Abstract
Objective To study the efficacy and tolerability of borage oil, which contains a high concentration of γ linolenic acid, in children and adults with atopic eczema.
Design Single centre, randomised, double blind, placebo controlled, parallel group trial.
Setting Acute district general hospital in Nuneaton, England.
Participants 151 patients, of whom 11 failed to return for assessment, leaving an evaluable population of 140 (including 69 children).
Intervention Adults received four capsules of borage oil twice daily (920 mg γ linolenic acid), and children received two capsules twice daily, for 12 weeks.
Main outcome measures Change in total sign score at 12 weeks measured with the six area, six sign, atopic dermatitis (SASSAD) score (primary endpoint); symptom scores, assessed on visual analogue scales; topical corticosteroid requirement, assessed on a five point scale; global assessment of response by participants; adverse events and tolerability.
Results The mean SASSAD score fell from 30 to 27 in the borage oil group and from 28 to 23 in the placebo group. The difference between the mean improvements in the two groups was 1.4 (95% confidence interval -2.2 to 5.0) points in favour of placebo (P = 0.45). No significant differences occurred between treatment groups in the other assessments. Subset analysis of adults and children did not indicate any difference in response. The treatments were well tolerated.
Conclusion γ linolenic acid is not beneficial in atopic dermatitis.
Introduction
Essential fatty acids are long chain fatty acids that are needed for normal cutaneous function and cannot be synthesised by human metabolism. They can be classified into series according to the position of the first double bond in the molecule relative to the N-terminal carbon atom. Most important are the n6 series fatty acids, which include γ linolenic acid, and the n3 series, which include eicosapentaenoic acid. Many mechanisms have been proposed whereby supplementation with essential fatty acids might prove effective in treating atopic dermatitis.1 For example, atopic eczema has been suggested to result partly from defective conversion of linoleic acid, the major dietary component of n6 series essential fatty acids, to metabolites such as the anti-inflammatory prostaglandin E1, as a result of low levels of activity of the enzyme Δ6-desaturase.1,2 This disease might therefore respond to fatty acid supplementation, which would bypass this metabolic step.
The most investigated form of supplementation has been with the n6 series fatty acid γ linolenic acid.3-8 Although this treatment has consistently proved safe and well tolerated, efficacy has been inconsistent.9 One possible explanation for the inconsistent results may be that the dose used has been too low in some trials. The most investigated source of essential fatty acid has been evening primrose oil, which contains 9% γ linolenic acid. Large numbers of capsules containing the oil have been needed to provide moderate doses (up to 12×500 mg capsules daily, providing 6 g total oil and 540 mg γ linolenic acid.
Purified borage oil contains a minimum of 23% γ linolenic acid. Borage oil is used to fortify infant foodstuffs with essential fatty acid and is available over the counter in chemists and in health food shops, where it is sold as “starflower oil.” The γ linolenic acid from borage is considered to be identical to that from evening primrose oil.10 Use of borage oil thus allows a higher dose of γ linolenic acid to be administered, with a reduction in the number of capsules that must be taken daily.
Only one large well reported randomised controlled trial of borage oil in atopic eczema has been published. Henz et al reported a four centre study on 160 patients aged 14-65, with results that are difficult to interpret.11 A response to borage oil could not be confirmed for the overall population with the primary response criterion. This primary endpoint was unconventional—the quantity of topical steroid needed before response, which was defined as 50% reduction in the Costa severity score. The results differed between centres; an apparently significant response to borage oil occurred in the patients in Berlin, whereas placebo was superior in Warsaw. Furthermore, several signs and symptoms (erythema, vesiculation, crusting, excoriation, lichenification, and insomnia) improved significantly during treatment with borage oil, but pruritus and the total severity score did not.
Four smaller studies have been reported, two suggesting an improvement and two suggesting none.12 The numbers of participants in these studies ranged from 12 to 32, so they were not adequately powered to detect anything other than a very large treatment effect. We report here a further trial investigating the efficacy and tolerability of borage oil in the treatment of atopic eczema in both adults and children.
Methods
Design
The study was a prospective, randomised, double blind, placebo controlled trial of parallel group design, done in a single centre. We assessed participants at baseline and at 2, 4, 8, and 12 weeks.
Participants
We recruited male and female patients aged over 2 years attending our department for treatment of atopic dermatitis. To ensure that the age structure of the treatment groups was reasonably balanced, we stratified participants into two groups of equal size: those aged up to 12 and those aged over 12. We based the diagnosis of atopic dermatitis on the criteria of Hanifin and Rajka.13 We excluded pregnant and lactating women, and fertile women were required to use effective contraception. All volunteers (or parents for those under 16 years) provided written informed consent to participation in the study. We obtained verbal or written agreement from all children considered by the investigators and their parents to be old enough to understand the issues.
Treatment
Essential Nutrition allocated borage oil or placebo treatment by using computer generated random numbers. Treatments were supplied in containers labelled with randomisation numbers in separate sequences for adults and children. These were allocated to patients in strict numerical sequence. Borage oil (Ropufa 25 N6, Roche Lipids Ltd) and placebo treatments were provided in matching capsules; placebo capsules contained liquid paraffin for adults and olive oil for children. Adults received four 500 mg capsules twice daily, providing 920 mg of γ linolenic acid for those receiving borage oil. Children received half this dose. We used liquid paraffin as placebo in adults, as this is considered to be the most inert placebo available and the dose was not large enough to act as a laxative. In children we used olive oil instead, to avoid any laxative effect of liquid paraffin. Neither placebo is considered likely to exert any beneficial or adverse effect on atopic dermatitis. To facilitate recruitment, we reduced the proportion of participants receiving placebo to 40%, and 60% received borage oil. We retained the treatment code in individual sealed envelopes for each patient. We did not break the blinding for any patient until the trial and all data entry were completed.
We allowed patients to use conventional treatment for atopic eczema throughout the study. We permitted no changes in concomitant treatment for two weeks before the study or for the duration of the study, except in the quantity and frequency of topical steroid application, which could be adjusted as needed in relation to the severity of the disease. We encouraged patients to apply the topical steroids only when needed. Permitted concomitant medications included emollient creams, bath oils, soap substitutes, and topical steroids classified in the British National Formulary as “mild,” “moderately potent,” or “potent” (but not “very potent” topical steroids). “Potent” topical steroids were prohibited for children. We allowed systemic antihistamines, but no other systemic treatment or ultraviolet light treatment was permitted. A minimum four week washout period was needed for patients who had received systemic steroids, psoralen plus ultraviolet A (PUVA), or other oral immunosuppressive treatment.
Assessments
We assessed disease activity objectively at each visit by using the six area, six sign, atopic dermatitis (SASSAD) score.14 This well validated scoring system, which has been used in many trials on atopic dermatitis, involves assessment of six signs (erythema, exudation, excoriation, dryness, cracking, and lichenification) at six sites (hands, feet, arms, legs, head and neck, and trunk). Each sign is graded at each site on a four point scale (0-3, representing grades of none, mild, moderate, and severe). The maximum score theoretically possible is therefore 108.
To assess the severity of symptoms, patients used horizontal 10 cm visual analogue scales marked none at the left hand end and worst ever at the right. We assessed itching, sleep disturbance, and irritability in this way. We measured scores in millimetres from the left hand end, so that the maximum severity was 100. Participants made an overall assessment of response to treatment at the end of the treatment relative to the baseline, on a five point scale: worse, same, improved, much improved, or cleared. These assessments were also done on discontinuation of treatment in patients who were withdrawn. We recorded the need for topical steroid at each visit by using a five point scale: 1 = none, 2 = occasionally, 3 = alternate days, 4 = once daily, 5 = twice daily.
Participants assessed overall tolerability of the treatment on a four point scale: very good, good, fair, or poor. We elicited information on adverse events by using non-leading questions at each visit. In adults, we obtained blood samples for haematology, biochemistry (urea, electrolytes, and liver function tests), fasting serum cholesterol, and triglycerides on screening, at week 2, and the end of treatment.
Statistical analysis
We defined the primary response criterion prospectively as the mean change in total sign score at the end of treatment. We compared mean changes in score between baseline and end of treatment for each treatment arm by using a two tailed t test. Although these data are approximately normally distributed, we corroborated results with non-parametric methods (the Wilcoxon rank sum test).
The study was designed to have 80% power to detect a treatment response of 20% (that is, above the response to placebo), with a standard deviation (derived from existing data) of 35%, at a significance level of 0.05. We estimated that 120 participants would be needed. We anticipated that around 20% of participants would be withdrawn and therefore aimed to recruit 152 participants, comprising 76 adults and 76 children.
We did the analysis with the last available data for each participant who returned at least once after randomisation. We also did identical analyses on the protocol correct population comprising those participants who completed the trial. In figures 2 and 3 the last observations have been carried forward for patients who withdrew.
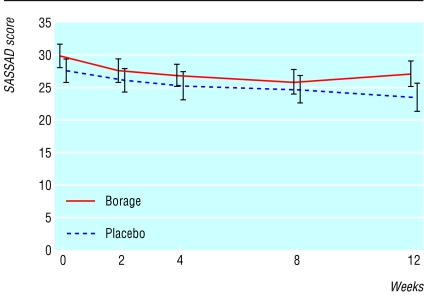
Fig 2.
Six area, six sign, atopic dermatitis (SASSAD) scores throughout the trial (n=140; error bars show SE)
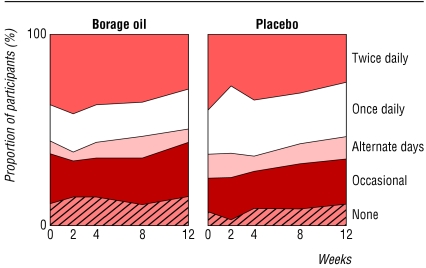
Fig 3.
Use of topical corticosteroids assessed at each visit on a five point scale (n=140)
Results
Demographics
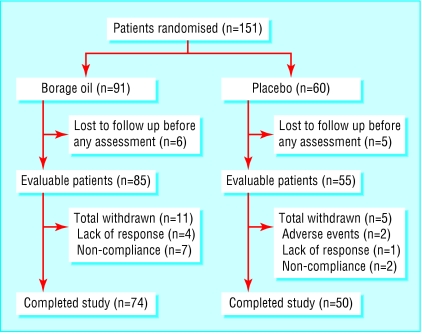
We recruited 151 participants between February 1997 and July 2001. We believe that the patient sample investigated is representative of the population of patients with atopic dermatitis treated at our department. The most common reason for declining to participate was inability to attend the clinic as required. Eleven patients failed to return for assessment at week two, leaving an evaluable population of 140 patients with at least one assessment after randomisation. Of these, we randomised 85, including 40 children, to receive borage oil and 55, including 29 children, to placebo. A further 16 participants withdrew during the trial, leaving a protocol correct population of 124. Figure 1 summarises the flow of patients through the study. Only two patients (both in the placebo arm) were withdrawn owing to adverse events—one developed a blotchy erythematous rash; the other developed diarrhoea and vomiting, headache, fever, and worsening of asthma. Disease severity was comparable in the two groups at baseline (table 1).
Fig 1.
Flow chart of trial participants
Table 1.
Mean (SD) SASSAD scores, symptom scores, and topical steroid requirement and differences between treatment groups at the end of treatment
|
Borage oil (n=85)
|
Placebo (n=55)
|
Difference (95% CI) at end of treatment
|
|||
|---|---|---|---|---|---|
| Baseline | End of treatment | Baseline | End of treatment | ||
| SASSAD | 30 (16) | 27 (17) | 28 (14) | 23 (16) | 3.6 (−2.1 to 9.3) |
| Pruritus | 50 (24) | 42 (28) | 56 (24) | 42 (30) | −0.46 (−10.3 to 9.4) |
| Sleep disturbance | 30 (25) | 28 (27) | 44 (28) | 33 (30) | −5.3 (−14.9 to 4.3) |
| Irritability | 40 (29) | 35 (27) | 45 (27) | 34 (31) | 0.87 (−8.9 to 10.7) |
| Topical steroid requirement | 3.4 | 3.2 | 3.7 | 3.3 | 0.1 |
SASSAD=six area, six sign, atopic dermatitis.
Efficacy
Table 1 and figure 2 show mean SASSAD scores and symptom scores. The mean SASSAD score fell from 30 to 27 in the borage oil group and from 28 to 23 in the placebo group at the end of treatment. The difference between the mean improvements in the two groups was 1.4 (95% confidence interval -2.2 to 5.0) points, with a marginally greater improvement in the placebo group (P = 0.45). Results were all similar in the protocol correct population—for example, the mean SASSAD score fell from 30 at baseline to 27 at week 12 in the borage oil group and from 28 to 24 in the placebo group. The difference in mean change between the two groups was 0.6 (-3.1 to 4.3), with a marginally greater improvement in the placebo group (P = 0.75).
Symptom scores all fell in both treatment groups during the study (table 1). Pruritus, sleep disturbance, and irritability all improved slightly more in the placebo group than in the borage oil group. These differences between the two groups were not significant for any of the symptoms. Table 2 shows patients' assessments of overall response to treatment. Table 1 and figure 3 show data on the frequency of application of topical corticosteroids at baseline and throughout the study. Subset analysis of adults and children yielded no suggestion of any difference between them in any of the parameters studied.
Table 2.
Overall assessments of response. Values are numbers (percentages)
| Borage oil (n=81) | Placebo (n=52) | |
|---|---|---|
| Worse | 15 (19) | 6 (12) |
| Same | 28 (35) | 20 (39) |
| Improved | 26 (32) | 14 (27) |
| Much improved | 11 (14) | 11 (21) |
| Cleared | 1 (1) | 1 (2) |
P=0.28, χ2 test for trend; data not recorded for seven participants.
Tolerability
Both treatments were generally well tolerated. Table 3 shows patients' assessments of tolerability at the end of treatment. Table 4 lists all adverse events reported by more than one patient. Analysis of biochemical data revealed no significant changes in the haematological or biochemical parameters monitored. In no patient did any haematological or biochemical monitoring reveal changes causing clinical concern.
Table 3.
Patients' assessment of tolerability of treatment. Values are numbers (percentages)
| Borage oil (n=81) | Placebo (n=52) | |
|---|---|---|
| Very good | 32 (40) | 22 (42) |
| Good | 37 (46) | 25 (48) |
| Fair | 10 (12) | 5 (10) |
| Poor | 2 (3) | 0 |
P=0.41, χ2 test for trend; data not recorded for seven participants.
Table 4.
Adverse events reported by more than one participant: number (percentage) of participants reporting
| Event | Borage oil (n=85) | Placebo (n=55) |
|---|---|---|
| Upper respiratory tract infection | 22 (26) | 21 (38) |
| Diarrhoea | 6 (7) | 6 (11) |
| Nausea, vomiting, or both | 3 (4) | 5 (9) |
| Abdominal pain | 5 (6) | 2 (4) |
| Episodes of asthma | 1 (1) | 4 (7) |
| Episodes of allergic rhinitis | 1 (1) | 1 (2) |
| Episodes of urticaria | 2 (2) | 1 (2) |
| New rash | 0 | 2 (4) |
| Musculoskeletal pains | 3 (4) | 3 (6) |
| Skin sepsis | 6 (7) | 7 (13) |
| Glandular fever | 2 (2) | 0 |
| Headache | 1 (1) | 4 (7) |
Discussion
This study has not yielded any evidence for efficacy of borage oil in the treatment of atopic dermatitis. We found no significant benefit over placebo in the sign score, symptom scores, global assessments of response by patients, or corticosteroid dose sparing effect. Subset analysis of adults and children did not indicate any difference in response between these groups. Both borage oil and placebo were well tolerated, with no significant differences between the treatment groups.
The greatest level of response to borage oil likely to be compatible with these data is a two point improvement in the SASSAD score. If a benefit of this magnitude occurred (approximately 7% of the baseline sign score), this would be of limited clinical value. A two point improvement might represent, for example, an improvement of a single sign by one grade in two areas or of two different signs in one out of the six areas assessed. However, the data do not exclude the possibility of such a small degree of benefit. The data do not suggest that a longer duration of treatment would have been more likely to have yielded a positive outcome. Indeed, figure 2 would suggest that, if anything, the difference in favour of placebo was widening.
The design and method of this trial worked well. The narrow confidence intervals for the improvement in sign score, seen in both the intention to treat and protocol correct populations, indicate that the sample size was correctly estimated and that the study was adequately powered. Treatment allocation remained effectively double blinded.
These data are compatible with results from the largest studies in which evening primrose oil was used as a supplement of γ linolenic acid.7,8 The results are also compatible with the results of previous trials on supplementation with borage oil.11,12 The dose of γ linolenic acid administered in this study was the highest used in any trial to date. The results would therefore seem to refute any contention that the response is dose related or that the lack of response, or the inconsistent response, seen in some previous trials has resulted from the dose being too low in those trials.
We found no evidence of a steroid sparing effect from borage oil treatment. The recording of topical steroid requirement has always been one of the most difficult aspects of trials on atopic dermatitis, but the five point scale used in this study proved useful and simple to apply. The result seems incompatible with the 60% reduction in steroid requirement reported in a previous small study on evening primrose oil.4
In conclusion, it seems unlikely that dietary supplementation with γ linolenic acid is beneficial in management of atopic dermatitis.
What is already known on this topic
The essential fatty acid γ linolenic acid has been investigated in many small trials as a supplementary treatment for atopic eczema
Results have so far been conflicting and inconclusive, but some studies have suggested a dose related benefit
What this study adds
The dose of γ linolenic acid in this study was the highest so far investigated
The symptoms and signs of atopic dermatitis improved to a similar degree in both groups, with the minimal difference being in favour of placebo
This study was well powered and confidence intervals were narrow, so γ linolenic acid is unlikely to offer any useful benefit in treatment of atopic dermatitis
Editorial by Williams
Contributors: JB-J, TC, and JRT designed the protocol. JB-J, AT, ET, SA, GB, IA, and KH recruited patients and conducted the study. All authors contributed to analysis and interpretation of the data and production of the manuscript. JRT provided statistical advice on the design and analysis of the trial. JB-J is the guarantor.
Funding: This study was sponsored by Essential Nutrition Ltd.
Competing interests: TC is a director of Essential Nutrition Ltd.
Ethical approval: Warwickshire Research Ethics Committee approved the protocol.
References
- 1.Wright S. Essential fatty acids and the skin. Br J Dermatol 1991;125: 503-15. [DOI] [PubMed] [Google Scholar]
- 2.Melnik BC, Plewig G. Are disturbances of omega-6-fatty acid metabolism involved in the pathogenesis of atopic dermatitis? Acta Derm Venereol 1992;suppl 176: 77-85. [PubMed]
- 3.Wright S, Burton JL. Oral evening primrose seed oil improves atopic eczema. Lancet 1982;2: 1120-3. [DOI] [PubMed] [Google Scholar]
- 4.Schalin-Karrila M, Mattila L, Jansen CT, Uotila P. Evening primrose oil in the treatment of atopic eczema: effects on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol 1987;117: 11-9. [DOI] [PubMed] [Google Scholar]
- 5.Bordoni A, Biagi BL, Masi M, Ricci G, Fanelli C, Patrizi A, et al. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Exp Clin Res 1988;14: 291-7. [PubMed] [Google Scholar]
- 6.Morse PF, Horrobin DF, Manku MS, Stewart JC, Allen R, Littlewood S, et al. Meta-analysis of placebo-controlled studies on the efficacy of Epogam in the treatment of atopic eczema: relationship between plasma essential fatty acid changes and clinical responses. Br J Dermatol 1989;121: 75-90. [DOI] [PubMed] [Google Scholar]
- 7.Berth-Jones J, Graham-Brown RAC. Placebo controlled trial of essential fatty acid supplementation in atopic dermatitis. Lancet 1993;341: 1557-60. [DOI] [PubMed] [Google Scholar]
- 8.Bamford JTM, Gibson RW, Reiner CM. Atopic eczema unresponsive to evening primrose oil (linoleic and gamma-linolenic acids). J Am Acad Dermatol 1985;13: 959-65. [DOI] [PubMed] [Google Scholar]
- 9.Berth-Jones J, Graham-Brown RAC. A review of the use of essential fatty acid supplementation in atopic dermatitis with emphasis on the methodology of trial design. In: Maibach HI, Schwindt DA, eds. Cutaneous biometrics. New York: Plenum Publishing Corporation, 2000;ch 9: 109-17.
- 10.Raederstorff D, Moser U. Borage or primrose oil added to standardised diets are equivalent sources for gamma-linolenic acid in rats. Lipids 1992;27: 1018-23. [DOI] [PubMed] [Google Scholar]
- 11.Henz BM, Jablonska S, Van der Kerkhof PCM, Stingl G, Blaszczyk M, Vandervalk PG, et al. Double-blind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br J Dermatol 1999;140: 685-8. [DOI] [PubMed] [Google Scholar]
- 12.Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4(37): 1-91. [PMC free article] [PubMed] [Google Scholar]
- 13.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980;92(suppl): 44-7. [Google Scholar]
- 14.Berth-Jones J. Six area six sign AD (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996;135(suppl 48): 25-30. [DOI] [PubMed] [Google Scholar]