diameter in the adult. However, while Clic4 is located on chromosome 4, for which no significant or suggestive QTL was identified (7), there is evidence that CLIC4 resides within the VEGF signaling pathway (4).
Our findings in CXB-F1 mice also agree in part with data obtained in the same F1 cross generated by Dokun et al. (7). They found that the C57BL/6 genome contributes dominant protective alleles for recovery of perfusion and necrosis, a finding confirmed by others for perfusion recovery (25). Similarly, we found dominance of the C57BL/6 genome for all traits measured in hindlimb and brain, with the exception of pial collateral diameter, where BALB/c was dominant.
Among the candidate genes identified by Dokun et al.(7), the Bcl-associated athanogene 3 (Bag3) locus resides on the peak of the region they identified on chromosome 7. Bag3 is known to regulate Hsp70 chaperone proteins (24), to increase in ischemia (14), and to promote Bcl-2 inhibition of apoptosis (15, 18). It is highly expressed in skeletal, cardiac, and smooth muscle, where it colocalizes with α-actinin and desmin (11). As well, Bag3 is required for maintenance of striated muscle cells and for regeneration after injury (11). Homozygous deletion results in myopathy and myofibrillar degeneration beginning by approximately postnatal day 12 (although knockouts are indistinguishable from wild type during the first week of life), resulting in death approximately 2 wk later, presumably from cardiac and respiratory muscle failure (11). Given these functions, we postulated that a deficiency in Bag3 expression due to a polymorphism shared by BALB/c and A/J could 1) affect recovery of perfusion after FAL and ischemic injury by impairing regeneration and thus blood flow demand and/or 2) result in reduced formation of collaterals in the embryo. However, we found no evidence for either hypothesis. Adult heterozygotes were only slightly lower in body weight (10%, P < 0.05) and did not differ from wild type in perfusion at any time point after FAL. Furthermore, neither adult Bag3+/− nor postnatal day 7 Bag3−/− mice evidenced alterations in cerebral collateral number or diameter. Thus, although unlikely, it remains possible that BALB/c and A/J strains may harbor a variant of Bag3 that leads to overexpression or altered activity/function, resulting in impaired collateral formation.
Building on a previous study showing that BALB/c have a very sparse native cerebral collateral circulation compared with C57BL/6, resulting in much larger infarct volumes after MCA ligation (1), Keum and Marchuk (12) recently sought to identify QTL associated with infarct volume in an F2 mapping population of these two strains. Interestingly, they identified the same QTL on chromosome 7 that was obtained in their hindlimb study (7). Furthermore, infarct volume varied by >30-fold among these and 13 additional inbred strains (12). We recently examined the same 15 strains for cerebral collateral function (26) and reported similar wide variations in native collateral number and diameter, which highly correlated inversely with the reported values (12) for infarct volume. Moreover, measurement of additional traits that also exhibited significant variation among the strains (collateral length, blood viscosity, MCA tree territory), followed by combining them with collateral number and diameter into a quasi-Poiseuille-like equation for collateral conductance, yielded predicted infarct volumes that closely matched the directly measured infarct volumes (26).
Our findings suggest that a locus(loci) on chromosome 7 contributes much, but not all, of the magnitude of differences in collateral traits among C57BL/6, BALB/c, and A/J and possibly other strains of mice. Moreover, taken together with the major role of the collateral circulation in determining recovery of blood flow and tissue injury after artery occlusion (1, 26), our results suggest that a genetic element(s) within the recently identified chromosome 7 locus (7, 12) is a key regulator of formation of the collateral circulation, and that variants in it are a major contributor to the wide genetic variation in the extent of this circulation among healthy individuals. Genomewide linkage analysis for collateral traits will be required to test these hypotheses. Furthermore, the significant differences in collateral traits between BALB/c and A/J identified in the present study indicate that multiple strains will be required for interval-specific SNP haplotype analysis or kinship association mapping to narrow down the list of candidate genes responsible for formation of the collateral circulation and its wide variation. The present findings help establish a framework for future work to define the molecular pathways underlying formation of the collateral circulation and the genetic polymorphisms responsible for its variation in healthy individuals. A more thorough understanding of these mechanisms will provide a better foundation for development of therapies to promote collateral formation and remodeling in individuals with or prone to occlusive vascular disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-62584 and HL-090655 (J. E. Faber) and F32-HL-080847 and K99-HL-093609 (D. Chalothorn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank K. Kirk McNaughton for histology.
REFERENCES
- 1.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 30: 179–191, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol 49: 251–259, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol 289: H947–H959, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res 105: 89–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 103: 1027–1036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res(April29, 2010). doi:10.1161/CIRCRESAHA.109.212746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 117: 1207–1215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun 310: 143–147, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol 156: 1741–1748, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol 26: 520–526, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol 169: 761–773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keum S, Marchuk DA. A locus mapping to mouse chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circ Cardiovasc Genet 2: 591–598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res 78: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol 175: 338–346, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Buchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett 503: 151–157, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 116: 975–983, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24: 221–225, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiurazzi F, Storti G, Volpe S, Venuta S, Turco MC, Leone A. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ 10: 383–385, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Schaper W. Collateral circulation: past and present. Basic Res Cardiol 104: 5–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 23: 1143–1151, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34: 775–787, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol 98: 1194–1197, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Silver LM. Mouse Genetics: Concepts and Applications. New York: Oxford Univ. Press, 1995 [Google Scholar]
- 24.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol 3: E237–E241, 2001 [DOI] [PubMed] [Google Scholar]
- 25.van Weel V, Toes RE, Seghers L, Deckers MM, de Vries MR, Eilers PH, Sipkens J, Schepers A, Eefting D, van Hinsbergh VW, van Bockel JH, Quax PH. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol 27: 2310–2318, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 30: 923–934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]