Abstract
It has been known for decades that ionizing radiation (IR) promotes carcinogenesis and high-linear energy transfer (LET) IR has a higher risk than low-LET IR for carcinogenesis; however, the mechanism remains unclear. MicroRNAs (miRNAs) have a critical effect on carcinogenesis through post-transcriptional modification. In this study, our purpose is to explore whether miRNAs are involved in IR-(especially high-LET IR) promoted liver carcinogenesis. We showed here that among several hundred miRNAs, miR-21 was the only one that increased 6 folds in high-LET IR-promoted mouse liver tumors when compared with that in the non-irradiated liver tissues. We also showed that miR-21 was up-regulated in human or mouse hepatocytes after exposure to IR, as well as in liver tissues derived from whole body irradiated mice. The increased level of miR-21 was more significant in high-LET irradiated cells or liver tissues. After the non-irradiated, low-LET or high-LET irradiated human hepatocytes were over-expressed with miR-21, these cells became tumorigenesis in nude mice. The tumors derived from high-LET-irradiated-cells were largest, and accompanied by more significant changes in the miR-21-targets: PTEN and RECK. In addition, we showed that IR-induced up-regulation of miR-21 depended on the up-regulation/activation of AP-1 (at an earlier time, within 2 h) and the ErbB/Stat3 pathway (at a later time, more than 2 h), which was also IR dose dependent. Taken together, we conclude that IR-induced up-regulation of miR-21 plays an important role in IR (especially high-LET IR)-promoted liver carcinogenesis.
Keywords: microRNA, miR-21, ionizing radiation, carcinogenesis
Introduction
It is known that terrestrial ionizing radiation (IR), such as γ- or x-ray (possibly above certain doses, such as 0.1 Gy [1-3]), increases the age-related risk of most common human cancers [4, 5]. People are constantly exposed to cosmic radiation and to γ-rays from naturally occurring radionuclides in the ground and building materials. Natural background radiation delivers an average individual dose of about 0.02-0.03 Gy/annum and variations of a factor of three are frequent around the world, but it is not difficult to find areas with doses 10 times greater or more, mainly due to high levels of radon gas and its decay products [5]. By far the largest component of anthropogenic radiation exposure comes from medical uses, with much smaller contributions from other sources such as nuclear weapons testing fallout, discharges from nuclear installations and miscellaneous uses of radiation (e.g. industrial radiography). IR-promoted carcinogenesis is initiated by IR-induced DNA damage that involves multi-pathways and affects genomic instability; however, the whole picture remains unclear. Compared with γ- or x-ray (low linear energy transfer (LET) radiation), high-LET radiation-induced DNA damage (generated by high charge energy (HZE) particles, high-energy ions in space radiation or by a special radiotherapy machine that is being increasingly and effectively used for targeted cancer therapy) is more difficult to be repaired, which reflects a higher relative biological effectiveness (RBE). Due to a lack of epidemiologic and mechanical data, it is highly uncertain how high the risk is for increasing carcinogenesis in astronauts following exposure to space radiation [6]. The results from animal studies showed that high-LET IR promoted more tumorigenesis than low-LET IR [7, 8], indicating an excess relative risk (ERR) from high-LET IR for promoting carcinogenesis. Although it is known that most cases of hepatocellular carcinoma (HCC) are secondary to either a hepatitis viral (B or C) infection or alcoholism cirrhosis [9], animal experiments have demonstrated that IR could increase the HCC frequency [3]. Recently, it was reported that HZE particle-irradiated mice had an even higher frequency of HCC than γ-ray irradiated mice [10], suggesting that people who exposed to IR, especially high-LET IR (such as people who received radiotherapy or astronauts) might increase the frequency of HCC, however, the mechanism remains unclear.
MicroRNAs (miRNAs) are a class of small non-coding RNAs (ncRNAs) with ∼22 nucleotides in length. To date, more than 1000 miRNAs have been identified and more miRNA genes in the human genome are estimated to be identified. In general, miRNAs post-transcriptionally negatively regulate gene expression by binding to the 3'-untranslatated region (UTR) of the targeted messenger RNAs (mRNAs) to inhibit the genes translation [11]. One miRNA can target multiple mRNAs and one mRNA can be targeted by multiple miRNAs [12]. Most mammalian mRNAs are conserved targets of miRNAs [13]. The expressions of miRNAs are usually tissue or developmental stage specific, and the change in the miRNA expression pattern is already found in many different cancers, such as brain, breast, lung, colon, prostate, bladder and pancreatic tumors including HCC [14-24]. Now more and more data has demonstrated that miRNAs play an important role in carcinogenesis. There are several possible mechanisms for miRNAs to affect tumorigenesis [16, 25]. Up-regulation and amplification of a miRNA that targets one or more tumor suppressors might lead to increasing oncogene expression and carcinogenesis; and loss or epigenetic silencing of a miRNA that targets one or more oncogenes could trigger multiple oncogenic pathways.
Based on the information above, it is possible that IR-modulated expression of miRNA contributes to IR-promoted carcinogenesis. In this study we were interested in testing this hypothesis and focused on studying whether miRNAs are involved in IR-promoted liver carcinogenesis. We show here for the first time that miR-21 is involved in IR-promoted hepatocellular carcinogenesis. We demonstrate that IR induced the up-regulation of miR-21 through the up-regulation and/or activation of AP-1 and ErbB/Stat3. In addition, our results indicate that high-LET IR is more efficient than low-LET IR for promoting miR-21-induced tumorigenesis.
Materials and methods
Cell lines, mice and irradiation
Immortalized human hepatocytes (LO2 cells) were obtained from Dr. Rui-Xia Sun's laboratory in the Shanghai Institute of Liver Disease and immortalized mouse hepatocytes were obtained from Dr. Philip Troilo's laboratory [26]. These cells were adapted to grow in DMEM medium supplement with 10% calf serum. The mice (CBA/CaJ, 6-8 week or 6-8 month old, purchased from Jackson Laboratory) were exposed to IR. Low-LET radiation was carried out using an x-ray machine (X-RAD 320, N. Branford 320 kV, 10 mA, 2-mm aluminum filtration for cells and the filter with 1.5 mm aluminum, 0.8 mm tin and 0.25 mm copper for mice) in our laboratory and high-LET radiation was carried out using an alternating-gradient synchrotron (Fe ions, 1 GeV/amu) at Brookhaven National Laboratory (BNL). The dose rates for both high-LET IR and low-LET IR were about 1 Gy/min. The L05 cells were generated by collecting the surviving LO2 cells after exposure to 0.5 Gy of x-ray (low-LET) and the H05 cells were the surviving LO2 cells after exposure to 0.5 Gy of Fe ions (high-LET). These cells had been subcultured for more than 10 generations before using in any experiment.
Plasmid constructs and transfection
The sequence of pri-miR-21 shown below was amplified from HepG2 cell (human hepatocellular carcinoma cells, purchased from ATCC) ge-nomic DNA by using the primer pairs: 5'-TAG AAG CTT TTA ACA GGC CAG AAA TGC CTG-3’ and 5'-TAT CTC GAG AGG ACC AGA GTT TCT GAT TA-3’ and inserted into pC-DNA3 plasmid (Invitrogen) at HindIII and XhoI sites.
TTAACAGGCCAGAAATGCCTGGGTTTTTTTGGTTTGTTTTTGTTTTTGTTTTTTTATCAAATCCTGCCTGACTG TCTGCTTGTTTTGCCTACCATCGTGACATCTCCATG GCTGTACCACCTTGTCGGGTAGCTTATCAGACTGAT GTTGACTGTTGAATCTCATGGCAACACCAGTCGATGGGCTGTCTGACATTTTGGTATCTTTCATCTGACCATC CATATCCAATGTTCTCATTTAAACATTACCCAGCATC ATTGTTTATAATCAGAAACTCTGGTCCT
The underlined is the precursor of hsa-miR-21. The non-irradiated LO2 cells (NR) or irradiated LO2 cells (L05 and H05, as described above) were transfected individually with pcDNA3.0 plasmid alone or the plasmid encoding miR-21 by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Stable cell lines in 500 μg/ml of G418 were selected and expression of miR-21 was confirmed by qRT-PCR analysis.
miRNAs microarray and qRT-PCR
Total RNA was isolated from the hepatocytes, fresh liver tissue or paraffin-embedded liver tissue by using a miRNeasy MINI kit or a miR-Neasy FFPE kit(Qiagen) according to the manufacturer's instructions. The paraffin-embedded liver tissues were kindly provided by Dr. Ullrich (University of Texas Medical Branch) and Dr. Weil (Colorado State University). These tissues were derived from mice 2 years after receiving 1 Gy of Fe ion (high-LET) IR. The miRNA microarray was analyzed by LC Science Inc with miRBase Version 13.0. Additional paraffin-embedded liver tissues were kindly provided by Drs. Wolo-schak and Paunesku from the Northwestern University Tissue Bank. These liver tissues were derived from mice 2 years after receiving 0.5 Gy neutron (high-LET) IR. cDNA was synthesized from 0.5 μg RNA with gene specific primer in 15 μl reaction using the TaqMan MicroRNA Reverse Transcription Kit and TaqMan® MicroRNA Assay hsa-miR21# and RNU48 (Applied Biosystems) according to the manufacturer's instructions. A product from the RT reaction was diluted 1:20 and performed in triplicate with a TaqMan Universal PCR Master Mix for qRT-PCR analysis according to the manufacturer's instructions. Each qRT-PCR experiment was repeated at least twice.
Western blot analysis
Whole cell lysates were prepared with RIPA buffer. Standard Western blot was carried out. The antibodies against ErbB2, c-Jun, c-fos, Stat3, phospho-Stat3 (tyr705), PTEN and RECK were purchased from Cell Signaling Technology. The antibodies against EGFR, p-Tyr(PY99) and beta-actin were purchased from Santa Cruz Biotechnology. The Western blot signals were analyzed by using a PhosphorImager (GE Healthcare).
siRNA transfection
The siRNAs against human ErbB2, c-Jun or c-Fos, as well as a control RNA, were purchased from Santa Cruz Biotechnology. LO2 cells were transfected with these siRNAs, respectively, with a siRNA transfection reagent from Santa Cruz Biotechnology according to the manufacturer's instructions. The cells were irradiated at 24 h after transfection. The cells were collected at 1 h and 72 h after IR, respectively.
Tumor xenografts
Thirty six 4-5 week old female athymic nude mice (Harlan Laboratories) were divided into 6 groups (6 mice/group) and injected with different stable cell lines as follows: NR-vand NR-miR -21 (non-irradiated human hepatocytes (LO2) transfected with vector alone or the vector containing miR-21); L05-v and L05-miR-21 (L05 cells transfected with vector alone or the vector containing miR-21); H05-v and H05-miR-21 (H05 cells transfected with vector alone or the vector containing miR-21). Each mouse was subcutaneously injected with 4 × 106 cells in 50 μl of sterile PBS at two sides of the body. The mice were sacrificed at four weeks after injection and tumors were weighed.
Results
miR-21 is highly-expressed in hepatocellular carcinomas from irradiated mice
To investigate whether miRNAs were involved in IR-promoted hepatocellular carcinomas, we compared the miRNA expression profiles in the liver carcinoma from mice at 2 years after exposure to 1 Gy of Fe ions (high-LET IR) and in the liver tissues from control mice. The results showed that among more than 700 mouse miRNAs, miR-21 was the only one with expression levels ∼6 folds higher in the liver carcinoma from high-LET irradiated mice versus in liver tissues from the control mice, although some miRNAs had minor differences in expression levels (less than 2 folds) (Figures 1A, B). The qRT-PCR data confirmed the big difference in the miR-21 expression between the two group samples (data not shown). To exclude the possibility that the results might only reflect specific incidents in a few samples (three hepa-tocellular carcinomas from three irradiated mice), we collected 10 additional mouse liver tumor tissues from the Northwestern University Tissue Bank, the mice were sacrificed 2 years after exposure to 0.5 Gy of neutron (high-LET) IR. The qRT-PCR data showed that miR-21 was over-expressed in all the liver tumor tissues from irradiated mice when compared with the liver tissues from non-irradiated controls (Figures 1C, D). These results indicate that over-expression of miR-21 is a common feature in IR-promoted liver cancers and suggest a functional link between miR-21 over-expression and IR-promoted liver tumors.
Figure 1.
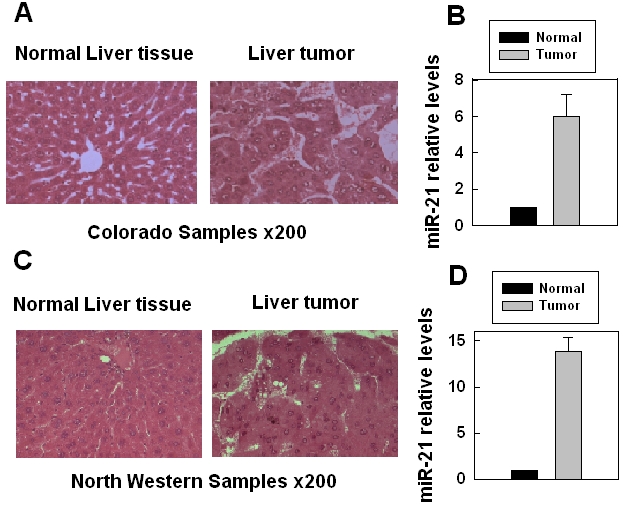
Over-expression of miR-21 in liver carcinoma from irradiated mice. (A) Histology of liver tissues or tumors (H&E, × 200) from non-irradiated or high-LET irradiated (1 Gy) mice. (B) The miR-21 expression level was derived from the microarray data (LC, Sciences, LLC). The mice were irradiated with 1 Gy high-LET IR (iron) and the mice were sacrificed two years after IR. The tumor data were mean + SE obtained from three mice. (C) Histology of liver tissues or tumors (H&E, × 200) from non-irradiated or high-LET irradiated (1 Gy) mice. (D) The miR-21 expression level was analyzed by qRT-PCR. The mice were irradiated with 0.5 Gy high-LET IR (neutron) and the mice were sacrificed two years after IR. The tumor data were mean +SE obtained from ten mice.
Ionizing radiation promotes miR-21 expression in hepatocytes and liver tissues
To study whether there was a functional link between IR-promoted hepatocellular carcinogenesis and the change of the miRNAs expression, we compared the microarray data from the mouse or the human hepatocytes that survived 0.5 Gy high or low-LET IR. The results showed that among several hundred mouse or human miRNAs, only the miR-21 level consistently increased in both irradiated mouse hepatocytes and human hepatocytes when compared with their non-irradiated counterparts (Figure 2A). The miR-21 level was higher in high-LET irradiated cells (Figure 2A). Similar results were observed in liver tissues derived from the mice at one day or 20 days after 0.5 Gy low or high-LET IR: only the miR-21 level consistently increased in all irradiated samples (Figure 2B), especially in high-LET irradiated samples (Figure 2B). These results indicate that IR could promote miR-21 expression in hepatocytes or liver tissues, even though the dose was only 0.5 Gy. High-LET IR dramatically increased the miR-21 level when compared with low-LET IR, particularly in the liver tissues from the mice at 20 days after IR (Figure 2B). Recently, two reports from different groups also showed that the miR-21 expression increased in irradiated human cells [27, 28], confirming that the miR-21 expression is stimulated by IR. miR-21 targets multiple pathways that are involved in p53, TGF-β, mitochondrial apoptosis and tumor-suppressor etc to promote carcinogenesis [24, 29-37]. It is reasoned that IR-stimulated miR-21 expression plays a very important role in promoting carcinogenesis. Here, we focused on elucidating how IR promotes miR-21 expression.
Figure 2.
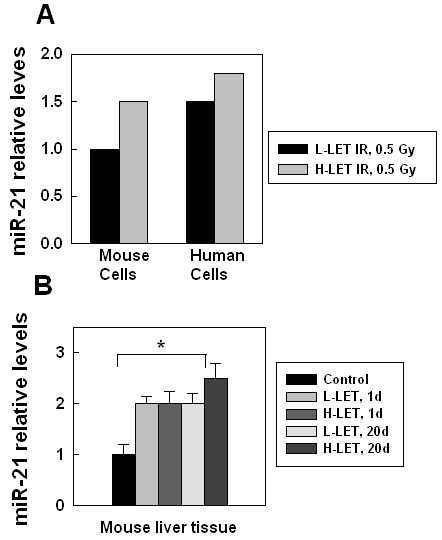
IR-stimulated miR-21 expression in human or mouse hepatocytes and liver tissue. (A) miR-21 expression in mouse or human hepatocytes survived 0.5 Gy low-LET or high-LET IR after more than 10 generations. The miR-21 level was derived from the microarray data (LC, Sciences, LLC). The analysis is based on the non-irradiated controls (as 1). (B) miR-21 expression in liver tissue from mice (6-8 month old) at 1 day or 20 days after low or high-LET IR (0.5 Gy). The miR-21 level was derived from the microarray data (LC, Sciences, LLC). Each group had 3 mice, the analysis is based on the non-irradiated controls (as 1) *, p < 0.05.
IR stimulated/activated AP-1 and Stat3, which correlates with the up-regulation of miR-21
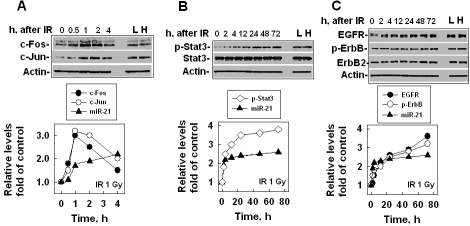
Similar to mRNA expression, miRNA expression requires transcriptional factor regulation. By searching literature, we found that two transcriptional factors, AP-1 and Stat3, could trigger miR-21 expression [37-40]. We were interested in studying whether IR-induced miR-21 expression was via stimulating these transcriptional factors/pathways and, if so, which transcriptional factor/pathway, AP-1, Stat3 or both was essential for IR-induced miR-21 expression. For this purpose, we compared miR-21, AP-1 and Stat3 expression/activation in human hepatocytes at different time points after IR. We also compared the data from the hepatocytes that survived (more than 10 generations) 0.5 Gy low-LET (L) or high-LET (H) IR exposures. The results showed that AP-1 (c-Fos and c-Jun) expression increased at 0.5 h, reached the maximum at 2 h and then decreased to the baseline at 4 h after IR (1 Gy, x-ray) exposure (Figure 3A). The AP-1 expression pattern induced by IR only matched the miR-21 expression within 2 h after IR (Figure 3A), suggesting that besides AP-1, other factor(s) also contributed to the increased miR-21 level at a later time (more than 2 h) after IR. This hypothesis is supported by the Stat3 data, which showed that the phosphorylated levels of Stat3 (at the Tyr705 site) increased at 2 h after IR (1 Gy) and continually increased until 72 h after IR (Figure 3B). The increased phosphorylation levels of Stat3 matched the IR-induced miR-21 expression, although the Stat3 protein level did not change much (Figure 3B). Since this phosphorylated Stat3 represents the activation of Stat3, these data suggest that Stat3 might be the major transcriptional factor for stimulating the expression of miR-21 at a later time (more than 2 h) after IR. Stat3 could be phosphorylated by ErbB1 (EGFR) or ErbB2 [41, 42]. Next, we examined which ErbB family member, EGFR or ErbB2 was the major activator for IR-stimulated Stat3 phosphorylation. The results showed that the EGFR level started to increase at 2 h and continually increased at 72 h after IR (Figure 3C). The ErbB2 level did not increase as dramatically as the EGFR level did; however, the phosphorylation level of ErbB (including EGFR and ErbB2) clearly increased with time after IR (Figure 3C), suggesting that both EGFR and ErbB2 involved IR-stimulated Stat3 phosphorylation. Although we could not measure these protein levels/activations for a longer time (more than 72 h) after radiation because of the cell growth situation, the increased or activated levels for these proteins in H05 (H) or L05 (L) cells (Figures 3B, C) suggest that the expression of miR-21 in the cells at a later time (more than 72 h) after IR could be continually stimulated by the ErbB/Stat3 pathway. These AP-1 and ErbB/Stat3 data indicate that miR-21 might be stimulated by AP-1 at an earlier time (within 2 h) and by the ErbB/Stat3 pathway at a later time (more than 2 h).
Figure 3.
IR-stimulated miR-21 expression correlates with up-regulation/activation of AP-1 and ErbB/Stat3. (A) The levels of AP-1 (c-Fos and c-Jun) or miR-21 in LO2 (human hepatocytes) cells at different times after 1 Gy exposure. Actin was used as an internal loading control. L: the cells (LO2) that survived 0.5 Gy low-LET IR. H: the cells (LO2) that survived 0.5 Gy high-LET IR. Top panel: the image from Western blot. Bottom panel: analysis of the protein levels shown in the top panel by ImageQuant. The miR-21 level was measured by qRT-PCR. (B) The levels of Stat3, phosphorylated Stat3, and miR-21 in LO2 cells at different times after 1 Gy exposure. (C) The levels of ErbB (EGFR and ErbB2), phosphorylated ErbB and miR-21 in LO2 cells at different times after 1Gy exposure.
AP-1 and ErbB/Stat3 are essential for IR-stimulated miR-21 expression
To verify our prediction, we measured the levels of miR-21 and its targets after knocking down AP-1 or ErbB/Stat3. Our AP-1 data showed that when either the c-Fos or c-Jun level was knocked down with the siRNAs (Figure 4A), the miR-21 level decreased at 1 h after IR (Figure 4B) and the protein levels of PTEN and RECK (two of the miR-21 targets) increased (Figure 4A). Knocking down both c-Fos and c-Jun enhanced the changed levels of miR-21 and its two targets when compared with knocking down c-Fos or c-Jun alone (Figures 4A, B), confirming that both c-Fos and c-Jun are required for stimulating the up-regulation of miR-21 by IR at an earlier time. The ErbB/Stat3 data showed that after EGFR or ErbB2 was knocked down by the siRNAs, the phosphorylation level of Stat3 decreased (Figure 4C) and, most importantly, the miR-21 level also decreased (Figure 4D) and the miR-21 targets (PTEN and RECK) increased (Figure 4C). Knocking down both EGFR and ErbB2 specifically enhanced the changed levels of miR-21, Stat3 phosphorylation and its two targets when compared with knocking down EGFR or ErbB2 alone (Figures 4C, D), confirming that both EGFR and ErbB2 are required for stimulating the up-regulation of miR-21 by IR at a later time. These results clearly indicate that IR-stimulated miR-21 expression is via IR-induced expression/activation of AP-1 (at an earlier time) and ErbB/Stat3 (at a later time).
Figure 4.
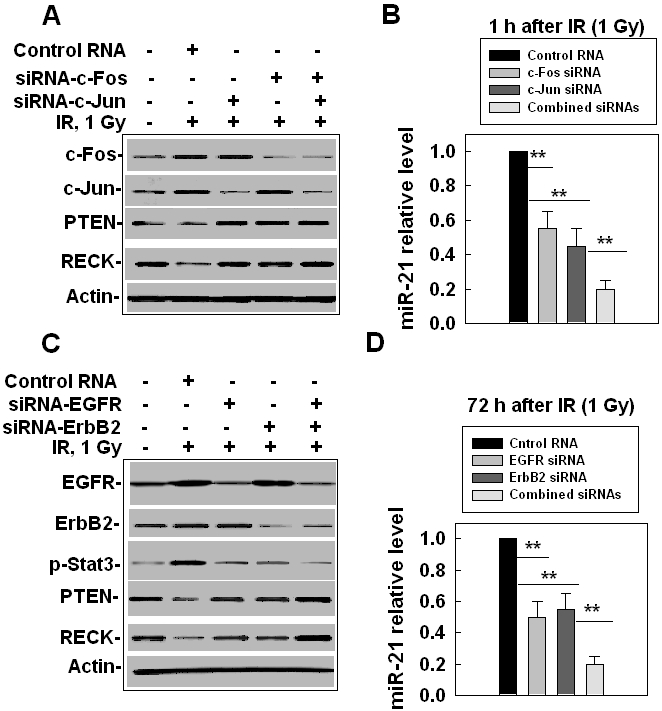
IR-stimulated miR-21 expression is via AP -1 at an earlier time and ErbB/Stat3 at a later time. (A) The effects of AP-1 siRNAs on the expression of miR-21 targets. LO2 cells were treated with c-Jun siRNA, c-Fos siRNA or both for 24 h and were then irradiated with 1 Gy. The cells were collected at 1 h after IR and the protein levels were measured by Western blot. Actin was used as an internal loading control. (B) The effects of AP-1 siRNA on the expression of miR-21. The cells were collected at 1 h after IR, RNA was prepared and the miR-21 levels were detected by qRT-PCR. The results were obtained from two separate experiments with 6 samples of each treatment, **, p< 0.01. (C) The effects of EGFR siRNA and ErbB2 siRNA on Stat3 activation and the expression of miR-21 targets. LO2 cells were treated with EGFR siRNA, ErbB2 siRNA, or both for 24 h and were irradiated with 1 Gy. The cells were then collected at 72 h after IR and the protein levels were measured by Western blot. (D) The effects of EGFR siRNA and ErbB2 siRNA on the miR-21 expression. The cells were treated and collected as described above, RNA was prepared and the miR-21 levels were detected by qRT-PCR. The results were obtained from two separate experiments using a total of 6 samples for each treatment, **, p< 0.01.
miR-21 promotes IR-induced hepatocellular carcinogenesis
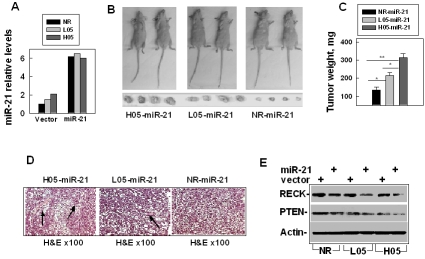
Although over-expression of miR-21 was accompanied with many kinds of cancer [14-23] and up-regulating the miR-21 expression promoted tumor growth [43, 44], it remains unclear whether up-regulating miR-21 in non-tumorigenesis cells could promote such cells becoming tumorigenesis. To answer this question, we made the miR-21 construct and transfected the plasmid encoding the pri-miR-21 in the following three human hepatocyte lines: NR (the non-irradiated LO2 cells, control human hepatocytes), L05 (the human hepatocytes that survived 0.5 Gy low-LET IR) or H05 cells (the human hepatocytes that survived 0.5 Gy high-LET IR). We then examined the miR-21 levels in the colonies from each transfection. The results showed that the transfection of the vector alone did not affect the miR-21 level, and the miR-21 level increased differently in the cells trans-fected with the miR-21 construct. We screened the cell lines (NR-miR-21, L05-miR-21 or H05-miR-21) and selected the cell lines with the similar high levels of miR-21 for the xenograft animal experiments (Figure 5A). Next, we examined the tumorigenesis of these cell lines: NR-v, L05-v or H05-v cells (transfected with vector alone) or NR-miR-21, L05-miR-21 or H05-miR-21 cells were subcutaneously injected into the nude mice. One month following the injection, we sacrificed the mice and examined the tumorigenesis frequency and tumor weight which indirectly reflected the tumor size. The results showed that all the vector cell lines did not show any tumor growth in the mice; however, all the cell lines over-expressed with miR-21 developed tumors (Figure 5B). To our knowledge, this is the first study to have showed that over-expressing miR-21 could make immortalized human cells become tumorigenesis. These results indicate that miR-21 plays an essential role in promoting tumorigenesis in these cells.
Figure 5.
Up-regulation of miR-21 contributes to IR-promoted hepatocyte tumorigenesis. (A) The levels of miR-21 in NR (non-irradiated LO2 cells), L05 or H05 cells that were transfected with miR-21 or a control vector. (B) Imaging of the sizes of tumors that developed in the nude mice from the cells over-expressed with miR-21: NR-miR-21, the non-irradiated LO2 cells that were over-expressed with miR-21; L05-miR-21, the LO2 cells that survived 0.5 Gy low-LET IR and over-expressed with miR-21; H05-miR-21, the LO2 cells that survived 0.5 Gy high-LET IR and over-expressed with miR-21. Each mouse was injected subcutaneously with the same type cells at two sides of the body. Each group contained 6 mice and 12 injections. (C) Analysis of the tumor weight in the mice from different types of cell lines: NR-miR -21, L05-miR-21 or H05-miR-21: *, P< 0.05; **, P<0.01. (D) Histopathological image of tumors derived from NR-miR-21, L05-miR-21 or H05-miR-21 cells. The tumor tissues were prepared for histopathological slides with hematoxylin and easin (H&E, × 100) staining. The black arrows indicated the small blood vessels. (E) Compare the levels of miR21 targets (RECT and PTEN) in NR-miR-21, L05-miR-21 or H05-miR-21 cells versus that in NR-v, L05-v and H05-v cells. Western blot was performed and Actin was used as an internal loading control.
Interestingly, the tumors that developed from the irradiated cells (L05-miR-21 or H05-miR-21) were bigger than those from the non-irradiated cells (NR-miR-21) (Figures 5B, C). The tumors that developed from the high-LET irradiated cells (H05-miR-21) were even bigger than those from the low-LET irradiated cells (L05-miR-21) (Figures 5B, C). The histopathological slides showed that more small vessels formed in the tumors developed from the irradiated cells (L05 -miR-21 and H05-miR-21), especially from the high-LET irradiated cells (H05-miR-21) than in the tumors developed from the non-irradiated cells (NR-miR-21) (Figure 5D). Since the levels of miR-21 are similar among all these cell lines (Figure 5A), the results of the different tumor sizes derived from these different cell lines suggest that IR (especially high-LET IR) induced other biological changes in the cells that provided an environment that facilitated miR-21 promoting liver tumorigenesis. To test the hypothesis, we compared the levels of PTEN and RECK (the targets of miR-21) in NR-miR-21, L05 -miR-21 and H05-miR-21 cells. The results showed that the levels of PTEN and RECK were the lowest in H05-miR-21 cells and the highest in NR-miR-21 cells (Figure 5E); although, the up-regulated levels of miR-21 in these cell lines were similar (Figure 5A). These results suggest that radiation, especially high-LET IR, could activate other pathways that facilitate the role of miR-21 in liver carcinogenesis.
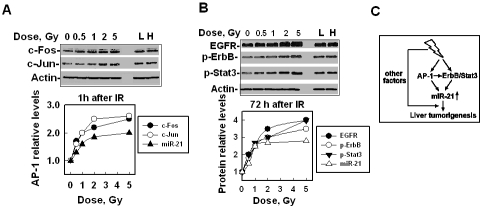
IR-promoted expression of miR-21 depends on the radiation dose
The size of the tumors developed from high-LET irradiated cells is bigger than that from low-LET irradiated cells (Figures 5B, C), which confirms the fact that high-LET radiation has an excess relative risk (ERR) when compared with low-LET IR to promote carcinogenesis [6, 8]. The ERR of carcinogenesis is believed to be related to the fact that the DNA damage induced by high-LET IR is more difficult to repair than by low-LET IR. To examine whether high-LET IR-stimulated expression of miR-21 was also related to ERR, we performed dose-response experiments because it is known that DNA damage induced by IR is linearly dose-dependent. The results showed that the expression/activation levels of AP-1, ErbB/Stat3 and miR-21 upregulated with increasing doses up to 2 Gy and then reached a plateau level until 5 Gy, at an earlier time (1 h) or at a later time (72 h) after IR (Figures 6A, B). These results suggest that IR-induced miR-21 expression is dose dependent under a certain dose (∼ 2 Gy). At this dose range, more damaged DNA might stimulate the activation of multiple carcinogenesis-relevant pathways (including the AP-1 and ErbB/Stat3 pathways) and when the activation reaches the maximal level, more DNA damage cannot further trigger the activation including the expression of miR-21 in the short term. These dose response curves of AP-1, ErbB/Stat3 and miR-21 also match the dose response curve for IR-promoted carcinogenesis [45] that revealed that high doses kill more cells and could not further increase carcinogenesis. After combining all our results, we formed the following model: IR-stimulated AP-1 and ErbB/Stat3 up-regulates miR-21 in the human hepatocytes, which resulted in these cells becoming tumorigenerable. IR, especially high-LET IR, facilitates the function of miR-21, which speeds up the process of tumorigenesis (Figure 6C).
Figure 6.
The effects of IR with different doses on AP-1, ErbB/Stat3 and miR-21 expression/activation. (A) The levels of AP-1 (c-Fos and c-Jun) or miR-21 in LO2 cells at 1 h after different doses of IR exposure. L and H are the same as described in Figure 3. Top panel: the image from Western blot. Bottom panel: analysis of the protein levels shown in the top panel by ImageQuant. The miR-21 level was measured by qRT-PCR. (B) The levels of EGFR, phosphorylated ErbB or Stat3 and miR-21 in LO2 cells at 72 h after different doses of IR exposure. L and H are the same as described above. Top panel: the image from Western blot. Bottom panel: analysis of the protein levels shown in the top panel by ImageQuant. The miR-21 level was measured by qRT-PCR. (C) A summary of the effects of IR-promoted liver carcinogenesis is as follows: IR stimulates miR-21 expression through the up-regulation or activation of AP-1 (at an earlier time) and ErbB/Stat3 (at a later time) pathways. AP-1 could also directly or indirectly activate the ErbB/Stat3 pathways. The over-expression of miR-21 promotes liver tumorigenesis and other IR-stimulated factors that may also facilitate the function of miR-21 and contribute to liver tumorigenesis.
Discussion
In this study, we reported for the first time that IR-stimulated miR-21 expression plays an important role in hepatocellular carcinogenesis. This stimulation depends on IR-induced/activated AP-1 and Stat3. IR could stimulate AP-1 in the following several ways: up-regulating expression [46], enhancing the DNA binding activity of AP-1 [47] and via JNK pathways [48]. We believe that IR-promoted miR-21 expression at an earlier time following IR is mainly due to IR -stimulated AP-1 expression. Our data showed at 4 h after 1 Gy irradiation, the AP-1 level decreased, which is consistent with the previous report [46]. However, at this time, IR-induced JNK activity is still much higher (data not shown) [49], suggesting that the activated JNK has less effects on the decreased AP-1. It is reasoned that AP-1 transcriptional activity depends on its DNA binding activity, which explains that AP-1 triggers miR-21 transcription via several AP-1-binding sites in the miR-21 promoter [39]. AP-1 could also directly or indirectly activate the ErbB pathways. The ErbB receptor tyrosine kinase is a major up-stream regulator of Stat3 that can be activated by mediated multiple signaling pathways to promote carcinogenesis in different organs. Stat3 could stimulate miR-21 expression by binding to its transcriptional enhancer sites [38] and the ErbB/Stat3 activation plays an important role in the regulation of miR-21 expression [37, 40]. The ErbB family includes four members: ErbB 1, 2, 3 and 4. The ErbB activation is mainly reflected by its autophos-phorylation. It is possible that the phosphorylational level of ErbB stimulated by IR could come from all the 4 members. Although we did not measure the levels of ErbB3 and ErbB4, the EGFR (ErbB1) siRNA and ErbB2 siRNA significantly inhibited the IR-stimulated miR-21 expression, excluding the possible involvement of ErbB3 or ErbB4. Based on these results we believe that increased phosphorylational level of ErbB might mainly represent EGFR (ErbB1) and ErbB2.
It is known that IR (might be above certain doses such as 0.1 Gy [1-3]) promotes carcinogenesis in multi-organs, which involves a long term and complicated process. Our results showed that over-expression of miR-21 alone could make immortalized human hepatocytes become tumorigenesis, which was promoted by IR, indicating the importance of miR-21 in carcinogenesis including IR-promoted carcinogenesis. The miR-21 level in IR-promoted liver tumors was ∼6 folds higher than that in non-tumor liver tissues, and up-regulating miR-21 up to 6 folds higher in the hepatocytes than in their counterpart controls makes the human hepatocytes become tumorigenesis. These results suggest that the tumorigenesis requires an increased expression of miR-21 up to a certain level. IR-stimulated miR-21 expression was only ∼2 folds in hepatocytes for the short term, and it gradually increased in the liver tissues at 20 days after IR (especially high-LET IR). We believe that IR-stimulated miR-21 requires a long term accumulation to a certain level. In fact, the levels of miR-21 and its upstream regulators in the hepatocytes that survived IR (L or H) after 10 generations was higher than in the cells just exposed to irradiation (Figures 2B, 3, 4, 6A, B), which supports our hypothesis. We believe that irradiated cells/tissues gradually auto-select the changed cells to better facilitate their survival and growth, which contributes to carcinogenesis. Of course, this prediction requires more experiments to detect miR-21 levels in liver tissues from the mice at different times (1, 3, 6, 12, 18 months) after IR. Our results showed that even miR-21 levels are similar among non-irradiated, low-LET irradiated and high-LET irradiated cells; the tumor size is much bigger when developed from irradiated cells especially from high-LET irradiated cells. These results indicate that IR-stimulated miR-21 up-regulation is not the only reason for IR-promoted tumors; other IR-stimulated pathway changes may collaborate together during the long term process of the cell selection to facilitate the tumorigenesis. The detailed mechanism needs more work to elucidate in the near future.
In summary, our results for the first time establish a link between IR-stimulated miR-21 expression and IR-promoted liver carcinogenesis. We demonstrate that IR-induced up-regulation of miR-21 depends on AP-1 and ErbB/Stat3. IR-promoted carcinogenesis is a long term complicated process that involves the cell selection with changed factors to facilitate survival and division. Our results suggest that miR-21 is one of the factors. We believe that these results will contribute to our understanding of liver carcinogenesis, and will be useful in preventing IR-induced liver carcinogenesis.
Acknowledgments
We thank Dr. Robert L. Ullrich (University of Texas Medical Branch), Dr. Michael Weil (Colorado State University), Dr. Gayle Woloschak and Dr. Tanja Paunesku (Northwestern University) for providing the paraffin-embedded liver tissues from irradiated mice; Dr. Rui-Xia Sun (Fudan University, Shanghai, China) for the immortalized human hepatocytes, Dr. Philip Troilo (Merck Inc) for the immortalized mouse hepatocytes; the support team at Brookhaven National Laboratory for their help with the high-LET IR and Ms Doreen Theune for editing this manuscript.
This work is supported by a NASA grant (NNX09AF24GtoY.W).
References
- 1.Redpath J, Antoniono R. Induction of an Adaptive Response against Spontaneous Neoplastic Transformation In Vitro by Low-Dose Gamma Radiation. Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- 2.Mitchel R, Jackson J, Carlisle S. Upper Dose Thresholds for Radiation-Induced Adaptive Response against Cancer in High-Dose-Exposed, Cancer-Prone, Radiation-Sensitive Trp53 Heterozygous Mice. Radiat Res. 2004;162:20–30. doi: 10.1667/rr3190. [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Lu L, Wen S, Wang Y. The effects of Fhit on tumorigenesis after multi-exposure to low dose radiation. IJCEM. 2009;2:348–353. [PMC free article] [PubMed] [Google Scholar]
- 4.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 5.Wakeford R. The cancer epidemiology of radiation. Oncogene. 2004;23:6404–6428. doi: 10.1038/sj.onc.1207896. [DOI] [PubMed] [Google Scholar]
- 6.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nature Rev Cancer. 2008;8:465–472. doi: 10.1038/nrc2391. [DOI] [PubMed] [Google Scholar]
- 7.Fry RJ, Powers-Risius P, Alpen EL, Ainsworth EJ, Ullrich RL. High-LET radiation carcinogenesis. Adv Space Res. 1983;3:241–248. doi: 10.1016/0273-1177(83)90194-1. [DOI] [PubMed] [Google Scholar]
- 8.Fry RJ, Powers-Risius P, Alpen EL, EJ A. High-LET radiation carcinogenesis. Radiat Res. 1985;8:S188–S195. [PubMed] [Google Scholar]
- 9.Thomas MB, Zhu AX. Hepatocellular Carcinoma: The Need for Progress. J Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich RL. NSCOR: Radiation Leukemogenesis NASA Task Book. 2008.
- 11.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Ann Rev Plant Biol. 2006;57:19. doi: 10.1146/annurev.arplant.57.032905.105218. LP -53. [DOI] [PubMed] [Google Scholar]
- 12.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farh KK-H, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 15.Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2005;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 16.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nature Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 17.Akao Y, Nakagawa Y, Naoe T. let-7 MicroRNA Functions as a Potential Growth Suppressor in Human Colon Cancer Cells. Biol Pharm Bull. 2006;29:903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 18.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–1641. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 19.Lanza G, Ferracin M, Gafa R, Veronese A, Spizzo R, Pichiorri F, Liu C-g, Calin G, Croce C, Negrini M. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol Cancer. 2007;6:54. doi: 10.1186/1476-4598-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and Prognostic MicroRNAs in Stage II Colon Cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotech. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 22.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 23.Ji J, Yamashita T, Budhu A, Forgues M, Jia H, Li C, Deng C, Wauthier E, Reid L, Ye Q, Qin L, Yang W, Wang H, Tang Z, Croce C, Wang X. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu W, Yang K, He X, Chen S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatology. 2010;53:98–107. doi: 10.1016/j.jhep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Ventura A, Jacks T. MicroRNAs and Cancer: Short RNAs Go a Long Way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rokos CL, Ledwith BJ. Peroxisome Prolifera-tors Activate Extracellular Signal-regulated Kinases in Immortalized Mouse Liver Cells. J Biol Chem. 1997;272:13452–13457. doi: 10.1074/jbc.272.20.13452. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry MA, Sachdeva H, Omaruddin RA. Radiation-Induced Micro-RNA Modulation in Glioblastoma Cells Differing in DNA-Repair Pathways. DNA and Cell Biology. 2010;00:1–9. doi: 10.1089/dna.2009.0978. [DOI] [PubMed] [Google Scholar]
- 28.Wagner-Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol. 2010;5:1–10. doi: 10.1186/1748-717X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Si M-L, Wu H, Mo Y-Y. MicroRNA-21 Targets the Tumor Suppressor Gene Tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 30.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;2:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel L, Christoffersen N, Jacobsen A, Lindow M, Krogh A, Lund A. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 32.Asangani I, Rasheed S, Nikolova D. MicroRNA-21 (miR-21) post-transcriptionally down-regulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 33.Gabriely G, Wurdinger T, Kesari S. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayed D, Rane S, Lypowy J, He M, Chen I-Y, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 Targets Sprouty2 and Promotes Cellular Outgrowths. Mol. Biol. Cell. 2008;19:3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 Targets a Network of Key Tumor-Suppressive Pathways in Glioblastoma Cells. Cancer Res. 2008;68:8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 36.Qi L, Bart J, Tan L, Platteel I, Sluis T, Huitema S, Harms G, Fu L, Hollema H, Berg A. Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC Cancer. 2009;9:163. doi: 10.1186/1471-2407-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natil Acad Sci USA. 2009;106:12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, Burger R, Gramatzki M, Blumert C, Bauer K, Cvijic H, Ullmann AK, Stadler PF, Horn F. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of mi-croRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 39.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. miR-21 Gene Expression Triggered by AP-1 Is Sustained through a Double-Negative Feedback Mechanism. Journal of Molecular Biology. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Huang T-H, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo Y-Y, Goga A, McManus MT. Up-regulation of miR-21 by HER2/neu Signaling Promotes Cell Invasion. J Biol Chem. 2009;284:18515–18524. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo H-W, Hsu S-C, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih J-Y, Hung M-C. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Ren Z, Schaefer TS. ErbB-2 Activates Stat3 in a Src- and JAK2-dependent Manner. J Biol Chem. 2002;277:38486–38493. doi: 10.1074/jbc.M112438200. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ, Jr, Lazo JS, Wang Z, Zhang L, Yu J. microRNA -21 Negatively Regulates Cdc25A and Cell Cycle Progression in Colon Cancer Cells. Cancer Res. 2009;69:8157–8165. doi: 10.1158/0008-5472.CAN-09-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE. miR-21: An Androgen Receptor-Regulated MicroRNA that Promotes Hormone-Dependent and Hormone-Independent Prostate Cancer Growth. Cancer Res. 2009;69:7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray L. Radiation biology and cancer. Cellular Radiation Biology: A Sympothsium Considering Radiation Effects in the Cell and Possible Implications for Cancer Therapy; A Collection of Papers. Published for the University of Texas MD Anderson Hospital and Tumor Institute. 1965:8–25. [Google Scholar]
- 46.Sherman ML, Datta R, Hallahan DE, Weichselbaum RR, Kufe DW. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natil Acad Sci, USA. 1990;87:5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YJ, Galoforo SS, Berns CM, Erdos G, Gupta AK, Ways DK, Corry PM. Effect of Ionizing Radiation on AP-1 Binding Activity and Basic Fibroblast Growth Factor Gene Expression in Drug-sensitive Human Breast Carcinoma MCF-7 and Multidrug-resistant MCF-7/ADR Cells. J. Biol. Chem. 1995;270:28790–28796. doi: 10.1074/jbc.270.48.28790. [DOI] [PubMed] [Google Scholar]
- 48.Kanzawa T, Iwado E, Aoki H, Iwamaru A, Hollingsworth EF, Sawaya R, Kondo S, Kondo Y. Ionizing radiation induces apoptosis and inhibits neuronal differentiation in rat neural stem cells via the c-Jun NH2-terminal kinase (JNK) pathway. Oncogene. 2006;25:3638–3648. doi: 10.1038/sj.onc.1209414. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y-R, Wang X, Templeton D, Davis RJ, Tan T-H. The Role of c-Jun N-terminal Kinase (JNK) in Apoptosis Induced by Ultraviolet C and î3 Radiation. J. Biol. Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]