Abstract
Objective
To compare body mass index (BMI), percent body fat (PBF), and fat mass index (FMI) and to investigate the accuracy of FMI as a convenient tool for assessing obesity.
Design
Anthropometric measurements and bioelectrical impedance analyses were performed on 538 Mexican Americans (373 women and 165 men). Correlations between BMI and PBF and between FMI and PBF were investigated. The percentage of persons misclassified as obese using different classifications was calculated. Multiple linear regression analysis was performed to generate predictive models of FMI for males and females separately.
Results
BMI and PBF were correlated in men (rho = 0.877; p < 0.0001) and women (rho = 0.966; p < 0.0001); however, 20% and 67.2% of the men and 9.2% and 84.2% of women, classified as normal weight and overweight by BMI, respectively, were diagnosed as obese by PBF. FMI and PBF were also correlated in men (rho = 0.975; p < 0.0001) and women (rho = 0.992; p < 0.0001). Four percent of the men classified as normal weight and 65.5% classified as overweight by BMI were obese by FMI, while 71.3% of women classified as overweight by BMI were obese by FMI. Misclassification of obesity between FMI and PBF categories was observed in 5.4% of men and 7.8% and women.
Conclusions
The discrepancy observed between BMI and PBF reflects a limitation of BMI. Conversely, FMI accurately assessed obesity in our study of Mexican Americans, but further studies are necessary to confirm our findings in different ethnic groups.
Keywords: Fat mass index, body composition, obesity
Introduction
In recent decades, there has been an alarming increase in the rates of overweight and obesity on a global scale (World Health Organization, 2000). Due to the health risks associated with obesity, efforts have been made to properly quantify body fat in individuals and in different populations (Stein and Colditz, 2004). Body adiposity cannot be measured directly, and although there are numerous techniques for accurately assessing total body fat such as underwater weighing (UWW) (Segal et al., 1988) and dual energy X-ray absorptiometry (DEXA) (Lohman et al. 2000; Kontogianni et al., 2005) these methods are not practical for use in large epidemiological studies.
Body mass index (BMI) which is defined as weight in kilograms divided by height in meters squared, is commonly used to determine overweight and obesity in clinical and field research settings (World Health Organization, 2000; Stein and Colditz, 2004; Snijer et al., 2006; McTigue et al., 2003). However, BMI does not distinguish between lean and fat body mass (Stein and Colditz, 2004; Frankenfield et al., 2001; Snijder et al., 2006; Peltz et al. 2007). Various methods that are simple to perform have been used to estimate body adiposity. For instance, skinfold thickness measurement, a method that assesses body fatness through the use of calipers at particular body sites, has showed a strong correlation with body adiposity measured by reference methods (Heimmel et al., 2007; Nooyens et al., 2007; Webber et al., 1994; Chumlea et al., 2000; Pollock and Jackson, 1984; Lohman, 1981; Martin et al., 1985). By using generalized equations for men and women, skinfold measures can predict estimates of body fat from body density (Jackson and Pollock, 1985; Durnin and Womersley, 1974; Brozek et al., 1963; Siri, 1993). Limitations of this method include reproducibility, inter- and intra-individual variation (Martin et al., 1985; Himes et al., 1979), and the difficulty in obtaining accurate measures in very obese subjects (Webber et al., 1994; Chumlea and Guo, 2000; Mei et al., 2002; Bray et al., 1978). In addition, site-specific selection for skinfold thickness measurements based on sex has been recommended (Nooyens et al., 2007; Kagawa et al., 2007; Marini et al., 2007; Lee and Nieman, 2002).
The distribution of body fat is also a well-established predictor of cardiovascular disease, metabolic disturbances, certain cancers, and premature mortality (Snijder et al., 2005; Zhang et al., 2007; Zhu et al., 2003a, Calling et al. 2006; Sanchez-Castillo et al., 2003; van der Brandt and Goldbohm, 2006). There are sex differences between fat depots, as women have greater abdominal subcutaneous fat and far less intra-abdominal fat than men (Schreiner et al., 1996). Waist circumference (WC) and waist-to-hip ratio (WHR) are accurate predictors of disease and have shown to be better markers of all-cause mortality than BMI (vander Brandt and Goldbohm, 2006; Calling et al., 2006; Simpson et al., 2001; Zhang et al., 2007). A limitation of this method is that established cutoff points for abdominal obesity as set out by the World Health Organization (2000) may not be appropriate for non-Caucasian or non-adult age groups (Snijder et al., 2005). Still the method is simple and easy to perform, requiring only measurements of the appropriate body sites with a non-elastic measuring tape.
Another, more sophisticated, approach to assessing body fatness is bioelectrical impedance analysis (BIA). This method is convenient for body composition analysis and is widely implemented in clinical and research settings (Pietrobelli et al., 2004; Ellis et al., 1999; Sun et al., 2003). Impedance is the frequency-dependent opposition of a conductor to the flow of an alternating electric current. Resistance is the pure opposition of the conductor to the alternating current, and reactance is the dielectric component of impedance (Chumlea and Sun, 2005). Estimates of total body water (TBW), fat-free mass (FFM) and fat mass (FM) can be made using predictive equations that include impedance values (Ellis et al., 1999; Kyle et al., 2004). The percentage of body fat (PBF) can then be calculated using FM and body weight.
BIA has been validated as a measure of body adiposity when compared to reference methods such as UWW and DEXA (Segal et al., 1988; Pietrobelli et al., 2004; Sun et al., 2003; Kyle et al., 2001). Although BIA has been shown to be a suitable, efficient, and safe method for clinical and field epidemiologic research (Kontogianni et al., 2005; Frankenfield et al., 2001; Peltz et al., 2007; Sun et al., 2003; Barbosa Silva, et al., 2005; Heymsfield et al., 1997), one of the major limitations of this method is that it requires predictive equations for the determination of FFM that are specifically developed for different populations, ethnicities, age groups, and both sexes (Peltz et al., 2007; Chumlea et al., 2000; Kyle et al., 2001; Heymsfield et al., 1997).
There is still a need for an even more simplified approach to assessing body fat content in large epidemiological studies and in the clinical setting as well. BMI is routinely and very conveniently used, requiring only height and weight to calculate, but the categorization of obesity is broad and imprecise. On the other hand, BIA is considered to be reliable but not very convenient. Although BIA is portable and less costly than DEXA and UWW, the logistics of the procedure must be planned in advance. Different types of BIA analyzers are available and certain devices are multi-frequency whereas others operate by single-frequency. Single-frequency instruments operate at 50 kHz, and although multi-frequency instruments provide a better estimate of intracellular (ICW) and extracellular (ECW) water, an improvement in body composition estimates with the use of multi-frequency instruments when compared to single-frequency instruments has not been consistently shown (Dittmar and Reber, 2001; Simpson et al., 2001). Nonetheless, multi-frequency analyzers are more useful in research and clinical settings involving end-stage renal disease and dialysis evaluation (Chumlea and Sun, 2005). Some instruments are tetrapolar and require subjects to lie down on an examining bed or stand on a scale whereas some are octapolar and require subjects to grasp grips in each hand while standing on a scale. Results of some BIA instruments are presented as TBW, FFM, and FM based on proprietary, built-in predictive equations that preclude the investigator from using equations that better fit the specific population under study. In addition to logistics, BIA requires dietary restrictions that if not strictly adhered to will produce imprecise results.
A potential indicator of body adiposity that may prove to be highly useful in field research is the fat mass index (FMI). This index was first introduced in a study involving nutritional assessment (VanItallie, 1990) and is calculated by taking the body fat mass component from BIA and dividing by height squared. This study examines the relationship among BMI, PBF, and FMI in a population of Mexican American college students and proposes FMI cut-off points for this population. In addition, we developed a predictive model of FMI that can be used in field studies requiring body composition assessment in Mexican American young adults without the need for sophisticated equipment or techniques subject to operator error. Although it can be argued that FMI, BMI, and PBF are composed of the same variables and are one and the same, they each categorize overweight and obesity differently. BMI takes into account body weight and body height while FMI requires information on body weight, body height, and fat mass content. Height is positively correlated with weight, although for calculation of BMI and FMI, this confounding variable is removed (Wells et al., 2007). Therefore, the comparison between BMI and FMI is directly influenced by fat mass content, which is the desired variable to be measured in obesity studies. On the other hand, in studies on body composition, FMI has shown superiority over PBF since PBF is not corrected by height (VanItallie, 1990). Because FMI takes height into account, it reduces the bias associated with PBF.
Materials and Methods
Subjects
Participants for this study consisted of a convenience sampling of 538 Mexican American college students (373 women and 165 men) attending the University of Texas at Brownsville & Texas Southmost College (UTB/TSC) and recruited from September 2004 through December 2005. Volunteers were recruited through classroom presentations and flyers posted throughout campus by research staff. Subjects were defined as Mexican Americans by using self-reported ancestry information. A participant was eligible for enrollment, if all four grandparents were of Mexican ancestry. The sole criterion for exclusion of a participant was pregnancy. The response rate was 90% among those who indicated they were interested in participating in the study. The study protocol was approved by the UTB/TSC Institutional Review Board. A signed written informed consent was required from all participants before participating in the study. All anthropometric measurements and BIA were performed in duplicate during weekdays from 7:30 to 10:30 AM at the Student Health Services, the UTB/TSC campus student health clinic, by trained research staff. Participants were asked to refrain from eating, drinking, and exercising for 6 hours prior to being examined and were also asked to abstain from consuming alcohol within 48 hours or diuretics within 7 days of their appointment at the clinic. Only those individuals who reported good health and followed the dietary recommendations participated in this study, while those who did not follow protocol were rescheduled. Women who perceived they were retaining fluids due to their menstrual cycle did not undergo testing. Participants were asked to urinate within 30 minutes of the measurements.
Weight and height measurements
Body weight in kilograms and body height in meters were measured for each subject while they wore an examining gown and no shoes. Body weight was measured to the nearest 0.1 kilogram with portable electronic digital scales (Tanita BWB-800S, Arlington Heights, IL). Body height was measured using a vertical wall-mounted stadiometer (Tanita HR-100), Arlington Heights, IL) and was recorded to the nearest 0.001 meter. BMI was calculated as weight in kilogram divided by height in meters squared.
Waist circumference and waist-to-hip ratio
Waist circumference (WC) and hip circumference (HC) were taken with a non-elastic tape measure in meters. WC was measured at the smallest circumference between the costal margin and the iliac crest, and HC was measured at the widest circumference between the waist and the thigh. Waist-to-hip ratio (WHR) was calculated as WC divided by HC.
Bioelectrical impedance analysis
A BIA analyzer (BIA Quantum II, RJL, Systems, Inc., Detroit, MI) was used to measure resistance (R) and reactance (Xc) at 50 kHz frequency at controlled room temperature. Subjects were placed in a supine position with arms and legs abducted approximately 45° to each other assuring no contact between the thighs and between the arms and the trunk. Shoes and socks were removed, and contact areas were scrubbed with alcohol immediately before electrode placement. Source electrodes were placed proximal to the metacarpo-phalangeal joint on the dorsal surfaces of the right hand and distal to the transverse arch on the superior surface of the right foot. Sensor electrodes were placed at the midpoint between the styloid processes and between the medial and lateral malleoli on the right ankle. R and Xc were recorded to the nearest ohm (Ω). The following FFM predictive equations validated for Mexican Americans (Sun et al., 2003) were applied to individual BIA resistance data in order to estimate FFM for each subject:
Men: FFM = -10.68 + (0.65 height2) / resistance + (0.26 weight) + (0.02 resistance)
Women: FFM = -9.53 + (0.69 height2)/ resistance + (0.17 weight) + (0.02 resistance)
where FFM is measured in kilogram, height2 / resistance in 0.01 m2 / Ω, and resistance in Ω. FM and percent body fat (PBF) were calculated as follows:
FM (kg) = body weight (kg) − FFM (kg)
PBF = [FM (kg) / body weight (kg)] × 100.
Definitions of obesity
A BMI of 25-29 kg/m2 was considered overweight, and a BMI ≥ 30 kg/m2 was considered obese for both men and women (World Health Organization, 2000). In agreement with previous studies, a PBF of ≥ 25% in men and ≥ 35% in women was considered obese (Deurenberg, et al., 1998; World Health Organization, 1995; De Lorenzo, et al., 2003). ROC analyses were performed to generate FMI cutoffs for men and women, and cut-off values of ≥ 6.6 kg/m2 in men and ≥ 9.5 kg/m2 in women were found to be indicative of obesity. Values of WC ≥ 1.02 m (102 cm) in men and ≥ 0.88 m (88 cm) in women indicate abdominal obesity (World Health Organization, 2000).
Statistical analysis
All statistical analyses were performed using SPSS, version 16.0. The distribution of all variables was tested using the One-Sample Kolmogorov-Smirnov Test. Since the majority of variables were non-normally distributed, summary statistics are presented as median and interquartile range (IQR). Statistical analyses are presented by sex and include the Mann-Whitney U test and chi-square test for comparing the medians between groups. Spearman correlation coefficients (rho) were used to examine the correlations between BMI, PBF, and FMI. Multiple linear regression analysis was performed with FMI as the dependent variable to create predictive models of FMI for Mexican Americans. The following independent variables were assessed: age, WC, BMI, BMI2, HC, and WHR. Analyses were performed separately for men and women because of the higher fat mass at any given height seen in women compared to men. The coefficients of determination (R2) were reported as a measure of the proportion of variability of FMI explained by the independent variables. To test whether the equations adequately predicted FMI in our entire study sample, we randomly split the dataset into two in order to (a) derive predictive equations in one dataset and (b) predict FMI in the other dataset. Statistical significance was set using a type I error level of 0.05.
Results
From Table 1, the median age in men and women was 22.0 years. Men were taller, weighed more, had greater FFM, and had slightly greater average BMI than women. Both men and women were below the cutoff points for abdominal obesity. Women had significantly greater PBF (35.6% versus 27.1%, p<0.0001) and FMI (9.0 versus 7.3, p<0.0001) than men. BMI distribution was relatively similar in men, with the lowest percentage in the normal weight category (30.0%), while in women the highest percentage was found in the normal weight category (46.6%). PBF cutoffs placed 64.2% of the men and 53.5% of the women in the obese category, while FMI cutoffs showed that 58.8% of the men and 46.7% of the women were obese.
TABLE 1.
Comparison of men and women for anthropometric measures1
| Men (n=165) | Women (n=373) | P-value | |
|---|---|---|---|
| Median (IQR) Number (%) |
Median (IQR) Number (%) |
||
| Age (years) | 22 (9.0) | 22 (9.0) | NS |
| Height (m) | 1.729 (.080) | 1.60(0.085) | <0.0001 |
| Weight (kg) | 79.8 (27.5) | 64.5 (21.4) | <0.0001 |
| WC (m) | 0.90 (0.195) | 0.808 (0.182) | <0.05 |
| HC (m) | 1.022 (0.14) | 1.022 (0.152) | NS |
| WHR | 0.8693 (0.0911) | 0.7843 (0.0957) | <0.001 |
| FFM (kg) | 59.2 (11.7) | 41.9 (7.7) | <0.0001 |
| FM (kg) | 22.2 (14.1) | 22.8 (14.9) | NS |
| BMI (kg/m2) | 27.3 (7.2) | 25.4 (8.2) | <0.05 |
| Normal | 50 (30.0) | 174 (46.6) | <0.001 |
| Overweight | 58 (35.0) | 101 (27.1) | <0.01 |
| Obese | 57 (35.0) | 98 (26.3) | <0.05 |
| PBF (%) | 27.1 (9.2) | 35.6 (11.5) | <0.0001 |
| Non-obese | 59 (35.8) | 173 (46.5) | <0.001 |
| Obese | 106 (64.2) | 199 (53.5) | <0.001 |
| FMI (kg/m2) | 7.3 (4.5) | 9.0 (5.9) | <0.0001 |
| Non-obese | 68 (41.2) | 202 (54.3) | <0.001 |
| Obese | 97 (58.8) | 170 (46.7) | <0.01 |
Differences between men and women using Mann-Whitney U Test for continuous variables and chi-square test for categorical variables.
BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio; FFM, fat-free mass; FM, fat mass; PBF, percent body fat; FMI, fat mass index; IQR, interquartile range; NS, not statistical significant.
As seen in Table 2, among men, correlation coefficients between BMI categories and PBF decrease as BMI categories increase (rho = 0.644 in normal weight, rho = 0.516 in overweight, and rho = 0.492 in obese categories) (p < 0.0001). In women, the correlation was strongest in the normal weight category (rho = 0.890), slightly weaker in the obese category (rho = 0.819) and weakest in the overweight category (rho = 0.715) (p < 0.0001). In addition, correlation coefficients between BMI and PBF across all BMI categories were higher in women than in men. Among men and women correlation coefficients between FMI and PBF were relatively similar (rho = 0.926 in normal weight and rho = 0.914 in obese categories in men, and rho = 0.978 in normal weight and rho = 0.961 in obese categories in women) (p < 0.0001), with women appearing to have a slightly higher correlation in the obese category than men.
TABLE 2.
Spearman correlation coefficients between BMI or FMI and PBF
| Men1 | Women2 | |||
|---|---|---|---|---|
| rho | P | rho | P | |
| BMI | ||||
| Overall | 0.877 | <0.0001 | 0.966 | <0.0001 |
| Normal | 0.644 | <0.0001 | 0.890 | <0.0001 |
| Overweight | 0.516 | <0.0001 | 0.715 | <0.0001 |
| Obese | 0.492 | <0.0001 | 0.819 | <0.0001 |
| Men | Women | |||
| rho | P | rho | P | |
| FMI | ||||
| Overall | 0.975 | <0.0001 | 0.992 | <0.0001 |
| Non-obese 1 | 0.926 | <0.0001 | 0.978 | <0.0001 |
| Obese 2 | 0.914 | <0.0001 | 0.961 | <0.0001 |
FMI non-obese cut-off in men <6.6; obese ≥6.6
FMI non-obese cut-off in women <9.5; obese ≥9.5
Table 3 shows that the percentage of men classified by BMI as being normal body weight and as overweight but classified as obese by PBF was 20.0% and 67.2%, respectively. In women, 9.2% and 84.2% of those classified by BMI as being normal body weight and as overweight, respectively, were found to be obese by PBF. In both sexes, 100% of those classified as obese by BMI were also obese by PBF. When comparing BMI and FMI categories, 4% and 65.5% of the men in the normal and overweight BMI categories, respectively, were obese by FMI, whereas 71.3% of the women in the overweight BMI category were obese by FMI. In men, 5.4% classified as non-obese by FMI were classified as obese by PBF, and in women, 7.8% classified as non-obese by FMI were classified as obese by PBF (data not shown).
TABLE 3.
Number and percentage of participants obese by PBF and FMI in each BMI category
| Men | Women | |||
|---|---|---|---|---|
| BMI (kg/m2) | Number obese by PBF |
Percentage obese by PBF |
Number obese by PBF |
Percentage obese by PBF |
| Normal | 10 | 20.0% | 16 | 9.2% |
| Overweight | 39 | 67.2% | 85 | 84.2% |
| Obese | 57 | 100.0% | 98 | 100.0% |
| Number obese by FMI |
Percentage obese by FMI |
Number obese by FMI |
Percentage obese by FMI |
|
| Normal | 2 | 4% | 0 | 0% |
| Overweight | 38 | 65.5% | 72 | 71.3% |
| Obese | 57 | 100.0% | 98 | 100.0% |
BMI shows a slightly curvilinear relationship with PBF (rho =0.877, p < 0.0001), and a more linear relationship with FMI (rho = 0.956, p < 0.0001) in men (Figure 1). The curvilinear relationship between BMI and PBF is more accentuated in women than in men (rho = 0.966, p < 0.0001), with BMI showing a distinct linear relationship with FMI (rho = 0.990, p < 0.0001) (Figure 2).
FIGURE 1.
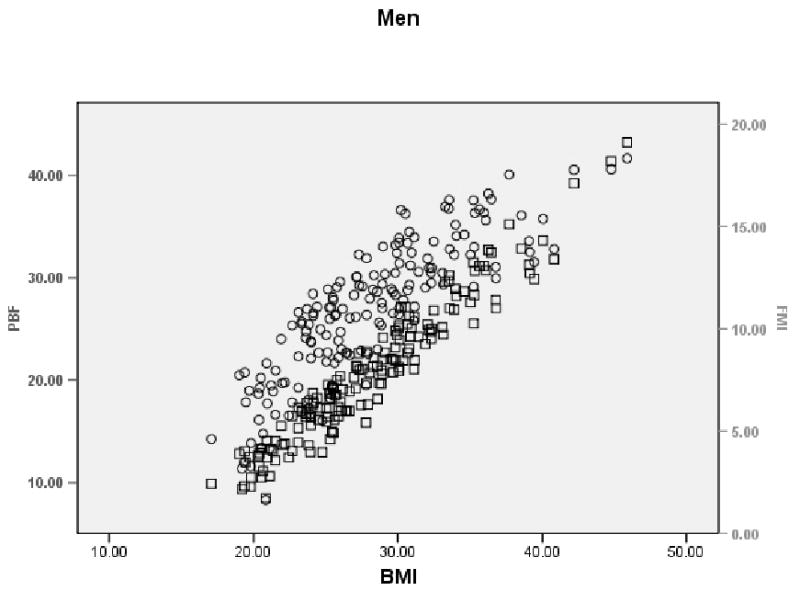
Scatterplot showing the relationship between PBF, BMI, and FMI in Mexican-American men. Circles show PBF vs. BMI and squares show FMI vs. BMI.
FIGURE 2.
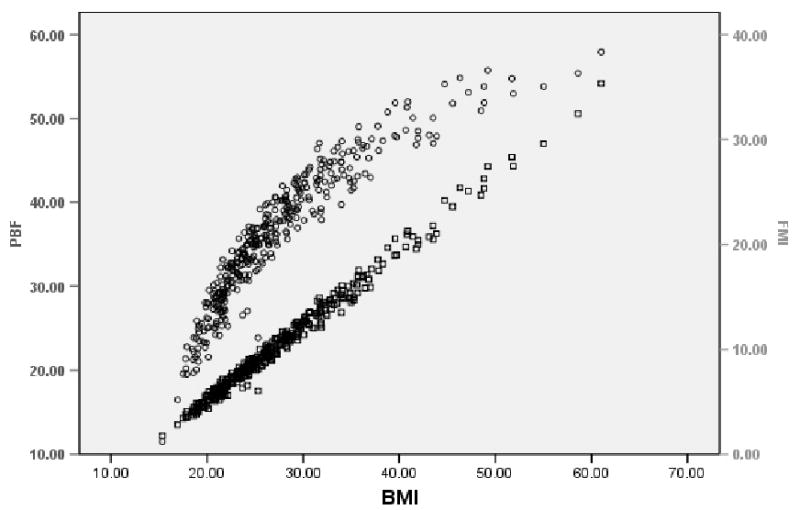
Scatterplot showing the relationship between PBF, BMI, and FMI in Mexican-American women. Circles show PBF vs. BMI and squares show FMI vs. BMI.
From Table 4, FMI was significantly predicted by BMI and BMI2 in both men and women. The following predictive equations explained approximately 92% of the variance of FMI in men and approximately 99% of the variance of FMI in women.
TABLE 4.
Multiple linear regression analysis of FMI for Mexican American men and women
| Variable | Coefficient | SE | P-value | R2 | |
|---|---|---|---|---|---|
| Men | |||||
| Model 1 | BMI | .600 | .165 | .001 | 0.92 |
| BMI2 | - .001 | .003 | |||
| Model 2 | WC | .146 | .033 | .000 | 0.94 |
| BMI | .170 | .173 | |||
| BMI2 | .002 | .003 | |||
| Age | - .011 | .011 | |||
| HC | - .015 | .014 | |||
| Model 3 | WC | .142 | .032 | .000 | 0.94 |
| BMI | .293 | .067 | |||
| Age | - .011 | .011 | |||
| HC | - .013 | .014 | |||
| Model 4 | WC | .136 | .032 | .000 | 0.94 |
| BMI | .282 | .066 | |||
| Age | - .010 | .011 | |||
| Model 5 | WC | .122 | .028 | .000 | 0.94 |
| BMI | .307 | .060 | |||
| Model 6 | BMI | .552 | .021 | .000 | 0.92 |
| WHR | 1.921 | 1.166 | |||
| Women | |||||
| Model 1 | BMI | .682 | .031 | .000 | 0.99 |
| BMI2 | .001 | .000 | |||
| Model 2 | WC | .024 | .009 | .000 | 0.99 |
| BMI | .597 | .042 | |||
| BMI2 | .001 | .000 | |||
| Age | - .010 | .006 | |||
| HC | .021 | .008 | |||
| Model 3 | WC | .023 | .009 | .000 | 0.99 |
| BMI | .646 | .024 | |||
| Age | - .011 | .006 | |||
| HC | .021 | .008 | |||
| Model 4 | BMI | .722 | .007 | .000 | 0.99 |
| WHR | - .058 | .735 | |||
FMI = -8.350 + 0.6 (BMI) − 0.001 (BMI2) for men
FMI = -8.657 + 0.682 (BMI) + 0.001 (BMI2) for women.
Adding age, WC, and HC to the model marginally improved the model's predictability. A Bland-Altman plot comparing measured and predicted FMI for both men and women, shows good agreement between the methods with a mean difference of -0.2900 (Figure 3).
FIGURE 3.
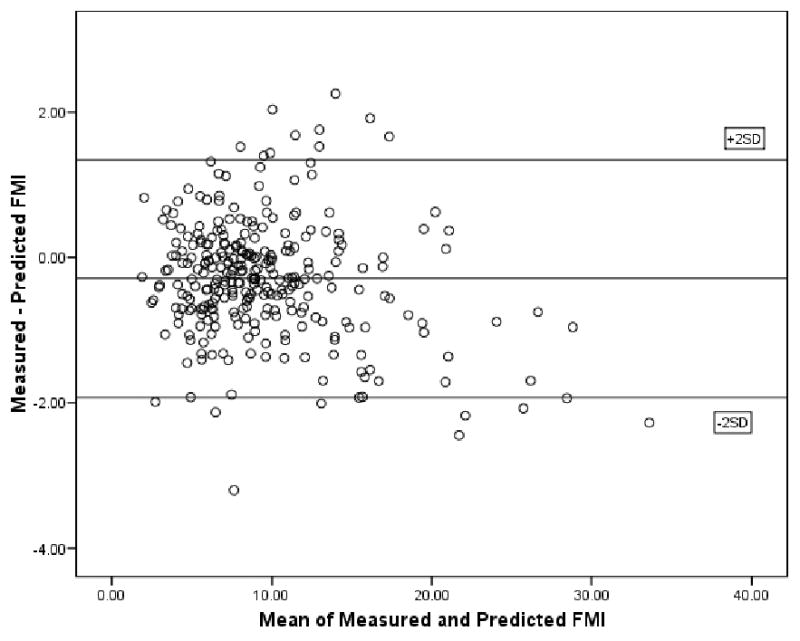
Bland-Altman plot showing the agreement between FMI based on measured fat mass/(height)2 and predicted fat mass/(height)2 in Mexican American men and women.
Discussion
Faced with the current, global epidemic of obesity, it has become necessary to validate simple and accurate tools for the diagnosis of obesity. Many health problems are associated with obesity, especially cardiovascular disease, type-2 diabetes, metabolic disorders, and certain cancers (Snijder et al., 2006; van den Brandt and Goldbohm, 2006; Flegal et al., 2004; Hogan et al., 2003), and approximately 300,000 deaths each year in the U.S. have been attributed to obesity (Stein and Colditz, 2004). It is of high relevance to investigate the rates of obesity in the Lower Rio Grande Valley LRGV), a four county area of almost 1 million inhabitants in the southern most region of Texas that is 81.4% Mexican American (U.S. Census Bureau, 2005). The rates of chronic diseases associated with obesity in the LRGV are among the highest in the U.S. For instance, the prevalence of type-2 diabetes in Mexican Americans in the LRGV has been estimated to range from 15.9% to 39% (Bastida et al., 2001; Hanis et al., 1983; Mier et al., 2007; Villas, 1999).
The ability to estimate or quantify fat stores accurately is central to the prevention and treatment of obesity-related conditions. Of the most common methods used, we chose to compare BMI and BIA in a Mexican American population and were able to demonstrate a strong correlation between BMI and PBF as estimated by BIA. BMI, however, cannot differentiate between FM and FFM. This is strongly evidenced by the fact that 46.2% of the men and 50.7% of the women categorized as being normal body weight or as overweight by BMI were actually obese when examined by BIA. Although all of the methods commonly used in assessing body fat content are intrinsically limited (Chumlea and Guo, 2000), this underestimation of obesity by BMI is a critical limitation of this method.
To our knowledge, this is the first study that shows discrepancies between BMI and PBF estimated by BIA in Mexican American college students. This unequivocal discrepancy between the two methods was previously observed in four distinct populations. In a random study of 141 predominantly white American adult men and women conducted in the New England area, 30% of the men and 46% of the women with a BMI below 30 were found to have PBF beyond the obesity cutoff points when analyzed by BIA (Frankenfield et al., 2001). In a study conducted in Greece, of 115 perimenopausal women considered obese by BIA, only 30.6% were classified as obese by BMI (Kontogianni et al., 2005). In a study of 637 women in Iran, 18.3% were found to be obese by BMI, whereas 39.4% were actually obese by BIA (Amani, 2007). In a study conducted in Chile of 433 women and 264 men, 64% of the women and 23.6% of the men with a BMI below 30 were found to be obese by BIA (Carrasco et al., 2004).
In addition to underestimating obesity, it appears that the discrepancies between BMI and PBF are more pronounced in women than in men across ethnic groups. The higher PBF normally observed in women is not accounted for when using BMI. For instance, in our study, the median BMI for men was 27.3 and the median PBF was 27.1, whereas in women, the median BMI was 25.4 and the median PBF was 35.6. Similarly, in a study of 8,259 non-Hispanic white and non-Hispanic black adults who participated in the Third National Health and Nutrition Examination Survey (NHANES III), women showed a higher percentage of body fat when compared to men of a comparable BMI. Non-Hispanic white men had a mean BMI of 26.7 and a mean PBF of 22.1, whereas non-Hispanic white women had a mean BMI of 26.1 and a mean PBF of 32.0. Non-Hispanic black men had a mean BMI of 26.7 and a mean PBF of 22.4, whereas non-Hispanic black women had a mean BMI of 28.8 and a mean PBF of 35.9 (Zhu et al., 2003b). In another study of 15,912 non-Hispanic white, non-Hispanic black, and Mexican Americans who participated in the same survey, Mexican American men and women in the 20-29.9 year age group had a mean BMI of 25.6 and 26.1 respectively, whereas their mean PBF was 24.1 and 35.8, respectively (Chumlea et al., 2002). The accepted BMI cutoff points as defined by the World Health Organization (2000) are the subject of controversy, prompting the suggestion of lower cutoffs for BMI by sex and for specific ethnic groups (Frankenfield et al., 2001; Snijder et al., 2006; Amani, 2007; Sanchez-Castillo et al., 2000; Deurenberg, 2001; Zhu et al., 2003b; Shiwaku et al., 2004).
Despite its limitations, BMI is continually used in clinical and epidemiological studies and is considered an accurate predictor of morbidity and mortality associated with obesity (Kontogianni et al., 2005; Frankenfield et al., 2001). In a mortality study of 8,029 predominantly white American women, aged 65 years and older, it was determined that BIA was not better than BMI in predicting obesity-related mortality (Dolan et al., 2007). Moreover, a study conducted with more than 12,000 men and women who participated in the NHANES III that examined the correlations between both BMI and BIA with four biological markers known to reflect obesity-related medical conditions, found that BMI had the highest correlations across biological markers. Although no direct comparison between BIA and BMI in assessing body adiposity was conducted during the study, the authors suggested that BIA is not superior to BMI as a predictor of overall adiposity in a general population (Willett et al., 2006).
Predictive equations for the assessment of FFM validated for Mexican-Americans were used in this study (Sun et al., 2003). Although BIA is not a reference method to evaluate adiposity, we previously observed a strong agreement between BIA using RJL Quantum II and air-displacement plethysmography (BOD POD) in Mexican Americans (Peltz et al., 2007). The Lin's concordance correlation coefficient between FM in kilograms by BIA and FM in kilograms by BOD POD was 0.96 (95% limits of agreement: -5.99, 7.12) and 0.93 (95% limits of agreement: -5.62, 7.59) for men and women, respectively.
Our results showed the correlation between BMI and PBF to be in agreement with previous studies in several populations. BMI and PBF have been shown to be strongly correlated in Greek women (rho = 0.83, p < 0.001) (Kontogianni et al., 2005), and in Iranian women (rho = 0.77, p < 0.001) (Amani, 2007), as well as in a NHANES III study of multiethnic American men and women (rho = 0.65 and rho = 0.74 in men and women, respectively) (Willett et al., 2006). A study of predominantly white women in the New England area, aged 65 years and older showed a strong correlation (rho = 0.87, p < 0.001) between BMI and PBF (Dolan et al., 2007), and a Swedish follow-up cancer study also showed a strong correlation in women (rho = 0.83, p < 0.001) with a weaker correlation in men (rho = 0.59, p < 0.001) (Calling et al., 2006).
Alternative simple and inexpensive approaches for assessing body adiposity have been suggested. For instance, some authors have used FM and FFM corrected by body height (FM index and FFM index, respectively) in studies evaluating morbidity and mortality related to obesity (Frankenfield et al., VanItallie et al., 1990; Zhu et al., 2003a). FM and FFM are known to change with height, weight, sex, and age (Forbes and Reina, 1970; Eto et al., 2004; Kyle et al., 2003). Increased height or weight may result in an increase in both compartments. Because PBF provides a good estimate of body adiposity, we chose to compare FMI to PBF. Further, we tested the accuracy of predictive equations of FMI to categorize obesity.
FMI accurately classified obesity when compared to PBF in 94.6% of the men and in 92.2% of the women. Our ROC analysis between FMI and PBF showed an area under the curve of 0.99 for men and 0.99 for women. The validity of using FMI to categorize obesity in men and women was determined using recommendations proposed by the WHO for selecting cutoffs (World Health Organization, 1995). The sensitivities of FMI for men and women were 92% and 87%, respectively for PBF. Specificities were 100% for both men and women. FMI was predicted using only BMI and BMI2 as independent variables with an R2 value of 0.92 in men and 0.99 in women (p < 0.0001). Adding WC and HC to the model marginally increased R2 among men. Since the median values for WC in men and women were well below established cutoff points for abdominal obesity (World Health Organization, 2000), this may explain the minimal contribution of these variables. In order to maintain the simplicity of the model, BMI and BMI2 were selected as independent variables. A non-randomized study conducted with 2,986 white men and 2,649 white women, from 15 to 98 years of age in Geneva, Switzerland, which also used BMI and BMI2 as independent variables, showed an R2 value for men of 0.77 and 0.88 for women (p<0.001) (Kyle et al., 2003). In a separate study of the same Swiss group, R2 values of 0.77 in men and 0.88 (p < 0.001) in women were found using only BMI as the independent variable (Schutz et al., 2002). In a New England study with 53 men and 88 women, using regression equations that predicted FMI with BMI and BMI2 as independent variables, R2 values of 0.99 in men and 1.00 in women were reported which are very similar to our findings (Frankenfield et al., 2001).
To our knowledge, few studies have explored the use of FMI as proxy of obesity. A study of Japanese children ages 3 to 5 years, evaluated the validity of FMI and BMI against PBF by BIA as the reference method (Eto et al., 2004). Correlations between FMI and BMI resulted in correlation coefficients of 0.472 in boys (p < 0.001) and 0.442 in girls (p < 0.001). However, similar to our findings, the correlations between FMI and PBF were higher (rho = 0.96, p < 0.001) in both boys and girls (Eto et al., 2004). In the aforementioned Swiss study, the mean FMI in the overall age group was 4.9 ± 1.8 kg/m2 in men and 6.6 ± 2.4 in women (Kyle et al., 2003), compared to our results, of 7.8 ± 3.3 kg/m2 in men and 10.3 ± 5.2 kg/m2 in women. In the same study, men with non-obese PBF values of 9.0% to 21.2% had corresponding FMI values of 1.8 to 5.2 kg/m2 and women with non-obese PBF values of 20.6% to 33.7% had corresponding FMI values of 3.9 to 8.2 kg/m2 (Kyle et al., 2003). Our study shows that in men, the non-obese PBF mean was 19.5% with a corresponding FMI mean of 4.8 kg/m2, and in women the non-obese PBF mean was 28.9% with a corresponding FMI mean of 6.8 kg/m2. Our FMI means fell within the upper range of the Swiss normal FMI ranges in both men and women. This was not unexpected, as Mexican Americans have the highest percentage of body fat across all age groups when compared to non-Hispanic whites and non-Hispanic blacks (Chumlea et al., 2002). In a subsequent study from the same group, obesity was defined as FMI greater than 8.2 kg/m2 in men and 11.8 kg/m2 in women (Schutz et al., 2002) which are greater than our FMI cutoffs of ≥ 6.6 kg/m2 in men and ≥ 9.5 kg/m2 in women in our study. This difference can be explained by the fact that FMI cutoffs in the Swiss group were derived from BMI and not from PBF cutoffs (Schutz et al., 2002).
FMI increased linearly with BMI in the Swiss study as well as in our study. The overall sensitivity of FMI vs. BMI of the Swiss study was 77.4% and the specificity was 84% compared to our sensitivity of 77% and specificity of 76%. These findings, although limited, show an interesting agreement with our results, which collectively contribute to the concept of developing one set of recommended ranges that are not affected by height (Kyle et al., 2003).
The usefulness of FMI has not been fully explored, conceivably due to the lack of specific cutoffs (Frankenfield et al., 2001; VanItallie et al., 1990; Eto et al., 2004; Schutz et al., 2002). In addition, there is a general acceptance that BMI is the simplest and most convenient method of assessing body adiposity. However, since BMI is mathematically equal to the sum of FMI and FFMI (Eto et al., 2004; Schutz et al., 2002; Wells et al., 2002), the use of FMI as a measure of adiposity is more appropriate and relates to the adiposity content of BMI.
Our study emphasizes three key points. First, BMI is not reliable or sufficient for identifying individuals with obesity. Given that our findings are in agreement with other similar studies conducted in different populations, we speculate that any report addressing the incidence of obesity based solely on BMI has the potential to be substantively biased. Second, PBF is superior to BMI in correctly classifying obesity based on accurate estimates of body fatness. Third, FMI can be accurately predicted from BMI and BMI2, and is as convenient to use as BMI and PBF in epidemiological studies. Aside from the potential benefits to epidemiological studies of obesity, results from this study might ignite exploratory research in other areas of biomedicine and anthropology. For instance, the observation of ongoing changes in body composition across different ethnicities and its relationship with obesity has demonstrated that obesity affects individuals no matter their cultural background and economic status.
This study is not without limitations. It may not reflect the results of a similar study conducted by random sampling. Risk factors for obesity were not examined. Direct comparison to reference methods for the assessment of body adiposity was not performed, which limits the study conclusions. Women outnumbered men by 2.26:1. This skew towards females was also observed in a feasibility study of screening for Chlamydia trachomatis conducted among college students attending the University of Antwerp, Belgium, in which participation was composed of 77.8% females and 22.2% males (Colliers A et al., 2009). In our study, most of the recruiters were females, and this may have biased the recruiting process. Equal attention was given to males and females during recruitment, and future studies should focus on enhancing male recruitment. A report from the National Academy of Sciences (NAS) on women and health research showed an unconscious bias toward inclusiveness (i.e., male investigator may tend to include people who are more like the investigator; similarly, women may feel uncomfortable with male researchers). Societies are stratified by sex and race, and different groups within societies also stratify along various lines. The report notes the difficulties (if not near impossibility) of disentangling the research process from the world within which it is conducted. Thus, one way to avoid the subtle bias of inclusion in a white, male–dominated scientific research community is to encourage more women and minorities to become researchers (Executive Summary, 1994).
Limitations of BIA include the assumption that the human body is analogous to a geometric model that is uniform in cross-sectional area and composition (Lukaski, 1989; Lukaski et al., 1985). Total body water is estimated at 73% of fat free mass, but this is not a constant between individuals of different race, sex, age, and health status (Lukaski, 1987; Lohman, 1986). Therefore, predictive equations must be appropriately developed to avoid misclassification (Kontogianni et al., 2005). Body fat is overestimated in lean individuals and underestimated in very obese individuals (Chumlea and Guo, 1994).
Conclusion
Although BMI is the most common tool used to evaluate obesity in clinical and epidemiological studies, it cannot directly measure body fat. The large discrepancy observed between BMI and PBF clearly reflects the major limitation of BMI. Although it may be a convenient method, it cannot be completely relied upon as a measure of adiposity, and its limitations must be taken into account when interpreting body weight classifications based on BMI. It is of critical importance that a standardized method that is also accurate, economical, and convenient for measuring body fat and assessing obesity be globally implemented in field research. BIA appears to be an acceptable alternative provided specific predictive equations are applied accordingly, and FMI appears to provide not only accuracy, but an economical advantage together with convenience.
Acknowledgments
Grant information: This study was supported by the U.S. Hispanic Nutrition Research and Education Center (USHNREC), Grant No. D52MP03115-02-0-HALE (GP) and by the NIH-MBRS RISE Program, Grant No. R25 GM065925-01A1.
Footnotes
Disclosure statement: The authors have no affiliations with any organization that, to their knowledge, has a direct interest, particularly a financial interest, in any of the subject matter or in any of the materials discussed herein.
References
- Amani R. Comparison between bioelectrical impedance analysis and body mass index methods in determination of obesity prevalence in Ahvazi women. Eur J Clin Nutr. 2007 Apr;61(4):478–482. doi: 10.1038/sj.ejcn.1602545. [DOI] [PubMed] [Google Scholar]
- Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- Bastida E, Cuéllar I, Villas P. Prevalence of diabetes mellitus and related conditions in a South Texas Mexican American sample. J Community Health Nurs. 2001;18:75–84. doi: 10.1207/S15327655JCHN1802_01. [DOI] [PubMed] [Google Scholar]
- Bray GA, Greenway FL, Molitch ME, Dahms WT, Atkinson RL, Hamilton K. Use of anthropometric measures to assess weight loss. Am J Clin Nutr. 1978;31:769–773. doi: 10.1093/ajcn/31.5.769. [DOI] [PubMed] [Google Scholar]
- Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- Calling S, Hedblad B, Engström G, Berglund G, Janzon L. Effects of body fatness and physical activity on cardiovascular risk: risk prediction using the bioelectrical impedance method. Scand J Public Health. 2006;34:568–575. doi: 10.1080/14034940600595621. [DOI] [PubMed] [Google Scholar]
- Carrasco F, Reyes E, Rimler O, Rios F. Predictive accuracy of body mass index in estimating body fatness measured by bioelectrical impedance. Arch Latinoam Nutr. 2004;54:280–286. [PubMed] [Google Scholar]
- Chumlea WC, Guo SS. Bioelectrical impedance and body composition: present status and future directions. Nutrition Reviews. 1994;52(4):123–131. doi: 10.1111/j.1753-4887.1994.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Guo SS. Assessment and prevalence of obesity: application of new methods to a major problem. Endocrine. 2000;13:135–142. doi: 10.1385/endo:13:2:135. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Sun SS. Bioelectrical Impedance Analysis. In: Heymsfield Steven B, Lohman Timothy G, Wang ZiMian, Going Scott B., editors. Human Body Composition. Champaign, Illinois: Human Kinetics; 2005. pp. 79–88. [Google Scholar]
- Colliers A, Verster A, Van Pueynbroeck K, Stalpaert M, Van Royen P, Verhoeven V. Screening Belgian university students for Chlamydia trachomatis infection: a feasibility study. Int J Adolesc Med Health. 2009;21(3):343–346. doi: 10.1515/ijamh.2009.21.3.343. [DOI] [PubMed] [Google Scholar]
- De Lorenzo A, Deurenberg P, Pietrantuono M, Di Daniele N, Cervelli, Andreoli A. How fat is obese? Acta Diabetol. 2003;40:S254–S257. doi: 10.1007/s00592-003-0079-x. [DOI] [PubMed] [Google Scholar]
- Deurenberg P. Universal cut-off BMI points for obesity are not appropriate. Br J Nutr. 2001;85:135–136. doi: 10.1079/bjn2000273. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Yap M, Van Stevens WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- Dittmar M, Reber H. New equations for estimating body cell mass from bio-impedance parallel to models in healthy older Germans. Am J Physiol Endocrinol Metab. 2001;281(5):E1005–14. doi: 10.1152/ajpendo.2001.281.5.E1005. [DOI] [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:7–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Dolan CM, Kraemer H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. Am J Public Health. 2007;97:913–928. doi: 10.2105/AJPH.2005.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ, Bell SJ, Chertow GM, Chumlea WC, Knox TA, Kotler DP, Lukaski HC, Schoeller DA. Bioelectrical impedance methods in clinical research: a follow-up to the NIH technology assessment conference. Nutrition. 1999;15:74–880. doi: 10.1016/s0899-9007(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Eto C, Nakao T, Kikkawa K. Validity of the body mass index and fat mass index as an indicator of obesity in children aged 3-5 year. J Physiol Anthropol Appl Human Sci. 2004;23:25–30. doi: 10.2114/jpa.23.25. [DOI] [PubMed] [Google Scholar]
- Mastroianni AC, Faden R, Federman D, editors. Women and health research: ethical and legal issues of including women in clinical studies, volume 1. Washington, DC: Institute of Medicine National Academy Press; 1994. Executive Summary; pp. 1–26. [PubMed] [Google Scholar]
- Flegal KM, Ogden CL, Carroll MD. Prevalence and trends in overweight in Mexican-American adults and children. Nutr Rev. 2004;62:S144–S148. doi: 10.1111/j.1753-4887.2004.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–663. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17:26–30. doi: 10.1016/s0899-9007(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Hanis CL, Ferrell RE, Barton SA, Aguilar L, Garza-Ibarra A, Tulloch BR, Garcia CA, Schull WJ. Diabetes among Mexican Americans in Starr County, Texas. Am J Epidemiol. 1983;118:659–672. doi: 10.1093/oxfordjournals.aje.a113677. [DOI] [PubMed] [Google Scholar]
- Heimmel J, Patel S, Cody R, Bachmann G. Evaluation of physical fitness in an ambulatory setting. Am J Obstet Gynecol. 2007;196:522.e1–522.e4. doi: 10.1016/j.ajog.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: Advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- Himes JH, Roche AF, Siervogel RM. Compressibility of skinfolds and the measurement of subcutaneous fatness. Am J Clin Nutr. 1979;32:1734–1740. doi: 10.1093/ajcn/32.8.1734. [DOI] [PubMed] [Google Scholar]
- Hogan P, Dall T, Nikolov P. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML. Practical assessment of body composition. The Physician and Sportsmedicine. 1985;13:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- Kagawa M, Binns CB, Hills AP. Body composition and anthropometry in Japanese and Australian Caucasian males and Japanese females. Asia Pac J Clin Nutr. 2007;16(Suppl 1):31–36. [PubMed] [Google Scholar]
- Kontogianni MD, Panagiotakos DB, Skopouli FN. Does body mass index reflect adequately the body fat content in perimenopausal women? Maturitas. 2005;51:307–313. doi: 10.1016/j.maturitas.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition. 2001;17:248–253. doi: 10.1016/s0899-9007(00)00553-0. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Schutz Y, Dupertuis YM, Pichard C. Body composition interpretation: contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19:597–604. doi: 10.1016/s0899-9007(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Bosaus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clinical Nutrition. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Lee R, Nieman DC. Anthropometry. In: Lee R, Nieman DC, editors. Nutritional Assessment. New York, NY: McGraw-Hill; 2002. pp. 223–288. [Google Scholar]
- Lohman TG. Skinfolds and body density and their relation to body fatness: a review. Hum Biol. 1981;53:181–225. [PubMed] [Google Scholar]
- Lohman T. Applicability of body composition techniques and constants for children and youths. Exercise and Sport Sciences Reviews. 1986;14:325–357. [PubMed] [Google Scholar]
- Lohman TG, Harris M, Teixeira PJ, Weiss L. Assessing body composition and changes in body composition: another look at dual-energy X-ray absorptiometry. Ann N Y Acad Sci. 2000;904:45–54. doi: 10.1111/j.1749-6632.2000.tb06420.x. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–817. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- Lukaski HC. Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr. 1987;46:537–556. doi: 10.1093/ajcn/46.4.537. [DOI] [PubMed] [Google Scholar]
- Lukaski H. Applications of bioelectrical impedance analysis: a critical review. In: Yasamura S, Harrison JE, McNeill KE, Woodhead AD, Dilmanian FA, editors. In vivo Body Composition Studies: Recent Advances. New York: Plenum; 1989. pp. 365–374. [Google Scholar]
- Marini E, Cabras S, Rebato E, Buffa R, Salces I, Borgognini-Tarli S. Sex differences in skinfold variability across human populations and during the life cycle. Ann Hum Biol. 2007;34:377–392. doi: 10.1080/03014460701367942. [DOI] [PubMed] [Google Scholar]
- Martin AD, Ross WD, Drinkwater DT, Clarys JP. Prediction of body fat by skinfold caliper: assumptions and cadaver evidence. Int J Obes. 1985;9(Suppl 1):31–39. [PubMed] [Google Scholar]
- McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, Lohr KN. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139:933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- Mier N, Medina AA, Ory MG. Mexican Americans with type 2 diabetes: perspectives on definitions, motivators, and programs of physical activity. Prev Chronic Dis. 2007;4:1–8. [PMC free article] [PubMed] [Google Scholar]
- Nooyens AC, Koppes LL, Visscher TL, Twisk JWR, Kemper HCG, Schuit AJ, van Mechelen W, Seidell JC. Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr. 2007;85:1533–1539. doi: 10.1093/ajcn/85.6.1533. [DOI] [PubMed] [Google Scholar]
- Peltz G, Sanderson M, Pérez A, Sexton K, Ochoa-Casares D, Kay-Fadden M. Serum leptin concentration, adiposity, and body fat distribution in Mexican-Americans. Arch Med Res. 2007;38:563–570. doi: 10.1016/j.arcmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Peltz G, Sanderson M, Wittenburg D, Bailey M, Aguirre K, Aguirre M. Body Composition by Bioelectrical Impedance Analysis and Air-Displacement Plethysmography: a Comparative Study. Obes Res. 2007;15(Suppl):A135. [Google Scholar]
- Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr. 2004;58:1479–1484. doi: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Jackson AS. Research progress in validation of clinical methods of assessing body composition. Med Sci Sports Exerc. 1984;16:606–615. [PubMed] [Google Scholar]
- Sanchez-Castillo CP, Velazquez-Monroy O, Berber A, Lara-Esqueda A, Tapia-Conyer R, James WP. 2000 Anthropometric cutoff points for predicting chronic diseases in the Mexican. National Health Survey Obes Res. 2003;11:442–451. doi: 10.1038/oby.2003.60. [DOI] [PubMed] [Google Scholar]
- Schreiner PJ, Terry JG, Evans GW, Hinson WH, Grouse II, Jr, Heiss G. Sex specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric indices. Am J Epidemiol. 1996;144:335–345. doi: 10.1093/oxfordjournals.aje.a008934. [DOI] [PubMed] [Google Scholar]
- Schutz Y, Kyle UUG, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasions aged 18-98 y. Int J Obes. 2002:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- Segal K, Van Loan M, Fitzgerald P, Hodgdon J, Van Itallie T. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr. 1988;47:7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- Shiwaku K, Anuurad E, Enkhmaa B, Nogi A, Kitajima K, Shimono K, Yamane Y, Oyunsuren T. Overweight Japanese with body mass indexes of 23·0 to 24·9 have higher risks for obesity-associated disorders: a comparison of Japanese and Mongolians. Int J Obes Relat Metab Disord. 2004:152–158. doi: 10.1038/sj.ijo.0802486. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Lobo DN, Anderson JA, Macdonald IA, Perkins AC, Neal KR, Allison SP, Rowlands BJ. Body water compartment measurements: a comparison of bioelectrical impedance analysis with tritium and sodium bromide dilution techniques. Clin Nutr. 2001;20:339–343. doi: 10.1054/clnu.2001.0398. [DOI] [PubMed] [Google Scholar]
- Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9:480–491. [PubMed] [Google Scholar]
- Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, Kuczmarski RJ, Flegal KM, Johnson CL, Hubbard RS. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–340. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. State and County Quick Facts: Texas. [08/02 2007];Version current. 2005 Internet: htt://quickfacts.census.gov/qfd/states/48/48000.html.
- van den Brandt PA, Goldbohm RA. Nutrition in the prevention of gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2006;20:589–603. doi: 10.1016/j.bpg.2006.04.001. [DOI] [PubMed] [Google Scholar]
- VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- Villas P. Utilizing capture-recapture techniques to determine the diabetes prevalence in the Rio Grande Valley. Texas J Pub Health. 1999;17:42–57. [Google Scholar]
- Webber J, Donaldson M, Allison SP, Macdonald IA. A comparison of skinfold thickness, body mass index, bioelectrical impedance analysis and dual-energy X-ray absorptiometry in assessing body composition in obese subjects before and after weight loss. Clin Nutr. 1994;13:177–182. doi: 10.1016/0261-5614(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Wells JCK, Cole TJ, ALSPAC study team Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- Wells JCK, Williams JE, Fewtrell M, Singhal A, Lucas A, Cole TJ. A simplified approach to analyzing bio-electrical impedance data in epidemiological surveys. Int J Obes. 2007;31:507–514. doi: 10.1038/sj.ijo.0803441. [DOI] [PubMed] [Google Scholar]
- Willett K, Jiang R, Lenart E, Spiegelman D, Willett W. Comparison of bioelectrical impedance and BMI in predicting obesity-related medical conditions. Obesity. 2006;14:480–490. doi: 10.1038/oby.2006.63. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Technical Report Series 854. Geneva: 1995. Physical Status: The use and interpretation of anthropometry. Report of a WHO Consultation. [PubMed] [Google Scholar]
- World Health Organization. WHO Technical Report Series 894. Geneva: 2000. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. [PubMed] [Google Scholar]
- Zhang X, Shu XO, Gong Y, Honglan L, Hui C, Yu-Tang G, Wei Z. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167:886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- Zhu S, Heo M, Plankey M, Faith MS, Allison DB. Associations of body mass index and anthropometric indicators of fat mass and fat free mass with all-cause mortality among women in the first and second National Health and Nutrition Examination Surveys follow-up studies. Ann of Epidemiol. 2003a;13:286–293. doi: 10.1016/s1047-2797(02)00417-9. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S. Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988-1994) Am J Clin Nutr. 2003b;78:228–235. doi: 10.1093/ajcn/78.2.228. [DOI] [PubMed] [Google Scholar]


