Abstract
The reproducibility of urinary phthalate metabolite concentrations has not been well characterized in nonpregnant women of reproductive age. Our primary study objectives were to describe the distribution of urinary phthalate metabolites concentrations among a population of Hmong women of reproductive age, and to evaluate intra- and inter-individual variability of phthalate metabolite concentrations. Ten phthalate metabolites were measured in first morning urine samples collected from 45 women and 20 of their spouses who were members of the Fox River Environment and Diet Study cohort in Green Bay, Wisconsin. Repeated first morning urine samples were collected and analyzed from 25 women who provided up to three samples over approximately one month. Measurement variability was assessed using intraclass correlations (ICCs) and surrogate category analysis. Linear mixed models were used to evaluate the associations between participant characteristics and phthalate metabolite concentrations. Nine of the 10 phthalate metabolites were detected in > 80% of all samples analyzed, of which seven were detected in all samples. As a measure of reliability, ICCs were strongest for monobenzyl phthalate (0.64) and weakest for the metabolites of di(2-ethylhexyl)phthalate (DEHP) (ranging from 0.13 to 0.22). Similarly, surrogate category analysis suggested that a single urine sample characterized average one-month exposure with reasonable accuracy across low, medium and high tertiles for all metabolites except the DEHP metabolites. Geometric mean concentrations of monoethyl phthalate increased with age, but patterns by education, income, body mass index, environmental tobacco smoke or season were not observed when measures were adjusted for urinary dilution. Our results suggest that the participant characteristics assessed in this study have limited influence on inter-individual variability of phthalate metabolite concentrations. With regard to intra-individual variability, our results suggest that urinary concentrations of some phthalate metabolites are more reproducible over time and less subject to exposure misclassification than others (e.g., metabolites of DEHP).
Keywords: phthalates, variability, exposure assessment, Hmong, women, reproductive age
Introduction
The diesters of 1,2-benzenedicarboxylic acid, referred to as phthalates, are industrial chemicals widely used in commercial, medical and personal care products to impart flexibility in plastics, retain color and fragrance in perfumes and cosmetics, add a gloss to lacquers, or provide time release for pharmaceuticals. High molecular weight phthalates such as di(2-ethylhexyl)phthalate (DEHP) are commonly used as plasticizers in vinyl flooring and medical devices, while low molecular weight phthalates such as diethyl phthalate (DEP) and dibutyl phthalate (DBP) are used as solvents in toiletries and lacquers (ATSDR, 1995; ATSDR, 2001; ATSDR, 2003). The ubiquitous use of phthalates results in human exposure through food sources, dermal absorption, inhalation, and parenteral use of medical devices (Hauser and Calafat, 2005).
Exposure to phthalates among the general United States (U.S.) population is widespread (Silva, et al., 2004). The high potential for the developing fetus to be exposed has raised concern for human health given the reproductive and developmental toxicity demonstrated in laboratory animals (Davis, et al., 1994; Gray, et al., 2000; Gray, et al., 2006; Mylchreest, et al., 1998; Mylchreest, et al., 1999; Mylchreest, et al., 2000). The epidemiologic investigation of potential phthalate-related health effects involves the use of biomarkers to quantify individual-level exposures from multiple sources. As non-persistent chemicals with short half-lives, urinary measurements of phthalate metabolites characterize recent exposures. It is unclear to what extent metabolite concentrations detected in a single sample may reliably characterize exposure patterns over longer time intervals. A limited number of studies have evaluated the temporal variability of phthalate metabolites in serial urine samples collected over time (Adibi, et al., 2008; Fromme, et al., 2007; Hauser, et al., 2004; Hoppin, et al., 2002; Teitelbaum, et al., 2008), but additional investigation is needed to elucidate patterns of within- and between-person variability in diverse populations. Given the paucity of exposure assessment studies in women of reproductive age, we conducted a study in a unique understudied population of Hmong females. The Hmong came to the U.S. as refugees from Laos after the Vietnam War, settling primarily in Wisconsin, Minnesota and California. Because they have an unusually high birth rate compared to other segments of the U.S. population, they are particularly at risk from chemical exposures that affect reproductive health or fetal development. The goals of this study were 1) to evaluate variability in urinary phthalate metabolites across three samples collected over approximately one month from reproductive age Hmong women and 2) to assess characteristics associated with phthalate exposures.
Methods
Study population
Forty-five Hmong women and 20 spouses from Green Bay, Wisconsin who were enrolled in the Fox River Environment and Diet Study (FRIENDS) between September 1999 and November 2005 were recruited to participate in a sub-study to evaluate phthalate exposures. The primary focus of FRIENDS was to evaluate the impact of polychlorinated biphenyl and methyl mercury exposures on reproductive health as well as neuropsychological and auditory function in children and adults. Informed consent was obtained from all subjects in this study, which was reviewed and approved by the Institutional Review Boards at the University of Illinois at Urbana/Champaign, Texas A&M Health Science Center, Michigan State University and the University of Oklahoma Health Sciences Center. The involvement of the CDC laboratory was limited and determined not to constitute engagement in human subjects research.
The eligibility criteria and recruitment methods for FRIENDS have been previously described (Kornosky, et al., 2008). Briefly, relationships with local Hmong associations were developed to assist in gaining the trust and cooperation required for successful recruitment in this minority population. Given the Hmong have a limited number of clan names that were adopted as surnames, and assurances that virtually all Hmong households have telephones, Hmong households in the Green Bay area were identified by surname in a continuously updated telephone directory (Metronet) and were contacted to participate by telephone. When contact could not be made by phone, interviewers visited the home to conduct eligibility screening. Eligibility was restricted to married couples in which the woman was between the ages of 18 and 46 at FRIENDS enrollment and the husband was at least 18 years of age. Because Hmong women have been reported to continue childbearing at later ages (Helsel, et al., 1992), the baseline enrollment criterion for women was extended beyond the traditional upper limit of age 40 commonly used in studies of reproductive outcomes. Through November 2005, a total of 175 enrolled couples completed baseline questionnaires which collected information on demographic and lifestyle factors, fish consumption patterns, exposure histories, and reproductive history at enrollment.
Sub-study
This sub-study had two primary goals. The first objective was to describe the distribution of urinary concentrations of phthalate metabolites among Hmong couples of reproductive age and explore the variability of the phthalate metabolite concentrations within households. The second objective was to evaluate intra- and inter-individual variability in urinary metabolite concentrations. Recruitment occurred between May and November 2005 and all participants were asked to provide a first morning urine sample and complete an exposure assessment questionnaire. For the first objective we recruited 20 women and their spouses and a single first morning urine sample was collected from each participant. For the second objective an additional 25 women were requested to provide three serial first morning urine samples approximately every two weeks over a one month time period. The median interval between the first and second sample was 15 days, and 29.5 days between the first and third sample. Since our focus in this report is on reproductive health among women, we restricted our analyses to females only, with the exception of the assessment of variability within households which evaluated agreement between samples collected from 20 women and their husbands. Thus, this study population includes 45 women providing one to three urine samples (totaling 92 samples) and 20 men providing one sample each. A total of 71 FRIENDS female participants were approached for participation resulting in a response rate of 63.4%. The primary reason provided for not participating was lack of time and interest. When the baseline characteristics of sub-study participants were compared to the remaining FRIENDS cohort, no differences were observed by age, body mass index (BMI), or education.
Data collection
Participants were provided with a urine collection kit containing instructions for the collection of a first morning void and a polypropylene plastic urine collection cup that had been prescreened for phthalate metabolites. Participants were instructed to record the time of collection, place the sample immediately in the freezer, and call the research staff on the day of collection to arrange for sample pick-up. When the research staff member visited the home to retrieve the urine specimen, an exposure assessment questionnaire was administered. Due to participant availability, the home visits occurred between 0 and 17 days (mean=1.2 d, sd=3.1 d) following urine sample collection. The questionnaire obtained information on age, weight, height, fish consumption, home furnishings, and use of products that may contain phthalates. Additional demographic and health behavior data such as education, household income and smoking status were available from baseline questionnaires administered at enrollment into FRIENDS.
Measurement of phthalate metabolites
Urine samples were retrieved from participants’ home freezers and stored frozen in the field office until shipped on dry ice to the Centers for Disease Control and Prevention (CDC) Division of Laboratory Sciences for processing and analysis. Phthalate metabolites were measured in urine to avoid potential contamination from the ubiquitous parent diesters and because the metabolites are considered to be the bioactive compounds (Hauser and Calafat, 2005). The analytical method for measuring the following ten phthalate monoesters in urine has been described in detail (Kato, et al., 2005): monomethyl phthalate (MMP), monoethyl phthalate (MEP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), and mono(3-carboxypropyl) phthalate (MCPP). Briefly, the analytical methodology involved enzymatic deconjugation of the phthalate metabolites from their glucuronidated form, followed by on-line solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry. Quality control and reagent blank samples were included in each analytical batch along with the study samples. Limits of detection (LOD) were: MMP 1.0 μg/L, MEP 0.4 μg/L, MBzP 0.11 μg/L, MnBP 0.4 μg/L, MiBP 0.26 μg/L, MEHP 0.9 μg/L, MEOHP 0.45 μg/L, MEHHP 0.32 μg/L, MECPP 0.25 μg/L, and MCPP 0.16 μg/L. CDC analysts were blind to participant characteristics.
Statistical Analysis
Statistical analyses were performed using the Statistical Analysis System (SAS), version 9.1 (SAS Institute, Cary, NC). Phthalate metabolite concentrations below the LOD were imputed by dividing the LOD by the square root of 2 (Hornung and Reed, 1990). MMP, detected in only 17 of the 92 samples (18.5%), was not evaluated further in the analyses. The metabolites of DEHP (MEHP, MEOHP, MEHHP, and MECPP) were evaluated individually and as the sum of the four (ΣDEHP). We calculated descriptive statistics including geometric means and percentiles for the nine metabolites which were detected in > 80% of samples and for ΣDEHP. The distribution of urinary concentrations of phthalate metabolites are reported in μg/L as well as in μg/g creatinine to adjust the measurement for urine dilution (Jackson, 1966). Because some women provided up to three samples, the descriptive statistics are based on the mean concentrations for the 45 women.
Measurement variability was assessed using intraclass correlations (ICC) and surrogate category analysis. ICCs were calculated using random effects models, which were applied to the natural log-transformed data to estimate the within-subject and between-subject variance for each phthalate metabolite (Rosner, 2000). The ICC is computed by dividing the estimate of the between-subject variance by the estimated total variance. As an indication of common household sources of phthalate exposures, we also calculated ICCs to assess the correlation between the 20 women’s phthalate metabolite concentrations and their spouses’. Surrogate category analyses were limited to the 22 women who had provided a set of three samples. This analysis was conducted by first defining tertiles (low, medium, high) based on the distribution of the log-transformed concentrations of a single sample (referred to as the surrogate category). For each subject, the phthalate metabolite concentrations (log-transformed) were averaged across all three samples to represent the “actual” exposure over a one month time period. The mean of the average concentrations was then calculated separately for each tertile and back-transformed to display the geometric mean. A monotonic increase in geometric means across tertiles demonstrates that categorical rankings from a single sample are representative of average exposure over the one month period (Hauser, et al., 2004). The analyses were repeated using each of the three samples as the surrogate category.
To account for the lack of independence across samples collected on the same woman, we used linear mixed models to evaluate the association between participant characteristics and log-transformed phthalate metabolite concentrations. Associations were estimated by entering each characteristic into the model one at a time. Characteristics evaluated in this analysis included age at time of specimen collection (19–29 y, 30–39 y, ≥ 40 y), BMI (kilograms per meter squared; ≤ 25 kg/m2, >25 to 30 kg/m2, >30 kg/m2), education (no formal education, less than high school, high school graduate, more than high school), income (≤ $24 999, $25 000 – $39 999, $40 000–$59 999), ever smoker (yes/no), smoker in the home (yes/no), and season of urine collection (May–August, September–November). Because of very small numbers reporting fish consumption within the 48 hours preceding urine collection (n=3), we did not investigate this factor further. We performed tests for trend by entering ordinal variables into the linear mixed model to evaluate incremental changes in phthalate metabolite concentrations across consecutive categories of age, BMI, education and income.
Creatinine levels were examined to identify urine samples considered extremely dilute (<50 mg creatinine/dl) or concentrated (>300 mg creatinine/dl) (Alessio, et al., 1985). Separate analyses were conducted after excluding 16 samples (among 11 women) with creatinine levels outside the specified range. Because the patterns of association were generally consistent, we report the results of analyses for all 92 urine samples contributed by 45 women, except where assessment of variability was restricted to the 22 women providing three samples or the 20 women whose spouses also provided specimens, as noted. All results are reported for creatinine-adjusted (μg/g creatinine) phthalate concentrations. Supplemental tables displaying results for unadjusted phthalate concentrations are available at the Journal’s website.
Results
Characteristics of the study population are presented in Table 1. At the time of this sub-study in 2005, participants ranged in age from 19 to 51 years (mean 34.8 y, sd 8.4 y) and 60% (n=27) were overweight or obese, according to the BMI cut points of >25kg/m2 or >30 kg/m2, respectively. Their spouses were similar in age (mean 35.3 y, sd 7.6, range 24–51) and mostly overweight (70%). A total of 60% of this Hmong female population reported less than a high school education, with nearly 40% reporting no formal education. Of those with a formal education, the average education level was less than high school (mean years of education 11.4 y, sd 4.5 y). Correspondingly, household income did not exceed $25 000 for most women (56%). Smoking is uncommon among Hmong women. Only one participant reported ever smoking more than 100 cigarettes in her lifetime and she was no longer a current smoker. Approximately 16% (n=7), however, lived with someone who smoked in the home.
Table 1.
Characteristics of 45 female Hmong study participants
| n | % | n | % | ||
|---|---|---|---|---|---|
| Age | Ever Smoked | ||||
| 19–29 y | 14 | 31.1 | Yes | 1 | 2.2 |
| 30–39 y | 19 | 42.2 | No | 42 | 93.3 |
| ≥ 40 y | 12 | 26.7 | Missing | 2 | 4.4 |
| Education | Lives with a Smoker | ||||
| No formal educ | 17 | 37.8 | Yes | 7 | 15.6 |
| < High School | 10 | 22.2 | No | 36 | 80.0 |
| High School | 11 | 24.4 | Missing | 2 | 4.4 |
| >High School | 6 | 13.3 | Body Mass Index | ||
| Missing | 1 | 2.2 | ≤ 25 kg/m2 | 18 | 40.0 |
| Household Income | >25 to ≤ 30 kg/m2 | 18 | 40.0 | ||
| <$24 999 | 25 | 55.6 | >30 kg/m2 | 9 | 20.0 |
| $25 000–$39 999 | 12 | 26.7 | Season of First Urine Collection | ||
| $40 000–$59 999 | 6 | 13.3 | Summer | 21 | 46.7 |
| Missing | 2 | 4.4 | Fall | 24 | 53.3 |
Urine samples were collected between May and November of 2005, with 66% (n=61) obtained during the fall months (September through November). Twenty women provided a single urine sample, three women provided two samples and 22 women provided three samples. Seven of the 10 phthalate metabolites were detected in all 92 samples (Table 2). These included four hydrolytic monoesters (MEP, MnBP, MiBP and MBzP) and three oxidative metabolites of DEHP (MECPP, MEHHP, and MEOHP). MEHP and MCPP were detected in 81% and 93% of samples, respectively. Selected percentiles of the distribution of creatinine-adjusted and unadjusted phthalate metabolites concentrations are reported in Table 2. We observed a large degree of variability in phthalate metabolite concentrations across all samples. Geometric mean concentrations were highest for MEP, followed by MECPP, MnBP, MEHHP, and MBzP. The rank-order of geometric mean concentrations was identical for creatinine-adjusted and unadjusted values. Supplementary information on the correlations between metabolites is available at the Journal’s website.
Table 2.
Distribution of creatinine-adjusted (μg/g creatinine) (in bold) and unadjusted (μg/L) phthalate metabolite concentrations in urine samples from 45 women*
| %> LOD | Geometric Mean | Geometric Std Dev | Percentiles | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | Min | Max | ||||
| MEP | 100 | 59.6 | 2.7 | 29.7 | 62.3 | 103.4 | 355.7 | 13.3 | 3866.6 |
| 63.9 | 3.0 | 32.9 | 63.0 | 116.9 | 313.5 | 6.9 | 5424.9 | ||
| MnBP | 100 | 25.2 | 1.9 | 17.5 | 25.4 | 40.0 | 83.9 | 6.9 | 127.1 |
| 27.1 | 2.6 | 14.1 | 26.1 | 55.1 | 122.7 | 3.6 | 287.7 | ||
| MiBP | 100 | 7.5 | 1.7 | 5.5 | 7.9 | 11.3 | 18.1 | 2.2 | 20.1 |
| 8.0 | 2.2 | 5.5 | 8.9 | 13.5 | 29.5 | 1.4 | 41.8 | ||
| MBzP | 100 | 20.9 | 2.2 | 15.6 | 23.0 | 38.1 | 65.4 | 2.8 | 90.1 |
| 22.4 | 2.6 | 12.8 | 30.1 | 48.9 | 105.2 | 3.6 | 191.7 | ||
| MCPP | 93 | 1.6 | 2.6 | 1.3 | 1.8 | 3.1 | 4.9 | 0.1 | 19.7 |
| 1.8 | 3.0 | 1.2 | 2.2 | 3.9 | 9.1 | 0.1 | 14.7 | ||
| MEHP | 81 | 3.4 | 2.4 | 2.3 | 3.5 | 9.1 | 21.3 | 0.5 | 36.0 |
| 3.6 | 2.6 | 2.6 | 4.5 | 7.7 | 22.2 | 0.6 | 30.8 | ||
| MECPP | 100 | 29.5 | 2.2 | 17.5 | 32.6 | 52.7 | 164.4 | 6.3 | 243.4 |
| 31.7 | 2.5 | 16.9 | 36.0 | 82.5 | 192.7 | 6.2 | 226.5 | ||
| MEHHP | 100 | 19.5 | 2.2 | 10.7 | 20.3 | 36.8 | 138.4 | 3.9 | 251.3 |
| 20.9 | 2.4 | 11.3 | 21.4 | 51.6 | 156.6 | 4.6 | 268.2 | ||
| MEOHP | 100 | 13.0 | 2.1 | 7.6 | 13.4 | 25.3 | 83.1 | 3.1 | 103.9 |
| 13.9 | 2.4 | 8.6 | 13.5 | 36.5 | 84.1 | 2.6 | 164.3 | ||
| ΣDEHP | - | 67.3 | 2.1 | 41.0 | 68.9 | 128.7 | 462.1 | 14.5 | 540.9 |
| 72.1 | 2.4 | 41.2 | 71.6 | 180.6 | 452.9 | 14.5 | 686.5 | ||
Values based on mean concentrations for 45 women providing one to three urine samples
ICCs for the phthalate metabolites are presented in Table 3. As measures of reliability, the ICCs for samples collected over time were strongest for MBzP, MEP, MCPP, MnBP and MiBP and weakest for the metabolites of DEHP. Spearman correlations between samples showed the same trend (data not shown). Although the rank order of ICCs differed for creatinine-adjusted and unadjusted concentrations, the metabolites with the top five ICCs remained the same. For these metabolites, the ICCs for the creatinine-adjusted concentrations were consistently stronger (0.51 to 0.64) than the unadjusted values (0.38 to 0.51). Of note, when analyses were restricted to samples with creatinine concentrations between 50 and 300 mg creatinine/dl, the magnitude of the ICCs increased substantially for creatinine-adjusted and unadjusted measures of MBzP (0.84 and 0.72) and MCPP (0.73 and 0.69). Exclusion of dilute (n=14) and concentrated (n=2) samples had little effect on the ICCs computed for the remaining phthalate metabolites (data not shown). Correlations between spouses’ creatinine-adjusted phthalate concentrations were strongest for MnBP, followed by MCPP, MBzP and MEP (Table 3).
Table 3.
Intraclass correlation coefficients (ICCs) for creatinine-adjusted phthalate metabolite concentrations in samples collected within women* and within households**
| Phthalate Metabolite | Woman ICC* (95% CI) | Household ICC** (95% CI) |
|---|---|---|
| MEP | 0.61 (0.53, 0.66) | 0.51 (0.29, 0.62) |
| MnBP | 0.55 (0.51, 0.58) | 0.69 (0.65, 0.73) |
| MiBP | 0.51 (0.48, 0.54) | 0.28 (0.18, 0.35) |
| MBzP | 0.64 (0.61, 0.67) | 0.51 (0.43, 0.56) |
| MCPP | 0.59 (0.51, 0.65) | 0.57 (0.34, 0.68) |
| MEHP | 0.22 (0.00, 0.38) | 0.21 (0.00, 0.51) |
| MECPP | 0.18 (0.00, 0.33) | 0.18 (0.00, 0.41) |
| MEHHP | 0.13 (0.00, 0.30) | 0.38 (0.03, 0.54) |
| MEOHP | 0.19 (0.00, 0.33) | 0.39 (0.10, 0.54) |
| ΣDEHP | 0.16 (0.00, 0.30) | 0.32 (0.00, 0.49) |
Measured in 92 urine samples which includes one to three samples from 45 female participants
Measured in 40 samples from 20 women and their husbands
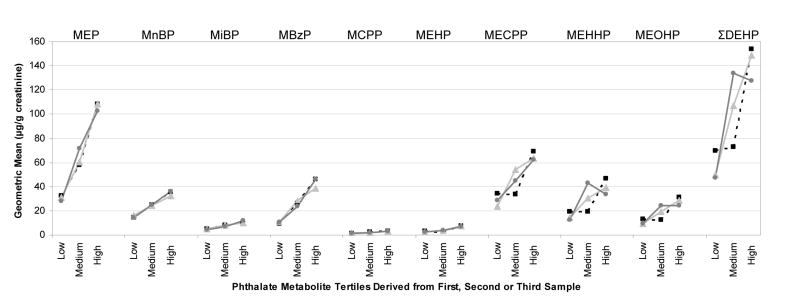
The results of the surrogate category analyses for the 22 women who provided three urine samples are displayed in Figure 1. For five phthalate metabolites (MEP, MnBP, MiBP, MBzP, MCPP), the results show that using one urine specimen, whether the first, second or third sample collected over the one month time period, characterized average one-month exposure with reasonable accuracy across low, medium and high tertiles. In other words, when subjects were categorized into exposure tertiles using a single sample, the actual geometric mean concentrations of all three available samples were lowest for subjects placed in the bottom tertile, highest for subjects placed in the highest tertile, and somewhere in between for subjects assigned to the middle tertile. The four metabolites of DEHP and their sum (ΣDEHP) each had one to two surrogate samples with non-monotonic increases in geometric means, although in each case the geometric mean for the highest tertile was more than two-fold greater than the geometric mean for the lowest tertile.
Figure 1.
Creatinine-adjusted geometric means for exposure tertiles determined by single surrogate samples for 22 women providing three urine samples
Following the assessment of temporal variability within individuals, we went on to explore patterns of geometric mean phthalate concentrations by participant characteristics, passive tobacco smoke exposure in the home and season of specimen collection (Table 4). Geometric mean concentrations of creatinine-adjusted MEP increased with age (p for trend = 0.03). No other phthalate concentrations were associated with age when adjusted for urinary dilution. Clear patterns by education and season were not apparent for creatinine-adjusted concentrations, but non-statistically significant patterns were observed for body mass index, environmental tobacco smoke and income. Except for MEP and MiBP, concentrations of all metabolites were lowest among the obese group, but these differences were statistically significant only when evaluating concentrations unadjusted for urinary dilution. Since only nine women were classified as obese, comparisons combining overweight and obese categories were also conducted but no differences in phthalate concentrations were detected. When compared to women in the highest income group ($40 000–$59 999), creatinine-adjusted concentrations of MECPP, MEHHP, MEOHP and ΣDEHP were marginally higher among women in the middle income category ($25 000–$39 999). Creatinine-adjusted concentrations of all metabolites were also somewhat lower among women reporting a smoker in the home, but marginally statistically significant differences were only detected for MEOHP and ΣDEHP.
Table 4.
Geometric meansa for creatinine-adjusted (μg/g creatinine) phthalate metabolite concentrations by characteristics of the study population
| No. of samples | MEP | MnBP | MiBP | MBzP | MCPP | MEHP | MECPP | MEHHP | MEOHP | ΣDEHP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age(μg/g creatinine) | |||||||||||
| 19–29 b | 25 | 40.7 | 24.9 | 7.5 | 20.5 | 1.7 | 4.8 | 36.6 | 26.4 | 18.6 | 83.7 |
| 30–39 | 36 | 59.2 | 25.8 | 7.0 | 20.0 | 1.6 | 2.7 | 28.5 | 17.9 | 12.0 | 60.7 |
| ≥ 40 | 31 | 91.5* | 23.9 | 8.2 | 24.0 | 1.9 | 3.9 | 38.5 | 23.1 | 14.4 | 87.3 |
| Body Mass Index (μg/g creatinine) | |||||||||||
| ≤ 25 b | 37 | 48.1 | 26.1 | 7.6 | 21.1 | 1.7 | 3.4 | 34.0 | 22.0 | 15.5 | 77.1 |
| >25 to ≤ 30 | 33 | 72.3 | 27.3 | 7.4 | 27.4 | 1.9 | 4.9 | 43.2 | 28.7 | 18.3 | 98.9 |
| >30 | 22 | 65.2 | 19.6 | 7.4 | 13.1 | 1.4 | 2.4 | 23.7 | 14.5 | 9.2** | 51.1 |
| Education (μg/g creatinine) | |||||||||||
| No formal educ | 41 | 81.5 | 26.1 | 8.2 | 25.5 | 1.8 | 3.9 | 32.9 | 21.1 | 13.2 | 73.1 |
| < HS | 18 | 33.5** | 26.1 | 6.7 | 16.0 | 1.1** | 3.8 | 33.5 | 22.8 | 16.2 | 77.8 |
| HS | 17 | 48.4 | 24.5 | 7.7 | 24.1 | 1.8 | 3.0 | 29.8 | 19.0 | 13.0 | 67.1 |
| >HS b | 14 | 78.1 | 24.3 | 7.2 | 18.0 | 2.7 | 4.2 | 46.7 | 28.9 | 19.9 | 103.7 |
| Income (μg/g creatinine) | |||||||||||
| <$24,999 | 48 | 58.1 | 28.7 | 7.5 | 22.9 | 1.8 | 3.1 | 28.7 | 17.7 | 11.8 | 63.4 |
| $25,000–$39,999 | 29 | 78.4 | 23.4 | 8.3 | 18.0 | 2.0 | 5.0 | 54.2** | 36.3** | 23.3** | 122.6** |
| $40,000–$59,999 b | 12 | 37.7 | 18.6 | 6.2 | 25.6 | 1.1 | 4.0 | 27.5 | 18.4 | 12.5 | 63.2 |
| Smoker in Home (μg/g creatinine) | |||||||||||
| Yes | 17 | 36.5 | 22.4 | 5.8 | 21.2 | 1.6 | 2.9 | 25.2 | 15.4 | 9.8** | 53.9** |
| No b | 72 | 65.4 | 25.9 | 8.0 | 21.7 | 1.8 | 4.0 | 37.7 | 24.7 | 16.4 | 85.9 |
| Season (μg/g creatinine) | |||||||||||
| Summer | 31 | 50.7 | 27.9 | 8.2 | 20.2 | 1.7 | 3.9 | 29.1 | 21.1 | 13.9 | 70.5 |
| Fall b | 61 | 69.3 | 22.8 | 7.0 | 22.2 | 1.7 | 3.4 | 36.9 | 22.0 | 14.6 | 78.5 |
Geometric mean concentrations were calculated from the linear mixed model containing the variable of interest
Referent group
p-value < 0.05 from univariate mixed model
0.05 ≤ p ≤ 0.10 from univariate mixed model
Discussion
Phthalate exposure is of potential concern for reproductive age women because the developing fetus may be susceptible to endocrine modulating effects. However, phthalate exposures in women of child-bearing age are not well characterized, particularly among underserved populations and in specific ethnic groups that cannot be captured in nationally representative surveys such as the National Health and Nutrition Examination Survey (NHANES). In our study, we investigated temporal variability of phthalate exposures in a socio-economically disadvantaged minority population of Southeast Asian women living in the Green Bay, Wisconsin area who emigrated to the U.S. from Laos and Thailand in the 1970’s and thereafter. We found detectable urinary concentrations of 9 of the 10 phthalate metabolites measured in more than 80% of the samples analyzed with the highest concentrations observed for MEP.
Five previous studies have evaluated the temporal variability of phthalate metabolites in various populations over time periods ranging from days to months (Adibi, et al., 2008; Fromme, et al., 2007; Hauser, et al., 2004; Hoppin, et al., 2002; Teitelbaum, et al., 2008). Hoppin et al. (2002) assessed agreement of phthalate measurements using first-morning urine samples collected on two consecutive days from 46 African-American women. Hauser et al. (2004) reported the temporal variability of phthalate concentrations among 11 men providing 9 samples each over a 3 month period. These earlier studies each assessed MEP, MBP, MBzP and MEHP but did not measure the oxidative metabolites of DEHP. More recently, Fromme et al. (2007) determined phthalate metabolites in the morning urine of 50 German men and women age 14–60 across eight consecutive days. Teitelbaum et al. (2008) collected two to seven urine samples over 6 months in 35 Hispanic and Black children age 6 to 10 years old. Adibi et al. (2008) described phthalate measurements in 28 pregnant Dominican and African-American women who gave two to four urine samples over a six week period during the third trimester. In accordance with ICCs reported previously for creatinine-adjusted MEHP, MEHHP, MEOHP, and MECPP over 8 days to 6 months (Adibi, et al., 2008; Fromme, et al., 2007; Teitelbaum, et al., 2008), our evaluation of measurement agreement over a one month period found these metabolites to have poor reliability (ICC<0.22). We found MBzP to be the most reproducible metabolite. Furthermore, the magnitude of the ICC for MBzP has been relatively consistent across previous studies (range 0.53 – 0.64) suggesting that exposures to BBzP may be fairly consistent over time and within particular age and racial/ethnic groups. Since BBzP is commonly used in home furnishings such as vinyl floor tile, vinyl wallpaper and carpet backing, it would seem reasonable that leaching or evaporation into indoor air would be a relatively consistent source of exposure via inhalation and ingestion for individuals living or working around such materials. Compared to MBzP, the reproducibility of measurements for MEP, MnBP and MiBP is less consistent across previous studies, but patterns of decreasing ICCs with increasing sampling intervals are not observed across studies. Our results, however, show moderately strong reproducibility for these biomarkers over a one month sampling interval. Furthermore, the similarly strong magnitude of the MnBP, MCPP, MBzP and MEP correlations between spouses suggests that the primary sources of such exposures may originate from the shared home environment and common lifestyle habits.
In agreement with the surrogate category analyses reported by Hauser et al. (2004) and Teitelbaum et al. (2008), a single urine sample was found to provide a reasonable prediction of high, medium and low categories of exposure to MEP, MBzP, MnBP, and MiBP as well as MCPP, which was only assessed by Teitelbaum. Unlike Teitelbaum, our results did not provide good support for the use of a single sample to indicate accurate exposure categories for DEHP metabolites. This may reflect differences in the timing of urine collection (first morning versus convenience sampling) or differences in phthalate exposure patterns among children compared to women of reproductive age. Although Hauser et al. (2004) did not measure the oxidative metabolites of DEHP, MEHP was reported as the least predictive metabolite of those evaluated. The MEHP monoester of DEHP is further metabolized by oxidation to several oxidative metabolites including MEOHP, MEHHP, and MECPP. Thus, the more complex metabolism of higher molecular weight phthalates such as DEHP could lead to greater within-subject variability.
While caution needs to be exercised in making comparisons across studies that employ different study designs or target different populations, our study confirms previous reports of detectable concentrations of urinary phthalate metabolites for the general U.S. population (Centers for Disease Control and Prevention, 2005; Silva, et al., 2004) and other female populations which included pregnant women (Adibi, et al., 2003; Adibi, et al., 2008; Swan, et al., 2005; Wolff, et al., 2008; Ye, et al., 2008), middle-aged African-American women (Hoppin, et al., 2002), young girls (Wolff, et al., 2007), and German females (Koch, et al., 2003) (Table 5). Consistent with these findings, MEP was the phthalate metabolite detected in urine at the highest median concentrations, although in our study median MEP concentrations (60.6 μg/g creatinine) were markedly lower than background levels in the U.S., averaging one-third the concentrations reported for females (≥ age 6 y) in NHANES 2001–2002 (171 μg/g creatinine) (Centers for Disease Control and Prevention, 2005). Increased MEP concentrations have been previously linked with smoking and use of personal care products such as perfumes (Duty, et al., 2005). While Hmong women have a lower prevalence of smoking compared to other ethnic groups, the degree to which lower MEP exposures in this population may be attributed to less frequent use of fragranced or other personal care products was not evaluated since identifying sources of phthalate exposure was beyond the scope of this paper. While MEP concentrations increased markedly with age in a clear dose-response fashion, we did not observe similar trends for any of the other phthalate metabolites. Although age patterns are not directly evaluated in the NHANES data, our data are consistent with the suggestion of an age trend as demonstrated by increasing MEP concentrations across children, adolescent and adult categories (Centers for Disease Control and Prevention, 2005).
Table 5.
Median urinary phthalate metabolite concentrations (μg/g creatinine) reported in female populations
| No. | Year | Location | MEP | MnBP | MiBP | MBzP | MEHP | MECPP | MEHHP | MEOHP MCPP | MMP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls ages 6–8 a (Wolff et al. 2007) | 90 | 2004–2005 | NY, OH, CA | 83.2 | 37.4 | 7.7 | 22.2 | 3.2 | 53.2 | 25.9 | 17.8 | 6.3 | < LOD |
| Pregnant women ages 18–35, African American and Dominican (Adibi et al. 2003) | 30 | 2000 | NY | 236 | 42.6 b | b | 12.1 | 4.6 | - | - | - | - | - |
| Pregnant women a, African American and Dominican (Adibi et al. 2008) | 246 | 1999–2005 | NY | 202 | 35.5 | 10.2 | 17.2 | 4.8 | 37.1 | 19.9 | 17.5 | 2.0 | - |
| Pregnant women ≥ age 18 a (Swan et al. 2005) | 85 | 1999–2002 | CA, MN, MO | 128.4 | 13.5 | 2.5 | 8.3 | 3.3 | - | 11.4 | 11.1 | 2.1 | 0.7 |
| Pregnant women a (Wolff et al. 2008) | 382 | 1998–2002 | NY | 380 | 36 | 6.2 | 22 | 6.0 | 35 | 20 | 17 | 3.2 | 1.6 |
| Pregnant women a ages 18–41 (Ye et al. 2008) | 100 | 2004–2006 | The Netherlands | 117 | 42.7 | 42.1 | 7.5 | 6.9 | 18.4 | 14.0 | 14.5 | 1.0 | < LOD |
| German females ages 7–64 (Koch et al. 2003) | 53 c | 2002 | Germany | 94.3 | 184 | - | 19.7 | 9.5 | - | 36.0 | 29.3 | - | - |
| Women ages 35–49 years African American (Hoppin et al 2002) | 46 c | 1996–1997 | Washington DC | 134.8 | 43.4 b | b | 21.6 | 6.4 | - | - | - | - | - |
| NHANES females ≥ age 6 (CDC 2005) | 1326 | 1999–2000 | US | 157 | 28.6 | - | 14.7 | 3.33 | - | - | - | - | - |
| NHANES females ≥ age 6 (CDC 2005) | 1411 | 2001–2002 | US | 171 | 21.5 | 2.83 | 15.1 | 4.43 | - | 17.6 | 12.0 | 2.75 | 1.45 |
| Hmong females ages 18–46 (current study) | 45 c | 2005 | WI | 60.4 | 23.5 | 7.3 | 24.1 | 3.6 | 32.2 | 18.4 | 12.8 | 1.9 | 2.14 d |
Median values are not corrected for creatinine, reported in μg/L
Monobutyl phthalate concentration reported as combination of mono-n-butyl phthalate and mono-isobutyl phthalate
First morning voids
Detected in only 17 of 92 samples (18.5%)
In contrast to our observations for MEP, median concentrations of MiBP and MBzP were approximately 2.7-fold (7.3 compared to 2.7 μg/g creatinine) and 1.6-fold (24.1 compared to 15.1 μg/g creatinine) greater in our study population compared to the general U.S. population. All other phthalate metabolites were present in our study population at concentrations similar to or slightly lower than those reported for the NHANES 2001–2002 population, except MECPP which was not measured in NHANES 2001–2002. Our median MECPP concentrations, however, were comparable to median MECPP concentrations calculated from 2003–2004 NHANES Laboratory Files data provided at http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/lab03_04.htm (31.1 μg/g creatinine compared to 28.7 μg/g creatinine).
Consistent with previous reports (Barr, et al., 2003; Koch, et al., 2003) the oxidative metabolites of DEHP exceeded the urinary concentrations of MEHP by four- to ten-fold. All DEHP metabolites were strongly correlated (r≥0.92) (Barr, et al., 2003; Koch, et al., 2003; Silva, et al., 2006) as expected since they derive from a common parent compound. Similarly, the positive correlation we observed between MnBP and MBzP concentrations (r=0.54) is consistent with evidence that these metabolites arise from a common diester, BBzP. BBzP predominantly metabolizes to MBzP, with lesser quantities eliminated as MnBP (Anderson, et al., 2001). DBP, however, is the primary source of MnBP excretion (Anderson, et al., 2001); thus, exposure to common sources of BBzP and DBP may contribute to the observed correlation. Likewise, the strong positive correlation between MnBP and its structural isomer MiBP (r=0.54) may be attributed to the use of DBP and di-isobutyl phthalate in similar commercial applications such as in paints, printing inks, adhesives, insecticides, nail polish and cosmetics. Although the magnitude of associations are somewhat attenuated, MnBP (r=0.44) and MiBP (r=0.44) are also positively correlated with MCPP concentrations which reflects the metabolic breakdown of DBP into MnBP and small quantities of MCPP (Centers for Disease Control and Prevention, 2005; Silva, et al., 2007).
Data regarding sociodemographic characteristics associated with urinary phthalate metabolite concentrations are scarce. Using data collected as part of the NHANES III examination of urinary phthalate monoester concentrations (Blount, et al., 2000), Koo et al. examined the association between various sociodemograhic characteristics and phthalate exposures (Koo, et al., 2002). Lower household income levels, defined as less than $1500 during the month prior to sampling, were associated with increased estimates of exposure to DEHP and BBzP. In our sample, in which over half of the women (n=25) reported household incomes of less than $25,000 annually, there was no clear pattern between income and creatinine-adjusted phthalate metabolite concentrations, with the exception of marginally significant increases in the concentrations of DEHP oxidative metabolites MECPP, MEHHP, and MEOHP in the middle income group.
Only a few studies have reported on the association between various phthalate monoesters and anthropometric measures, and two were restricted to male subjects (Duty, et al., 2005; Hatch, et al., 2008; Stahlhut, et al., 2007). One study examining the relationship between usage of various personal products and phthalate exposures among men reported a weak correlation between BMI and MEP concentrations (Spearman correlation coefficient = 0.1, p < 0.05) (Duty, et al., 2005). A recent study also found statistically significant positive correlations between the urinary concentrations of four phthalate metabolites, MBzP, MEHHP, MEOHP, and MEP, and increased abdominal adiposity in male NHANES participants (Stahlhut, et al., 2007). In a separate analysis of NHANES data, Hatch et al. (2008) reported increasing BMI and waist circumference with increasing MEP quartiles in adolescent girls, with a similar but somewhat weaker association observed in women between the ages of 20 and 59. In our sample of women with 60% (n=27) classified as overweight or obese, we observed an association between BMI and MEP (Spearman correlation = 0.26, p=0.08), utilizing the mean MEP concentrations for each woman. Correlations between BMI and other phthalate metabolites were not observed. There was a pattern of slightly higher geometric mean concentrations (creatinine-adjusted) for most of the metabolites, most notably for MEP, among overweight versus normal weight women. The results, however, may be the result of overweight women having less muscle mass and therefore lower creatinine levels. This pattern was not sustained among women classified as obese; although, this may be due to the very small numbers in this category (n=9). Future investigations evaluating larger sample sizes are needed to further explore the relationship between BMI and phthalate metabolites.
This study has several important strengths. Our study population is a distinct minority population in the U.S., which is characterized by a unique set of cultural factors including an unusually high birth rate that make this group an important population in which to investigate the effects of environmental exposures that may impair reproductive health or fetal development. To our knowledge, this is the first study to evaluate phthalate exposure among the Hmong population. In restricting the window of urine sample collection to first morning voids, we minimized a potential source of inter-individual variability in concentrations of phthalate metabolites. We also collected repeated measurements on over one-half of our study participants; thus, we could examine temporal variability and the effect of exposure determinants on urinary phthalate metabolites while incorporating intra-individual variability in phthalate concentrations in the analyses that were conducted. Finally, a greater number of urinary phthalate metabolites were evaluated in our investigation as compared to several previous studies (see Table 5), which provided an opportunity to better characterize phthalate exposure in this understudied population.
While it is informative to compare the distribution of phthalate metabolites concentrations observed in this minority population with reference levels for females in the general U.S. population, these comparisons should be interpreted with caution due to our small sample size, differences in the timing of urine collection, and the restricted age distribution of our sample. Differences in phthalate metabolite concentrations between Hmong women and the general population could be attributed to differences in exposure patterns, geographic or diurnal variations, population characteristics or pharmacokinetic factors influenced by age or race/ethnicity. The Hmong population was largely comprised of nonsmokers with limited educational attainment and low household income. The lack of variability by smoking status and recent fish consumption restricted our ability to assess these factors. Furthermore, insufficient proportions of individuals with more than a high school education or household income of $40 000 or above limited our assessment of phthalate exposure patterns across all levels of education and income. Finally, assessment of environmental tobacco smoke exposure was self-reported and limited to exposure in the home.
The findings of this study support evidence that environmental phthalate exposures are also prevalent among women of reproductive age in underserved populations. Phthalate metabolites concentrations are reproducible over a one month sampling interval for most metabolites measured, but caution should be exercised when using single samples to estimate exposure to DEHP. Sociodemographic and lifestyle factors that increase the likelihood of exposure have not been well delineated and should be further explored. Given the limited number of studies in reproductive-aged women, future investigations are required to determine if adverse reproductive outcomes are associated with phthalate exposures at levels that have been commonly observed in the population.
The authors would like to thank Dr. Jane Hoppin for sharing phthalate exposure assessment questionnaires for adaptation for this study. We would also like to thank Donna Gasior and the staff of the FRIENDS study for their data collection and data management efforts, and Ella Samandar, James Preau and John A. Reidy (CDC, Atlanta, GA) for technical assistance in measuring the concentrations of phthalate metabolites. This research was supported by grants P30-ES09106 and ES011263 from the National Institute of Environmental Health Sciences, R82939001 from the U.S. Environmental Protection Agency, TS000008 from the Agency for Toxic Substances and Disease Registry, and the Women’s Studies Program Women’s Interdisciplinary Seed Grant funded by the Texas A&M University Office of the Vice President for Research. The authors declare no conflicts of interest.
Supplementary Material
Footnotes
Supplementary information is available at Journal of Exposure Science and Environmental Epidemiology’s website.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, Whyatt RM. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environmental Health Perspectives. 2003;111(14):1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R. Characterization of Phthalate Exposure among Pregnant Women Assessed by Repeat Air and Urine Samples. Environmental Health Perspectives. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio L, Berlin A, Dell’Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55(2):99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18(12):1068–1074. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Diethyl Phthalate. Atlanta, GA: 1995. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Di-n-butyl Phthalate. Atlanta, GA: 2001. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Di(2-ethylhexyl) Phthalate. Atlanta, GA: 2003. [PubMed] [Google Scholar]
- Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environmental Health Perspectives. 2003;111(9):1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environmental Health Perspectives. 2000;108(10):979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. CDC; Atlanta, GA: 2005. [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128(2):216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental Health Perspectives. 2005;113(11):1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, Mayer R, Liebl B. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. International Journal of Hygiene and Environmental Health. 2007;210(1):21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Laskey J, Ostby J. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci. 2006;93(1):189–195. doi: 10.1093/toxsci/kfl035. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental Health Perspectives. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel D, Petitti DB, Kunstadter P. Pregnancy among the Hmong: birthweight, age, and parity. Am J Public Health. 1992;82(10):1361–1364. doi: 10.2105/ajph.82.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environmental Health Perspectives. 2002;110(5):515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentrations in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Jackson S. Creatinine in urine as an index of urinary excretion rate. Health Phys. 1966;12(6):843–850. doi: 10.1097/00004032-196606000-00014. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical Chemistry. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Koch HM, Rossbach B, Drexler H, Angerer J. Internal exposure of the general population to DEHP and other phthalates--determination of secondary and primary phthalate monoester metabolites in urine. Environmental Research. 2003;93(2):177–185. doi: 10.1016/s0013-9351(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL, Portier CJ. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environmental Health Perspectives. 2002;110(4):405–410. doi: 10.1289/ehp.02110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornosky JL, Peck JD, Sweeney AM, Adelson PL, Schantz SL. Reproductive characteristics of Southeast Asian immigrants before and after migration. J Immigr Minor Health. 2008;10:135–143. doi: 10.1007/s10903-007-9064-8. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43(1):47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999;156(2):81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55(1):143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 5. Duxbury Press; Pacific Grove, CA: 2000. [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006;11(1):1–13. doi: 10.1080/13547500500382868. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Reidy JA, Hauser R, Needham LL, Calafat AM. Metabolite profiles of di-n-butyl phthalate in humans and rats. Environ Sci Technol. 2007;41(21):7576–7580. doi: 10.1021/es071142x. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environmental Health Perspectives. 2007;115(6):876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental Health Perspectives. 2007;115(1):116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environmental Health Perspectives. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, Steegers EA, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environmental Research. 2008;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.