Abstract
Previous studies demonstrated that hepatic matrix metalloproteinase-9 (MMP-9) activity increased following cecal ligation and puncture (CLP) in rats, indicating liver injury in sepsis. The activity of MMP-9 in degrading extracellular matrix is controlled by activation of proenzymes and inhibition of tissue inhibitor of MMPs (TIMP-1). To further assess the proteolytic cascade imbalance in sepsis, hepatic MMP-9 and TIMP-1 expressions were examined in CLP rats. In this study, sepsis was induced in rats by CLP, and at 10 and 20 h after sepsis induction, liver samples were collected and MMP-2, MMP-9, and TIMP-1 gene and protein expressions were evaluated by real time PCR and Western blot analysis, respectively. Gene expression of MMP-9 was increased by 6.4-fold and 3.0-fold at 10 h and 20 h after CLP as compared to sham group, respectively. Likewise, MMP-9 protein expression was also significantly increased at both time points. In contrast, MMP-2 gene expression was not altered at 10 h and 20 h after CLP as compared to sham controls. Interestingly, TIMP-1 gene expression was elevated to 89-fold and 46-fold from sham levels at 10 h and 20 h after CLP, respectively. Similarly, TIMP-1 protein levels were also significantly increased at both time points. In addition, MMP-9/TIMP-1 protein ratio was lower at both 10 h and 20 h after CLP compared to sham rats. Results demonstrated an imbalance between MMP and TIMP, with a more evident role for MMP-9 than MMP-2, and high value of TIMP-1 was particularly evident in CLP rats. Our results indicate that MMP-9 and TIMP-1 expressions are increased and they may serve as useful markers to predict the outcome of sepsis.
Keywords: MMP, TIMP-1, sepsis, MMP-9/TIMP-1 ratio, MMP-2, matrix metalloproteinase, inhibitors, liver, sepsis
Introduction
The physiological response to sepsis appears to be an exaggeration of normal immune function, in which the severity of stress overcomes counter regulatory processes resulting in unrestricted disturbances that are frequently fatal. For example, release of proinflammatory mediators (cytokines, arachidonic acid, metabolites, complements) in sepsis causes a systemic inflammatory response that results in sequestration of polymorphonuclear neutrophils (PMNs), and the effectors (proteases, MMPs) released from activated PMNs causes tissue injury [1]. The subsequent release of inflammatory mediators perpetuates a vicious cycle of inflammation and tissue destruction. We have reported that hepatic MMP-9 and gelatinase activity increased significantly after sepsis, and that pre-treatment with chemically modified tetracycline-3 (CMT-3), an MMP inhibitor, blocked these activities, as well as the ensuing septic shock [2]. In addition, the molecular mechanism underlying the MMP-9 response to sepsis was studied in our laboratory, demonstrating that sepsis affects hepatic MMP-9 activity by modulating cytokine and mitogen activated protein kinase mechanism [3]. Furthermore, these results are consistent with MMP-9 induced caspase-3 activation in response to CLP. CMT-3 post treatment increased TIMP-1 level and thereby inhibited MMP-9, which in turn decreased TGF-β1 and caspase-3 signaling pathways and improved survival in septic rats [4].
MMPs are released as inactive precursors that are activated in the extracellular compartment by proteolytic cleavage [5]. After activation, MMPs are regulated by their interaction with TIMPs and alpha-2 macroglobulin [6]. Since the integrity of tissue architecture is closely dependent on delicate balance between the activation of MMPs and their inhibition by TIMPs, any alteration in this balance is linked to tissue damage. MMP-9 is stored in the tertiary granules of polymorphonuclear leukocytes which are key effectors in acute inflammatory diseases such as sepsis. Specifically, MMP-9 has been shown to mediate vascular leakage [7] and to induce the migration of inflammatory cells to sites of inflammation to induce wound repair [8]. Elevated levels of MMP-9 and TIMP-1 were reported in septic patients, and that higher TIMP-1 levels at the beginning of severe sepsis were predictive of death [9]. The development of specific inhibitors of MMPs or TIMPs as a new class of drugs for sepsis therapy is challenging and future clinical trials have to clarify their role within the treatment regimen of septic patients [9].
The purpose of the present study was to understand the modulation of MMPs and TIMP-1 in septic rats and whether these parameters determine severity in mortality associated with sepsis.
Materials and methods
Experimental animals
Male adult Sprague-Dawley rats (250–320g) purchased from Charles River Laboratories (Wilmington, MA) were used in this study. All rats were housed in a temperature controlled room on a 12-hr light/dark cycle and fed a standard Purina rat chow diet for at least one week before the experiment. The experiments described here were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee (IACUC) of The Feinstein Institute for Medical Research.
Animal model of sepsis
Sepsis was induced by cecal ligation and Puncture (CLP) as previously described by us [10]. Briefly, the rats were anesthetized with isoflurane inhalation and a 2-cm ventral midline abdominal incision was performed. The cecum was then exposed, ligated just distal to the ileo-cecal valve to avoid intestinal obstruction, punctured twice with an 18-guage needle, and returned to the abdominal cavity. The incision was then closed in layers. Sham operated animals underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The animals were resuscitated with 3 ml/100 g body wt normal saline subcutaneously immediately after surgery.
Detection of mRNA expression of MMPs using quantitative real time PCR
Total RNA (4 μg) extracted from liver tissues were reverse transcribed to cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City,CA). The resulting cDNA was diluted 1:30 fold and the PCR reaction was performed with 2.5 μl cDNA, 0.2 μM each forward and reverse primers, 12.5 μl SYBR Green PCR Master Mix (Applied Biosystems) in a final volume of 25 μl. The thermal profile for the real-time Q-PCR was 50°C for 2 min, 95°C for 10 min and followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 min. The gene expression was expressed as fold change from the GAPDH level which is calculated as 2-ΔΔCt. In addition, melting curve analysis was performed to assure the specificity of PCR product in this experiment. The following rat primers were used: MMP-9 (NM_031055): 5'-TCGAAGGCG ACCTCAAGTG-3’ (forward), 5'-TTCGGTGTAGCTT TGGATCCA-3’ (reverse). MMP-2 (NM_031054): 5'-ACCGTCGCCCATCATCAA-3’ (forward), 5'-TTGC ACTGCCAACTCTTTGTCT-3’ (reverse); TIMP-1 (N M_053819): 5'-CGCAGCGAGGAGGTTTCTCAT-3’ (forward), 5'-GGCAGTGATGTGCAAATTTCC-3’ (reverse); GAPDH (AF 106860): 5'-ATG ACT CTA CCC ACG GCA AG-3’ (forward), 5'-CTG GAA GAT GGT GAT GGG TT-3’ (reverse).
Western blot analysis of MMP-9 and TIMP-1
Total proteins (50 μg) from hepatic tissues were loaded on 4-12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and electrophoretically fractionated in MES-SDS running buffer (Invitrogen). The protein on the gel was then transferred to a 0.45-μm nitrocellulose membrane, and blocked with 5% nonfat dry milk in 10 mM Tris-HCl with 0.1% Tween 20, pH 7.5 (TBST). The membrane was incubated with 1:1000 dilution of anti-MMP-9 or anti-TIMP-1 antibody (Calbiochem, Gibbstown, NJ) overnight at 4°C followed by incubation in 1:5,000 dilution of HRP-linked anti-mouse IgG for 1 h at room temperature. Mouse anti-β-actin monoclonal antibody (1:10,000; Sigma, Saint Louis, MO) was used as the loading control in this experiment. To reveal the reaction bands, the membrane was reacted with ECL Western blot detection system (Amersham, Piscataway, NJ) and exposed on X-ray film. Bio-Rad GS-800 Calibrated Densitometer analysis system (Bio-Rad, Hercules, CA) was used to quantitate the Western blots. This system can select the contour of the band, subtract the background and calculate the density.
Statistical analysis
All data were expressed as mean ± SEM. The statistical analysis methods are one-way ANOVA with Student-Newman-Keuls test. Differences in values were considered significant if P < 0.05.
Results
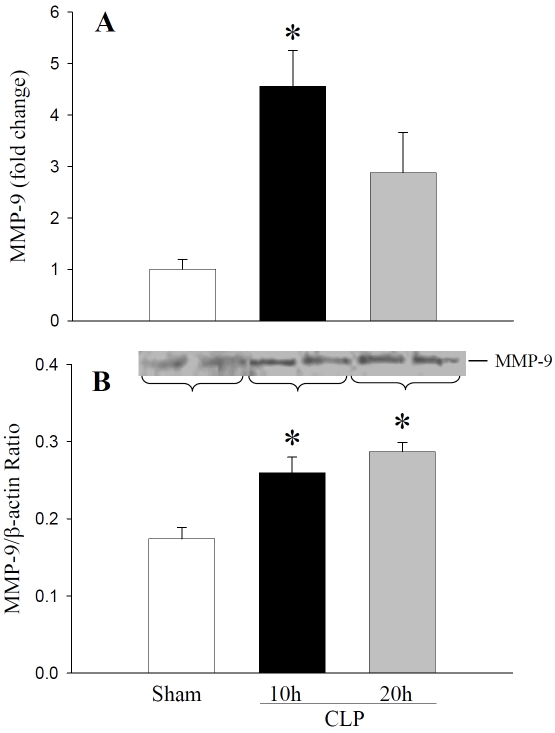
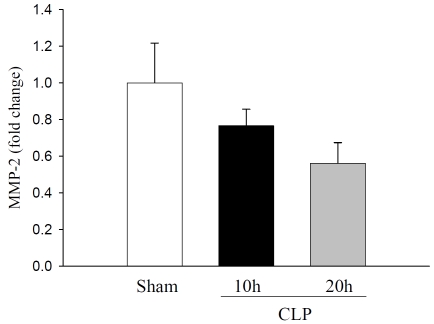
In this study, we focused on the modulation of MMP-9 and TIMP-1 in liver tissues of septic rats. Sepsis was produced by the CLP method. Changes in mRNA and protein expressions of liver MMP-9, MMP-2, and TIMP-1 were assessed at 10 h and 20 h after CLP. At 10 h after CLP, MMP-9 mRNA expression was increased 4.6-fold compared to sham control and remained elevated 3.0-fold at 20 h CLP (Figure 1A). Similarly, total immunoreactive MMP-9 was increased to1.5-fold and 1.7-fold at 10 h and 20 h after CLP as compared to sham rats, respectively (Figure 1B). Liver tissue samples showed a basal level of MMP-9 protein in sham animals. In contrast, MMP-2 mRNA expression was not significantly altered either at 10 h or 20 h after CLP (Figure 2).
Figure 1.
Hepatic MMP-9 mRNA and protein expressions in septic rats: Liver tissues from sham rats, and rats subjected to sepsis by CLP for 10h and 20 h were examined for MMP-9. (A) MMP-9 mRNA expression was assessed by quantitative real time PCR using primers specific for rat MMP-9. Results are shown as fold change over GAPDH levels. (B) MMP-9 protein levels were measured by Western blotting using anti-MMP-9 antibodies. Antibody to β-actin is used as the internal control. Results are shown as MMP-9/β-actin ratio in arbitrary densitometric units. Data are presented as mean ± SE (n=4) and compared by oneway analysis of variance and Student-Newman-Keuls method: *P < 0.05 versus Sham group.
Figure 2.
Hepatic MMP-2 mRNA expression in septic rats: Liver tissues from sham rats and septic rats were examined for MMP-2 mRNA expressions by quantitative real time PCR using primers specific for rat MMP-2 and results are expressed as fold change over GAPDH. Data are presented as mean ± SE (n=4) and compared by one-way analysis of variance and Student-Newman-Keuls method.
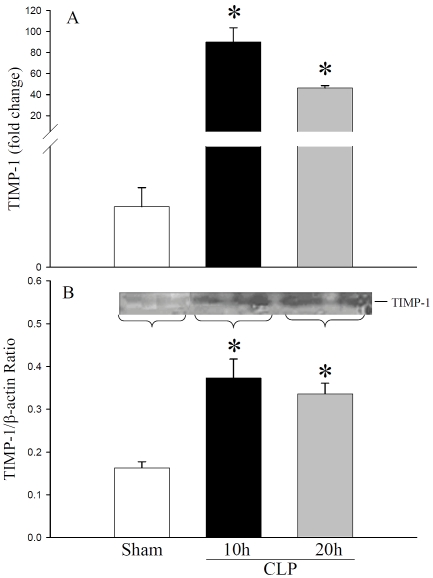
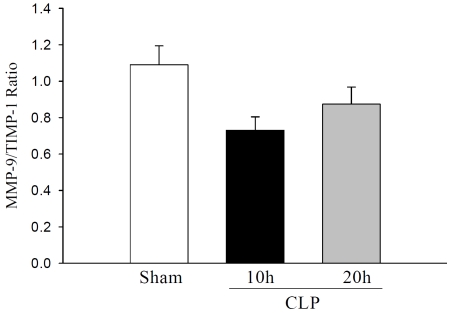
Interestingly, TIMP-1 mRNA expression was significantly increased to 89-fold at 10 h CLP and remained elevated to 46-fold at 20 h CLP as compared to sham control (Figure 3A). Immuno-reative TIMP-1 was also detected in sham rats, and was increased to 3.0-fold and 2.0-fold at 10 h and 20 h after CLP, respectively (Figure 3B). A recent study in septic patients showed that MMP-9 to TIMP-1 ratio is lower in septic patients than healthy controls [11]. Therefore, we compared MMP-9 and TIMP-1 protein levels in septic rats. The MMP-9 to TIMP-1 ratio in sham animals were 1.09 ± 0.10. At 10 h and 20 h after CLP, this ratio decreased to 0.73 ± 0.07 and 0.87 ± 0.09, respectively (ANOVA, P=0.059, Figure 4). These results indicated the elevation of hepatic MMP-9 and TIMP-1 level following CLP sepsis and that MMP-9/TIMP-1 ratio may be used as indictators of severity of sepsis.
Figure 3.
Hepatic TIMP-1 mRNA and protein expressions in septic rats: Liver tissues from sham and septic rats were examined for TIMP-1. (A) TIMP-1 mRNA expressions were determined by quantitative real time PCR using primers specific for TIMP-1 and results are expressed as fold change over GAPDH. (B) TIMP-1 protein levels were assessed by Western blotting using anti-TIMP-1 antibodies. Results are shown as TIMP-1/β-actin ratio. Data are presented as mean ± SE (n=4) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. *P < 0.05 versus Sham group.
Figure 4.
Ratio between MMP-9 and TIMP-1 protein in septic rats: Results are expressed as MMP-9/TIMP -1 ratio in arbitrary densitometric units. Data are presented as mean ± SE (n=4) and compared by one -way analysis of variance (ANOVA) and Student-Newman-Keuls method.
Discussion
Within the extracellular matrix, MMPs are a major group of enzymes that regulate extra cellular matrix (ECM) composition and are critical for the normal development and function of the organism. By regulating the integrity and composition of the ECM structure, these enzyme systems play a pivotal role in the control of signals elicited from matrix molecules, which regulate cell proliferation, differentiation, and cell death. In the immune system, the cleavage of denatured collagens of the extracellular matrix, which are present in the basement membrane, helps lymphocytes and other leukocytes to enter the blood and lymph circulations. Similarly, MMPs assist in the peripheralization of leukocytes in response to chemokines into sites of infection [12]. MMP-9 has been found to process cyto-kines and chemokines, resulting in modulation of immune functions [12].
Ample evidence is available regarding the involvement of MMP pathways in the inflammatory response; therefore, they are of particular interest in shock pathophysiology. Elevated levels of MMP-9 have been measured in plasma and corresponding monocyte messenger RNA in patients with septic shock and that treatment with polymyxin B immobilized on fibers (PMX-F), a reportedly effective treatment for septic shock, attenuated these effects on MMP-9 in the patients [13]. Previous results indicate that TNF-α, p38 and p42/44 MAPK, and MMP-9 increased significantly in CLP septic rats and that pretreatment with CMT-3, a cytokine and protease inhibitor, blocked these activities as well as the ensuing septic shock [2, 3]. Additional results also showed for the first time increases in hepatic MMP-9, TGF-β, and caspase-3 activity, indicating hepatic injury during CLP [4]These alterations were attenuated by post treatment with CMT-3 which increased TIMP-1 level and thereby inhibited MMP-9, which in turn decreased TGF-β1 and caspase-3 signaling pathways along with improvement in survival [4].
Inhibition of MMP-2 and MMP-9 by CMT-3 in a clinically relevant model of sepsis induced lung injury, reduces pulmonary injury and improves survival in a dose dependent fashion [14]. LPS-induced cardiac dysfunction is associated with a loss in ventricular MMP-2 activity and the release of MMP-9 from the heart. MMP inhibitors can significantly preserve cardiac mechanical function during septic shock [15]. All these findings together with our present observation of increased MMP-9 level in CLP septic rats strongly suggest that MMP-9 plays a major role in the pathogenesis of shock. In our studies, unlike MMP-9, MMP-2 levels were not significantly altered. This is consistent with previous studies where there were no differences of MMP-2 levels detected between healthy controls and either septic patients or healthy volunteers infused with low dose LPS [9, 16].
Extracellular matrix degeneration and deposition is tightly controlled by MMPs and their inhibitors (TIMPs). Therefore, MMPs and TIMPs play a critical role in the immune response during inflammatory diseases associated with extracellular matrix degradations [17]. The imbalance between MMPs and TIMPs is considered to be important in the inflammatory process. TIMPs have biological activities which are independent of MMP-inhibitory activities [18]. In the present study, we have observed elevated levels of TIMP-1 at 10 h and 20 h after CLP sepsis. Our results are consistent with a recent study which demonstrates that levels of TIMP-1 are significantly higher in septic patients compared to healthy controls. Moreover, non-survivors of severe sepsis have higher TIMP-1 levels compared to survivors [9]. In another study it was shown that non-survivors of sepsis patients had lower levels of MMP-9, higher levels of TIMP-1 than that of survivors [11]. Interestingly, MMP-9/TIMP-1 ratio was lower in non-survivors than either healthy controls or survivors of sepsis. These studies and our current study suggest elevated level of TIMP-1 and lower MMP-9/TIMP -1 ratio is an important predictor of mortality in severe sepsis.
In conclusion, our results showed elevation of hepatic MMP-9 and TIMP-1 level following CLP. The data obtained from this investigation provide valuable information and insight regarding septic shock. Further studies with new inhibitors of MMP-9, and TIMP-1 are needed to elucidate their role to prevent hepatic disorder and mortality during sepsis.
Acknowledgments
This investigation was partially supported by National Institutes of Health (NIH) grants.
References
- 1.Gadek JE, Pacht ER. The interdependence of lung antioxidants and antiprotease defense in ARDS. Chest. 1996;110:273S–277S. doi: 10.1378/chest.110.6_supplement.273s. [DOI] [PubMed] [Google Scholar]
- 2.Maitra SR, Bhaduri S, Valane PD, Tervahartiala T, Sorsa T, Ramamurthy N. Inhibition of matrix metalloproteinases by chemically modified tetracyclines in sepsis. Shock. 2003;20:280–285. doi: 10.1097/00024382-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Maitra SR, Bhaduri S, Chen E, Shapiro MJ. Role of chemically modified tetracycline on TNF -alpha and mitogen-activated protein kinases in sepsis. Shock. 2004;22:478–481. doi: 10.1097/01.shk.0000140298.40440.51. [DOI] [PubMed] [Google Scholar]
- 4.Maitra SR, Shapiro MJ, Bhaduri S, El-Maghrabi MR. Effect of chemically modified tetracycline on transforming growth factor-beta1 and caspase-3 activation in liver of septic rats. CritCare Med. 2005;33:1577–1581. doi: 10.1097/01.ccm.0000169880.82060.f7. [DOI] [PubMed] [Google Scholar]
- 5.Woessher J. The family of metalloproteinases. Ann NY Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- 6.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 7.Keck T, Balcom JHt, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188–201. doi: 10.1053/gast.2002.30348. [DOI] [PubMed] [Google Scholar]
- 8.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17:519–528. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman U, Bertsch T, Dvortsak E, Liebetrau C, Lang S, Liebe V, Huhle G, Borggrefe M, Brueckmann M. Matrix metalloproteinases and their inhibitors are elevated in sepsis. Prognostic value of TIMP-1 in severe sepsis. Scandinv. J. Infect. Disease. 2006;38:867–872. doi: 10.1080/00365540600702058. [DOI] [PubMed] [Google Scholar]
- 10.Wu R, Dong W, Zhou M, Cui X, Simms HH, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Lorente L, Martin MM, Labarta L, Diaz C, Sole-Violan J, Blanquer J, Orbe J, Rodriguez JA, Jimenez A, Borreguero-Leon JM, Belmonte F, Medina JC, Lliminana MC, Ferrer-Aguero JM, Ferreres J, Mora ML, Lubillo S, Sanchez M, Barrios Y, Sierra A, Paramo JA. Matrix metalloproteinase-9, -10, and tissue inhibitor of matrix metalloproteinases-1 blood levels as biomarkers of severity and mortality in sepsis. Crit Care. 2009;13:R158. doi: 10.1186/cc8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Ebihara I, Shimada N, Shoji H, Koide H. Modulation of plasma metalloproteinase-9 concentrations and peripheral blood monocyte mRNA levels in patients with septic shock: effect of fiber-immobilized polymyxin B treatment. Am J Med Sci. 1998;316:355–360. doi: 10.1097/00000441-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg J, Halter J, Schiller HJ, Dasilva M, Landas S, Gatto LA, Maisi P, Sorsa T, Rajamaki M, Lee HM, Nieman GF. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. 2003;111:185–195. doi: 10.1016/s0022-4804(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 15.Lalu MM, Gao CQ, Schulz R. Matrix metalloproteinase inhibitors attenuate endotoxemia induced cardiac dysfunction: a potential role for MMP-9. Mol Cell Biochem. 2003;251:61–66. [PubMed] [Google Scholar]
- 16.Albert J, Radomski A, Soop A, Sollevi A, Frostell C, Radomski MW. Differential release of matrix metalloproteinase-9 and nitric oxide following infusion of endotoxin to human volunteers. Acta Anaesthesiol Scand. 2003;47:407–410. doi: 10.1034/j.1399-6576.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 17.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 18.Chromek M, Tullus K, Lundahl J, Brauner A. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun. 2004;72:82–88. doi: 10.1128/IAI.72.1.82-88.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]