Abstract
Saccadic eye movements rapidly orient the line of sight towards the object of interest. Pre-motor burst neurons (BNs) controlling saccades receive excitation from superior colliculus and cerebellum, but inhibition by omnipause neurons (OPNs) prevents saccades. When the OPNs pause, BNs begin to fire. It has been presumed that part of the BN burst comes from post-inhibitory rebound (PIR). We hypothesized that in the absence of prior inhibition from OPNs there would be no PIR, and thus the increase in initial firing rate of BNs would be reduced. Consequently, saccade acceleration would be reduced. We measured eye movements and showed that sustained eye closure, which inhibits the activity of OPNs and thus hypothetically should weaken PIR, reduced the peak velocity, acceleration, and deceleration of saccades in healthy human subjects. Saccades under closed eyelids also had irregular trajectories; the frequency of the oscillations underlying this irregularity was similar to that of high-frequency ocular flutter (back-to-back saccades) often seen in normal subjects during attempted fixation at straight ahead while eyes are closed. Saccades and quick phases of nystagmus are generated by the same pre-motor neurons, and we found that the quick-phase velocity of nystagmus was also reduced by lid closure. These changes were not due to a mechanical hindrance to the eyes, because lid closure did not affect the peak velocities or accelerations of the eyes in the “slow-phase” response to rapid head movements of comparable speeds to those of saccades. These results indicate a role for OPNs in generating the abrupt onset and high velocities of saccades. We hypothesize that the mechanism involved is PIR in pre-motor burst neurons.
Keywords: Omnipause neurons, Burst neurons, Oscillations, Ballistic movement, Post-inhibitory rebound
1. Introduction
Rapidly reorienting the line of sight towards an object of interest with saccades is a vital visual-motor behavior. Close correlations between the activity of pre-motor burst neurons within the pontine reticular formation and the duration and velocity of horizontal saccades suggest that excitatory burst neurons (EBNs) provide the saccadic drive to the agonist eye muscles and inhibitory burst neurons (IBNs) inhibit the drive to the antagonist muscles (Keller, 1974; Scudder, Fuchs, & Langer, 1988; Strassman, Highstein, & McCrea, 1986; Van Gisbergen, Robinson, & Gielen, 1981). EBNs for horizontal saccades project to the ipsilateral abducens nucleus, and IBNs for horizontal saccades project to the contralateral abducens nucleus (Buttner-Ennever & Buttner, 1988; Hikosaka, Igusa, Nakao, & Shimazu, 1978; Scudder et al., 1988; Strassman et al., 1986), facilitating Sherrington’s second law of reciprocal innervation. Furthermore, the IBNs ipsilateral to the movement inhibit the contralateral EBNs and IBNs. Thus, IBNs must reciprocally inhibit each other. At the end of a saccade, neurons in the ipsilateral deep cerebellar nuclei fire, exciting the contralateral IBNs. They help stop the saccade by inhibiting the excitatory drive for saccades coming from the ipsilateral side. The reciprocal inhibition between IBNs on both sides produce a pre-motor circuit that is inherently unstable (Ramat, Leigh, Zee, & Optican, 2005; Shaikh et al., 2007, 2008).
Omnipause neurons (OPNs) are also important for the control of saccades. OPNs send inhibitory projections to the EBNs and IBNs. When stable fixation is desired OPNs are constantly active, but the tonic activity of the OPNs ceases just before and during saccades (Cohen & Henn, 1972; Keller, 1974; Luschei & Fuchs, 1972). OPNs resume firing at the end of the saccade, but many of them do not turn on until after saccade ends. Thus, the end of the saccade may be determined by both the IBNs and OPNs. The sudden removal of inhibition by the OPNs from the burst neurons could facilitate a rebound increase in the firing rate of the burst neurons – post-inhibitory rebound (PIR) (Enderle & Engelken, 1995; Miura & Optican, 2006; Ramat et al., 2005; Shaikh et al., 2007). PIR is presumed to be a major contributor to the early acceleration of saccades: it would ensure a rapid increase in burst neuron discharge at the onset of saccades, which in turn would lead to the strong contraction of the eye muscles that is necessary to overcome the viscous forces in the orbit hindering rotation of the globe.
In non-human primates pharmacological inactivation of the pontine raphe interpositus nucleus, where OPNs reside, leads to slow saccades with irregular trajectories but normal latencies (Kaneko, 1996; Soetedjo, Kaneko, & Fuchs, 2002). Based on simulations of a conductance-based computational model of saccadic burst neurons Miura and Optican (2006) attributed the slow saccades to reduced inhibitory neurotransmission mediated by glycine, and the normal latencies to a neuronal threshold that kept burst neurons from responding to small inputs. In the model of Miura and Optican (2006), lower glycine levels reduced PIR by reducing low-threshold calcium currents, and also reduced NMDA currents, because glycine is the NMDA co-agonist along with glutamate (Miura & Optican, 2006). Sustained inhibition of OPNs (i.e., in absence of OPN induced inhibition of the burst neurons) slows saccades, so to maintain accuracy saccade duration must increase. This might allow more time for reciprocally innervated IBN circuits time to oscillate and create high-frequency ocular flutter even when saccades are slow (i.e., small, back-to-back saccades without an intersaccadic interval) (Ramat et al., 2005; Shaikh et al., 2007, 2008; Zee & Robinson, 1979).
These observations motivate the hypothesis that transient inhibition of OPNs could affect saccades in several ways: (1) by reducing peak acceleration and peak velocity, due to the lack of an abrupt increase of the EBN firing rate in the absence of PIR; (2) reducing deceleration, since OPNs cannot turn on toward the end of a saccade to help stop it; and (3) adding oscillations during the saccades.
Here we tested the effects of eye closure on saccades made by healthy human subjects. In non-human primates inhibition of OPN activity has been reported during transient eyelid closures or blinks (Mays & Morrisse, 1995). The same probably happens in human subjects, because high-frequency ocular flutter has been noted during sustained eye closure and blinks (Hain, Zee, & Mordes, 1986; Shaikh et al., 2007). The relationship between closing the lids and OPN inhibition gave us the opportunity to investigate the effect of OPN inhibition on saccades. We recorded eye movements in healthy subjects during fixation and saccades under (actively) closed eyelids.
2. Methods
Fifteen healthy subjects (age range: 25–65 years; four women and 11 men) were studied. Written informed consent was obtained. The study was approved by The Johns Hopkins Institutional Review Board.
2.1. Experimental setup
In eleven subjects, horizontal and vertical eye movements were recorded with scleral annuli (Chronos Vision, Berlin, Germany) using the magnetic-field, search coil system (Robinson, 1963). During the experiment the subject’s head was stabilized with a bite-bar. Raw search coil signals were sampled at 1000 Hz with 12 bit resolution and filtered in hardware (90-Hz low-pass Butterworth). The data were further processed and analyzed with custom software prepared in Matlab.
In four subjects, eye movements were recorded with infrared oculography using an Ober saccadometer (Ober Consulting, Poland). The apparatus normally tracks the limbus, but when the eyes are closed it can track the corneal bulge. This system averages the position of both eyes producing a conjugate trace. The voltages generated by the infrared sensor were digitized with a sampling rate of 1000 Hz. In vivo calibration was performed prior to each experimental session.
Three eye movement paradigms were used. Visually-guided saccade paradigm: The room was dark except for a laser target. The subjects looked at a target that was projected straight ahead or at 5°, 10°, 20° or 30° to the right and left. Saccades to the remembered location of a target: This paradigm was different from the traditional memory-guided saccades. The subjects were asked to make saccades in complete darkness (without a target that disappears prior to a saccade, as in a traditional memory-guided saccade paradigm). The approximate amplitude (estimated by the subjects, based on the remembered target locations from the preceding visually-guided saccade paradigm) was 10° and 20° to the right and left of straight ahead. Some saccades were randomly made from the remembered right or left targets, rather than from straight ahead, resulting in amplitudes up to 50°. Saccades under closed eyelids: Subjects performed the same eye movements as in the remembered saccade task, but under actively closed eyelids.
The vestibulo-ocular reflex (VOR) was recorded in 10 subjects seated in a completely dark room, first with the eyes open and then with the eyes closed. Passive rotations were either manually imposed, high-velocity, horizontal head impulses or motorized, constant-velocity chair rotations (60°/s). Weissman and colleagues (1989) reported that eye closure reduces the VOR gain during passive, constant velocity rotations. They suggested that the reduction in gain was related to reduced arousal under closed eyelids, because they also noticed that the VOR gain with eyes closed increased with vocalization (Weissman, DiScenna, Ekelman, & Leigh, 1989). In our experiments, we attempted to keep the subjects alert by asking them to constantly imagine a central target during head impulses, and by frequently alerting them verbally.
2.2. Analysis of saccades and VOR
The variables of interest were the amplitude, peak velocity, peak acceleration, and peak deceleration of saccades or VOR slow phases during head impulses. Eye position was differentiated and smoothed with a Savitzky-Golay filter (polynomial order: 3; frame length: 21) to compute eye velocity. Acceleration was also computed by differentiating and smoothing eye velocity with the same filter. A velocity criterion (40°/s) was used to determine the start and the end of visually-guided saccades. However, because of the irregular trajectory and variable velocity and acceleration profiles, the onset and offset of the remembered saccades (with eyes open and closed) were interactively confirmed by visual inspection. The start was when eye position shifted 2° away from the steady baseline; the end was when eye position reached the new baseline. VOR responses were identified interactively by using the eye and head position traces. VOR gain was calculated as peak eye velocity/peak head velocity or peak eye acceleration/peak head acceleration. Matlab® toolboxes were used for the statistical analyses and curve fitting.
2.3. Analysis of eye oscillations
Eye oscillations were best characterized on the eye velocity trace. Oscillations were separated interactively. Cycle-by-cycle analysis was performed as follows. The offset was removed from the data by subtracting the mean velocity from the actual velocity (i.e., adjusted velocity = actual velocity − mean velocity). This process allowed the velocity to align on the abscissa with the peaks of the cycles remaining positive and the troughs negative. The point of intersection of the velocity data trace to the zero line, moving from the negative value to the positive value with the abscissa was recorded. The ‘x’ coordinate of these values was called a positive zero-crossing. The cycle duration was calculated from the distance between two positive zero-crossings. The inverse of the cycle duration yields the cycle frequency.
2.4. Analysis of quick phases of nystagmus
Constant velocity (60°/s), on-axis, en bloc chair rotations were used to evoke pre- and post-rotatory nystagmus in three healthy subjects. These experiments were performed in a completely dark room; the experiments were repeated twice – first to measure nystagmus with eyes open and then with the eyes actively closed. Quick phases of nystagmus were interactively selected from the eye position trace.
3. Results
3.1. Saccades: representative traces
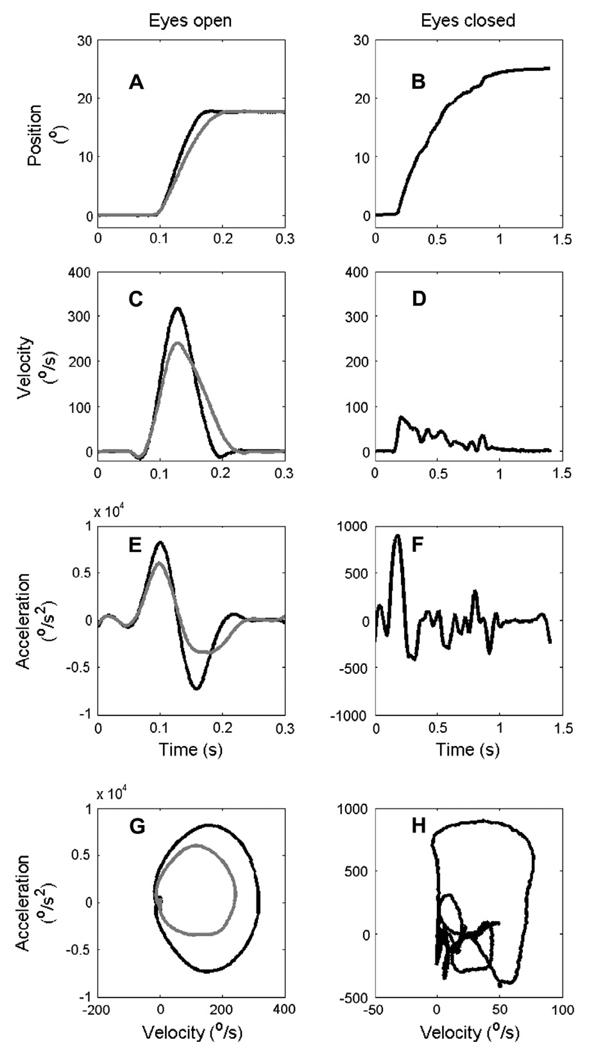
Fig. 1A illustrates visually- and memory-guided saccades in a representative subject. In this example, the peak eye velocity during a visually-guided saccade, 316°/s, was moderately reduced to 235°/s when a saccade of similar amplitude was made to the remembered location of the target with eyes open (Fig. 1C). A reduction in the saccade velocity to the remembered location of targets is a well known phenomenon, presumably related to reduction in pre-motor saccade-related activity in the superior colliculus (Edelman & Goldberg, 2001). Fig. 1B shows a memory-guided saccade with eyes closed (note different time scales in left and right columns). The peak velocity of the saccade under closed eyelids was strikingly diminished to 71°/s (Fig. 1D). Peak acceleration and deceleration during the visually-guided saccade (8156°/s2 and −7273°/s2, respectively) were larger than for the saccade made to the remembered locations (5961°/s2 and −3377°/s2, respectively; Fig. 1E). There was a further, more substantial, reduction in the peak acceleration and deceleration when the saccade was made under closed eyelids (Fig. 1F; acceleration: 853°/s2; deceleration: −427°/s2).
Fig. 1.
Examples of a representative saccade from one subject. (A and B) Eye positions (ordinate) are plotted versus time (abscissa). (A) Black trace represents visually-guided saccade and gray trace the remembered saccade. (B) Saccade made under closed eyelids. (C) Eye velocities of visually-guided saccades (black) and of saccades to the remembered location (gray) are plotted versus time. (D) Eye velocity of saccade under closed eyelids is plotted versus time. (E and F) Eye acceleration (and deceleration) during a visually-guided saccade, saccade to a remembered location, and saccade under closed eyelids are plotted versus time. Notice the fivefold greater time scale in panels B, D, and F as compared to A, C, and E. (G) Phase-plane plot comparing eye velocity with eye acceleration during visually-guided (black) and memory-guided (gray) saccades. (H) Phase-plane plot of the saccade made under closed eyelids.
The movement trajectory was irregular for saccades under closed eyelids. The irregularity of the trajectory can be clearly appreciated in the phase-plane plots (acceleration on ordinate and velocity on abscissa). For visually-guided saccades and saccades to remembered locations, the phase plane trajectory was smooth (Fig. 1G). In contrast, the trajectory became irregular for saccades under closed eyelids (Fig. 1H).
3.2. Summary for all saccades in one subject
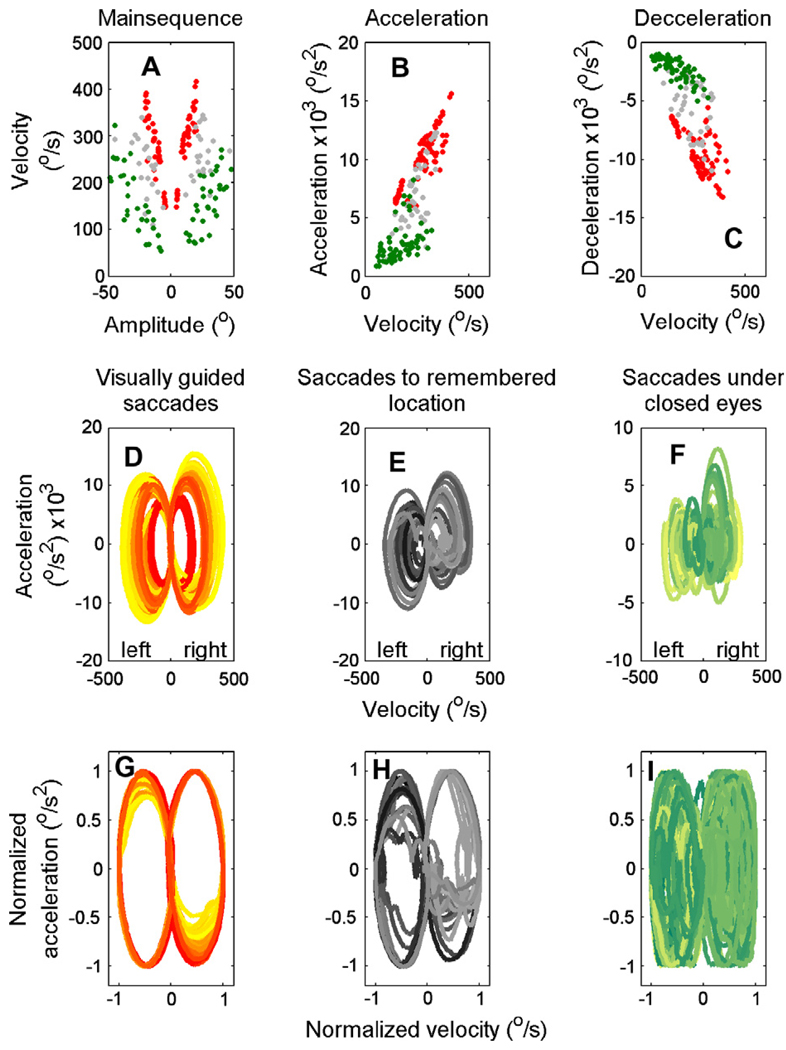
Fig. 2 summarizes the metrics (or main sequence) of all saccades (visually guided, memory guided, and under closed eyelids) for the subject whose data from a single saccade is shown in Fig. 1. Fig. 2A shows the peak velocity versus amplitude relationship for each type of saccade (visually-guided saccades, red symbols; memory-guided saccades with eyes open, gray symbols and saccades under closed eyelids, green symbols). Saccades under closed eyelids have much lower peak eye velocities than visually-guided saccades and saccades to remembered locations with eyes open. The difference was quantified further by fitting the data points during each of the three conditions with a power law function between amplitude (A) and peak velocity (Vp)1 depicting the main-sequence relationship. The slopes and the intercept of the best fit line to the data in log–log coordinates during all three conditions were calculated. Higher saccade velocities would yield a steeper slope and vice versa. The slope (i.e., the power law’s exponent) for the visually-guided saccades was 2.3, for the saccades to the remembered locations 1.8, and for the saccades under closed eyelids 1.5.
Fig. 2.
An example of all visually-guided saccades, saccades to the remembered locations, and saccades under closed eyelids in one subject. (A) An example of the main-sequence relationship for horizontal visually-guided saccades (red symbols), saccades to the remembered location (gray symbols), and saccades under closed eyelids (green symbols). Each symbol represents one saccade. Peak saccade velocity is plotted along the ordinate, saccade amplitude along the abscissa. Values of peak acceleration (B) and deceleration (C) are plotted versus the corresponding saccade velocity (abscissa). The phase plane trajectories depicting the relationship between eye velocity and acceleration are plotted separately for the three conditions (D–F). Lighter shades of color depict smaller amplitudes of saccades, with increases in the amplitude the color shade gets darker. Phase-plane plots comparing the velocity normalized to its peak value and the corresponding acceleration normalized to its peak value for the three conditions in panels G–I. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Likewise, peak acceleration and deceleration of the visually-guided saccades were also reduced during saccades to the remembered locations and even more so during saccades under closed eyelids (Fig. 2B and C). There was a linear relationship between acceleration (or deceleration) and saccade velocity. The slopes of the linear fit for the saccade to the visual target and to the remembered saccade were 0.04 and 0.02, respectively. There was a further reduction in the slope to 0.01 when the subject made saccades under closed eyelids. The slopes of the fitted linear function in deceleration phase were 0.03 (visually-guided saccades), 0.02 (saccades to remembered target), and 0.01 (saccade under closed eyelids).
The effect of eye closure on the trajectories of the eye movements during all saccades is shown in the phase plane trajectories plotted separately for the three conditions in Fig. 2D–F. Since saccade amplitude determined the size of the ellipse, the phase plane trajectories of the velocities and accelerations were normalized by their corresponding peaks (Fig. 2G–I) before further analysis. The irregularity in the shape of the phase plane trajectory was quantified by fitting an elliptical function to the normalized phase-plane plot. The value of the average sum of the squared error (mSSE; sum of squared error/number of saccades) was computed to quantify the goodness-of-fit. Larger mSSE suggested more irregularity in the saccade trajectory and vice versa. The value of mSSE was 0.001 during visually-guided saccades, 0.009 for saccades to remembered locations, and 0.018 during saccades under closed eyelids.
3.3. Summary of observations in all subjects
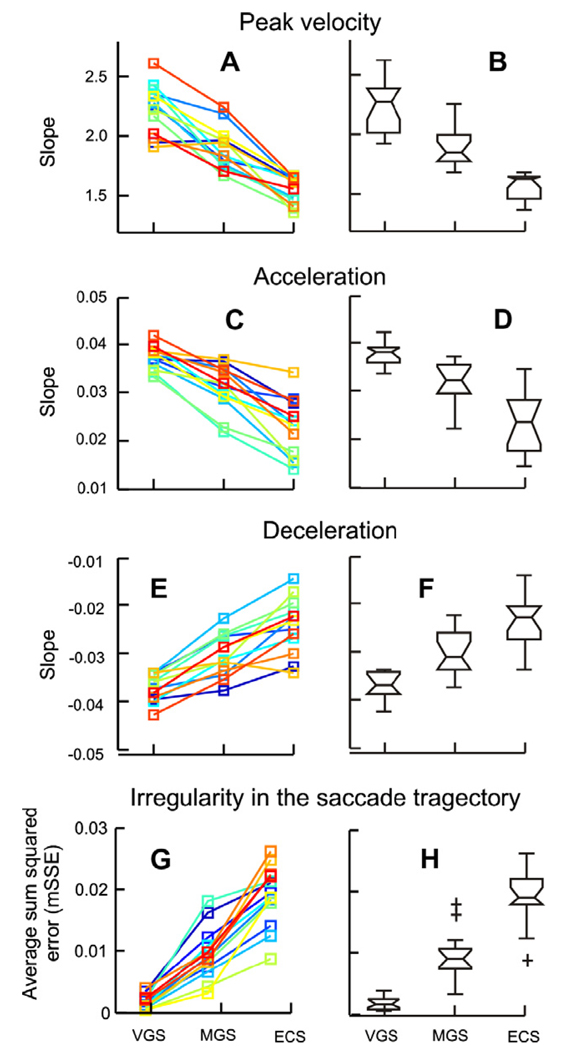
The results were consistent across all 15 subjects. Fig. 3A illustrates the slopes of the fitted main-sequence relationships. The slope of the main-sequence for saccades to remembered locations was lower than that for visually-guided saccades. There was a further decrease in the slope for the saccades made under closed eyelids. Box and whisker plots in Fig. 3B summarize the statistics; the slopes were significantly different among the three paradigms (one-way ANOVA, p < 0.001).
Fig. 3.
(A) Summary of the slopes of the fitted main-sequence equation in all subjects (visually guided saccade = VGS, memory guided saccade = MGS, saccade with eyes closed = ECS). Each of the three conditions is sorted along the x-axis and the corresponding slope is plotted along the y-axis. Each color depicts one subject. (B) Box and whisker plots summarize the slopes of the fitted main-sequence equation in all subjects. The horizontal line in the center of the notch represents the median slope; the notches represent the 95% confidence interval; the length of the box represents the inter-quartile difference; and the whiskers depict the range. Non-overlapping notches of the box-whisker plot suggest that the corresponding median values are significantly different (one-way ANOVA, p < 0.05). Plus symbols represent outliers. (C) Summary of slopes of the linear fit to the relationship between the peak acceleration and peak velocity. (E) Summary of the slopes of the linear fit to the relation between peak deceleration and the peak velocity. (D and F) Box and whisker plots representing the statistically significant effect of eye closure on the slopes. (G) Summary of goodness-of-fit of the fitted elliptical function to the phase plane trajectory during the three conditions and the summary of their statistics (H).
Fig. 3C and E summarize the slopes of the linear functions fitted through the scatter comparing peak acceleration versus peak velocity and peak deceleration versus peak velocity (e.g., Fig. 2B and C) for all subjects. Larger slopes suggest higher relative peak acceleration (or peak deceleration) and vice versa. For saccades to remembered locations the slopes were less than those for visually-guided saccades. For saccades under closed eyelids the slopes were even less (Fig. 3C and E). The reductions in the slopes were statistically significant among the three paradigms (one-way ANOVA, p < 0.01; Fig. 3D and F).
The trajectories of the saccades made under closed eyelids were consistently irregular in all subjects; the mSSE (i.e., the goodness-of-fit of the elliptical function to the phase-plane curves) are summarized in Fig. 3G and H. The mSSE was significantly increased for saccades made under closed eyelids (one-way ANOVA, p < 0.001).
3.4. Reduction in the quick-phase velocity of pre and post-rotational nystagmus under closed eyelids
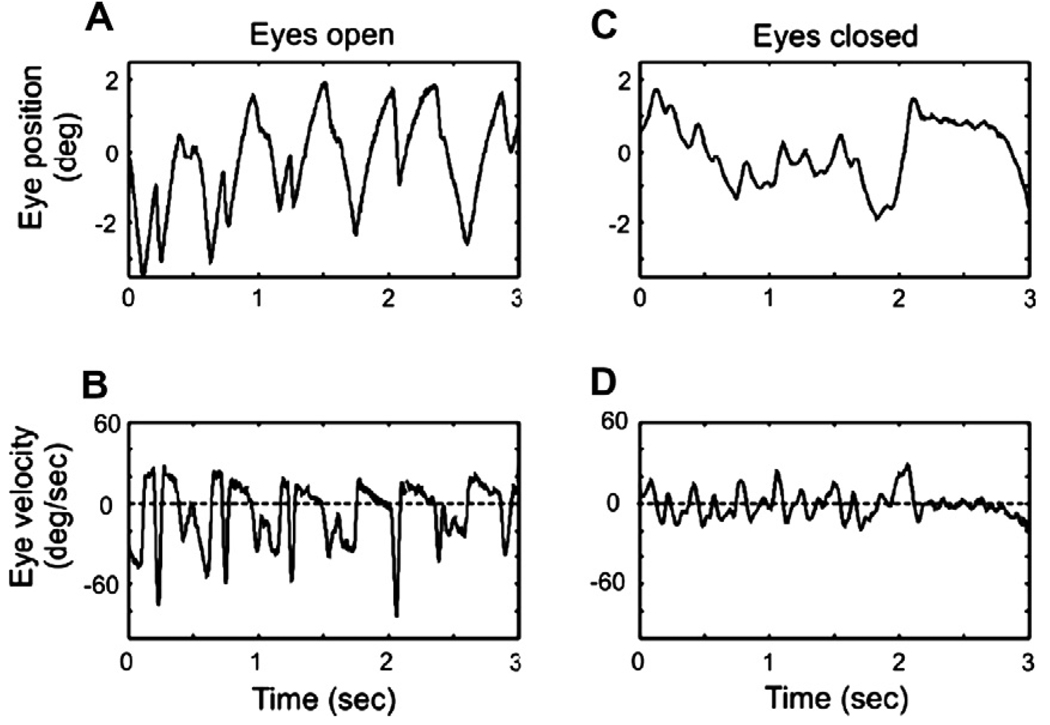
Pre-motor commands for quick phases of nystagmus and for voluntary saccades have the same neurophysiological substrate, we therefore hypothesized that eye closure would also reduce the velocity of the quick phases of nystagmus. Pre- and post-rotational vestibular nystagmus (evoked by 60°/s constant velocity step rotations in complete darkness) was recorded from three subjects, with eyes open and with eyes closed. As a consequence of the dynamic properties of the cupula-endolymph system, one would anticipate decay in the slow-phase velocity of the pre- and post-rotational nystagmus over time, however, we anticipated no change in the quick-phase velocity. The goal of this analysis was to investigate the effects of eyelid closure on the quick-phase velocity. Nevertheless, we only used nystagmus quick phases that were evoked within 6 s of stimulus onset. The first 3 s of raw-data from one of the three subjects is shown in Fig. 4. Note that eye closure does not affect slow-phase velocity of the rotational nystagmus; but significantly reduces the eye velocity of quick phases. This pattern was consistently noted in all subjects.
Fig. 4.
An example of rotational nystagmus evoked during constant velocity (60°/s) steps of en bloc chair rotation in complete darkness. Eye position during rotational nystagmus, with eyes open and closed, are plotted in panels ‘A’ and ‘C’, respectively. Panels ‘B’ and ‘D’ depict the eye velocities with eyes open and closed, respectively. Peaks in the eye velocity above the dashed, zero-line (positive peaks), in this example depict slow-phase velocity; while troughs in the eye velocity below the dashed zero line (i.e., negative peaks) in this example illustrate eye velocity during quick phase. Eye closure does not affect slow-phase velocity of the rotational nystagmus (i.e., no effects on the values of the positive peaks); but significantly reduced the eye velocity of quick phase (i.e., less negative troughs when eyes are closed).
3.5. Irregularity in the saccade trajectory under closed eyelids has the same frequency as saccadic oscillations with eyes open
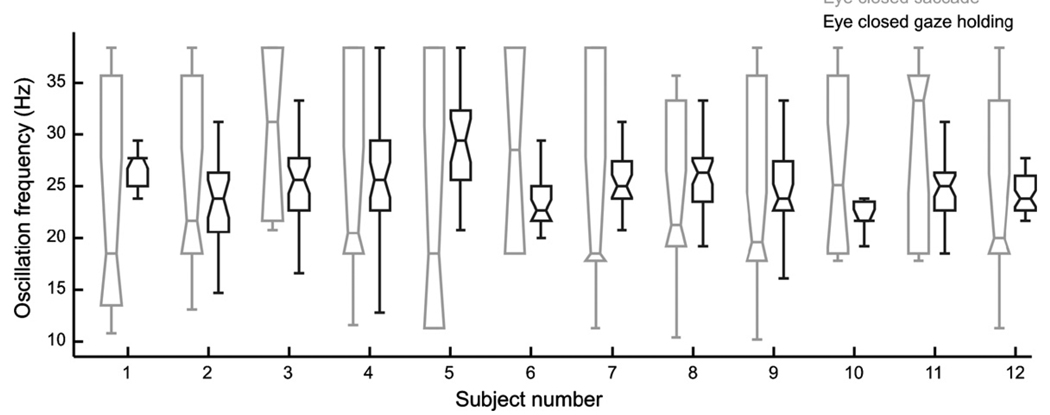
We recently proposed that sustained inhibition of OPNs can lead to disinhibition of the reciprocally innervated IBN circuit and in turn cause reverberations within that circuit. These reverberations cause ocular flutter, small back-to-back saccades without an intersaccadic interval (Shaikh et al., 2007, 2008). One possibility is that the ocular flutter induced by the OPN inhibition during slow saccades under sustained eye closure introduces irregularity in the saccade trajectory. In order to test this hypothesis we compared the oscillation frequency of the irregularity seen during slow saccades with the frequency of ocular flutter during attempted steady fixation under closed eyelids (Hain et al., 1986; Shaikh et al., 2007). For the latter, epochs of eye positions were interactively selected when the subjects attempted to hold gaze straight ahead under closed eyelids. The difference in the oscillation frequency between the two conditions was not significant. Fig. 5 illustrates the summary from all subjects. Note that the variance of oscillation frequency during slow saccades was larger than that during eye closure (note taller box length and wider notches for saccade as compared to gaze holding during eye closure).
Fig. 5.
Comparison of the frequency of saccadic oscillations seen during saccades under closed eyelids (gray box and whisker plots) with the saccadic oscillations seen during steady fixation under closed eyelids (black box and whisker plots). The analysis was performed in 12 subjects. The saccadic oscillation frequency during gaze holding was not significantly different from the frequency of oscillations during slow saccades.
3.6. What reduces the velocity of saccades under closed eyelids?
Several factors might affect the velocity of the saccades made under closed eyelids – (1) mechanical hindrance to the eye movement under closed eyelids, i.e., increased resistance to the rotating globe and the search coil, (2) slipping of the search coil annulus over the surface of the eye under closed lids or (3) a reduced neuronal drive. In order to address these possibilities we measured eye movements during passive, high-velocity, head impulses. The head impulse evokes compensatory slow-phase eye movements (vestibulo-ocular reflex; VOR) by a mechanism that involves a neural circuit that is independent of OPN and burst neurons. Therefore, if it is a mechanical hindrance to movements of the eyes under closed eyelids that reduces saccade velocities and accelerations, eye closure should also reduce the eye velocities and accelerations of the VOR during head impulses.
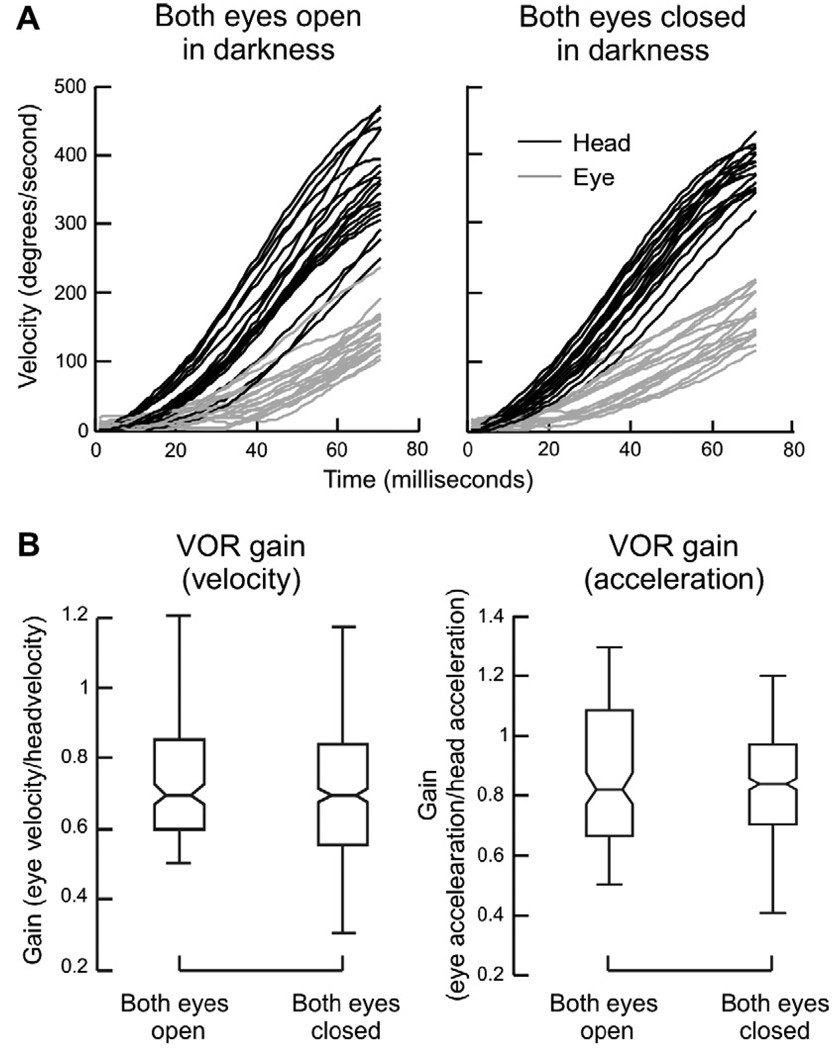
We used a range of velocities and accelerations of the head to induce compensatory slow phases of the VOR (with eyes open and closed) that were comparable to those of saccades with eyes open. Fig. 6A compares head velocity and the corresponding compensatory eye velocity during head impulses delivered in complete darkness while eyes were open, with those delivered with the eyes closed. Eye velocity (gray trace) and head velocity (black trace) are plotted along the y-axis; time is plotted along the x-axis. Notice, in contrast to saccades under closed lids, there is no irregularity in the trajectory of the VOR even when head impulses are delivered with eyes closed (Fig. 6A). Eye closure did not affect the gain of the VOR (computed as peak eye velocity/peak head velocity or peak eye acceleration/peak head acceleration). The box and whisker plots in Fig. 6B summarize this data for all subjects. There was no significant difference in VOR gain (one-way ANOVA, p > 0.05). Therefore, any mechanical hindrance to the movement of the eyes or to slipping of the search coil annulus over the surface of eye is unlikely to account for reduction of saccade velocity under closed eyelids.
Fig. 6.
The VOR elicited by head impulses delivered in complete darkness when the eyes were open and closed. (A) An example of eye and head velocities from one subject. To facilitate the comparison, the sign of the eye velocity is reversed. Velocity is plotted along the y-axis and time on the x-axis. Gray lines represent eye velocity, black represent head velocity. (B) Box and whisker plots summarize the VOR gain (eye velocity/head velocity as well as eye acceleration/head acceleration) in all subjects. Horizontal line in the center of the box-whisker plot represents median gain, the notches represent 95% confidence interval, the length of the box represents the inter-quartile difference, and the whiskers represent the range.
4. Discussion
Pre-motor excitatory burst neurons within the brainstem fire at high rates (up to 1000 spikes/s) to ensure that saccades rotate the globe at the high speeds necessary to rapidly change the line of sight. Reciprocal innervation, with a strong burst of firing in excitatory burst neurons projecting to the agonist muscles, and in inhibitory burst neurons projecting to the antagonist muscles, helps maximize the ability of the saccadic system to overcome the orbital viscous forces and rapidly move the eyes to the desired orientation. The EBNs and IBNs are driven by input from the superior colliculus and cerebellum, but a post-inhibitory rebound (PIR) mechanism could help ensure faster accelerations and higher peak velocities. Note that PIR alone is not sufficient, because OPNs affect both ipsi- and contralateral burst neurons equally. The drive from the SC and cerebellum to the ipsilateral burst neurons breaks the symmetry in the system, because the ipsilateral IBNs inhibit the contralateral burst neurons. Hence, whichever side receives the larger input dominates. Although PIR has not been reported in brainstem burst neurons, experimental lesions of the OPN area (Kaneko, 1996; Soetedjo et al., 2002) and models of ocular oscillations suggest its presence (Enderle & Engelken, 1995; Miura & Optican, 2006; Shaikh et al., 2007, 2008).
Experimental lesions, created by the injection of neurotoxins in the brainstem areas where OPNs are located cause slow saccades (Kaneko, 1996; Soetedjo et al., 2002). Although Kaneko (1996) did not report irregularity in the trajectory of these slow saccades, nor the appearance of oscillations when monkeys were fixing upon targets, the recordings of Soetedjo et al. (2002) do show irregularities in saccade trajectory (evident in velocity traces during deceleration, their Fig. 9). Slow saccades during eye blinks were noted in a number of other studies (Gossens & Van Opstal, 2000; Rambold, Sprenger, & Helmchen, 2002; Rottach, Das, Wohlgemuth, et al., 1998).
In our data, we noticed a dramatic decrease in the peak velocity, peak acceleration, and peak deceleration of the saccades made under closed eyelids as compared to when the eyes were open in darkness. We also noticed that the velocity of the quick phases of nystagmus, which are generated by the same pre-motor neurophysiological mechanism as voluntary saccades, also decreased with eye closure. These observations support the hypothesis that, in humans, long-term inhibition of OPNs reduce the strength of firing in burst neurons and consequently reduce the peak acceleration and the velocity of the saccade. The acceleration at the onset of the saccade is determined, at least in part, by the abruptness with which the excitatory and inhibitory burst neurons begin to fire. If the OPNs are already partially or fully off before the saccade is initiated, PIR would theoretically be weakened (Enderle & Engelken, 1995; Miura & Optican, 2006), which would reduce the abruptness with which the burst neurons begin to fire. Consequently, the peak acceleration of the saccade would be reduced. At the end of the saccade the contralateral IBNs normally resume firing abruptly, and OPNs turn on to inhibit the EBN discharge, and so aid the deceleration of the eyes (Lefevre, Quaia, & Optican, 1998; Quaia, Lefevre, & Optican, 1999). Of course, the above explanations for reduced acceleration and deceleration are hypothetical and awaiting experimental confirmation.
Saccade slowing might also be caused by a reduction of activity in superior colliculus neurons during movements in the dark, to remembered targets, and when the eyelids are closed. Closing the eyes with the orbicularis oculi muscle may even inhibit SC activity. Furthermore, sustained contraction of the orbicularis oculi may cause the globe to retract (by co-contracting the extraocular muscles, as humans have no retractor bulbi muscles). Such a co-contraction effect should show up as slowing in both saccades and head impulses. In our results, however, only saccades were affected.
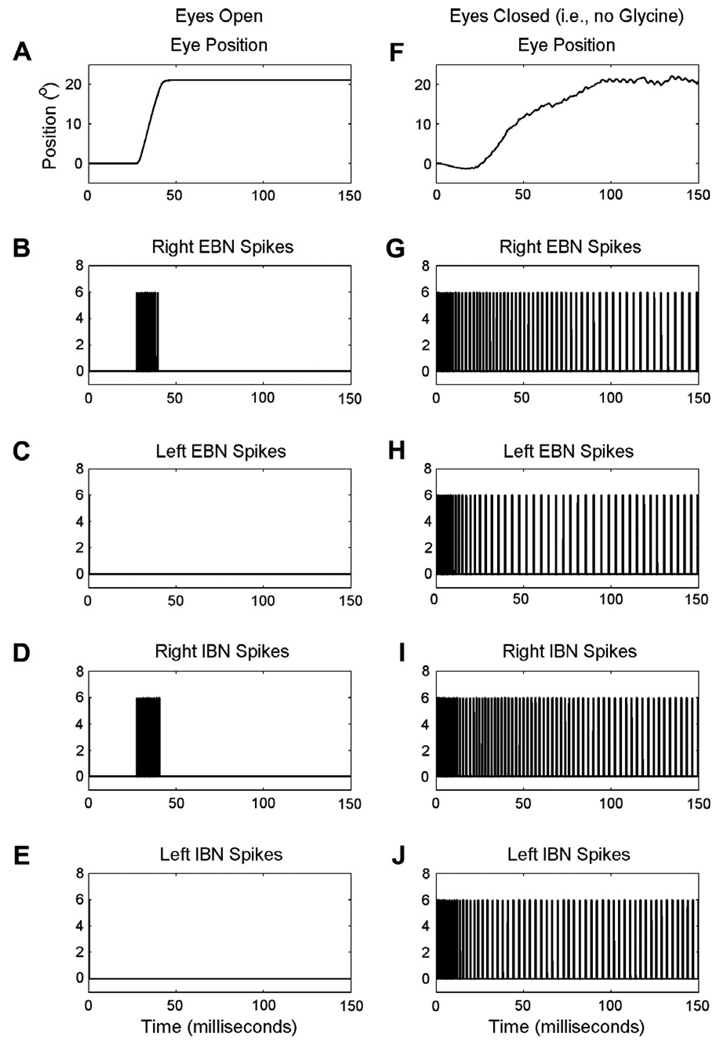
The removal of OPN inhibition could also cause disinhibition of the burst neurons and allow reverberations within the reciprocally innervated IBN circuit. We recently reported that sustained eye closure causes eye oscillations in the range of 20–40 Hz (Shaikh et al., 2007). A similar type of oscillation was seen in the irregularity of the trajectory of the eye movement when the saccades were made under closed eyelids. In support of this explanation, the frequency of the oscillations causing this irregularity is similar to that of high-frequency eye oscillations during eye closure while simply fixating and not attempting a change in gaze (Shaikh et al., 2007). Such oscillations are not seen during normal saccades (i.e., saccades made when eyelids are open) because during normal saccades the ipsilateral EBNs and IBNs are firing strongly and thus the contralateral IBNs are shut down and cannot oscillate. This phenomenon is clearly observed in the model simulations below (Fig. 8).
Fig. 8.
Simulation of the correlation of the dynamics of eye movements during normal (left panels) or slow (right panels) saccades with the firing activity in the right and left excitatory and inhibitory burst neurons. A and F: eye positions are plotted on the y-axis, time (milliseconds) on the x-axis. B–E and G–J: the remaining panels represent the spike trains of the excitatory and inhibitory burst neurons (EBNs and IBNs). Note that with the eyes open, only the right side burst neurons fire. When the eyes are closed, the lack of OPN-derived glycine allows cells on both sides to fire.
Here we emphasize that the proposed mechanisms for slow saccades and oscillations (irregular trajectory) are quite distinct from each other. We assume that the long-term loss of OPN inhibition reduces the hyperpolarization of the pre-motor burst neurons, thus reducing or eliminating PIR of the EBN. Thus, when the saccade occurs it is slower. However, because the ipsilateral IBN starts firing, the contralateral IBN gets inhibited (the contralateral IBN was not inhibited prior to this as there was no OPN activity). The contralateral IBN begins to fire with PIR with a drop in ipsilateral IBN activity. This in turn provides inhibition to the ipsilateral IBN, hyperpolarizing it. When the contralateral IBN slows down, the ipsilateral IBN can burst again. This cycle can be repeated, resulting in an oscillation. Thus, slow saccades are the result of the loss of OPN inhibition and in turn, the loss of PIR in the EBNs, while oscillations are the result of the loss of OPN inhibition but persistence of PIR in the reciprocally inhibited IBNs.
4.1. Model simulations
We simulated the previously reported conductance-based model of the saccade generator (Miura & Optican, 2006; Shaikh et al., 2007), which had been used to account for both slow saccades after the OPN lesions and saccadic oscillations. The OPNs send glycinergic inhibitory projections to the burst neurons (Horn, Buttner-Ennever, Suzuki, & Henn, 1995). The effect of sustained OPN inhibition was simulated by reducing the glycinergic output from the modeled OPN; no further changes were made to the model. The details of the model simulations and methodology were previously reported (Miura & Optican, 2006; Shaikh et al., 2007, 2008).
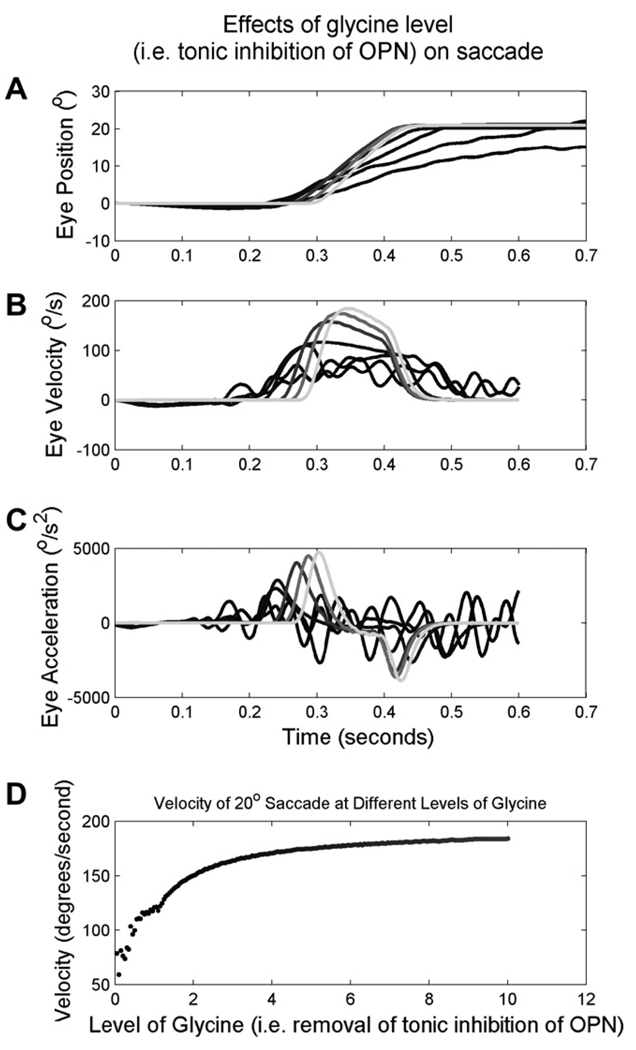
Fig. 7 illustrates the results of a simulated 20° saccade at different levels of glycinergic inhibition (intensity encoded by the shades of grey). Simulations of normally functioning OPNs and normal levels of glycine, generated saccades with normal speeds and a smooth trajectory (gray traces). A gradual reduction in glycine (i.e., turning off the inhibitory effect of modeled OPN) progressively reduces the eye velocity and introduces irregularity in the simulated saccade trajectory. Fig. 7B illustrates the velocities of the simulated saccades. The irregularity of the slow saccades is clearly seen (as high-frequency oscillations ranging from 15 to 30 Hz) in Fig. 7B. The acceleration and deceleration of the simulated saccade was reduced as the level of glycine was decreased (Fig. 7C). The model simulations also showed that any reduction in glycine levels led to slowing of the saccade. However, only after the reduction in glycine reached a certain threshold level did the eye oscillations (and irregularity in the saccade trajectory) appear. The peak velocity of the simulated saccade increased with increases in the glycine levels (Fig. 7D).
Fig. 7.
An example of a simulated saccade in presence of normal OPN activity (i.e., glycinergic inhibition) and when OPNs are inhibited (i.e., during eye closure). The level of glycine is color coded with the intensity of the gray-scale. Darker shades of the trace (black traces) illustrate reduced levels of glycine (i.e., OPN inhibition or simulating eye closure); while the lighter shades (gray traces) simulate normal conditions (i.e., simulating saccades with eyes open). Simulated eye position (A), velocity (B), and acceleration (C) are plotted on the y-axis, and time on the x-axis. (D) Illustration of the effects of the levels of glycine on peak saccade velocity. Each data point represent one saccade, peak eye velocity is plotted along the y-axis while the level of glycine during the given trial is plotted along the x-axis.
The model simulations further support the hypotheses that reduced abruptness in the change in the firing rate of the burst neurons causes reduced acceleration and deceleration; and that the irregularity in saccade trajectory is secondary to the disinhibition of the burst neurons causing reverberations in the burst generating circuit. Fig. 8A illustrates the example of a simulated normal rightward saccade. Fig. 8B and D illustrates right EBN and IBN spikes. There was a rapid change in the burst neuron firing during the saccade. When saccades were not desired, the burst neurons remained silent. This figure also shows that temporal synchrony in the firing of the EBN and ipsilateral IBN, and complete shut-off of the contralateral BNs is fundamental to prevent circuit reverberations. Fig. 8F illustrates a simulated slow saccade. As shown in Fig. 8G–J, the burst neurons were disinhibited and the abruptness of change in the firing at saccade onset was reduced compared to that for saccades shown in Fig. 8B. The reduction in the abruptness of the change in the firing was consistent with reduced acceleration and deceleration of the saccade. The reduced firing rate (i.e., spike density) was consistent with reduced saccade velocity. The disinhibition of the burst neurons and reverberations in the underlying circuit, which are evident as differences in the activity between the right and left EBN, can cause oscillations on top of an ongoing saccade and thus create the irregularity in the saccade trajectory.
4.2. Mechanisms for saccade slowing
These simulations also bear on the issue of why saccades are slower when made in the dark to remembered targets, even with eyes open. It is known that the superior colliculus (SC) output is weaker when saccades are made to remembered targets (Edelman & Goldberg, 2001). It has also been shown that firing of the OPNs slows down in darkness (Evinger, Kaneko, & Fuchs, 1982). Thus, saccade slowing during the memory-guided saccade task could be due to a reduction in SC and/or OPN output. However, reduction in SC output would only explain slowing of saccades; it would not explain ocular flutter, for which a reduction in OPN firing is necessary. Thus, our simulations suggest that reduced output from the OPN neurons plays an important role in saccade slowing.
4.3. Did mechanical hindrance by the closed eyelids affect the dynamics of the saccade?
Results in four subjects recorded with the infrared technique that followed the corneal bulge under closed lids were not different, therefore it is unlikely that any mechanical hindrance (i.e., resistance and friction) to the moving search coil (mounted on the eyeball) affected the peak eye velocity of the saccades made under closed eyelids. Furthermore, if any mechanical hindrance of the moving eyeball had reduced the eye velocity of the saccades under eye closure, the VOR gain (eye velocity/head velocity) would also have been reduced when measured under closed eyelids. However, neither the VOR acceleration nor the velocity gain was affected by eye closure. Thus, we consider it very unlikely that mechanical hindrance of the moving eyeball explains the reduced saccade velocities and accelerations observed under closed eyelids.
A few caveats and alternative mechanisms should be considered when interpreting the mechanism of saccade slowing under closed eyelids. Although in monkeys the OPNs stop during blinks, the effects of sustained eye closure (requiring the orbicularis oculi muscle) on neurons within the SC and on OPN are not known. We do not know whether or not OPNs remain completely inhibited during sustained eye closure. Turning on and off of the OPN during a saccade might lead to both saccade slowing and irregularity in the saccade trajectory. The saccade slowing during voluntary eyelid closure might also be an eyelid related suppressive effect of the superior colliculus. It is also possible that prolonged pre-motor activity in the SC before saccadic onset is transmitted downstream in a poorly synchronized fashion, because the OPN gating mechanism is turned off during eye closure. This in turn could slow saccades. Finally we emphasize the hypothetical nature of our model. Although it nicely explains many normal phenomena and our data, it cannot substitute for more definitive electrophysiological studies of neural activity within the brain stem during saccades with eyes open and eyes closed.
4.4. Implications
Our results emphasize a need for experimental electrophysiological research on pre-motor burst neurons to establish the hypothesized contribution of PIR to the control of saccades and perhaps other rapid ballistic movements. These results may also help us understand the pathophysiology of slow saccades seen in clinical disorders affecting the brainstem areas where OPNs, EBNs and IBNs are located (Leigh & Zee, 2006; Ramat, Leigh, Zee, & Optican, 2007).
Here we point out a fundamental difference between disorders that damage the pontine OPNs and/or burst neurons and those that alter the effects of inhibitory control of OPNs on the burst neurons. The disorders that reduce the effects of the inhibitory control of OPNs may cause saccadic oscillations (Shaikh et al., 2007, 2008; Zee & Robinson, 1979). In these disorders, PIR would be present, and perhaps stronger than normal (Shaikh et al., 2007). In contrast, PIR could be absent in disorders that damage (permanently) the OPNs. These disorders manifest as slow saccades. We emphasize that the lesions that affect the OPN area may also affect the burst neuron areas. Therefore, the slow saccades in these disorders may be a manifestation of a mixed deficit in OPN and burst neuron function. Indeed lesions in the OPN region have been associated with saccadic oscillations in some patients but not in others (Hormigo, Dalmau, Rosenblum, River, & Posner, 1994; Ridley et al., 1987; Schon, Hodgson, Mort, & Kennard, 2001; Wong, Musallam, Tomlinson, Shannon, & Sharpe, 2001).
Acknowledgment
This work was supported by grants from the Gustavus and Louise Pfeiffer Foundation, NIH EY01849, and Leon Levy Foundation.
Footnotes
(Vp = kAa) can be transformed for linear regression as: Log(Vp) = a * Log(A) + Log(k); where a is the slope and Log(k) is the intercept of the main-sequence in log–log coordinates.
References
- Buttner-Ennever JA, Buttner U. Neuroanatomy of the oculomotor system. The reticular formation. Reviews of Oculomotor Research. 1988;2:119–176. [PubMed] [Google Scholar]
- Cohen B, Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Research. 1972;46:403–410. doi: 10.1016/0006-8993(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Goldberg ME. Dependence of saccade-related activity in the primate superior colliculus on visual target presence. Journal of Neurophysiology. 2001;86:676–691. doi: 10.1152/jn.2001.86.2.676. [DOI] [PubMed] [Google Scholar]
- Enderle JD, Engelken EJ. Simulation of oculomotor post-inhibitory rebound burst firing using a Hodgkin–Huxley model of a neuron. Biomedical Sciences Instrumentation. 1995;31:53–58. [PubMed] [Google Scholar]
- Evinger C, Kaneko CR, Fuchs AF. Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. Journal of Neurophysiology. 1982;47:827–844. doi: 10.1152/jn.1982.47.5.827. [DOI] [PubMed] [Google Scholar]
- Gossens, Van Opstal Blink perturbed saccades in monkey. I. Behavioral analysis. Journal of Neurophysiology. 2000;83:3411–3429. doi: 10.1152/jn.2000.83.6.3411. [DOI] [PubMed] [Google Scholar]
- Hain TC, Zee DS, Mordes M. Blink-induced saccadic oscillations. Annals of Neurology. 1986;19:299–301. doi: 10.1002/ana.410190315. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Igusa Y, Nakao S, Shimazu H. Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. Experimental Brain Research. 1978;33:337–352. doi: 10.1007/BF00235558. [DOI] [PubMed] [Google Scholar]
- Hormigo A, Dalmau J, Rosenblum MK, River ME, Posner JB. Immunological and pathological study of anti-Ri-associated encephalopathy. Annals of Neurology. 1994;36:896–902. doi: 10.1002/ana.410360615. [DOI] [PubMed] [Google Scholar]
- Horn AK, Buttner-Ennever JA, Suzuki Y, Henn V. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. Journal of Comparative Neurology. 1995;359:350–363. doi: 10.1002/cne.903590212. [DOI] [PubMed] [Google Scholar]
- Kaneko CR. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. Journal of Neurophysiology. 1996;75:2229–2242. doi: 10.1152/jn.1996.75.6.2229. [DOI] [PubMed] [Google Scholar]
- Keller EL. Participation of medial pontine reticular formation in eye movement generation in monkey. Journal of Neurophysiology. 1974;37:316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Lefevre P, Quaia C, Optican LM. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Networks. 1998;11:1175–1190. doi: 10.1016/s0893-6080(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford Press; 2006. [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. Journal of Neurophysiology. 1972;35:445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Mays LE, Morrisse DW. Electrical stimulation of the pontine omnipause area inhibits eye blink. Journal of the American Optometric Association. 1995;66:419–422. [PubMed] [Google Scholar]
- Miura K, Optican LM. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. Journal of Computational Neuroscience. 2006;20:25–41. doi: 10.1007/s10827-006-4258-y. [DOI] [PubMed] [Google Scholar]
- Quaia C, Lefevre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. Journal of Neurophysiology. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Experimental Brain Research. 2005;160:89–106. doi: 10.1007/s00221-004-1989-8. [DOI] [PubMed] [Google Scholar]
- Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35. doi: 10.1093/brain/awl309. [DOI] [PubMed] [Google Scholar]
- Rambold H, Sprenger A, Helmchen C. Effects of voluntary blinks on saccades, vergence eye movements and saccade vergence interactions in humans. Journal of Neurophysiology. 2002;88:1220–1233. doi: 10.1152/jn.2002.88.3.1220. [DOI] [PubMed] [Google Scholar]
- Ridley A, Kennard C, Scholtz CL, Buttner-Ennever JA, Summers B, Turnbull A. Omnipause neurons in two cases of opsoclonus associated with oat cell carcinoma of the lung. Brain. 1987;110:1699–1709. doi: 10.1093/brain/110.6.1699. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Transactions on Biomedical Engineering. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rottach KG, Das VE, Wohlgemuth W, et al. Properties of horizontal saccades accompanied by blinks. Journal of Neurophysiology. 1998;79:2895–2902. doi: 10.1152/jn.1998.79.6.2895. [DOI] [PubMed] [Google Scholar]
- Schon F, Hodgson TL, Mort D, Kennard C. Ocular flutter associated with a localized lesion in the paramedian pontine reticular formation. Annals of Neurology. 2001;50:413–416. doi: 10.1002/ana.1140. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. Journal of Neurophysiology. 1988;59:1430–1454. doi: 10.1152/jn.1988.59.5.1430. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Miura K, Optican LM, Ramat S, Leigh RJ, Zee DS. A new familial disease of saccadic oscillations and limb tremor provides clues to mechanisms of common tremor disorders. Brain. 2007;130:3020–3031. doi: 10.1093/brain/awm240. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Ramat S, Optican LM, Miura K, Leigh RJ, Zee DS. Saccadic burst cell membrane dysfunction is responsible for saccadic oscillations. Journal of Neuro-Ophthalmology. 2008;28:329–336. doi: 10.1097/WNO.0b013e31818eb3a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko CR, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. Journal of Neurophysiology. 2002;87:679–695. doi: 10.1152/jn.00886.2000. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. Journal of Comparative Neurology. 1986;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen JA, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. Journal of Neurophysiology. 1981;45:417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- Weissman BM, DiScenna AO, Ekelman BL, Leigh RJ. Effect of eyelid closure and vocalization upon the vestibulo-ocular reflex during rotational testing. Annals of Otology Rhinology and Laryngology. 1989;98:548–550. doi: 10.1177/000348948909800710. [DOI] [PubMed] [Google Scholar]
- Wong AM, Musallam S, Tomlinson RD, Shannon P, Sharpe JA. Opsoclonus in three dimensions: Oculographic, neuropathologic and modelling correlates. Journal of the Neurological Sciences. 2001;189:71–81. doi: 10.1016/s0022-510x(01)00564-0. [DOI] [PubMed] [Google Scholar]
- Zee DS, Robinson DA. A hypothetical explanation of saccadic oscillations. Annals of Neurology. 1979;5:405–414. doi: 10.1002/ana.410050502. [DOI] [PubMed] [Google Scholar]