Abstract
Autologous bone marrow cell (BMC) transplantation has been shown as a potential approach to treat various ischemic diseases. However, under many conditions BMC dysfunction has been reported, leading to poor cell engraftment and a failure of tissue revascularization. We have previously shown that skeletal muscle angiogenesis induced by electrical stimulation (ES) is impaired in the SS/Mcwi rats and that this effect is related to a dysregulation of the renin angiotensin system (RAS) that is normalized by the replacement of chromosome 13 derived from the Brown Norway rat (SS-13BN/Mcwi consomic rats). The present study explored bone marrow-derived endothelial cell (BM-EC) function in the SS/Mcwi rat and its impact on skeletal muscle angiogenesis induced by ES. SS/Mcwi rats were randomized to receive BMC from: SS/Mcwi; SS-13BN/Mcwi; SS/Mcwi rats infused with saline or ANG II (3 ng·kg−1·min−1). BMC were injected in the stimulated tibialis anterior muscle of SS/Mcwi rats. Vessel density was evaluated in unstimulated and stimulated muscles after 7 days of ES. BMC isolated from SS/Mcwi or SS/Mcwi rats infused with saline failed to restore angiogenesis induced by ES. However, BMC isolated from SS-13BN/Mcwi and SS/Mcwi rats infused with ANG II effectively restored the angiogenesis response in the SS/Mcwi recipient. Furthermore, ANG II infusion increased the capacity of BM-EC to induce endothelial cell tube formation in vitro and slightly increased VEGF protein expression. This study suggests that dysregulation of the RAS in the SS/Mcwi rat contributes to impaired BM-EC function and could impact the angiogenic therapeutic potential of BMC.
Keywords: angiogenesis, endothelial progenitor cells, regenerative medicine
the formation of new blood vessels is essential for a variety of physiological processes as well as for tissue repair and remodeling during acute and chronic ischemic vascular diseases. Rapid revascularization of injured, ischemic, and regenerating organs is essential to restore organ function. Despite great advances in both medical and surgical management of patients with ischemic-related diseases, failure in the revascularization process is still considered a major issue and is frequently associated with a poor prognosis. Over the past decade, intensive efforts have been undertaken to better understand the underlying mechanisms regulating blood vessel growth and to develop new therapeutic options to promote revascularization of ischemic tissues.
Recently, new strategies using transplantation of autologous bone marrow cells (BMC) have been shown as an innovative and promising therapeutic approach to induce neovascularization in ischemic tissues in adults (19, 29, 33, 50, 51). Several studies have shown that endothelial progenitor cells (EPC), identified in the bone marrow, augment reparative neovascularization either through differentiation into mature endothelial cells (EC) or indirectly through paracrine stimulation of resident EC proliferation (43, 60). Numerous experimental studies have shown that the transplantation, as well as the therapeutic mobilization of the EPC to the sites of injury, restored tissue vascularization after ischemic events in limbs, retina, brain, and myocardium (17, 18, 28, 40, 53–55). These studies have created tremendous enthusiasm in the stem cell research field. However, many of the benefits found with the EPC therapy in experimental animals were not replicated in clinical trials (30, 37). This seems to be due to the fact that the primary studies showing the role of the EPC in neovascularization were performed in healthy animals with experimentally induced vascular injury, whereas vascular events often occur in patients with cardiovascular disease risk factors and endothelial dysfunction. Recent studies have shown reduced EPC availability and altered EPC function and differentiation in patients at increased cardiovascular disease risk may negatively influence current therapeutic strategies involving autologous stem cell transplantation (22, 58). EPC dysfunction has now been shown in a large number of diseases including diabetes, atherosclerosis, stroke, and hypertension (36, 42, 56, 58). The mechanisms underlying the EPC dysfunction are not clear. However, the impairment in EPC proliferation, adhesion, and angiogenic properties may underlie new mechanisms involved in disease pathogenesis or vascular complications. Development of new strategies to restore EPC function and consequently increase EPC engraftment and/or mobilization may substantially impact angiogenic stem cell-based therapy.
The present study takes advantage of the immune-compatible chromosome substitution rats to provide new insight into the mechanisms underlying reduced EPC availability and/or altered EPC function and differentiation. The Dahl salt-sensitive rat (SS/Mcwi) is an animal model for salt-sensitive hypertension and rarefaction. We have previously shown that the skeletal muscle angiogenesis induced by electrical stimulation is significantly impaired in the SS/Mcwi rats regardless of the salt intake (3, 9) and that this angiogenesis is restored in the consomic SS-13BN/Mcwi rat by a renin dependent mechanism (3). Although the dysregulation of the renin angiotensin system (RAS) has been implied in the poor angiogenesis response as well as vascular dysfunction in the SS/Mcwi rats (3, 9, 12, 13, 35, 38), little is know about the role of the RAS on bone marrow-derived endothelial cell (BM-EC) function and stem cell-based therapy. In the present study we hypothesized that low RAS activity present in SS/Mcwi rats will impair BM-EC function and consequently the angiogenic efficacy of bone marrow stem cell therapy. Understanding how the RAS affects the EPC function may help to identify optimal stem cell therapy to treat the reduction in vessel density that occurs in several forms of low renin hypertension in humans (20, 32, 47).
MATERIAL AND METHODS
Bone marrow cell isolation.
The Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee approved all animal protocols. The SS/Mcwi rat strain is an inbred substrain derived from the Dahl-S rat. The SS-13BN/Mcwi consomic rat strain was derived from BN/Mcwi rats and SS/Mcwi rats by replacement of SS chromosome 13 with BN chromosome 13 on the SS genetic background; the origin of both of these strains has been described previously (8). Animals were housed and cared for in the MCW Animal Resource Center and were given low salt diet (0.4% NaCl) and water ad libitum. Donor rats were killed with an overdose of pentobarbital, both femurs and tibias were surgically dissected, and the adhering tissues were completely removed. Both ends of the bones were excised, and bone marrow cells were harvested by flushing with RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 20% fetal calf serum, using a 23-gauge needle. The BMC were gently resuspended with an 18-gauge needle and filtered through sterile 100 μm nylon mesh. Red blood cells were lysed with red blood cell lysis buffer (BioLegend) for 5 min at room temperature. Cells were washed in PBS three times and then resuspended at 6 × 106 cells in 100 μl of PBS for transplantation or Western blot analysis.
Electrical stimulation surgery and BMC implantation.
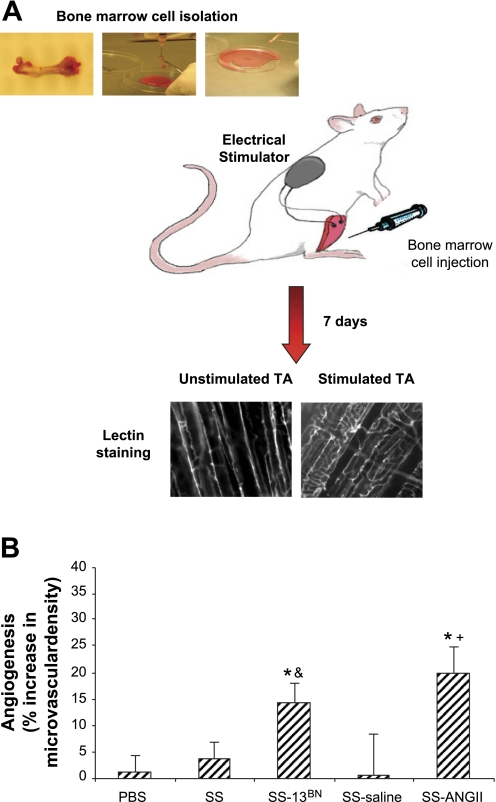
The tibialis anterior (TA) and extensor digitorum longus (EDL) muscles of the recipient and sham-injected animals were electrically stimulated for 8 h per day for 7 consecutive days as previously described (34). Briefly, SS/Mcwi rats were anesthetized with intramuscular injection of a mixture of ketamine (100 mg/kg), xylazine (50 mg/kg), and acepromazine (2 mg/kg). BMC (6 × 106 cells) or PBS was injected into three to four different areas of the right TA muscle of the SS/Mcwi rats. The recipient rats were randomly divided into five groups. 1) Control, SS/Mcwi rats that received 0.1 ml of PBS injection; 2) BMC-SS, SS/Mcwi rats that received BMC from SS/Mcwi rats; 3) BMC-SS13BN, SS/Mcwi rats that received BMC from the SS-13BN/Mcwi; 4) BMC-SS ANG II, SS/Mcwi rats that received BMC from the SS/Mcwi rats infused with a subpressor dose of ANG II; 5) BMC-SS saline, SS/Mcwi rats that received BMC from the SS/Mcwi rats infused with saline. Immediately after the cell or PBS injection a miniature battery-powered stimulator was implanted subcutaneously in the thoracolumbar region of the rats and a pair of electrodes was tunneled under the skin from the stimulator to the right lower hindlimb muscles to promote muscle contractions. On the following day the electrical stimulators were turned on. Animals were euthanized 7 days following the onset of stimulation for analysis of angiogenesis. A schematic illustration of the bone marrow injection protocol and vessel density analysis is given in Fig. 1A. A total of 61 animals were used in this study. Numbers of animals for each group varied according to the experiment design and are indicated in the figure legends.
Fig. 1.
Effect of bone marrow cell (BMC) injection on the skeletal muscle angiogenesis induced by electrical stimulation. A: schematic illustration of the bone marrow injection protocol and vessel density analysis. B: percent increase in vessel density from unstimulated to stimulated tibialis anterior (TA) muscle of SS/Mcwi rats that received intramuscular PBS injection (n = 14) or BMC from SS/Mcwi (n = 11), BMC from SS-13BN (n = 11), BMC from SS/Mcwi infused with saline (n = 5) or BMC from SS/Mcwi infused with ANG II (n = 9). SS/Mcwi BMC failed to restore angiogenesis induced by electrical stimulation in SS/Mcwi rats. In contrast there was a significant increase in skeletal muscle vessel density of SS/Mcwi rats that received BMC from SS-13BN and SS/Mcwi rats infused with ANG II. Values are expressed as means ± SE. Unstimulated and stimulated vessel densities within each treatment were compared by paired t-test, *P < 0.05. Percent increase in vessel density was compared across groups by 1-way ANOVA, &P < 0.05 vs. SS; +P < 0.05 vs. SS-saline.
Morphological analysis of vessel density.
The TA muscle from the unstimulated and stimulated hindlimbs were harvested and stained with rhodamine-labeled Griffonia simplicifolia I lectin (Sigma) as previously described (11, 46). Briefly, the TA muscles from the unstimulated and stimulated hindlimbs were harvested and fixed in 0.25% formalin for 24 h. Each muscle was longitudinally sliced on a sliding microtome to a thickness of ∼75 μM and stained in a 30 μg/ml rhodamine-labeled G. simplicifolia I lectin (Sigma) solution. After several rinses slices were mounted on microscope slides in water-soluble mounting media. Twenty images per muscle were taken at ×200 under fluorescent light (excitation 555 nm and emission wavelength 580 nm). Image files were analyzed by Metamorph software using an overlaid 10 × 13 grid, and vessel density is expressed as the mean number of vessel-grid intersections per microscopic field (0.077 mm2).
Monitoring of the implanted BMC in the ischemic hindlimbs.
To show the incorporation of the cells into the skeletal muscle vasculature and their differentiation into EC, BMC (2 × 107 cells/ml) were isolated from SS-13BN/Mcwi rat and labeled with of PKH-67 dye (Sigma) according to manufacturer's instructions. In brief, samples were incubated at room temperature with 4 μM PKH-67 dye for 5 min with gentle mixing. Staining was terminated by addition of equal volume of RPMI media containing 20% FBS; cells were collected by centrifugation (400 g, 10 min, 4°C) and washed three times with PBS. The cell viability was tested by the trypan blue exclusion test. Cells were resuspended in 0.1 ml of PBS and injected into three or four different areas of the TA muscle of SS/Mcwi rats. After 7 days of electrical stimulation the TA muscle of the SS/Mcwi rats was collected and processed for lectin or CD31 staining as previously described (11, 46).
In vivo ANG II infusion.
SS/Mcwi rats received ANG II at a subpressor dose of 3 ng·kg−1·min−1 or saline intravenously for 7 days as described previously (11, 21, 38, 41). Briefly, SS/Mcwi rats were anesthetized with intramuscular injection of a mixture of ketamine (100 mg/kg), xylazine (50 mg/kg), and acepromazine (2 mg/kg), a catheter was securely inserted into the left jugular vein, and the animals were allowed to recover for 24 h. Conscious rats were then connected to a multisyringe pump to provide a continuous intravascular infusion of saline alone or saline containing ANG II (3 ng·kg−1·min−1) for 7 days in their home cages. After 7 days the BMC were harvested and resuspended in PBS for implantation.
Vascular endothelial growth factor Western blotting.
Whole BMC were harvested and then washed three times in cold PBS and lysed in RIPA buffer (150 mM NaCl, 1% NP 40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris base, pH 8.0) containing protease inhibitors (10 mmol/l sodium pyrophosphate, 100 mmol/l NaF, 1 mmol/l Na3VO4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 2 mmol/l PMSF). Protein was separated in a denaturing SDS/4–20% polyacrylamide gel (20 μg per lane) and then blotted onto a nitrocellulose membrane. Membranes were incubated with a rabbit polyclonal antibody for vascular endothelial growth factor [VEGF (A-20), dilution 1:500; Santa Cruz, CA] for 4 h at room temperature and after serial washes (5 × 3 min in TBS-T), with the secondary antibody (anti-rabbit IgG, 1:3,000) for 1 h at room temperature. Immunoblots were visualized by chemiluminescence (Pierce Rockford, IL), followed by autoradiography. VEGF (A-20) is recommended for detection of the 189, 165, and 121 amino acid splice variants of VEGF. The 42 kDa band (VEGF dimer) was quantified. Membranes were stained with Ponceau S (Sigma) to confirm equal protein loading. A C6 tumor cell line was used as positive control. Results were normalized by Ponceau S and expressed as relative densitometric values using the Image Quant software.
BM-EC isolation, characterization, and tube formation assay.
Mononuclear cells (MNC) were isolated by gradient density centrifugation (Histopaque 1083, Sigma) according to the manufacturer's protocol. After purification with three washing steps, 107 MNC were plated on fibronectin-coated six-well plates. Cells were cultured in endothelial cell basal medium-2 (Clonetics) supplemented with EGM-2 single aliquots consisting of 10% FBS, VEGF, fibroblast growth factor-2, epidermal growth factor, insulin-like growth factor-1, ascorbic acid, and antibiotics. Adherent cells were washed on day 4 to remove unattached cells, and fresh growth medium was added. After 7 days in culture BM-EC were identified by typical EC morphology. However, it must also be acknowledged that the cell population used in the present study is heterogeneous but considered enriched in endothelial progenitor cells, which express EC markers including CD31 expression, BS-1 lectin, and incorporation of acetylated low-density lipoprotein with marked angiogenic properties. BM-EC were further imaged to confirm incorporation of acetylated low-density lipoprotein (Dil-ac-LDL, Biomedical Technologies) and binding of BS-1 lectin (FITC-labeled lectin from Bandeiraea simplicifolia, Sigma) as previously described (63). Immunofluorescence for detection of CD31 was performed using an anti-rat CD31 antibody (PharMingen) by a standard protocol as given by the manufacturer.
To investigate the ability of BM-EC to integrate into vascular structures, BM-EC (104 cells) from SS/Mcwi rats infused with saline or ANG II were cocultured with human umbilical vein endothelial cells (HUVEC, 104 cells) on Matrigel (BD Biosciences) as previously described (14). BM-EC were stained with Dil-Ac-LDL (Molecular Probes, 5 μg/ml) for 1 h and then coplated with HUVEC labeled with DAPI (20 μg/ml, Sigma) on Matrigel-coated 96-well plates. After 18 h the network formation was assessed using an inverted phase contrast microscope (Nikon, Tokyo, Japan). The total length of tubes was quantified in 2–5 random low-power fields per group. The incorporation of BM-EC was quantified by counting the number of Dil-ac-LDL-labeled cells incorporated into the tubes in 18 fields (×200) per group.
Data analysis and statistics.
The results are expressed as means ± SE. P < 0.05 was considered significant. To evaluate the significance of differences in vessel density between stimulated and unstimulated sides of the TA muscle, a paired t-test (unstimulated vs. stimulated) and one-way analysis of variance were performed. Significant differences between groups were further investigated using a post hoc Tukey's test. All data were tested to assure the normality of distribution requirement was met.
RESULTS
Attenuated therapeutic efficacy of SS/Mcwi BMC transplantation on skeletal muscle angiogenesis induced by electrical stimulation.
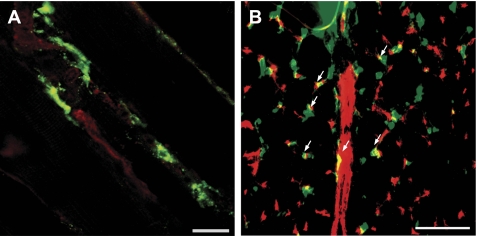
The effect of BMC injection on skeletal muscle vessel density was evaluated after 7 days of electrical stimulation. Figure 1B shows that PBS injection as well as the autologous transplantation of BMC derived from an SS/Mcwi rat did not restore skeletal muscle angiogenesis in SS/Mcwi rats after electrical stimulation. To investigate if genetic manipulations of the RAS would affect angiogenesis induced by stem cell therapy, whole BMC isolated from the SS-13BN consomic rats were injected into the stimulated leg of the SS/Mcwi rats. We have previously reported that the SS/Mcwi rat has an impaired regulation of the renin gene located on chromosome 13 (3, 8, 9). Consomic SS-13BN rats are compatible BMC donors since they have the entire SS/Mcwi genetic background with the exception of the chromosome 13 from the normotensive BN rat, which contains a functioning renin gene. In contrast to the BMC derived from the SS/Mcwi rats, BMC derived from the SS-13BN rats promoted a significant increase in the TA muscle vessel density of the SS/Mcwi rats after 7 days of electrical stimulation. To test the hypothesis that the low ANG II levels in the SS/Mcwi could impact the ability of the bone marrow stem cell therapy to restore skeletal muscle angiogenesis during electrical stimulation, BMC were isolated from SS/Mcwi rats that had been infused with low doses of ANG II for 7 days and then injected into the stimulated TA of another group of SS/Mcwi rats. As shown in Fig. 1B the BMC derived from the ANG II-infused SS/Mcwi rat restored the angiogenic response in the TA muscle after 7 days of electrical stimulation. Figure 2 confirms that the BMC labeled with PKH-67 dye actively incorporated into the skeletal muscle vasculature (Fig. 2A) and many of the labeled cells were colocalized with capillaries (Fig. 2B).
Fig. 2.
Representative images of the injection sites showing the incorporation of the PKH-67-labeled BMC into the skeletal muscle vasculature after 7 days of electrical stimulation. A: lectin staining in longitudinal section of the stimulated TA. Green, PKH-67-labeled BMC; red, lectin-labeled blood vessels; scale bar = 40 μm. B: CD31 staining in cross section of the stimulated TA; scale bar = 100 μm. Red, CD31-stained endothelial cells. Arrows indicate the colocalized BMC with skeletal muscle capillaries.
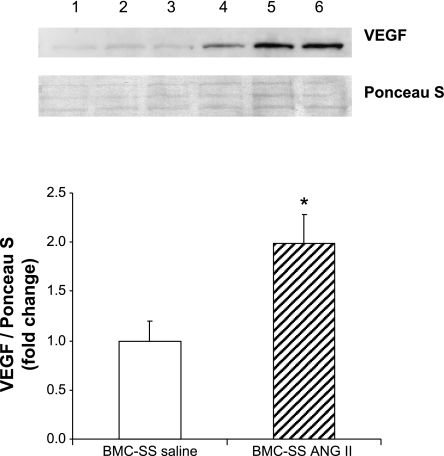
In vivo ANG II infusion increases VEGF protein levels in SS/Mcwi whole BMC.
To evaluate the effect of ANG II infusion on VEGF protein levels in the BMC, a quantitative Western blot analysis with densitometry was performed. Figure 3 shows that low-dose ANG II infusion significantly increased VEGF protein levels in the BMC of SS/Mcwi rats.
Fig. 3.
Representative Western blot (top) and corresponding quantitative densitometry (bottom) of VEGF expression in the whole BMC of SS/Mcwi rats infused with saline (lanes 1–3) or ANG II (lanes 4–6). The VEGF protein expression is presented as relative (fold change) to BMC-SS saline (control = 1). *P < 0.05 vs. BMC-SS saline. Data are presented as means ± SE. BMC-SS saline (n = 5), BMC-SS ANG II (n = 6).
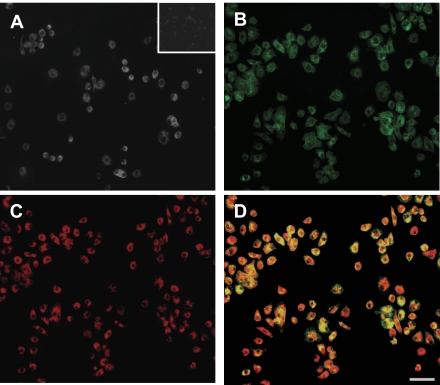
Bone marrow-derived endothelial characterization.
After 7 days in culture BM-EC exhibited cobblestone morphology, which is typical for confluent EC. The endothelial phenotype was further confirmed by the expression of CD31 (Fig. 4A), the ability to react with BS-1 lectin (Fig. 4B) and to uptake ac-LDL (Fig. 4C). Fluorescent microscopy identified double positive cells as BM-EC (Fig. 4D).
Fig. 4.
Characterization of bone marrow of the endothelial lineage. After 7 days in culture, the cells express CD31 (A; small frame: isotype control), react with BS-1 lectin (B), and uptake ac-LDL (C). The overlay image of B and C indicates double-positive cells in yellow (D); scale bar = 50 μm.
In vivo ANG II infusion improves SS/Mcwi BM-EC function potentiating HUVEC tube network formation.
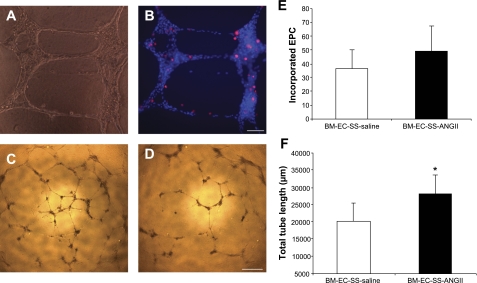
A Matrigel tube formation assay was performed to investigate whether the in vivo restoration of ANG II levels would affect the ability of SS/Mcwi derived BM-EC to integrate into vascular structures. As shown in Fig. 5A, HUVEC form vascular structures on Matrigel, and the BM-EC actively incorporate into the DAPI-labeled HUVEC network structure (Fig. 5B). Although the incorporation rate was not significantly higher in the BM-EC derived from SS/Mcwi rats infused with ANG II (Fig. 5E), the cells derived from ANG II infused SS/Mcwi rats significantly increased the total length of the vascular network structure formed by HUVEC compared with the BM-EC derived from SS/Mcwi infused with saline (Fig. 5, C, D, and F).
Fig. 5.
Effect of ANG II infusion on bone marrow-derived endothelial cell (BM-EC) function. Representative phase-contrast image (×200) showing the vascular network formed by the coculture of human umbilical vein endothelial cells (HUVEC) and BM-EC on Matrigel (A). Fluorescent image showing the incorporation of the BM-EC labeled with Dil-ac-LDL (red) into the HUVEC labeled with DAPI (blue) (B). Scale bar = 100 μm. Representative phase-contrast images (×40) of the network projections formed on Matrigel by the coculture of HUVEC and BM-EC derived from SS/Mcwi rats infused with ANG II (C) or saline (D). Scale bar = 600 μm. Quantitative analysis of the number of BM-EC incorporated into HUVEC for each experimental group (E). Quantitative analysis of total length of the tubes formed on Matrigel for each experimental group (F). *P < 0.05 vs. BM-EC from SS-saline. Data presented as means ± SE; n = 3–5 independent experiments performed in triplicate.
DISCUSSION
This study tested the hypothesis that low RAS activity in SS/Mcwi rats would impair BM-EC function and consequently angiogenic efficacy of bone marrow-derived stem cell therapy. Our results, using the SS rat, the consomic SS-13BN/Mcwi with a normal RAS and SS rats with ANG II replacement therapy support this hypothesis.
In the past decade studies have shown that the bone marrow derived EPC actively participate in adult angiogenesis. These studies have received a great deal of attention due to their potential for cell-based clinical therapies in many pathologies. A major therapeutic limitation is that EPC levels and function are reduced in patients with high cardiovascular risk, and angiogenesis efficacy in these patients is significantly impaired after autologous stem cell transplantation (22, 48, 58). Among the individual risk factors investigated, hypertension emerged as the strongest predictor of EPC migratory impairment (58). In addition, EPC senescence has been reported to be accelerated in both experimental hypertensive rats and patients with essential hypertension (26). Nevertheless, the mechanisms involved in the EPC dysfunction, as well as its time course during hypertension, it is not clear. Thus, a major remaining challenge is to elucidate the mechanisms underlying the EPC dysfunction and to develop new strategies to improve EPC properties and consequently the revascularization outcome after stem cell transplantation.
In the present study we used the SS/Mcwi rats as an animal model for low-renin hypertension and microvascular rarefaction (7) to explore the role of the RAS in bone marrow-derived EPC-mediated angiogenesis. The normotensive SS-13BN/Mcwi consomic served as a source of control cells for transplant and allowed us to investigate the hypothesis that impairments in the RAS may be responsible for the inability of SS/Mcwi cells to normalize angiogenesis.
The effect of ANG II on EPC function is controversial. Some studies have shown that ANG II diminishes telomerase activity of circulating EPC and accelerates the onset of EPC senescence through an increase in oxidative stress (24). In contrast, studies have shown that ANG II increases NO production, inhibits apoptosis, and enhances adhesion potential of bone marrow-derived EPC (62). Although ANG II inhibited EPC proliferation in one study, it enhanced VEGF-induced EPC proliferation in another (24, 25). Thus, further studies are necessary to define the mechanisms by which ANG II stimulates or inhibits EPC proliferation, adhesion, homing, and differentiation, as well as its impact in the angiogenesis stem cell therapy.
We have previously shown that changes in the ANG II levels, as well as genetic manipulation of the renin gene, have profound effects on skeletal muscle angiogenesis induced by exercise or electrical stimulation (1–3, 9–11, 41). In the present study we have shown that autologous bone marrow stem cell implantation failed to restore skeletal muscle angiogenesis induced by electrical stimulation in SS/Mcwi rats. We hypothesized that the failure in the bone marrow stem cell therapy to restore angiogenesis during electrical stimulation is related to a low RAS activity present in SS/Mcwi rats. Although RAS activity was not measured in the present study, we have previously demonstrated the renin activity profile of BN, SS/Mcwi, and consomic SS-13BN, as well as its correlation with the angiogenic phenotype in several studies (3, 8, 9). In contrast to the BN and SS-13BN rats, the renin activity was significantly lower in SS/Mcwi rats and is associated with an impaired angiogenesis response after electrical stimulation (8, 9). ANG II infusion at a very low dose, as well as the transfer of the entire chromosome 13 or even a small region of the chromosome 13 containing the renin gene, from the normotensive BN rat into the SS/Mcwi genetic background, completely restores the angiogenesis response after electrical stimulation (3, 9). Furthermore, the angiogenic response is inhibited in SS-13BN rats when the rats are treated with high-salt diet or the AT1 receptor blocker Losartan (9). These studies suggest that RAS has an important role in skeletal muscle angiogenesis; however, the mechanisms underlying the impaired RAS activation in the SS/Mcwi and the regulation of the capillary growth process are not totally understood. In the present study we show that low RAS activity observed in SS/Mcwi rats is associated with an impaired BM-EC function that may lead to failure in the angiogenesis process after stem cell therapy.
A significant increase in skeletal muscle vessel density was observed after BMC implantation derived from the SS-13BN rat, suggesting that the dysregulation of genes in the chromosome 13 may lead to an impaired bone marrow derived BM-EC function. Although there are many genes in the chromosome 13, previous studies from or laboratory indicate the renin gene as a strong candidate for the control of the angiogenesis response after electrical stimulation (3, 9). Using marker-assisted breeding four chromosome 13 congenic strains were developed that included or excluded the renin locus from the BN rat into the SS/Mcwi background (9). Microvessel density was markedly increased after stimulation in congenic strains that contained the renin gene from the BN rat and suppressed in control strains that carried regions of the BN genome just above or just below the renin gene. Renin is a key enzyme in the production of ANG II, and indeed low-dose ANG II infusion also restored angiogenesis induced by electrical stimulation in SS/Mcwi rats (9). The present study suggests that the restoration of ANG II levels in SS/Mcwi rats may also have an important effect on the bone marrow-derived stem cell function.
It is important to note that in this study only the donor SS/Mcwi rats and not the recipient rats received low dose ANG II infusion. Thus it was a restoration of ANG II in the donors that improved BM-EC function and consequently restored the stem cell therapeutic angiogenesis in the recipient SS/Mcwi rats. In agreement with the in vivo results we demonstrated that by restoring the circulating ANG II levels in the SS/Mcwi rats we improved the BM-EC function in vitro as measured by the vascular tube formation assay.
The slight increase in the VEGF protein levels in the BMC after ANG II infusion may improve BM-EC function. VEGF has been reported to increase the mobilization, proliferation, as well as the survival of the EPC (5, 57). In past few years, the use of stem cells overexpressing single or multiple growth factors is gaining popularity as therapeutic agents for neovascularization of ischemic tissue (43). Transplantation of the mesenchymal stem cells overexpressing VEGF in combination with cytokine therapy has shown superior BMC mobilization and EC proliferation, more pronounced angiogenic response, reduced tissue damage, and improved cardiac function (59). In vitro studies suggest that ANG II increases VEGF mRNA expression and protein synthesis in mesenchymal stem cells throughout activation of the AT1 receptor (49), and it is a potent stimulator of VEGF-induced proliferation and network formation of human EPC (25) via upregulation of VEGF receptor kinase domain-containing receptor (KDR). In agreement with these studies, we have demonstrated that systemic low-dose ANG II infusion increased VEGF protein level in the BMC of SS/Mcwi rats and also improved the ability of BM-EC to work in concert with the mature EC to form vascular tubes on Matrigel. Studies have shown that the efficiency of neovascularization may not solely be attributable to the incorporation of EPC in newly formed vessels but may also be influenced by the release of proangiogenic factors in a paracrine manner (61). The local release of growth factors by EPC may influence the classical process of angiogenesis, including the proliferation and migration as well as survival of mature EC (16). Therefore, the increase in skeletal muscle angiogenesis in vivo may be a result of the EPC paracrine effects in addition to the physical incorporation of EPC into newly formed capillaries.
Recent studies have shown that ANG II increases NO production, inhibits apoptosis, and enhances adhesion potential of bone marrow-derived EPC (62). This study shows that ANG II signaling through AT1 receptors activated PI3K/Akt pathways and significantly decreased EPC apoptosis. Although we haven't investigated the effect of ANG II on EPC survival in vivo it is possible that the increase in angiogenesis may be related to a greater survival of the transplanted EPC-derived BMC. We have previously shown that low-dose ANG II infusion in animals fed a high-salt diet significantly reduced vascular EC apoptosis and restored skeletal muscle angiogenesis induced by electrical stimulation (10). Since both EPC and mature EC share many of common features including the expression of endothelial-specific markers, VEGFR-2, Tie-1, Tie-2, and VE-cadherin (4, 27, 39, 60), the ability to uptake ac-LDL and to form vascular structure in vitro (27, 45), it is likely that EPC function is affected by common EC mediators. The present study suggests that besides the classic ANG II angiogenic mechanisms, including increase in mature EC survival, migration, adhesion, and proliferation,(6, 15, 23, 31, 52). ANG II infusion may also improve BM-EC function and, as such, may impact BM-EC biology and consequently the organ vascularization and the regeneration process induced by bone marrow stem cell based therapy. Further studies using ANG II receptor blockers would support this hypothesis.
GRANTS
This work was supported by National Institutes of Health Grants Support NIH P01-HL-082798; N01-HV-28182.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Justin Friske for expert technical assistance.
REFERENCES
- 1.Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 8: 57–67, 2001 [PubMed] [Google Scholar]
- 2.Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol Heart Circ Physiol 281: H1163–H1169, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension 37: 386–390, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell L, Madri JA. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol 137: 7–12, 1990 [PMC free article] [PubMed] [Google Scholar]
- 7.Boegehold MA, Kotchen TA. Arteriolar network morphology in gracilis muscle of rats with salt-induced hypertension. Microvasc Res 40: 169–178, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001 [DOI] [PubMed] [Google Scholar]
- 9.De Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics 33: 33–40, 2008 [DOI] [PubMed] [Google Scholar]
- 10.De Resende MM, Amaral SL, Munzenmaier DH, Greene AS. Role of endothelial cell apoptosis in regulation of skeletal muscle angiogenesis during high and low salt intake. Physiol Genomics 25: 325–335, 2006 [DOI] [PubMed] [Google Scholar]
- 11.De Resende MM, Greene AS. Effect of ANG II on endothelial cell apoptosis and survival and its impact on skeletal muscle angiogenesis after electrical stimulation. Am J Physiol Heart Circ Physiol 294: H2814–H2821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol 287: H957–H962, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Drenjancevic-Peric I, Phillips SA, Falck JR, Lombard JH. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am J Physiol Heart Circ Physiol 289: H188–H195, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, Chen Y, Lawton MT, Young WL, Yang GY. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 28: 90–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med 105: 141–145, 1985 [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O'Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke 36: 151–153, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8: 607–612, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Li TS, Ito H, Mikamo A, Hamano K. Comparison of intramyocardial and intravenous routes of delivering bone marrow cells for the treatment of ischemic heart disease: an experimental study. Cell Transplant 13: 639–647, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Henrich HA, Romen W, Heimgartner W, Hartung E, Baumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klin Wochenschr 66: 54–60, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Hernandez I, Cowley AW, Jr, Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol Heart Circ Physiol 263: H664–H667, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hu DE, Hiley CR, Fan TP. Comparative studies of the angiogenic activity of vasoactive intestinal peptide, endothelins-1 and -3 and angiotensin II in a rat sponge model. Br J Pharmacol 117: 545–551, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens 23: 97–104, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res 27: 101–108, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens 23: 1831–1837, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 7: 1035–1040, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7: 430–436, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Koshikawa M, Shimodaira S, Yoshioka T, Kasai H, Watanabe N, Wada Y, Seto T, Fukui D, Amano J, Ikeda U. Therapeutic angiogenesis by bone marrow implantation for critical hand ischemia in patients with peripheral arterial disease: a pilot study. Curr Med Res Opin 22: 793–798, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kuethe F, Richartz BM, Sayer HG, Kasper C, Werner GS, Hoffken K, Figulla HR. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol 97: 123–127, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Le Noble FA, Schreurs NH, van Straaten HW, Slaaf DW, Smits JF, Rogg H, Struijker-Boudier HA. Evidence for a novel angiotensin II receptor involved in angiogenesis in chick embryo chorioallantoic membrane. Am J Physiol Regul Integr Comp Physiol 264: R460–R465, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation 104: 735–740, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Lin GS, Lu JJ, Jiang XJ, Li XY, Li GS. Autologous transplantation of bone marrow mononuclear cells improved heart function after myocardial infarction. Acta Pharmacol Sin 25: 876–886, 2004 [PubMed] [Google Scholar]
- 34.Linderman JR, Kloehn MR, Greene AS. Development of an implantable muscle stimulator: measurement of stimulated angiogenesis and poststimulus vessel regression. Microcirculation 7: 119–128, 2000 [PubMed] [Google Scholar]
- 35.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J 39: 150–158, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension 27: 760–765, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen VA, Fürhapter C, Obexer P, Stössel H, Romani N, Sepp N. Endothelial cells from cord blood CD133(+)CD34(+) progenitors share phenotypic, functional and gene expression profile similarities with lymphatics. J Cell Mol Med 13: 522–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 98: 10344–10349, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol 291: H114–H120, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Pistrosch F, Herbrig K, Oelschlaegel U, Richter S, Passauer J, Fischer S, Gross P. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis 183: 163–167, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95: 9–20, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 109: 337–346, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rieder MJ, O'Drobinak DM, Greene AS. A computerized method for determination of microvascular density. Microvasc Res 49: 180–189, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Rieder MJ, Roman RJ, Greene AS. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension 30: 120–127, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 49: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Shi RZ, Wang JC, Huang SH, Wang XJ, Li QP. Angiotensin II induces vascular endothelial growth factor synthesis in mesenchymal stem cells. Exp Cell Res 315: 10–15, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776–2779, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 103: 897–903, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Stoll M, Meffert S, Stroth U, Unger T. Growth or antigrowth: angiotensin and the endothelium. J Hypertens 13: 1529–1534, 1995 [PubMed] [Google Scholar]
- 53.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106: 1913–1918, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360: 427–435, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Urbich C, Knau A, Fichtlscherer S, Walter DH, Bruhl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J 19: 974–976, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118: 489–498, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Yamahara K, Sone M, Itoh H, Yamashita JK, Yurugi-Kobayashi T, Homma K, Chao TH, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Fukunaga Y, Tamura N, Nakao K. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS ONE 3: e1666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res 18: 792–799, 2008 [DOI] [PubMed] [Google Scholar]
- 63.You D, Cochain C, Loinard C, Vilar J, Mees B, Duriez M, Levy BI, Silvestre JS. Combination of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide activate postnatal vasculogenesis in spontaneously hypertensive rats. J Pharmacol Exp Ther 325: 766–773, 2008 [DOI] [PubMed] [Google Scholar]