Abstract
Gender differences in the incidence of cardiovascular disease have been related to plasma estrogen levels; however, the role of vascular estrogen receptor (ER) subtypes in these sex differences is less clear. We tested whether the gender differences in vascular smooth muscle (VSM) function reflect differential expression/activity of ERα, ERβ and the newly-identified GPR30. Single aortic VSM cells (VSMCs) were freshly isolated from male and female Sprague-Dawley rats, and their contraction to phenylephrine (PHE, 10−5 M), Angll (10−7 M) and membrane-depolarization by KCl (51 mM) was measured in the absence or presence of 10−6 M 17β-estradiol (E2, stimulant of most ERs), PPT (ERα agonist), DPN (ERβ agonist), and ICI 182,780 (an ERα/ERβ antagonist with GPR30 agonistic properties). The cells were fixed and fluorescently labeled with ERα, ERβ or GPR30 antibody, and the subcellular distribution of ERs was examined using digital imaging microscopy. The mRNA expression and protein amount of aortic ER subtypes was examined using RT-PCR and Western blots. PHE, AngII, and KCl caused less contraction in VSMCs of females than males. Pretreatment of VSMCs with E2 reduced PHE-, AngII- and KCl-induced contraction in both males and females. PPT caused similar inhibition of PHE-, AngII- and KCl-induced contraction as E2, suggesting a role of ERα. DPN mainly inhibited PHE and KCl contraction, suggesting an interaction between ERβ and Ca2+ channels. ICI 182,780 did not reduce aortic VSMC contraction, suggesting little role for GPR30. RT-PCR and Western blots revealed greater expression of ERα and ERβ in VSMCs of females than males, but similar amounts of GPR30. The total immunofluorescence signal for ERα and ERβ was greater in VSMCs of females than males, and was largely localized in the nucleus. GPR30 fluorescence was similar in VSMCs of males and females, and was mainly in the cytosol. In PPT treated cells, nuclear ERα signal was enhanced. DPN did not affect the distribution of ERβ, and ICI 182,780 did not significantly increase GPR30 in the cell surface. Thus, ER subtypes demonstrate similar responsiveness to specific agonists in VSMCs of male and female rats. The reduced contraction in VSMCs of females could be due to gender-related increase in the expression of ERα and ERβ.
Key Words: Estrogen, Sex hormones, Blood vessels, Cardiovascular disease
Introduction
Cardiovascular disease is more common in men and postmenopausal women than in premenopausal women, suggesting vascular protective effects of endogenous estrogen (E2) [1-6]. The beneficial vascular effects of E2 have been ascribed to modification of circulating lipoproteins [3, 7], changes in blood coagulation [8], inhibition of intravascular accumulation of collagen [9], as well as genomic and non-genomic effects on the blood vessels [6, 10]. Genomic effects of E2 include stimulation of endothelial cell growth and inhibition of vascular smooth muscle (VSM) proliferation [2, 6]. E2 causes rapid non-genomic vasodilation of blood vessels via activation of endothelium-dependent vascular relaxation pathways [11, 12]. E2 also causes relaxation of endothelium-denuded blood vessels, suggesting direct action on VSM cells (VSMCs) [13, 14].
Many of the effects of E2 are mediated via estrogen receptors (ERs) [15]. ERs have been characterized in the female reproductive tract, mammary glands and other tissues including blood vessels [15-18]. Two major ERs, ERα and ERβ, have been suggested to mediate many of the genomic effects of E2 [15, 17, 18]. Surface membrane ERs have also been implicated in the rapid vasodilator effects of E2 [19]. Also, a novel transmembrane G protein-coupled receptor GPR30 has been shown to bind E2 and to mediate some of the rapid effects of E2 [20-23].
Sex differences in vascular function have been demonstrated in the aorta, coronary, mesenteric and renal arteries of mice, rats and pigs [24-31]. We have previously shown that the aortic contraction is reduced in female compared with male rats [25, 28]. Also, studies from our laboratory and others have suggested that the sex differences in arterial contraction are likely due to the high plasma levels of E2 in females [25, 28, 32, 33]. However, the role of vascular ER subtypes in these sex differences is less clear.
The purpose of this study was to test the hypothesis that the gender differences in VSM contraction reflect differences in the expression, subcellular distribution, and/or activity of specific ER subtypes. Because E2 may affect various types of vascular cells, studying the role of gender and ER in a multicellular vascular preparation could be difficult. Therefore, this study was performed on single VSMCs freshly isolated from the aorta of male and female rats.
Materials and Methods
Animals
Age-matched male and female Sprague-Dawley rats (12 weeks old, Charles River laboratory, Wilmington, MA) were housed in the animal facility and maintained on ad libitum standard rat chow and tap water in 12 hr/12 hr light/dark cycle. Adult female rats were randomly selected regardless of the stage of the ovarian cycle. Because the ovarian cycle in rats is frequent (every 4 to 5 days) and the estrous stage is short (12 hours), the average data from all adult female rats should cancel out any possible fluctuations in estradiol levels at the specific stages of the ovarian cycle and should roughly represent the average changes in VSMC contraction during all stages of the ovarian cycle. Rats were euthanized by inhalation of CO2, and the thoracic aorta was rapidly excised. All procedures followed the guidelines of the Animal Care and Use Committee at Harvard Medical School and the American Physiological Society.
Tissue preparation
The thoracic aorta was placed in oxygenated Krebs solution, cleaned of connective and adipose tissue, opened by cutting along its longitudinal axis, then sectioned into 2 × 2 mm strips.
Single cell isolation
Single aortic VSMCs were freshly isolated as previously described with a few modifications, specifically avoiding aspiration through a pipette or centrifugation [28, 34]. Rat aortic strips (50 mg) were placed in a siliconized flask containing a tissue digestion mixture of collagenase type II (236 U/mg protein activity, Worthington, Freehold, NJ), elastase grade II (3.25 U/mg protein activity, Boehringer Mannheim, Indianapolis, IN), and trypsin inhibitor type II-soybean (10,000 U/ml, Sigma, St. Louis, MO) in 7.5 ml of Ca2+- and Mg2+-free Hanks solution supplemented with 30% bovine serum albumin (Sigma). The tissue was incubated 3 consecutive times in the tissue digestion mixture to yield 3 batches of cells. For the first batch, the tissue was incubated with 6 mg collagenase, 4 mg elastase and 147 μl trypsin inhibitor for 90 min. For batch 2 and 3, the collagenase was reduced to 3 mg, the trypsin inhibitor was reduced to 122 μl and the incubation period was reduced to 30 min. The tissue preparation was placed in a shaking water bath at 34oC in an atmosphere of 95% O2 and 5% CO2. At the end of each incubation period, the preparation was rinsed with 12.5 ml of Hanks with albumin and poured over ice-cooled wells with glass bottom (Biotechs, Butler, PA) (for cell contraction studies), or over glass cover slips placed in 6-well plates (for immunofluorescence studies). By using the gravitational force, single VSMCs were allowed to settle and adhere to the glass coverslips. Ca2+ was gradually added back to the preparation in order to avoid the “calcium paradox” [35]. The cell isolation procedure consistently yielded spindle-shaped and viable VSMCs that showed significant contraction in response to contractile stimuli. Although differences between female and male cell adhesion to the glass coverslips may affect contraction, such possibility was not tested in the present study.
Cell contraction
Freshly isolated aortic VSMCs in the glass-bottom wells were placed on a slide warmer at 37°C (Biotechs) and on the stage of an inverted Nikon Eclipse-300 microscope. The cells were viewed using a 20X or 40X objective, and cell images were acquired using a Nikon digital camera and image acquisition software. Three different VSM activators were used. The α-adrenergic agonist phenylephrine (PHE) was used to stimulate both Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space [36]. AngII was used as another activator of receptor-mediated mechanisms via the AT1 receptor. Membrane depolarization by high KCl solution was used to activate Ca2+ entry from the extracellular space through voltage-gated Ca2+ channels [28, 36]. The changes in cell length in response to PHE (10−5 M), Ang II (10−7 M) and high KCl depolarizing solution (51 mM) were measured and the magnitude of cell contraction was expressed as ((Li-L)/Li)x100, where Li is the initial cell length and L is the final cell length. To test the role of ERs, the PHE, AngII and KCl contraction was measured in VSMCs pretreated with 10−6 M 17β-estradiol (E2, activator of most ERs). We have previously examined the dose-response curve for E2 in isolated vascular strips and VSMCs and demonstrated that E2 causes significant relaxation at 10−6 M concentration or higher [13, 28]. Based on these previous observations we used E2 (10−6 M) in the present study. We should note that these E2 concentrations are significantly higher than the nM plasma levels observed in vivo. However, E2 is a lipophilic compound, and the plasma levels of E2 may not reflect the concentration of E2 in the vascular tissue. To further examine the role of specific ER subtypes, the PHE, AngII and KCl contraction was measured in VSMCs pretreated with 10−6M 4,4′,4′’-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol (PPT, selective ERα agonist) [37, 38], diarylpropionitrile (DPN, selective ERβ agonist) [39], and ICI 182,780 (fulvestrant, an ERα/ERβ antagonist that also possesses agonistic activity at GPR30) [40, 41].
Immunofluorescence
VSMCs nontreated or treated with PPT, DPN, or ICI 182,780 were fixed for 10 min in 2% paraformaldehyde in PBS (pH 7.4). The excess fixative was quenched two times 5 min each with 0.1 mM glycine in 1% bovine serum albumin (BSA) Hanks. The cells were permeabilized with 0.1% Triton X-100 in 1% BSA Hanks for 10 min and then washed 3 times 5 min each with 0.05% Triton X-100 in 1% BSA Hanks. Cells were blocked in 1% BSA Hanks supplemented with 2% goat serum for 45 min and then reacted with polyclonal specific antibody to ERα (1:200), ERβ (1:500) or GPR30 (1:200) (Affinity Bioreagents, Golden, CO). Cells were washed three times 10 min each in 1% BSA Hanks, then incubated with fluorescein isothiocyanate (FITC)-labeled anti-rabbit immunoglobulin G (Sigma) for 30 min. For double staining of the nucleus the DNA marker ethidium dimer (1:1000, Invitrogen, Carlsbad, CA) was used. Excess label was removed by washing in 1% BSA Hanks. All procedures were performed at 22°C.
Fluorescently-labeled cells were viewed on a Nikon microscope using a 40X objective. For FITC, an excitation filter at 485 nm, a dichroic filter at 500 nm, and a longpass filter at 530 nm were used. For ethidium dimer, a Texas Red excitation filter at 488 nm, a dichroic filter at 500 nm, and a long-pass filter at 615 were used. An excitation light shutter was opened only when necessary for taking measurements. Fluorescent images were acquired using a Quantix cooled intensified charge-coupled device (CCD) camera (Photometrics, Tucson, AZ) and analyzed using Metamorph Imaging System software (Universal Imaging Corporation, West Chester, PA). Images were background subtracted. Average cell fluorescence intensity was measured by tracing the area along the borders of the cell image, integrating the total cell fluorescence intensity in the cell image, and dividing by the number of pixels in the cell image. To test for subcellular localization of ERs at the cell surface, a line was traced along the cell border and the average pixel intensity was determined. Nuclear staining was determined by tracing the area of ethidium dimer fluorescence. We have used a similar fluorescence microscopy approach to determine the subcellular distribution of protein kinase C (PKC) in VSMCs and demonstrated that PKC activation with PHE causes translocation of PKC from the cytosol to the vicinity of the surface membrane [42, 43]. In these VSMCs, the surface membrane area was identified using the cell membrane marker 7-decylBODIPY-1-propionic acid, and the nuclear area was identified using ethidium dimmer. The difference between the total fluorescence and the combined fluorescence in the surface membrane and nucleus represented the cytosolic fluorescence. To test for localization at the cell surface, the peripheral cell surface/cytosolic fluorescence ratio was measured. To test for localization in the nucleus, the nuclear/cytosolic fluorescence ratio was measured.
Real-Time RT-PCR analysis
RNA was isolated from endothelium-denuded aortic tissue samples using RNeasy Fibrous Tissue Mini Kit (QIAGEN, Valencia, CA). 1 μg of total RNA was used for reverse transcription to synthesize single-strand cDNA in a 20 µl-reaction mixture according to the protocol of First-Strand cDNA Synthesis Kit (Amersham Biosciences, Pittsburgh, PA). 2 µl of cDNA dilution (1:5 for ERα and ERβ, and 1:25 for α-actin) of reverse transcription (RT) product was applied to 20 µl RT-PCR reaction. Quantification of gene expression was performed using real-time quantitative RT-PCR machine (Mx4000 Multiplex Quantitative PCR System, Stratagene, La Jolla, CA) and employing published oligonucleotide primers specific for ERα and ERβ (Integrated DNA Technologies (IDT), Coralville, IA), and the Bio-Rad iQ SYBR Green Supermix, which employs the fluorescein compound SYBR-Green for amplicon detection (Bio-Rad, Hercules, CA). α-Actin primer with expected product length of 285 base pairs was included in the RT-PCR reaction as internal standard to normalize the results.
| Primers | Sequences |
| ERα | Forward 5′- AATTCTGACAATCGACGCCAG- 3′ |
| Reverse 5′- GTGCTTCAACATTCTCCCTCCTC- 3′ | |
| EPβ | Forward 5′- AAAGCCAAGAGAAACGGTGGGCAT- 3′ |
| Reverse 5′- GCCAATCATGTGCACCAGTTCCTT- 3′ | |
| α- actin | Forward 5′- GACACCAGGGAGTGATGGTT- 3′ |
| Reverse 5′- GTTAGCAAGGTCGGATGCTC- 3′ |
PCR was carried out with 1 cycle for 10 min at 95°C then 40-45 cycles of 30 sec denaturation at 95°C, 45 sec of annealing at 56°C, and 30 sec of extension at 72°C, followed by 1 min of final extension step at 95°C. The number of PCR cycles varies according to the expression level of the target gene. An appropriate primer concentration and number of cycles was determined to ensure that the PCR is taking place in the linear range and thereby guarantees a proportional relationship between input RNA and the cycles readout. The relative amount of gene expression was calculated by comparison of cycle thresholds with the housekeeping gene α-actin.
Western blot analysis
Endothelium-denuded aortic strips from the same rats used for the cell isolation studies were homogenized in a buffer containing 20 mM 3-[N-morpholino]propane sulfonic acid, 4% SDS, 10% glycerol, 2.3 mg dithiothreitol, 1.2 mM EDTA, 0.02% BSA, 5.5 μM leupeptin, 5.5 μM pepstatin, 2.15 μM aprotinin and 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, using a 2 ml tight-fitting homogenizer (Kontes Glass). The homogenate was centrifuged at 10,000g for 2 min and the supernatant was used as whole tissue fraction. Protein concentration was determined using a protein assay kit (Bio-Rad).
Aortic tissue homogenate was subjected to electrophoresis on 8% SDS polyacrylamide gel then transferred electrophoretically to nitrocellulose membrane. The membrane was incubated in 5% dried non-fat milk for 1 hr, then treated with polyclonal antibody to ERα, (1:100), ERβ (1:1000) and GPR30 (1:200) (Affinity bioreagents) for 24 hr. α-Actin was used as an internal control and was detected using a very specific monoclonal anti-actin antibody at an extremely low titer (1:500000, Sigma). The nitrocellulose membrane was washed 5 times 15 min each in TBS-Tween then incubated in horseradish peroxidase-conjugated secondary antibody (1:1000) for 1.5 hr. The blots were visualized with Enhanced Chemi Luminescence (ECL) Western Blotting Detection Reagent (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and the reactive bands were analyzed quantitatively by optical densitometry. The densitometry values represented the pixel intensity, and were normalized to α-actin to correct for loading.
Solutions
Normal Krebs solution was used for dissecting the tissue and contained (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2 at pH 7.4. Hank's solution was used for cell isolation and for performing the experiments and contained (in mM): 137 NaCl, 5.4 KCl, 0.44 KH2PO4, 0.42 Na2HPO4, 4.17 NaHCO3, 5.55 dextrose and 10 HEPES. Hank's solution was bubbled for 30 min with a 95% O2 5% CO2 mixture and the pH was adjusted to 7.4. The high KCl (51 mM) depolarizing solution had the same composition as normal Hank's solution with equimolar substitution of NaCl with KCl.
Drugs and chemicals
Stock solutions of PHE (10−1 M, Sigma) and AngII (10−2 M, Sigma) were prepared in distilled water. Stock solution of 17β-estradiol (2,3,5[10]-Estratriene-3,17β-diol, Sigma) was prepared as 5×10−2 M in 100% ethanol. Stock solutions of PPT, DPN, and ICI 182,780 (10−1 M, Tocris) were prepared in diemtheylsulfoxide (DMSO). The final concentration of DMSO in the experimental solution was <0.1%. All other chemicals were of reagent grade or better.
Statistical analysis
Data were collected from 2 to 7 cells from each single rat for each single experiment, and the cumulative data from the total number of cells “n” from 5 to 7 different rats for each experimental protocol were used for analysis. The data were first analyzed using ANOVA. When a statistical difference was observed, the data were further analyzed using Student's t-test for unpaired data for comparison of two means. Data were presented as means±S.E.M, and differences were considered statistically significant if p<0.05.
Results
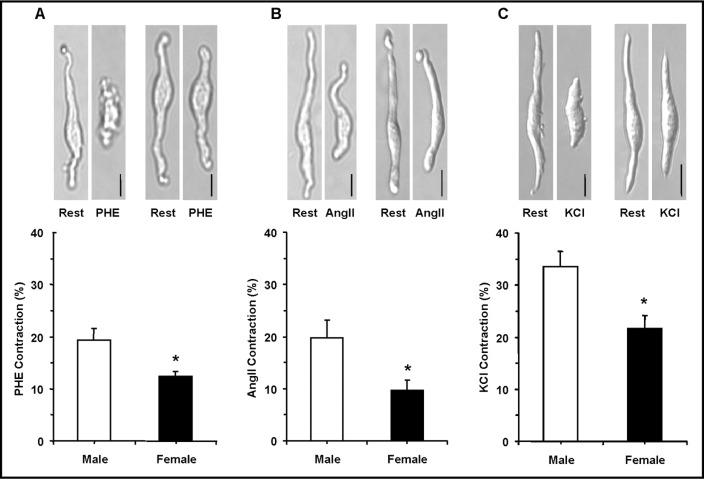
The cell isolation procedure produced cells of variable lengths. Only spindle-shaped cells ≥ 50 µm in length were selected for this study. In resting cells of male rats, the average cell length was 61.86±0.86µm, and was not significantly different from the resting cell length in female rats 60.17±0.91µm. Aortic VSMCs of male rats were responsive to contractile stimuli. PHE (10−5 M) and AngII (10−7 M) caused cell contraction that reached steady-state in ∼5 min (Fig. 1). Also, membrane depolarization by 51 mM KCl caused significant contraction in VSMCs of male rats (Fig. 1). The PHE, AngII and KCl-induced contraction was significantly reduced in VSMCs of female compared with male rats (Fig. 1).
Fig. 1.
Effect of PHE, AngII and membrane depolarization by high KCl on contraction of VSMCs of male and female rats. Freshly isolated aortic VSMCs from male and female rats were viewed on the stage of a Nikon microscope and images of the single cells at rest were acquired. The cells were stimulated with PHE (10−5 M) (A), AngII (10−7 M) (B) or KCl (51 mM) (C) for 10 min and the cell contraction was measured as described in the Methods. Total magnification = 400. Calibration bar in pictures = 10μm. Cumulative data (n= 11 to 28 cells from 5 to 7 different rats) were presented as the means±SEM in bar graphs. ∗ Measurement in females are significantly different (p<0.05) from corresponding measurements in males.
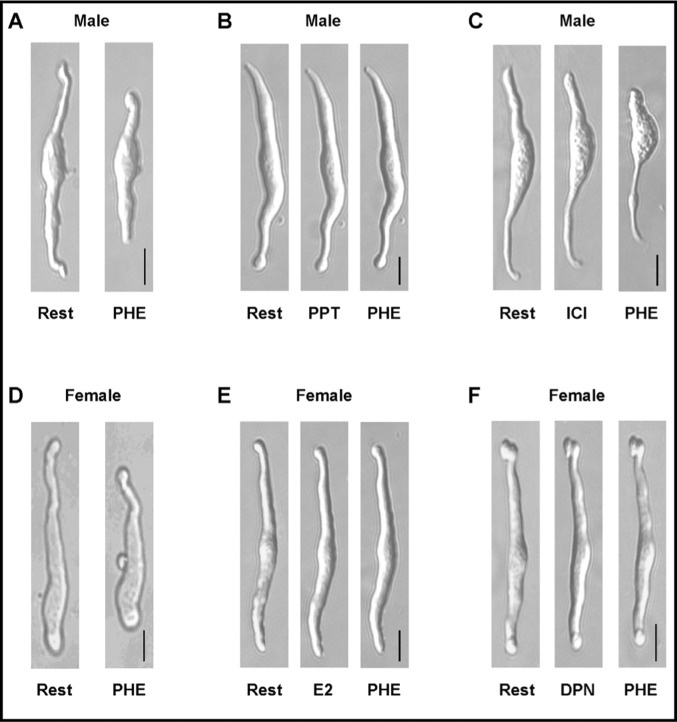
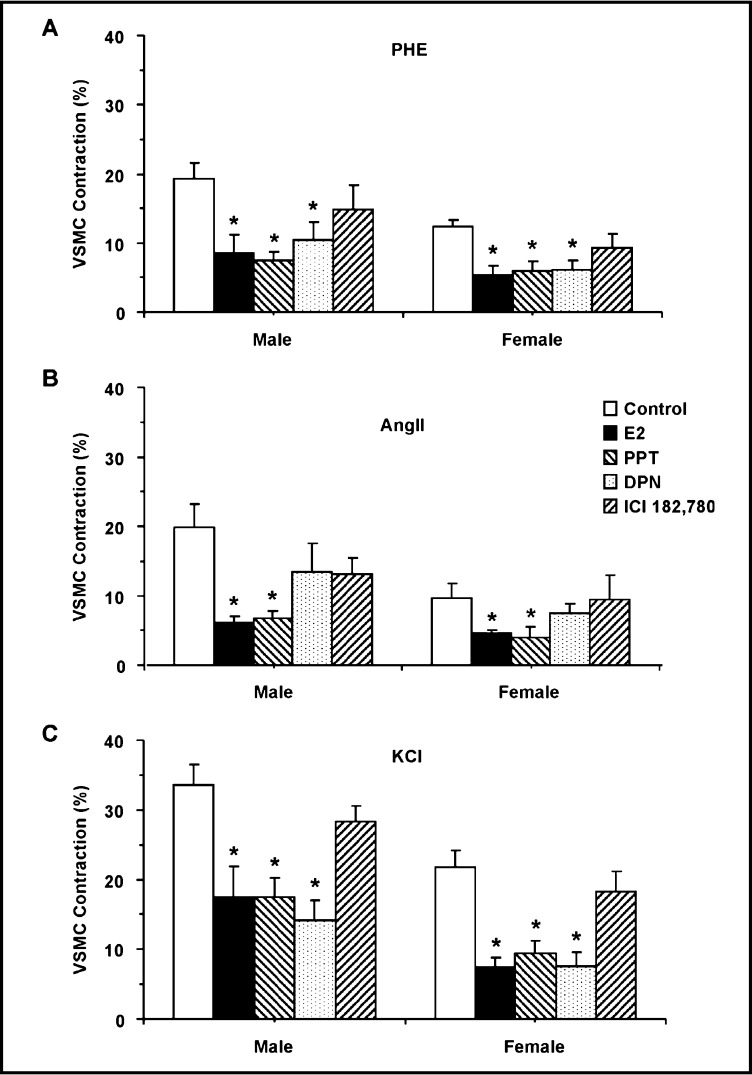
Pretreatment of VSMCs with E2 (10−6 M) or the ERα agonist PPT (10−6 M) for 10 min did not cause significant change in resting cell length, but significantly reduced PHE, AngII and KCl-induced contraction in cells of male and female rats (Fig. 2 and 3). Pretreatment of VSMCs with the ERβ agonist DPN (10−6 M) significantly reduced PHE and KCl contraction, but insignificantly reduced AngII-induced contraction in cells of male and female rats (Fig. 2 and 3). In contrast, pretreatment of the cells with ICI 182,780 did not significantly affect PHE, AngII or KCl-induced contraction in VSMCs of male or female rats (Fig. 2 and 3).
Fig. 2.
Representative images demonstrating the effect of ER modulators on cell contraction. VSMCs from male (A,B,C) or female rats (D,E,F) were either at rest (A,D) or pretreated with (10−6 M) of 17β-estradiol (E2, activator of most ERs) (E), PPT (ERα agonist) (B), DPN (ERβ agonist) (F), or ICI 182,780 (ERα/ERβ antagonist, and GPR30 stimulant) (C), for 10 min then stimulated with PHE (10−5 M) and the effects on cell contraction were observed. Total magnification = 400. Calibration bar in pictures = 10µm.
Fig. 3.
Effect of ER modulators on PHE, AngII and KCl-induced contraction in VSMCs of male and female rats. VSMCs of male (left panels) or female rats (right panels) were stimulated with PHE (10−5 M) (A), AngII (10−7 M) (B) or KCl (51 mM) (C) in the absence or presence of (10−6 M) of 17β-estradiol (E2, activator of most ERs), PPT (ERα agonist) (C), DPN (ERβ agonist) (D), or ICI 182,780 (ERα/ERβ antagonist, and GPR30 stimulant), and the cell contraction was measured. Data represent the means±SEM (n= 10 to 32 cells from 5 to 7 different rats). ∗ Measurements in the presence of E2, PPT, DPN, or ICI 182,780 are significantly different (p<0.05) from corresponding measurements in the absence of the ER modulator.
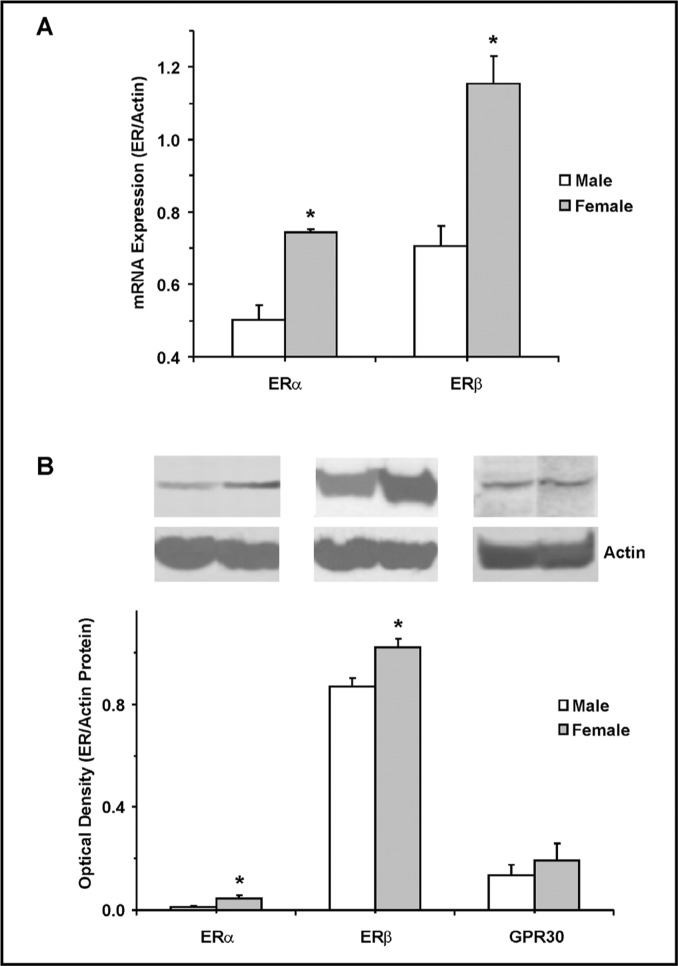
RT-PCR analysis revealed greater expression of ERα and ERβ in the aorta of female compared with male rats (Fig. 4A). Western blot analysis of aortic tissue homogenate from male and female rats revealed immunoreactive bands corresponding to ERα at 64 kDa, ERβ at 55 kDa, and GPR30 at 50 kDa. Optical density analysis revealed greater protein amount of ERα and ERβ, but similar amount of GPR30 in aortic tissue homogenate of female compared with male rats (Fig. 4B).
Fig. 4.
Expression and protein amount of ERα, ERβ and GPR30 in VSM of male and female rats. Real Time RT-PCR were used to measure the mRNA expression (A) and Western blot analysis was used to measure protein amount (B) of ERα, ERβ, and GPR30 in aortic tissue homogenate from male and female rats. Measurements were normalized to the house keeping gene or protein actin. Data represent the means±SEM (n= 3 to 4). ∗ p<0.05.
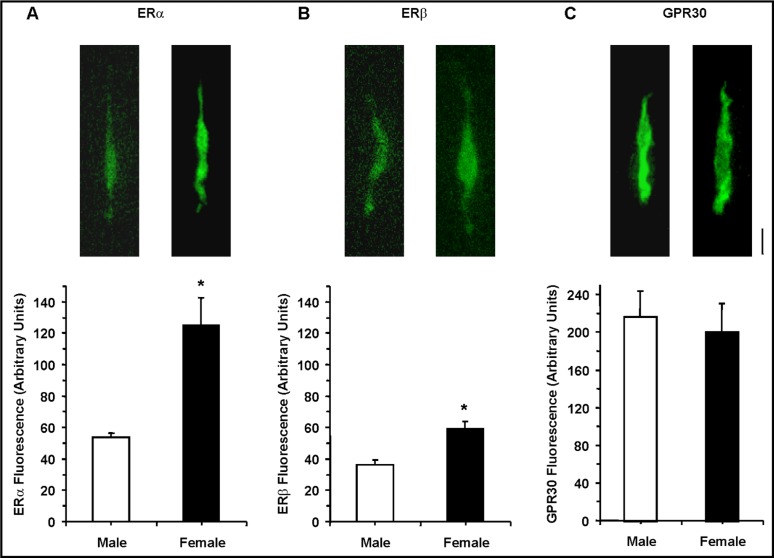
Immunofluorescence experiments indicated that in resting VSMCs the average fluorescence signal for ERα or ERβ was greater in cells of female compared with male rats. In contrast, the GPR30 signal was not significantly different in VSMCs of female and male rats (Fig. 5).
Fig. 5.
Immunofluorescence of ERα, ERβ and GPR30 in VSMCs of male and female rats. Resting aortic VSMCs from male and female rats were fixed, permeabilized and labeled with antibodies to ERα (1:200) (A), ERβ (1:500) (B) and GPR30 (1:200) (C). Images of fluorescently-labeled cells were acquired, the total cell fluorescence was divided by the number of pixels in the cell image, and the average cell fluorescence was measured. Total magnification = 400. Calibration bar in pictures = 10µm. Data represent the means±SEM (n= 15 to 41 cells from 5 to 7 different rats). ∗ p<0.05.
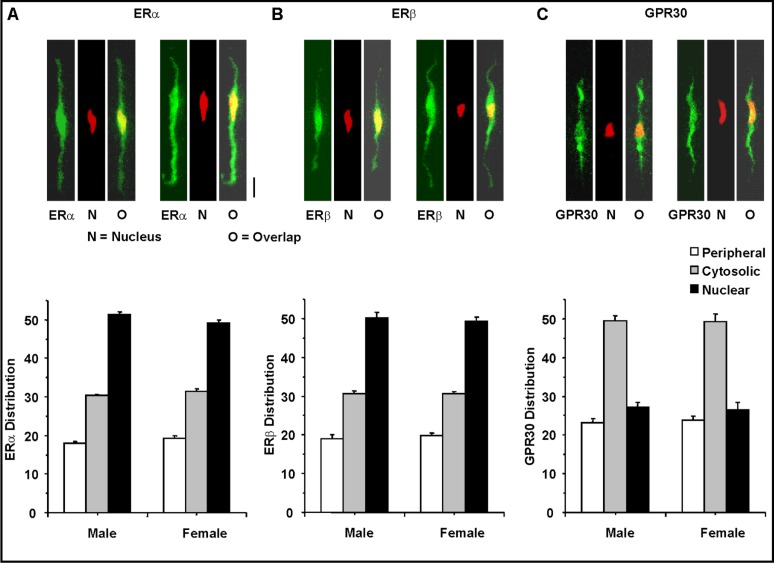
Optical dissection of the subcellular distribution of ERα and ERβ revealed a low fluorescence signal in the cell periphery, a greater fluorescence in the cytosol, and a prominent fluorescence signal in the center of the cell coinciding with the nucleus (Fig. 6A and 6B). In contrast, the subcellular distribution of GPR30 was mainly in the cytosol, with less fluorescence signal in the periphery and the center of the cells (Fig. 6C). The distribution pattern of ERα, ERβ and GPR30 in the periphery, cytosol, and nuclear segments was not significantly different in VSMCs of female and male rats.
Fig. 6.
Subcellular distribution of ERα, ERβ and GPR30 in VSMCs of male and female rats. Resting aortic VSMCs from male and female rats were fixed, permeabilized and labeled with primary antibodies to ERα (1:200) (A), ERβ (1:500) (B) and GPR30 (1:200) (C), and FITC-labelled IgG (Green). The center of the cell was determined by co-staining with the DNA marker ethidium dimer (Red). Co-localization of the specific ER with the DNA marker ethidium dimer in the nucleus is presented in yellow. The cell image was dissected into 3 components: the cell surface, the center (corresponding to the nucleus), and the cytosol (total fluorescence – combined peripheral and central fluorescence) and the average pixel intensity in each segment was measured. Total magnification = 400. Calibration bar in pictures = 10µm. Data represent the means±SEM (n= 15 to 41 cells from 5 to 7 different rats).
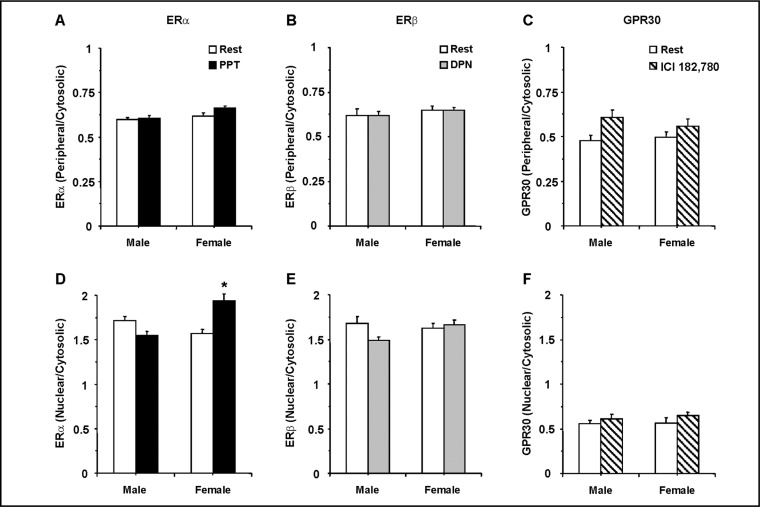
To test whether ER activation is associated with its translocation between the cell surface and the cytosol, the periphery/cytosol fluorescence ratio was measured at rest and during stimulation by a specific ER agonist. An increase in periphery/cytosol ratio would indicate increased localization at the cell surface. Also, to test whether ER activation is associated with its translocation from the cytosol to the nucleus, the nuclear/cytosol fluorescence ratio was measured at rest and during activation by a specific ER stimulant. An increase in the nuclear/cytosol ratio would indicate increased localization in the nucleus. The ERα agonist PPT did not cause significant change in the distribution of ERα at the cell surface of VSMCs of male or female rats (Fig. 7A). In contrast, PPT significantly increased the nuclear distribution of ERα in VSMCs of female rats (Fig. 7D). The ERβ agonist DPN did not significantly increase the distribution of ERβ at the cell surface (Fig. 7B) or the nucleus (Fig. 7E) of VSMCs of male or female rats. Also, no significant increase in the distribution of GPR30 at the cell surface (Fig. 7C) or the nucleus (Fig. 7F) was observed in VSMCs of male or female rats when treated with the GPR30 stimulant ICI 182,780.
Fig. 7.
Effect of specific ER modulators on the subcellular distribution of ERα, ERβ and GPR30 in VSMCs of male and female rats. VSMCs from male and female rats were nontreated or treated for 10 min with PPT (ERα agonist), DPN (ERβ agonist) or ICI 182,780 (ERα/ERβ antagonist, and GPR30 stimulant). The cells were fixed, permeabilized and labeled with antibody to either ERα (1:200) (A and D), ERβ (1:500) (B and E) or GPR30 (1:200) (C and F). The average fluorescence in the cell surface and cytosol was used to measure the periphery/cytosol ratio (Top panels). The average fluorescence in the nuclear area (as determined by ethidium dimer) and the cytosol was used to measure the nuclear/cytosol ratio (Bottom panels). Data represent the means±SEM (n= 14 to 43 cells from 5 to 7 different rats). ∗ p<0.05.
Discussion
The main findings of the present study are: 1) VSMC contraction to three different modes of stimulation is reduced in cells of female compared with male rats, 2) ERα and ERβ agonists cause similar inhibition of VSMC contraction in males and females, 3) The expression of ERα and ERβ is greater in VSMCs of females compared with males, and is localized mainly in the nucleus, 4) GPR30 demonstrates similar expression in males and females, is mainly in the cytosol, and its activation does not appear to affect VSM contraction or its subcellular location.
Gender differences in vascular function have been demonstrated in numerous blood vessels from various species [24-31]. We have previously demonstrated gender-related reduction in contraction of aortic strips isolated from female compared with male rats [25, 44]. The aortic VSM contraction was enhanced in ovariectomized compared with intact females, but not different in castrated compared with intact male rats. These studies suggested that the sex differences are more likely due to differences in the amount/activity of estrogen and ER, rather than testosterone and the androgen receptor. Also, several studies have examined the gender differences and the effects of E2 in arterial rings [13, 24, 27]. However, the multicellular nature of blood vessels has made it difficult to discriminate between the specific roles of the endothelium and VSM in the gender differences in vascular function. The present observations in freshly isolated single aortic VSMCs of Sprague-Dawley rats are consistent with our previous report in Wistar-Kyoto and spontaneously hypertensive rats [28] and demonstrate gender-specific reduction in contraction in VSMCs of female compared with male rats. The reduction in both PHE and AngII-induced contraction in female VSMCs suggests a reduction in a common-receptor mediated mechanism of VSM contraction. We have previously demonstrated that the transient cell contraction and [Ca2+]i in response to caffeine and PHE in Ca2+-free medium were not different in male and female WKY rat aortic VSMCs, suggesting that the gender differences in cell contraction are not related to differences in the intracellular Ca2+ stores. Membrane depolarization by high KCl is known to stimulate mainly Ca2+ entry from the extracellular space through voltage-gated Ca2+ channels [28, 36]. We have previously demonstrated that the maintained PHE and KCl-induced contraction and [Ca2+]i were reduced in VSMCs of female compared with male WKY rats, suggesting differences in the Ca2+-entry mechanisms from the extracellular space [28]. The observed reduction in KCl contraction in female Sprague-Dawley VSMCs is consistent with the possibility that the gender-specific reduction in VSMC contraction is related to differences in the Ca2+ entry mechanism of VSM contraction.
Gender differences in vascular contraction have been partly related to differences in the plasma levels of E2, being higher in females compared with males [25, 28, 32, 33]. Therefore, the results from the VSMC contraction experiments should be interpreted with caution as the adult female rats were randomly selected regardless of the stage of the estrous cycle. Because the plasma levels of E2 are known to change during various stages of the ovarian cycle and E2 could affect VSMC reactivity, studies on VSMCs isolated from female rats at the specific stage of the estrous cycle as monitored by vaginal cytology should be examined in future studies.
ER subtypes have also been identified in numerous vascular preparations [16-18, 21, 29, 45]. Previous imaging studies were able to localize ERα, but not ERβ, in en face arterial endothelium of female rat vessels [46]. Also, studies from our laboratory and others have shown that the interaction of E2 with ERs causes direct vasodilation of blood vessels via endothelium-dependent and endothelium-independent mechanisms [5, 6, 10, 19]. The present study provides evidence for gender-related difference in the amount of ERα and ERβ, being greater in VSMCs of females compared with males because: 1) RT-PCR analysis showed greater mRNA expression of ERα and ERβ in female aorta, 2) Western blot analysis revealed increased protein amount of ERα and ERβ in aortic tissue homogenate from female rats, and 3) Immunofluorescence experiments on single cells demonstrated greater total fluorescence signal for ERα and ERβ in female than male VSMCs. Although it is difficult to reach a definitive conclusion regarding the amount of ER based on any one of these techniques alone, the consistent findings using three different techniques raise the likelihood of meaningful data and support the conclusions regarding the amount of ER subtypes in VSM.
The present RT-PCR and Westren blot data in the rat aortic VSMCs are consistent with our previous report that the expression of ERα and ERβ is greater in venous tissues from female compared with male Sprague-Dawley rats [47]. We should note that some studies have reported that only the ERβ isoform level showed a clear gender difference, being lower in VSMCs from female than in cells derived from male rats [48]. Other studies have shown that VSMCs from female rat express less ERα and ERβ than VSMCs from male rats [49]. The causes of the differences in the results between those studies and the present study are unclear. However, in those reports the rat strain studied could not be verified. Also, those studies were performed on cultured aortic VSMCs, which are phenotypically different from the freshly isolated contractile VSMCs used in the present study.
Although the biochemical and immunofluorescence studies provide important information regarding the relative abundance of ERs in aortic VSM of female rats, they do not necessarily indicate whether these ERs are functional. ERα and ERβ in VSM could mediate multiple functions including: 1) genomic effects on the expression of membrane channel or contractile protein that eventually affect VSM contraction, 2) rapid non-genomic effects that directly affect the mechanisms of VSM contraction, and 3) genomic nuclear events that are unrelated to VSM contraction and likely involved in modulating cell growth and proliferation. The reduced VSMC contraction to PHE, AngII and KCl, and the increased expression of ERα and ERβ in female aorta are consistent with a possible genomic role of these ER subtypes on the Ca2+ entry mechanism of VSM contraction. To determine whether ERα and ERβ also mediate rapid non-genomic effects in VSM we sought to test the effects of modulators of specific ER subtypes on VSMC contraction. Previous studies to evaluate the effects of ER subtypes on arterial tone have shown variable results. It has been shown that activation of either ERα or ERβ decreases rat pulmonary arterial tone; however, the contribution of a specific ER subtype appears to be stimulus specific, with ERα primarily modulating PHE-induced vasoconstriction, while ERβ inhibiting hypoxia-induced pulmonary vasoconstriction [50]. It has also been shown that both PPT, activator of ERα [37, 38], and DPN, selective ERβ agonist [39], cause acute relaxation in isolated rat mesenteric arteries, but PPT has a significantly greater vasodilatory effect [51]. Other studies demonstrated that E2 causes acute relaxation of rat aorta solely through ERα [52]. Also, studies on subcutaneuous arteries from postmenopausal women have shown that ERβ expression was enhanced in the vascular wall from women with coronary heart disease, while ERα was predominant in control women [53]. In the present study, both E2, an activator of most ERs, and PPT, activator of ERα [37, 38], caused significant reduction of PHE, AngII and KCl contraction, suggesting a role for ERα in mediating the inhibition of a common VSM signaling pathway. Because KCl contraction is mainly due to Ca2+ entry from the extracellular space [36], the ERα-mediated inhibition of VSM contraction is likely due to inhibition of Ca2+ entry mechanisms. This is supported by our previous report that E2 decreases KCl-induced Ca2+ influx in endothelium-denuded coronary artery strips and in isolated rat aortic VSMCs [13, 28]. We do not wish to draw conclusions on whether the E2-or PPT-ERα interaction inhibits Ca2+ entry by direct or indirect action on plasmalemmal Ca2+ channels. Some studies have shown that E2 activates Ca2+-activated K+ channels, leading to membrane hyperpolarization, and inhibition of Ca2+ entry through voltage-gated channels [54, 55]. Other studies have shown that E2 blocks Ca2+ channels in cultured A7r5 and aortic VSMCs [56, 57]. The similarity in the inhibitory effects of E2 and the ERα agonist PPT on VSMC contraction suggests that ERα is a major mediator of the effects of E2 in VSM. Also, the prominent inhibitory effects of E2 and PPT on contraction of VSMCs of both males and females suggest that ERα is functional and mediates rapid non-genomic effects in VSM of both genders.
Another approach to test for functionality of the VSM ERs is to track the effects of modulators of specific ER subtypes on the distribution of ERs in VSMCs. The effects of E2 on target tissues have classically been thought to involve genomic actions mediated through interaction with cytoplasmic ERs and translocation of the E2-ER complex to the nucleus [58]. Although a genomic role of ERα on the expression of the Ca2+ channels might underlie the observed reduced contractility of female aortic VSMCs, it is less likely to account for the acute inhibitory effects of E2 or PPT on cell contraction. The acute nature of the vasorelaxant effects of E2 and PPT may represent additional non-genomic effects on the mechanisms of Ca2+ entry into VSM.
It is important to note that the distribution of ERα in resting VSMCs was mainly in the nucleus. Activation of ERα by PPT did not cause significant change in the distribution of ERα at the cell surface, suggesting that ERα-mediated inhibition of VSM contraction does not involve redistribution of ERα between the cytosol and the surface membrane. In contrast, treatment of VSMCs with PPT for 10 min caused significant increase in the nuclear localization of ERα. The rapid localization of ERα to the nucleus may not be related to its rapid inhibition of VSM contraction, but suggests potential ERα-mediated initiation of rapid nuclear events and genomic effects. This is supported by the recent report that estrogen directly and rapidly recruits specific transcriptional factors that then propagate distinct cascades of gene expression in blood vessels [59].
The ERβ agonist DPN inhibited PHE and KCl-induced contraction, suggesting rapid non-genomic effect of ERβ on Ca2+ entry mechanisms or downstream contraction pathways such as myosin light chain kinase. However, DPN did not inhibit AngII-induced contraction, suggesting that AngII could be activating other mechanisms that are not inhibited by ERβ. AngII is known to stimulate AT1 receptors and to activate tyrosine kinase-dependent pathways in VSM [60-66], and the effects of ERβ on these pathways need to be further examined. It is important to note that ERβ was mainly localized in the nucleus, and its activation by DPN was not associated with increased localization at the cell surface, suggesting that the inhibitory effects of DPN on VSM contraction do not involve redistribution of ERβ between the cytosol and the surface membrane. Also, DPN did not cause significant increase in the nuclear localization of ERβ within the 10 min exposure time, suggesting that any potential ERβ-mediated activation of nuclear events may be delayed beyond the 10 min of stimulation used in the present study.
GPR30 is a novel transmembrane G protein-coupled receptor that has been shown to bind E2 and to mediate some of its rapid non-genomic effects [20-23]. ICI 182,780 is a known ERα and ERβ antagonist, that also possesses GPR30 agonistic properties [40, 41]. These unique properties make ICI 182,780 an interesting compound to test the effects of GPR30 while simultaneously blocking unwanted effects on ERα or ERβ. The present experiments demonstrated that GPR30 is expressed at similar levels in male and female rats, is mainly distributed in the cytosol of VSMCs at rest, and its activation by ICI 182,780 did not affect its subcellular location or VSMC contraction. We have performed preliminary experiments to test the effects of the GPR30 agonist G1 on female rat aortic strips. Our preliminary findings suggest that PHE (3×10−7 M) caused significant contraction in female rat thoracic aorta (0.64±0.08 g/mg tissue weight), and that treatment with G1 (10−6 M) for 15 min did not cause any significant decrease in PHE contraction (0.61±0.09 g/mg tissue weight, 94.18±3.38%) (Reslan & Khalil, unpublished observations). While these data suggest a little role of GPR30 in the gender differences and the effects of E2 on rat aortic VSM function, the results do not rule out potential role of GPR30 on other vascular cell types or other vascular beds. Future studies with G1 and other GPR30 agonists such as STX are needed to further clarify the role of GPR30 in the regulation of VSM contraction in various vascular beds. We should also note that the present subcellular distribution of ERs was determined using fluorescence microscopy. Given the limited resolution of this technique, future experiments should further verify the subcellular distribution of ERs in VSMCs using confocal microscopy.
In conclusion, aortic VSMCs of female rats exhibit gender-related reduction in contraction to three different modes of stimulation. The reduced VSMC contraction in females is likely related to increased expression of ERα and ERβ. The localization of ERα and ERβ mainly in the nucleus suggests genomic effects on the mechanisms of VSM contraction, and perhaps other long-term nuclear events. ERα and ERβ demonstrate similar rapid responsiveness in male and female VSMCs, and specific ERα and ERβ agonists may be useful for rapid non-genomic reduction of VSM contraction in vascular diseases associated with severe vasoconstriction. It is important to note that while there was an increase in ER expression in aorta from female compared to male rats, the inhibition of the contractile response induced by ER activation was similar in male and female VSMCs. This is likely related to differences in the sensitivity of the ER to activation possibly due to the differences in exposure to endogenous estrogen in females compared with males, and should be further examined in future experiments. GPR30 demonstrates similar expression in males and females, is mainly in the cytosol, and its activation does not affect its subcellular location or VSM contraction. More studies in other vascular beds are needed to further evaluate the role of GPR30 in VSM function.
Abbreviations
AngII (angiotensin II); DPN (diarylpropionitrile); E2 (17β-estradiol); ER (estrogen receptor); PHE (phenylephrine); PPT (4,4′,4′’-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol); VSM (vascular smooth muscle); VSMC (vascular smooth muscle cell).
Acknowledgements
R. A. Khalil was partly supported by grants from National Heart, Lung, and Blood Institute (HL-65998 and HL-70659) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702). Dr. Y. Ma was a visiting scholar from West China Hospital, Sichuan University, and a recipient of a scholarship from the China Scholarship Council.
References
- 1.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861–1867. [PubMed] [Google Scholar]
- 2.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44:789–795. doi: 10.1161/01.HYP.0000145988.95551.28. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard M, Ganz P. How do we explain the clinical benefits of estrogen? From bedside to bench. Circulation. 1995;92:5–8. doi: 10.1161/01.cir.92.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Koledova VV, Khalil RA. Sex hormone replacement therapy and modulation of vascular function in cardiovascular disease. Expert Rev Cardiovasc Ther. 2007;5:777–789. doi: 10.1586/14779072.5.4.777. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 6.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza SG, Zerpa A, Carrasco H, Colmenares O, Rangel A, Gartside PS, Kashyap ML. Estradiol, testosterone, apolipoproteins, lipoprotein cholesterol, and lipolytic enzymes in men with premature myocardial infarction and angiographically assessed coronary occlusion. Artery. 1983;12:1–23. [PubMed] [Google Scholar]
- 8.Bing RJ, Conforto A. Reversal of acetylcholine effect on atherosclerotic coronary arteries by estrogen: pharmacologic phenomenon of clinical importance? J Am Coll Cardiol. 1992;20:458–459. doi: 10.1016/0735-1097(92)90117-6. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky H. Effects of estrogen and progestogen treatment on the response of the aorta of male rats to hypertension. Morphological and chemical studies. Circ Res. 1972;30:341–349. doi: 10.1161/01.res.30.3.341. [DOI] [PubMed] [Google Scholar]
- 10.Serock MR, Wells AK, Khalil RA. Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov. 2008;3:165–186. doi: 10.2174/157489008786263970. [DOI] [PubMed] [Google Scholar]
- 11.Gisclard V, Miller VM, Vanhoutte PM. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- 12.Herrington DM, Braden GA, Williams JK, Morgan TM. Endothelial-dependent coronary vasomotor responsiveness in postmenopausal women with and without estrogen replacement therapy. Am J Cardiol. 1994;73:951–952. doi: 10.1016/0002-9149(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Crews JK, Khalil RA. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19:1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- 14.Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17beta-oestradiol in vitro. Br J Pharmacol. 1991;104:1033–1307. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smiley DA, Khalil RA. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr Med Chem. 2009;16:1863–1887. doi: 10.2174/092986709788186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 17.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 20.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G proteincoupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 21.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17betaestradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 22.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 24.Barber DA, Miller VM. Gender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol. 1997;273:H2325–2332. doi: 10.1152/ajpheart.1997.273.5.H2325. [DOI] [PubMed] [Google Scholar]
- 25.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca2+ entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34:931–936. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Kakami M, Kobayashi T, Kamata K. Gender differences in vascular reactivity to endothelin-1 (1–31) in mesenteric arteries from diabetic mice. Peptides. 2008;29:1338–1346. doi: 10.1016/j.peptides.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Miller VM, Barber DA, Fenton AM, Wang X, Sieck GC. Gender differences in response to endothelin-1 in coronary arteries: transcription, receptors and calcium regulation. Clin Exp Pharmacol Physiol. 1996;23:256–259. doi: 10.1111/j.1440-1681.1996.tb02607.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000;278:C834–844. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- 29.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tostes RC, David FL, Carvalho MH, Nigro D, Scivoletto R, Fortes ZB. Gender differences in vascular reactivity to endothelin-1 in deoxycorticosterone-salt hypertensive rats. J Cardiovasc Pharmacol. 2000;36:S99–101. doi: 10.1097/00005344-200036051-00032. [DOI] [PubMed] [Google Scholar]
- 31.Varbiro S, Matrai M, Szekeres M, Nadasy GL, Szaky E, Mericli M, Banhidy F, Monos E, Szekacs B. Intramural coronary artery constrictor reactivity to thromboxane is higher in male than in female rats. Gynecol Endocrinol. 2006;22:44–47. doi: 10.1080/09513590500453759. [DOI] [PubMed] [Google Scholar]
- 32.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Krause DN, Doolen S, Duckles SP. Ovariectomy eliminates sex differences in rat tail artery response to adrenergic nerve stimulation. Am J Physiol. 1997;272:H1819–1825. doi: 10.1152/ajpheart.1997.272.4.H1819. [DOI] [PubMed] [Google Scholar]
- 34.Khalil RA, Morgan KG. Phenylephrine-induced translocation of protein kinase C and shortening of two types of vascular cells of the ferret. J Physiol. 1992;455:585–599. doi: 10.1113/jphysiol.1992.sp019317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nayler WG, Perry SE, Elz JS, Daly MJ. Calcium, sodium, and the calcium paradox. Circ Res. 1984;55:227–237. doi: 10.1161/01.res.55.2.227. [DOI] [PubMed] [Google Scholar]
- 36.Khalil RA, van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther. 1988;244:537–542. [PubMed] [Google Scholar]
- 37.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 39.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 40.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 41.Kleuser B, Malek D, Gust R, Pertz HH, Potteck H. 17-Beta-estradiol inhibits transforming growth factor-beta signaling and function in breast cancer cells via activation of extracellular signalregulated kinase through the G proteincoupled receptor 30. Mol Pharmacol. 2008;74:1533–1543. doi: 10.1124/mol.108.046854. [DOI] [PubMed] [Google Scholar]
- 42.Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol. 1992;263:C714–719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- 43.Khalil RA, Morgan KG. Imaging of protein kinase C distribution and translocation in living vascular smooth muscle cells. Circ Res. 1991;69:1626–1631. doi: 10.1161/01.res.69.6.1626. [DOI] [PubMed] [Google Scholar]
- 44.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 45.Lin AL, Shain SA. Estrogen-mediated cytoplasmic and nuclear distribution of rat cardiovascular estrogen receptors. Arteriosclerosis. 1985;5:668–677. doi: 10.1161/01.atv.5.6.668. [DOI] [PubMed] [Google Scholar]
- 46.Dan P, Cheung JC, Scriven DR, Moore ED. Epitope-dependent localization of estrogen receptor-alpha, but not -beta, in en face arterial endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1295–1306. doi: 10.1152/ajpheart.00781.2002. [DOI] [PubMed] [Google Scholar]
- 47.Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg. 2010;51:972–981. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malorni W, Straface E, Matarrese P, Ascione B, Coinu R, Canu S, Galluzzo P, Marino M, Franconi F. Redox state and gender differences in vascular smooth muscle cells. FEBS Lett. 2008;582:635–642. doi: 10.1016/j.febslet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Straface E, Vona R, Gambardella L, Ascione B, Marino M, Bulzomi P, Canu S, Coinu R, Rosano G, Malorni W, Franconi F. Cell sex determines anoikis resistance in vascular smooth muscle cells. FEBS Lett. 2009;583:3448–3454. doi: 10.1016/j.febslet.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 50.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR. Selective estrogen receptor-alpha and estrogen receptor-beta agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1486–1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montgomery S, Shaw L, Pantelides N, Taggart M, Austin C. Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol. 2003;139:1249–1253. doi: 10.1038/sj.bjp.0705368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203–1208. doi: 10.1124/jpet.104.082867. [DOI] [PubMed] [Google Scholar]
- 53.Cruz MN, Agewall S, Schenck-Gustafsson K, Kublickiene K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis. 2008;196:49–58. doi: 10.1016/j.atherosclerosis.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 54.Luksha L, Kublickiene K. The role of estrogen receptor subtypes for vascular maintenance. Gynecol Endocrinol. 2009;25:82–95. doi: 10.1080/09513590802485038. [DOI] [PubMed] [Google Scholar]
- 55.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 17beta-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 1995;294:625–635. doi: 10.1016/0014-2999(95)00602-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhang F, Ram JL, Standley PR, Sowers JR. 17beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994;266:C975–980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]
- 58.Landers JP, Spelsberg TC. New concepts in steroid hormone action: transcription factors, proto-oncogenes, and the cascade model for steroid regulation of gene expression. Crit Rev Eukaryot Gene Expr. 1992;2:19–63. [PubMed] [Google Scholar]
- 59.Schnoes KK, Jaffe IZ, Iyer L, Dabreo A, Aronovitz M, Newfell B, Hansen U, Rosano G, Mendelsohn ME. Rapid recruitment of temporally distinct vascular gene sets by estrogen. Mol Endocrinol. 2008;22:2544–2556. doi: 10.1210/me.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doan T, Farmer P, Cooney T, Ali MS. Selective down-regulation of angiotensin II receptor type 1A signaling by protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells. Cell Signal. 2004;16:301–311. doi: 10.1016/j.cellsig.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Ishihata A, Tasaki K, Katano Y. Involvement of p44/42 mitogen-activated protein kinases in regulating angiotensin II- and endothelin-1-induced contraction of rat thoracic aorta. Eur J Pharmacol. 2002;445:247–256. doi: 10.1016/s0014-2999(02)01790-9. [DOI] [PubMed] [Google Scholar]
- 62.Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 2007;105:74–81. doi: 10.1254/jphs.fp0070770. [DOI] [PubMed] [Google Scholar]
- 63.Rocic P, Jo H, Lucchesi PA. A role for PYK2 in ANG II-dependent regulation of the PHAS-1-eIF4E complex by multiple signaling cascades in vascular smooth muscle. Am J Physiol Cell Physiol. 2003;285:C1437–1444. doi: 10.1152/ajpcell.00075.2003. [DOI] [PubMed] [Google Scholar]
- 64.Sayeski PP, Bernstein KE. Signal transduction mechanisms of the angiotensin II type AT(1)-receptor: looking beyond the heterotrimeric G protein paradigm. J Renin Angiotensin Aldosterone Syst. 2001;2:4–10. doi: 10.3317/jraas.2001.007. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki H, Motley ED, Frank GD, Utsunomiya H, Eguchi S. Recent progress in signal transduction research of the angiotensin II type-1 receptor: protein kinases, vascular dysfunction and structural requirement. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:305–322. doi: 10.2174/156801605774322355. [DOI] [PubMed] [Google Scholar]
- 66.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97:829–836. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]