Abstract
Platelet-activating factor (PAF) is a potent bioactive lipid generated in the cornea after injury whose actions are mediated through specific receptors. Studies from our laboratory have shown that PAF interactions with its receptors activate several transmembrane signals involved in apoptosis. Continuous exposure to PAF during prolonged inflammation increases keratocyte apoptosis and inhibition of epithelial adhesion to the basement membrane. As a consequence, there is a marked delay in wound healing, which is not countered by the action of growth factors. While apoptosis of stroma cells is rapid and potent, epithelial cells as well as myofibroblasts, which appear in the stroma during the repair phase, are resistant to apoptosis. However, PAF accelerates apoptosis of corneal epithelial cells exposed to oxidative stress by stimulating phospholipase A2, producing an early release of cytocrome C from mitochondria and activating caspase-3. In myofibroblasts, PAF has a synergistic action with tumor necrosis factor-α (TNF-α), increasing apoptosis of the cells to 85%. PAF antagonists block the effects of PAF and could have a therapeutic role in maintaining a healthy and transparent cornea.
Keywords: Cornea, Platelet-activating factor, Wound healing, PAF antagonists, Alkali burn, Myofibroblasts, Tumor necrosis factor-α, Phospholipase A2
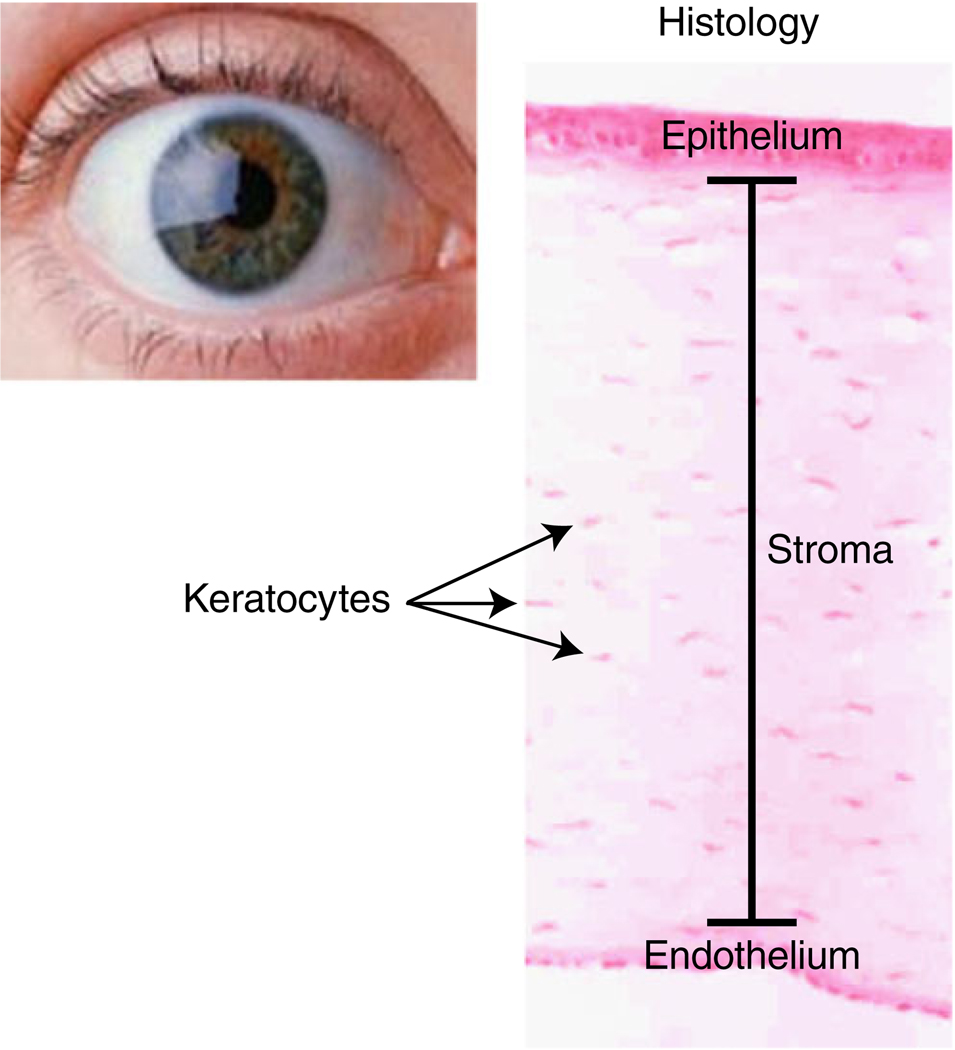
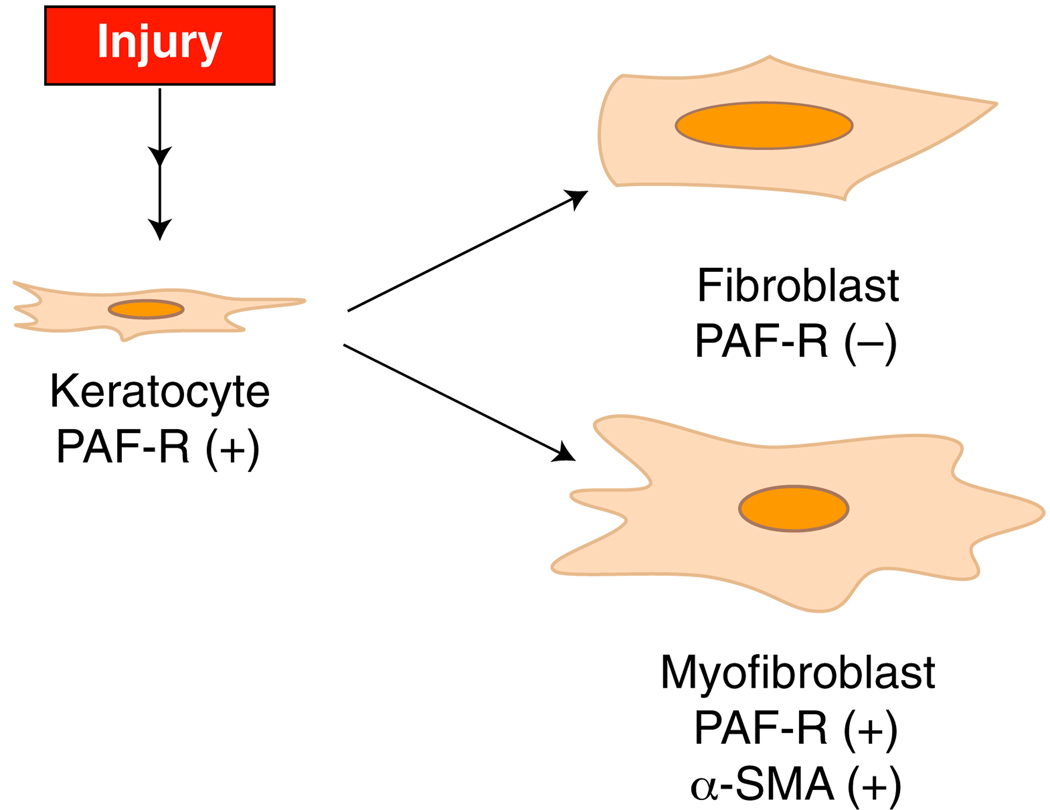
The main characteristic of the cornea is its transparency. The lack of blood elements in the tissue allows the light to pass through the cornea to the retina (Fig. 1). The cornea is the most refractive tissue in the eye and is mainly composed of three different cell layers. The first layer is the epithelium, which is the outer layer in contact with the tear film, composed of six to seven layers of cells. The epithelium, which is composed of basal cells, is actively proliferative, allowing the cornea to correct an epithelial defect very rapidly. The stroma, which is the thickest of the three layers, is composed of a mixture of collagen types I, V, VI and XII and proteoglycans possessing either keratin sulfate side chains or chondroitin–dermatan sulfate side chains [1]. The stroma has an amazing organization where the fibers are arranged in layers, forming the lamella, which maintains corneal transparency. Embedded within the lamella are the keratocytes, nearly quiescent cells that maintain collagen and other components of the extracellular matrix. In response to injury, the keratocytes become metabolically active and migrate to the damaged area, transforming into active fibroblasts and myofibroblasts (Fig. 2). Although both fibroblasts and myofibroblasts contribute to normal wound repair, myofibroblasts play a crucial role in tissue fibrosis, producing extracellular matrix components at a high rate and regulating contractile elements necessary for wound closure, thanks to the expression of α smooth muscle actin (α-SMA). Once a wound closes, myofibroblasts are no longer necessary and few, if any, myofibroblasts remain within the area. Excessive proliferation or persistent presence of these cells has been associated with pathologic fibrosis, contracture and corneal haze [2].
Fig. 1.
Diagram of the structural organization of the cornea
Fig. 2.
Transformation of stromal keratocytes after injury in fibroblasts and myofibroblasts and expression of PAF-R in the cells. α-SMA α-smooth muscle actin
Apoptosis has been suggested to play a central role in the deletion of myofibroblasts after the cornea had been repaired, but the mechanism by which myofibroblast apoptosis is regulated remains unclear. Finally, the inner-most layer is called the endothelium, a monolayer that acts as an important pumping system for regulating corneal hydration. In humans the endothelial cells have very limited mitosis, and with aging they elongate to cover the posterior part of the cornea.
The cornea is frequently exposed to injuries. The most common is dry eye, which produces desiccation and loss of epithelial cells. It is calculated that 75% of the population of those 60 years or older will suffer dry eye [3]. Infections by microorganisms, such as bacteria and viruses like the herpesvirus, are also common diseases [4]. Damage frequently occurs as a result of chemical exposure, mainly from industrial accidents. Chemical burns represent between 7% and 10% of eye injuries; of these, corneal alkali burns are the most dangerous because alkali penetrates the surface of the eye very quickly and induces a strong inflammatory reaction characterized by cell infiltration and production of proteolytic enzymes and cytokines [5]. Such injuries often lead to recurrent corneal erosions, ulceration and perforation of the eye.
Platelet-activating factor (PAF) (1-o-alkyl-2-acyl-sn-glycero-3-phosphocholine) is a bioactive lipid produced by a broad range of cells in a variety of tissues including the cornea in response to injury [6, 7]. It can cause microvascular leakage, vasodilatation and activation of several types of inflammatory cells such as neutrophils, eosinophils and macrophages [6, 8]. In the cornea, PAF stimulates several signal-transduction pathways and gene expression programs (for review, see Refs. [9, 10]). PAF exerts its actions through a seven-transmembrane G-protein-linked receptor (PAF-R) [11]. The PAF-R belongs to the family of coupled G protein receptors and is expressed in corneal epithelial cells, keratocytes and myofibroblasts, but not in fibroblasts [12, 13] (Fig. 2). We have suggested that the lack of PAF-R expression in fibroblasts could be a mechanism to avoid more inflammation during the corneal repair process. Studies using a porcine eye model with a PAF-R polyclonal antibody show that the receptor is expressed in both the plasma and nuclear membranes of myofibroblasts [13]. The PAF-R is also expressed in the limbal area of the epithelium and in the endothelium [12]. The endothelial expression of the receptor indicates that it affects some endothelial functions.
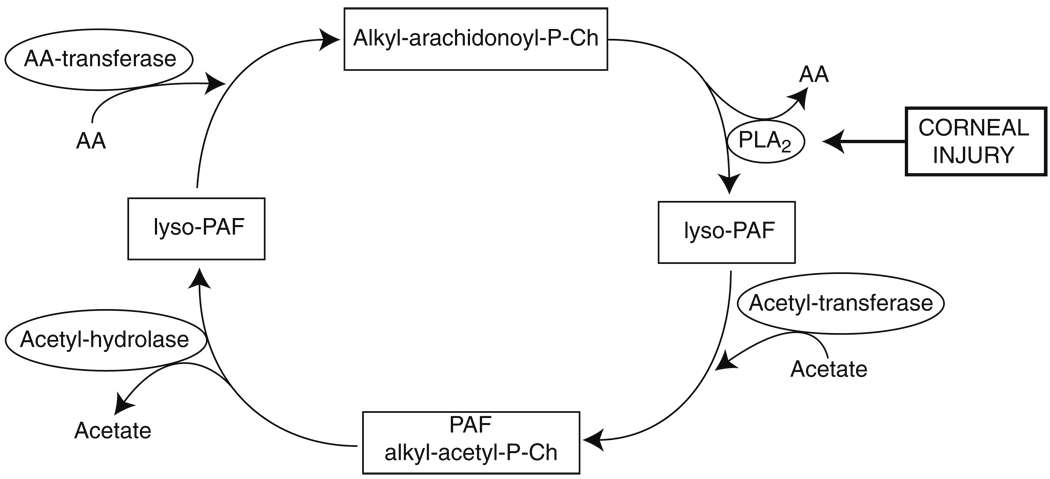
PAF synthesizes very rapidly after a corneal alkali burn and accumulates as soon as 30 min after the injury. It continues to increase in a linear fashion up to 4 h, when the contribution of PMNs arriving to the cornea increase PAF up to 24 h [7], the maximum time studied. Our results from isolated corneas stimulated in vitro with calcium ionophore A23187 suggest that PAF synthesis occurs through the remodeling pathway (Fig. 3), in which the activation of a phospholipase A2 (PLA2) mainly releases arachidonic acid (AA) from the second carbon of the glycerophospholipid alkyl-arachidonoyl-phosphorylcholine (alkyl-arachidonoyl-P-Ch). This results in the formation of the inactive compound lyso- PAF, which, by the action of an acetyltransferase, introduces an acetyl group to form PAF. PAF is degraded by acetylhydrolase, reverting again into lyso-PAF, and then by an arachidonoyl transferase is converted back to its precursor, alkyl-arachidonoyl-P-Ch. Because PAF is accumulated in vivo very soon after corneal injury [7], this lipid mediator seems to be synthesized by corneal cells and is not by recruited inflammatory cells, since these cells arrive later [5]. Moreover, if the injury causes stromal edema and endothelial damage, the amount of PAF accumulated is even greater.
Fig. 3.
Synthesis of PAF by the remodeling pathway. Corneal injury activates PLA2, which releases AA to form lyso-PAF, a biologically inactive intermediate that, by the action of an acetyltransferase, synthesizes PAF. During corneal injury, the rate of PAF synthesis is higher than the degradation and, as a consequence, PAF accumulates [7]
In this review, we summarize the actions of PAF in the cornea, particularly the ones that drive cells to apoptosis, and some of the signaling pathways involved in PAF-apoptotic response.
PAF Induces Apoptosis of Selective Stroma Cells
One of the interesting actions of PAF is its strong inhibitory action on epithelial wound healing. Our laboratory demonstrated a delay in epithelization in corneas in organ culture after injury in the presence of PAF [14]. This effect was accompanied by a strong apoptosis of stroma keratocytes. A significant number of TUNEL-positive cells were found in the stromal area, as well as in the area covering the wound. Very few TUNEL-positive cells were found in controls or in corneas incubated with PAF in the presence of PAF antagonists. To corroborate the action of the lipid mediator in the organ culture model, isolated keratocytes were incubated with PAF and 60% of cells underwent apoptosis after 6 h. The apoptosis was reversed when the PAF-R was blocked with an antagonist. PAF also caused between 35% and 56% inhibition of adhesion of epithelial cells to proteins of the extracellular matrix, such as collagen I and IV, fibronectin and laminin [14]. Lyso-PAF, the biologically inactive metabolite, does not have any effect. Our studies demonstrated that PAF plays an important role in delaying corneal wound healing by affecting adhesion of epithelial cells and increasing apoptosis of keratocytes, with consequences such as epithelial defects and, in more severe cases, corneal ulceration.
Several growth factors are known to promote corneal wound healing [15, 16]. Wound closure with growth factors such as epidermal growth factor, hepatocyte growth factor and keratinocyte growth factor in the presence of PAF is also greatly inhibited, and addition of the PAF antagonists blocks the inhibitor effect of PAF in the presence of these growth factors [14]. Further studies have shown that hepatocyte growth factor and keratinocyte growth factor increase the expression of PAF-R [12], thus promoting a feedback mechanism by which PAF can overcome the action of the growth factors in the corneal epithelium.
To study the effect of blocking PAF stimulation on corneal pathologies that affect stromal cells, a novel PAF antagonist, LAU-0901 (2,4,6-trimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid ester), was employed in two relevant models of injury.
The first model, diffuse lamellar keratitis (DLK), is an inflammatory condition associated with laser in situ keratomileusis (LASIK) surgery characterized by infiltration of inflammatory cells along the flap–stroma interface of the cornea produced during surgery. All the untreated eyes developed DLK 5 days post-LASIK surgery, while in eyes treated with a peribulbar injection of the PAF-R antagonist LAU-0901, only 30% developed a mild inflammation [17]. At 8 days, the treated eyes showed excellent transparency. TUNEL-positive cells were detected in the corneal stroma 2 days post-LASIK surgery. The apoptotic cells were located in the posterior and anterior areas to the lamellar interface created by the microkeratome. In the LAU-0901-treated corneas, very few TUNEL-positive cells were seen. Histopathological evaluation and immunohistochemical studies demonstrated that treatment with the PAF antagonist inhibits keratocyte apoptosis and chemotaxis of inflammatory cells. The inhibition of the inflammatory response induced by PAF promotes adequate healing of the flap interface and adjacent stroma after LASIK surgery.
The second model was an alkali burn produced on rabbit corneas. Chemical injuries represent almost 10% of all eye injuries treated in the emergency room, and as many as 20% of the chemical injuries result in significant visual and cosmetic disability [18]. In severe injuries, tissue granulation results in severe fibrosis and, if left untreated, can result in corneal ulcerations and even perforations of the ocular globe. Prevention of corneal ulceration will determine the prognosis for vision and retention of the globe. Current medical therapy remains controversial and an optimal procedure has not been firmly established. To assess the action of LAU-0901, rabbits with severe corneal alkali injury were treated with this antagonist for 8 weeks [19]. Controls were treated with a vehicle and all animals were followed for 8 weeks. Persistent epithelial defects were present in treated and control groups 5 days after the injury, but from day 9 onwards the average clinical scores of both epithelial defects and stromal ulcerations in the controls were significantly higher than those in the LAU-0901 treatment group. Eighteen days after injury, most of the nontreated animals showed perforation, while the animals treated with the PAF antagonist displayed intact corneas. At 4 weeks, 90% of vehicle-treated animals showed perforation, while only 20% of the LAU-0901-treated corneas showed ulceration, and none displayed perforation. Histological examination demonstrated complete re-epithelization in the treated group, with fewer inflammatory polymorphonuclear leukocytes and more repair fibroblasts (myofibroblasts) in the stroma as compared with controls.
Normally after a chemical burn, there is an intense apoptosis of keratocytes limiting the amount of cells that can transform into fibroblasts and myofibroblasts, which repopulate the stroma and promote wound healing. Our research found a significant decrease in keratocyte apoptosis in the stroma in corneas treated with the LAU-0901 [19]. In addition, the PAF antagonist increased the number of myofibroblasts in the limbal area. As mentioned, these cells are very important in maintaining the integrity of the tissue after such a severe injury; therefore, blocking the action of PAF inhibits corneal ulceration and perforation in this severe alkali-burn rabbit corneal model. In vivo experiments suggest that PAF plays a central role in apoptosis of keratocytes after injury and in the disappearance of myofibroblasts from the limbal area, which is necessary for the repair, suggesting that after such strong injury, there are not enough cells to be converted into myofibroblasts (Fig. 2). The novel PAF antagonist, LAU-0901, is able to prevent deep ulceration and perforation after a chemical burn, decrease inflammation and increase the number of myofibroblasts that can cope with the damage.
Myofibroblasts are more resistant than keratocytes to apoptosis by PAF and require higher concentration (300 nM) and longer time of incubation with the lipid mediator (24 h instead of 6 h) [13]. This may suggest that the signaling through PAF receptor is different in these two cells or that PAF requires activation of other cytokines to complement its action. This result led us to ask whether PAF is directly involved in the myofibroblast apoptosis noted in the previous experiments, or if it requires another inducer. It has been reported that PAF has synergistic or reciprocal effects when combined with known inducers of apoptosis [20, 21].
One possible inducer of apoptosis in myofibroblasts is TNF-α. This cytokine is well known for stimulating apoptosis in many cell types [22]. It is synthesized by activated macrophages, lymphocytes and neutrophils, and by non-immune cells, including corneal cells [22, 23]. TNF-α effects are mediated by TNF-RI and TNF-RII receptors. These receptors are expressed in corneal keratocytes and fibroblasts, and stimulation with TNF-α induces apoptosis in corneal fibroblasts when NF-κB activation is blocked [24]. We also found that myofibroblasts express TNFα-R1, a major signaling receptor for TNF-α [13]. In addition, Saika et al [25] demonstrated that TNF-α potentiates the undesirable, pathogenic response of wound healing in alkali-burned cornea in a mouse model, in which corneas were alkali-burned and myofibroblasts quantified at different times. After 2 weeks of injury, there were many more myofibroblasts in the TNF-α-null knockout mice than in the wild type, demonstrating the importance of TNF-α in inducing disappearance of myofibroblasts. These data are in agreement with our findings in the alkali-burn corneal model where we demonstrated the presence of myofibroblasts when animals were treated with the PAF-R antagonists, LAU-0901 [19].
Quantification of TUNEL staining in isolated myofibroblasts incubated for 24 h showed that 12% of cells enter apoptosis after PAF treatment and 28%after TNF-α (20 ng/ml) treatment [13]. A combination of both cytokines produced 77% apoptotic cells. The synergistic increase in apoptosis is through PAF-R stimulation since this cell death was inhibited by LAU-0901. Analysis by DNA laddering showed that PAF and TNF-α induced DNA fragmentation that was synergistically increased when both cytokines were added together.
Synergism between PAF and TNF-α in the response of myofibroblasts to apoptosis could suggest (a) existence of two independent pathways controlling apoptosis or (b) involvement of TNF-α in increasing PAF synthesis that amplifies the apoptotic response to TNF-α. With respect to the second possibility, there is evidence in other cell types showing that TNF-α stimulates PAF synthesis [26, 27]. However, we are more inclined to favor the first possibility since our experiments with LAU-0901 show no effect on apoptosis induced by TNF-α. In addition, several studies show that cPLA2 is a mediator in TNF-α-induced cell death [28, 29]. In the cornea, PAF is an activator of cPLA2 [30, 31]; therefore, the possibility that further increase in cPLA2 activity in the presence of PAF could amplify the response (as shown in stress-challenged epithelial cells, described below) must be considered as a mechanism that merits further study.
PAF Stimulates Epithelial Apoptosis by a PLA2 Mechanism
In the normal cornea, there is a cyclical epithelial cell loss (shedding), and a percentage of these cells shed following apoptosis [32]. Exposure to UV radiation, prolonged use of contact lenses and bacterial infections are some of the factors that increase epithelial cell apoptosis [33–35]. All these conditions have an inflammatory component; however, corneal epithelial cells are resistant to apoptosis by PAF [36]. One possible reason for this is that epithelial cells need to have some degree of injury (priming of the tissue) to be affected by PAF [37].
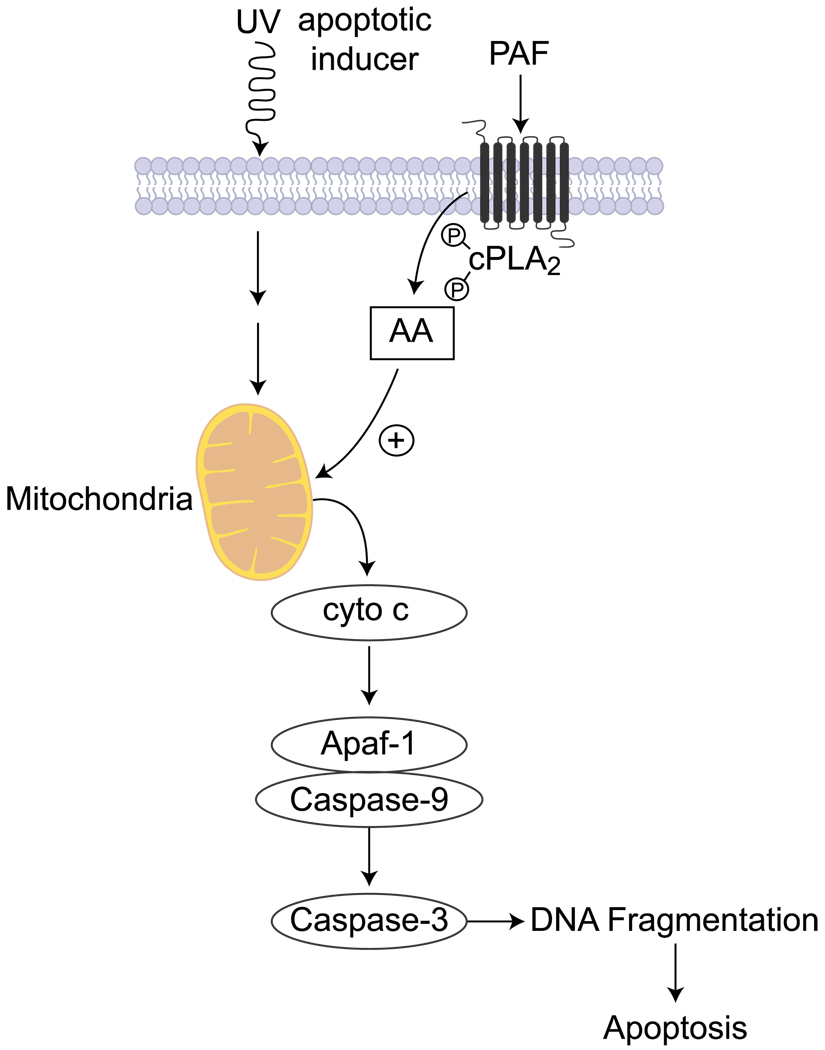
We investigated whether PAF modulates corneal epithelial cell apoptosis caused by UV radiation using rabbit corneal epithelial cells and human corneal epithelial cells exposed to UVC radiation and then to carbamyl PAF (a nonhydrozable form) for different increments in time [36]. The presence of the lipid mediator almost doubles the increase of mitochondrial cytochrome c release to the cytosol 30 min after UVC radiation. The amount increases up to 2 h, concomitant with a decrease in the mitochondria. PAF also enhances the cleavage of poly (ADP-ribose) polymerase PARP, a substrate for caspase-3, while pretreatment with the antagonists protects the cellular actions of PAF. So PAF accelerates the release of cytochrome c to the cytosol of corneal epithelial cells (Fig. 4), which drives the formation of an apoptosome, a mega complex of Apaf-1 (apoptotic protease-activating factor-1) and caspase-9. This results in caspase-9 activation, followed by a downstream activation of caspase-3, which carries the execution phase of apoptosis. The action of PAF is inhibited by LAU-0901. Also, in our experiments, no release of cytochrome c by PAF was seen in cells treated with higher concentrations of PAF (2 µM) that had not been previously irradiated, demonstrating the action of a first inducer in the effect. DNA degradation was observed in UV-irradiated cells 2 h after PAF exposure and DNA fragmentation increased in the presence of PAF at 4 and 8 h of treatment [36].
Fig. 4.
Signaling pathway of apoptosis stimulated by PAF in corneal epithelial cells. Apoptosis can be induced by UV irradiation. PAF accelerates the process of releasing cytochrome c and activating the caspase cascade that results in early DNA fragmentation and apoptosis. cPLA2 activation is involved in the process. Although the mechanism is not known, one possibility is that AA can regulate mitochondrial permeability and stimulate the release of cytochrome c. The cPLA2 inhibitor MAFP blocks the effect of PAF on the release of cytochrome c and, as a consequence, increases the time of DNA fragmentation after UV exposure [36]. Apaf-1 apoptotic protease-activating factor-1
Previous studies from our laboratory have shown that after 5 min of PAF stimulation, the cornea releases AA to the medium, most likely through cPLA2 activation [30, 31]. These observations led us to investigate the role of cPLA2 in the release of cytochrome c from mitochondria in the presence of the inhibitor methyl arachidonoyl fluorophosphate (MAFP). There was inhibition of cytochrome release in the presence of the PLA2 inhibitor [36].
The exact mechanism of the involvement of cPLA2 is yet unknown. One possibility is that release of AA by activation of the enzyme changes the mitochondria cell permeability and accelerates the release of cytochrome c induced by UV (Fig. 4). Other studies have shown involvement of a caspase-3-dependent activation of cPLA2 in apoptosis [29]. A recent study in human endothelial cells showed that pigment epithelial-derived growth factor (PEDF) induces caspase-3 activation and apoptosis that may be inhibited in the presence of MAFP and siRNA specific for cPLA2α [38], a PLA2 that releases AA from glycerolipids. Activation of caspase-3 results in cleavage and activation of cPLA2α. The investigators conducting this study also found that cPLA2α is localized in the perinuclear region and is phosphorylated by p38 MAPK when the cells are stimulated by PEDF [38]. They postulated that cPLA2α induces PPARγ-p53-mediated apoptosis. PAF also activates p38 MAPK in corneal epithelial cells; a similar mechanism could occur in these cells, but this will need to be tested in future studies.
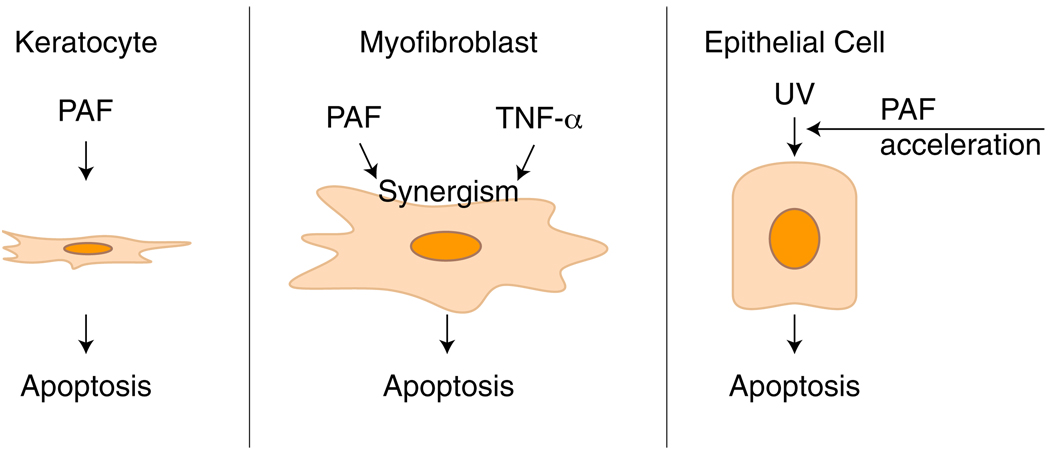
In conclusion, our work shows that the action of PAF, an inflammatory mediator released after corneal injury, is specific to corneal cell types (Fig. 5). While keratocytes enter into apoptosis in the presence of PAF, myofibroblasts act in a synergistic fashion with TNF-α, suggesting that during corneal wound healing, PAF acts in conjunction with other cytokines. As such, PAF and these cytokines may potentially play an important role in eliminating corneal stromal myofibroblasts and impairing the repair of the cornea during deep injuries that compromise the stroma. Epithelial cells, on the other hand, need to be “primed” (e.g., by UV), and PAF acts as an accelerator leading to apoptosis. Inhibition of cPLA2 activation plays a role in this apoptosis pathway, but future studies are needed to determine its role in the signaling that drives these cells to apoptosis. Finally, PAF-receptor antagonists, such as LAU-0901, may be a valuable therapeutic tool when inflammation is not controlled and when damage to the cornea compromises vision, as in alkali injuries. Future studies need to be done to determine the clinical use of this and other PAF antagonists for the treatment of eye injuries.
Fig. 5.
Scheme on the action of PAF in corneal cells. While stromal keratocytes enter into apoptosis in the presence of PAF, there is a synergistic effect in apoptosis of myofibroblasts when TNF-α is combined with PAF. Epithelial cells are resistant to PAF apoptosis, but the lipid mediator accelerates the process after UV irradiation
Acknowledgments
The studies described in this review were supported by NIH grant EY004928 and COBRE grant P20RR021970.
References
- 1.Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen type I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–1477. [PubMed] [Google Scholar]
- 2.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from physician’s health studies. Arch Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green M, Apel A, Stepleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–39. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 5.Pfister RR, Burnstein N. The alkali burned cornea: I: epithelial and stromal repair. Exp Eye Res. 1976;23:519–535. doi: 10.1016/0014-4835(76)90160-3. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–S301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 7.Bazan HEP, Reddy STK, Lin N. Platelet activating factor (PAF) accumulation correlates with injury in the cornea. Exp Eye Res. 1991;52:481–491. doi: 10.1016/0014-4835(91)90046-h. [DOI] [PubMed] [Google Scholar]
- 8.Lin N, Bazan HEP, Braquet P, Bazan NG. Prolonged effect of a new Platelet-activating factor antagonist on ocular vascular permeability in an endotoxin model of uveitis. Curr Eye Res. 1991;10:19–24. doi: 10.3109/02713689109007607. [DOI] [PubMed] [Google Scholar]
- 9.Bazan HEP, Ottino P. The role of platelet-activating factor in the corneal response to injury. Prog Retin Eye Res. 2002;21:449–464. doi: 10.1016/s1350-9462(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 10.Kenchegowda S, Bazan HEP. Significance of lipid mediators in corneal and repair. J Lipid Res. 2010;51:879–891. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumi T, Shimizu T. Platelet-activating factor receptor: gene expression and signal transduction. Biochim Biophys Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Bazan HE. Increased platelet activating factor receptor gene expression by corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2000;41:1696–1702. [PubMed] [Google Scholar]
- 13.He J, Bazan HEP. Synergistic effect of platelet-activating factor and tumor necrosis factor-α on corneal myofibroblast apoptosis. Invest Ophthalmol Vis Sci. 2006;47:883–891. doi: 10.1167/iovs.05-0581. [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekher G, Ma X, Lallier TE, Bazan HEP. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet activating factor. Invest Ophthalmol Vis Sci. 2002;43:1422–1428. [PubMed] [Google Scholar]
- 15.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotonozo C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Ret Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 16.Schultz G, Khaw PT, Oxford K, Ma Cauley S, Van Setten G, Chequini N. Growth factors and ocular wound healing. Eye. 1994;8:184–187. doi: 10.1038/eye.1994.43. [DOI] [PubMed] [Google Scholar]
- 17.Esquenazi S, He J, Bazan HEP, Bazan NG. Prevention of experimental diffuse lamellar keratitis using a novel platelet activating factor receptor antagonist. J Cataract Refract Surg. 2004;30:884–891. doi: 10.1016/j.jcrs.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald EC, Cauchi PA, Azuara-Blanco A, Foot B. Surveillance of severe chemical corneal injuries in the UK. Br J Ophthalmol. 2009;93:1177–1180. doi: 10.1136/bjo.2008.154831. [DOI] [PubMed] [Google Scholar]
- 19.He J, Bazan NG, Bazan HEP. Alkali induced corneal stromal melting prevention by a novel platelet activating factor receptor antagonist. Arch Ophthalmol. 2006;124:70–78. doi: 10.1001/archopht.124.1.70. [DOI] [PubMed] [Google Scholar]
- 20.Myers AK, Robey JW, Price RM. Relationship between tumor necrosis factor, eicosanoids and platelet-activating factor as mediator of endotoxin-induced shock in mice. Br J Pharmacol. 1990;70:449–502. doi: 10.1111/j.1476-5381.1990.tb12957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Chiarri M, Ortiz A, Lerma JL, et al. Involvement of tumor necrosis factor and platelet-activating factor in the pathogenesis of experimental nephrosis in rats. Lab Invest. 1994;154:3567–3581. [PubMed] [Google Scholar]
- 22.Ashkenazi A. Targeting death and decoy receptors of the tumor-necrosis factor superfamily. Nature Rev. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 23.He J, Ichimura H, Iida T, et al. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV infection. J Interferon Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- 24.Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF alpha induced apoptosis in corneal fibroblasts by transcription factor NF-KappaB. Invest Ophthalmol Vis Sci. 2000;41:1327–1336. [PubMed] [Google Scholar]
- 25.Saika S, Ikeda K, Yamanaka O, Flanders KC, Okada Y, Miyamoto T, Kitano A, Ooshima A, Nakajima Y, Ohnishi Y, Kao WW. Loss of tumor necrosis factor-α potentiates transforming growth factor β-mediated pathogenic tissue response during wound healing. Am J Pathol. 2006;168:1848–1860. doi: 10.2353/ajpath.2006.050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gountopoulou A, leondaritis G, Galanopoulou D, Mavri-Vavayanni M. TNFalpha is a potent inducer of platelet-activating factor synthesis in adipocytes but not in preadipocytes. Differential regulation by PI3K. Cytokine. 2008;41:174–181. doi: 10.1016/j.cyto.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Sun X-M, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988;81:1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voelkel-Johnson C, Thorne TE, Laster SM. Susceptibility to TNF in the presence of inhibitors of transcription or translation is dependent on the activity of cytosolic phospholipase A2 in human melanoma tumor cells. J Immunol. 1996;156:201–207. [PubMed] [Google Scholar]
- 29.Wissing D, Mouritzen H, Egeblad M, Poirier GG, Jaatela M. Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor induced apoptosis. Proc Natl Acad Sci. 1997;94:5073–5077. doi: 10.1073/pnas.94.10.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst JS, Bazan HE. Activation of the phospholipase/cyclooxygenase cascade in the rabbit cornea by platelet activating factor is challenged by PAF receptor antagonists. Ocul Pharmacol Ther. 1995;11:329–337. doi: 10.1089/jop.1995.11.329. [DOI] [PubMed] [Google Scholar]
- 31.Hurst JS, Bazan HEP. Platelet activating factor preferentially stimulates the phospholipase A/cyclooxygenase cascade in the rabbit cornea. Curr Eye Res. 14:769–775. doi: 10.3109/02713689508995798. [DOI] [PubMed] [Google Scholar]
- 32.Ren H, Wilson G. The cell shedding of the corneal epithelium-a comparison of collecting methods. Curr Eye Res. 1996;15:1054–1059. doi: 10.3109/02713689609017655. [DOI] [PubMed] [Google Scholar]
- 33.Pauloin T, Dutot M, Joly F, Warnet JM, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human corneal epithelial cells. Mol Vis. 2009;15:577–583. [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Zhang X, Liu M, Zhang J, Ye L, Lin Y, Luyckx J, Qu J. Trehalose protects against surface disorders in experimental murine dry eye through suppression of apoptosis. Exp Eye Res. 2009;89:311–318. doi: 10.1016/j.exer.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JL, Wang J, Dong Z, Mian S, Yu FS. Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells. Invest Opthalmol Vis Sci. 2004;45:2569–2576. doi: 10.1167/iovs.03-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Bazan HEP. Platelet activating factor (PAF) enhances apoptosis induced by ultraviolet radiation in corneal epithelial cells through cytochrome c-caspase activation. Curr Eye Res. 2001;23:326–335. doi: 10.1076/ceyr.23.5.326.5445. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Hsueh W, Torre-Amione G. Effects of in vivo “priming” on endotoxin-induced hypotension and tissue injury. The role of PAF and tumor necrosis factor. Am J Pathol. 1990;136:949–956. [PMC free article] [PubMed] [Google Scholar]
- 38.Ho TC, Chen SL, Yang YC, Lo TH, Hsieh JW, Cheng HC, Tsao YP. Cytosolic phospholipase A2-{alpha} is an early apoptotic activator in PEDF-induced endothelial cell apoptosis. Am J Physiol Cell Physiol. 2009;296:C273–C284. doi: 10.1152/ajpcell.00432.2008. [DOI] [PubMed] [Google Scholar]