Abstract
Persistent inflammation is often present in patients with lung diseases such as chronic obstructive pulmonary diseases (COPD) and pulmonary hypertension. Circulatory leukocyte migration through the lung vascular endothelium contributes to the structural destruction and remodeling seen in these chronic lung diseases. An inflammatory chemokine CX3CL1/fractalkine is associated with inflammatory lung diseases. Membrane-anchored CX3CL1 serves as an adhesion molecule to capture subsets of mononuclear leukocytes that express the sole receptor, CX3CR1. The extracellular chemokine domain of CX3CL1 can be cleaved/shed by a disintegrin and metalloproteinase domain (ADAM) from stimulus-exposed cells. Soluble CX3CL1 chemoattracts and activates CX3CR1+ leukocytes such as CD8+, CD4+, and γδ T lymphocytes, natural killer cells, dendritic cells, and monocytes/macrophages. CX3CR1+ leukocyte attachment to and migration through the lung vascular endothelium lead to mononuclear cell accumulation in the lung vessel walls and parenchyma. Infiltrated CX3CR1+ immune cells can release mediators to induce injury, stimulate proliferation, and/or chemoattract inflammatory cells. This contributes to structural destruction and remodeling in the development of inflammatory lung diseases. Limited clinical success in treating chronic pulmonary diseases-associated lung functional decline indicates the urgency and significance of understanding upstream signaling that triggers inflammation. This article reviews the advances in the CX3CL1-CX3CR1 axis-mediated modulation of mononuclear leukocyte adhesion and migration in inflammatory lung diseases such as COPD and pulmonary hypertension. Better understanding of the constant flow of circulating leukocytes into the lung vessel wall and parenchyma will help set a stage for the development of novel therapeutic approaches to treat or even cure chronic lung diseases including COPD and pulmonary hypertension.
Keywords: Chemokine, fractalkine, inflammation, pulmonary, COPD, endothelium, vasculature
Inflammatory lung diseases and CX3CL1/CX3CR1 expression
Persistent inflammation is often present in patients with lung diseases such as chronic obstructive pulmonary diseases (COPD) and pulmonary hypertension (PH)[1-15]. Infiltration and accumulation of immune cells in the lung contribute to structural destruction and remodeling in the pathogenesis of these chronic lung diseases [16-17]. Endothelial cells (EC) respond to stimuli and overproduce a proinflammatory chemokine, fractalkine/CX3CL1 [18-23]. This leads to the endothelial attachment of the subset mononuclear leukocytes that express the sole CX3CL1 receptor, CX3CR1 [23-29]. Membrane-anchored CX3CL1 has been reported to serve as a pro-adhesion molecule to capture mononuclear leukocytes rapidly and firmly under high blood flow [27, 30]. The extracellular chemokine domain of CX3CL1 can be cleaved/shed by a disintegrin and metalloproteinase domain 17 (ADAM 17) from stimulus-exposed endothelial cells [31-32]. ADAM 17 is a member of the ADAM family involved in proteolytic ecto-domain shedding [33-35]. ADAM 17 has been shown to cleave a variety of transmembrane proteins including TNFα precursor and CX3CL1 [31, 36-38]. ADAM 10 and ADAM 17 are involved in constitutive and inducible cleavage of CX3CL1, respectively [31-32]. The extracellular chemokine domain of CX3CL1 can be cleaved/shed by ADAM 17 from stimulus-exposed endothelial cells [31-32]. Soluble CX3CL1 chemoattracts and activates CX3CR1+ leukocytes such as CD8+, CD4+, and γδ T lymphocytes, natural killer (NK) cells, dendritic cells (DC), and mono-cytes [25, 31, 39-45]. Leukocyte trafficking is modulated by multiple signal transduction pathways including CX3CL1-CX3CR1 signaling.
A number of extracellular stimuli can trigger inflammatory responses in the lungs. For example, smoke-induced activation of the lung vascular endothelium is the initial event in a cellular cascade that transmits smoke stimulation to lung inflammatory responses in the pathogene-sis of COPD [46-47]. Persistent inflammation including vascular inflammation occurs in COPD [48], even when the vessels are distant from airways [47]. Endothelial dysfunction with impaired relaxation is characteristic of vascular lesions in COPD [49]. Activated EC release mediators to promote leukocyte trafficking in COPD [50-51]. T cells, predominantly CD8+ T cells, are present in the lung parenchyma of smokers with COPD. This can attract other inflammatory cells like neutrophils and macrophages. These infiltrated inflammatory cells play a critical role in vessel wall remodeling and parenchymal destruction seen in the lungs of COPD patients [3]. The early signaling events that transmit smoke-induced endothelial activation to immune cell/leukocyte infiltration in the lung are unclear. Gene profiling reveals an increase in CX3CL1 expression in the lung tissues of smokers who developed COPD when compared to smokers without COPD, suggesting upregulation of CX3CL1 expression plays a role in tobacco smoke-induced COPD [52]. Smoke stimulates CX3CL1 expression in the mouse pulmonary vasculature and lungs [52-53].
Chronic inflammation occurs in the lungs of PH patients, and elevation of CX3CL1 expression correlates with PH. Chronic alveolar hypoxia is present in persons living at high altitude as well as some patients with COPD [54-58]. The levels of plasma and cellular CX3CL1 are increased in the circulation and lungs with COPD and/or PH [39, 52, 59-62]. Exposure of human or animal lungs to low levels of oxygen often leads to overproduction of CX3CL1 and PH [39, 52, 59-62]. Systemic inflammation is linked to PH and fibro-sis [63-64]. The levels of serum CX3CL1 and CX3CR1 in monocytes/macrophages are increased in patients with systemic sclerosis [63]. These increases in CX3CL1/CX3CR1 levels are also correlated with the severity of pulmonary fibrosis. In addition, the frequencies of mutations in the CX3CR1 alleles are increased in a subgroup of patients with systemic sclerosis-associated PH [64]. The correlation of increased CX3CL1/CX3CR1 and inflammatory lung diseases is summarized in Table 1. These observations support the notion that overexpression of CX3CL1/CX3CR1 triggered by stimuli such as smoking and hypoxia contributes to the patho-physiology of inflammatory lung diseases.
Table 1.
Elevation of CX3CL1 and its receptor CX3CR1 in lung diseases
Stimulus-induced CX3CL1/CX3CR1 expression in lung cells
Upregulation of CX3CL1/CX3CR1 expression can be an upstream signaling event in the cell to transmit environmental stimulation to inflammatory responses. A variety of cells in the lung has been shown to respond to stimuli that overproduce CX3CL1/CX3CR1 (Table 2). For instance, exposure of human smooth muscle cells to high glucose results in upregulation of CX3CL1 [65]. Interferon-gamma stimulates CX3CL1 expression in bronchial epithelial cells [66]. The levels of CX3CL1 in bronchoalveolar lavage fluids are increased in patients with inflammatory diseases. Exposure of EC to inter-feron-gamma, resistin, cigarette smoke, or shear stress leads to upregulation of CX3CL1 [19, 22, 39, 67-68]. Cigarette smoke induces CX3CR1 expression in mononuclear phagocytes and T lymphocytes [39].
Table 2.
Upregulation of CX3CL1 and CX3CR1expression in the cells/tissues of the lungs
| Cell/tissue | CX3CL1/CX3CR1 | Reference |
|---|---|---|
| EC | Increased CX3CL1 | Hatakeyama et al[67]; Imaizumi[19]; Matsumiya et al[107]; Popovic et al[99]; Umehara et al[94] |
| Epithelial cells | Increased CX3CL1 | Lucas et al[108] |
| Smooth muscle cells | Increased CX3CL1/CX3CR1 | Ollivier et al[109]; Bursill et al.[110]; Chandrasekar et al.[111]; Chen et al.[112]; Dragomir et al[65]; Lucas et al[45]; Ludwig et al[113]; Perros et al[59]; Sukkar et al[114]; Yoshikawa et al[30] |
| Fibroblasts | Increased CX3CL1/CX3CR1 | Fahy et al[115]; Klosowska et al[116]; Sawai et al[117] |
| Monocytes/macrophages | Increased CX3CR1 | Ancuta et al[18]; Apostolakis et al[118]; Green et al[27]; Landsman et al[119] |
| Lymphocytes | Increased CX3CR1/CX3CL1 | Foussat et al[120]; Kobayashi et al[121]; McComb et al[39]; Muehlhoefer et al[122]; Nishimura et al[123]; Truman et al[43] |
| DC | Increased CX3CR1 | Auffray et al[79]; del Rio et al[124]; Dichmann et al[97]; Foussat et al[120]; Jung et al[125]; Kikuchi et al[126]; Niess et al[127]; Papadopoulos et al[128] |
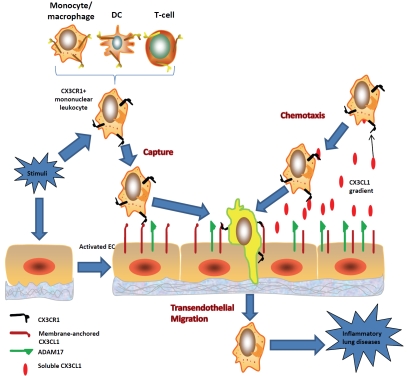
Mononuclear leukocyte recruitment in vascular lesions through CX3CL1-CX3CR1 signaling promotes obstructive remodeling [69]. Neointimal smooth muscle cells develop a proinflammatory phenotype via chemokine pathways including CX3CL1-CX3CR1 [70]. Since CX3CR1-/- mice do not show this recruitment [71], it indicates that the CX3CL1-CX3CR1 axis is critical to inflammatory remodeling. CX3CL1 expression is in-creased in COPD, which leads to the recruitment of CX3CR1+ cells into the lung parenchyma of mice chronically exposed to tobacco smoke [39]. Current studies suggest that endothelial CX3CL1 expression contributes to CX3CR1+ leukocyte adhesion, transendothelial migration, and chemotaxis in inflammatory lung diseases (Figure 1). The following observations support the notion. First, CX3CL1 serves as an adhesion molecule [27, 30]. Tobacco smoke-induced overproduction of CX3CL1 on the activated endothelium can capture CX3CR1+ leukocytes [72-77]. Second, tobacco smoke-induced CX3CL1-CX3CR1 interaction can enhance leukocyte transendothelial migration [39, 75]. Third, CX3CL1 shedded from the smoke-activated endothelium can act as a potent chemotactic agent for CX3CR1+ leukocytes [28, 31, 43].
Figure 1.
CX3CL1-CX3CR1 modulation of environmental stimulus-induced leukocyte trafficking in chronic lung diseases. Capture: Stimuli trigger endothelial CX3CL1 expression, which enhances CX3CR1+ leukocyte attachment to the activated lung vascular endothelium. This leads to CX3CR1+ leukocyte infiltration. Chemotaxis: Stimulus-induced CX3CL1 shedding by ADAM promotes CX3CR1+ leukocyte chemotaxis and inflammation. Transendothelial migration: Stimulus-induced CX3CL1 interaction with CX3CR1 promotes the transendothelial migration of CX3CR1+ leukocytes, inflammatory cell accumulation in vessel walls/parenchyma, and lung structural remodeling and destruction.
Modulation of leukocyte attachment via CX3CL1 interaction with CX3CR1
The first key step in lung inflammatory cell infiltration of the low resistance but high flow system is the attachment of circulatory leukocytes to the endothelium. Stimuli trigger endothelial activation and leukocyte adhesion. For instance, tobacco smoke has been shown to enhance the attachment of leukocytes to the vascular endothelium [72-78]. Exposure of mice to secondhand smoke promotes leukocyte adhesion on the lung microvascular endothelium [78]. CX3CR1 deficiency impairs monocyte accumulation in arterial intima, suggesting a key role of the CX3CL1-CX3CR1 axis in COPD immunopa-thology [27, 79-80]. Inflammatory chemokines regulate leukocyte trafficking and COPD-related remodeling [81-84]. Expression of CX3CL1 and its receptor in the lung endothelium and leukocytes are upregulated in COPD [39, 52]. Overex-pression of endothelial CX3CL1 following tobacco smoke exposure may act as a lung vascular gateway. CX3CR1+ leukocytes are captured rapidly and then migrate into the lung, contributing to inflammation in the lungs of patients with COPD.
Hypoxia-induced inflammatory response results in EC activation with enhanced lymphocyte and DC adhesion [17, 85-89]. Endothelial cells treated with TNF-alpha and hypoxia/reoxygenation induced a strong NK cell adhesion [90]. Idiopathic pulmonary arterial hypertension-related inflammatory infiltrates have been seen in the range of plexiform lesions with local expression of chemokines CCL2, CCL5, and CX3CL1 [55, 59, 91-93]. Expression of CX3CL1 and its receptor in the lung endothelium and T-lymphocytes are upregulated in PH [61, 94]. These observations support the notion that overexpression of endothelial CX3CL1 following hypoxia exposure is associated with CX3CR1+ leukocyte adhesion and transendothe-lial migration into the artery tissues, leading to vascular remodeling and progression of PH.
CX3CL1-promoted CX3CR1+ cell migration
Transendothelial migration of attached leukocytes is essential for immune cell trafficking and accumulation in the lungs. Tobacco smoke stimulates the migration of lung leukocytes including T-cells and monocytes in vitro and in vivo [39, 75]. McComb and his colleagues have reported that exposure of mice to acute or chronic tobacco smoke results in upregulation of CX3CL1 and CX3CR1, the influx of inflammatory cells including monocytes/macrophages and lymphocytes into the lungs, and the development of COPD [39]. Experimental results support the notion that tobacco smoke-induced activation of the endothelium and migration of monocytes play a role in the accumulation of lung monocytes/macrophages [75]. Smoke-enhanced interaction between the CX3CL1+ endothelium and CX3CR1+ leukocytes is expected to promote the onward transendothelial migration of the mononuclear leukocytes, resulting in inflammatory cell accumulation and the development of COPD.
Hypoxia constitutes a stimulus for EC activation and circulating monocyte/mononuclear fibro-cyte migration in the lung [17, 95]. Alveolar hypoxia is present in a variety of lung disorders such as COPD (blocked airways and destructed structures for the oxygen-CO2 exchange), lung cancers, chronic inflammation (systemic inflammation such as scleroderma, interstitial lung diseases, and bacterial infections). The local hypoxic microenvironment may serve as a stimulus to promote the transendothelial migration of leukocytes including monocytes/macrophages [95]. The recruited immune cells adapt to the hypoxic environment through the alteration of the gene expression [95]. Proin-flammatory mediators released from infiltrates contribute to structural modulations including cell differentiation, proliferation, and remodeling in the development and progression of PH [54]. Balabanian et al have found that the levels of CX3CL1/CX3CR1 in PH patients are higher than that in control subjects [61]. It is unclear about the link between upregulation of CX3CL1/CX3CR1 expression and the development of PH. However, CX3CL1 is known to mediate CX3CR1+ cell migration. For instance, CX3CL1 preferentially modulates the transendothelial migration of monocytes expressing CX3CR1 [96]. CX3CL1 induces chemotaxis of immature and mature DC [97]. The CX3CL1-CX3CR1 axis contributes to transmigration of neuroblastoma cells through bone-marrow endothelium [98]. In addition, thrombin-induced CX3CL1 expression is associated with an increase in monocyte transendothelial migration [99]. These observations support the notion that PH-related upregulation of CX3CL1 in the lung plays a critical role in leukocyte trafficking and monocyte/macrophage accumulation in vascular remodeling.
CX3CL1-stimulated chemotaxis and infiltration of leukocytes in lung inflammation
Accumulation of monocytes is an essential event in inflammatory responses of the lung [95]. Monocytes migrate along chemotactic gradients within the lung vasculature. Tobacco smoke stimulates leukocyte chemotaxis [100]. Smoke-increased CX3CL1 expression can promote lung mononuclear leukocyte chemotaxis via the CX3CL1-CX3CR1 axis. Leukocyte recruitment requires intercellular communication between infiltrating leukocytes and the endothe-lium through mediators such as CX3CL1. ADAM-induced cleavage of CX3CL1 is associated with the recruitment of CX3CR1+ leukocytes to CX3CL1-expressing cells [31-32]. Tobacco smoke exposure is linked to increases in ADAM 17 activity [101-102], which can enhance CX3CL1 shedding. Tobacco smoke-induced recruitment and accumulation of CX3CR1+ leukocytes can therefore contribute to alveolar wall destruction. Soluble CX3CL1 stimulates CX3CR1+ leukocyte chemotaxis [27, 42, 44, 103]. Extravasations of inflammatory cells are found in the alveolus [54, 104]. CX3CL1-mediated chemotaxis of CX3CR1+ leukocytes can contribute to accumulation of inflammatory cells in the lung and development of COPD. Tobacco smoke-increased CX3CL1 expression may promote lung mononuclear leukocyte chemotaxis via the CX3CL1-CX3CR1 axis in the development of emphysema.
Hypoxia promotes leukocyte chemotaxis [95, 105-106]. Soluble CX3CL1 stimulates CX3CR1+ leukocyte chemotaxis [27, 42, 44, 103]. Hy-poxic recruitment and accumulation of CX3CR1+ leukocytes contribute to the wall thickening and the “muscularization” of the precapillary segment. Extravasations of inflammatory and mes-enchymal precursor cells were found in the alveolus [54, 104]. These infiltrated cells can differentiate into smooth muscle-like cells and/or release mediators to promote cell proliferation, differentiation, and transdifferentiation in hy-poxic distal muscularization [54]. PH-associated elevation of CX3CL1/CX3CR1 appears to be correlated with monocyte infiltration in vascular remodeling processes.
CX3CL1-CX3CR1 signaling: a potential target for treatment of inflammatory lung diseases
Limited clinical success in treating chronic lung diseases-associated lung structural remodeling and functional decline indicates the urgency and significance of understanding upstream signaling that triggers inflammation. The constant flow of circulating leukocytes into the lung vessel wall and parenchyma contributes to cell proliferation, damage, and/or death in the pathogenesis of COPD and PH. CX3CL1 may act as a powerful gatekeeper that responds to stimuli, such as smoke/hypoxia, to allow CX3CR1+ leukocyte migration through the endothelial barrier. Elucidating the role of CX3CL1-CX3CR1 axis in environmental stimulus-induced leukocyte capturing, chemotaxis, and trafficking can help set a stage for the development of novel therapeutic approaches to treat or even cure COPD. For instance, antibodies or small molecules may be used to block the CX3CL1-CX3CR1 interaction. This may reduce or prevent leukocyte infiltration/accumulation, structural remodeling/destruction, and functional decline in the development and progression of chronic lung diseases including COPD and PH.
Acknowledgments
This work was supported in part by a Career Investigator Award from the American Lung Association of Florida, Inc (JZ), by a Clinical Innovator Award from the Flight Attendant Medical Research Institute (JZ), by the Medical Research Service of the Department of Veterans Affairs and by NIH grants HL68666 and HL85133 (JMP).
Reference
- 1.Agostini C, Trentin L, Adami F. Chronic obstructive pulmonary disease (COPD): new insights on the events leading to pulmonary inflammation. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:3–7. [PubMed] [Google Scholar]
- 2.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 3.Cosio MG, Majo J. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002;121:160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- 4.Joppa P, Petrasova D, Stancak B, Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130:326–333. doi: 10.1378/chest.130.2.326. [DOI] [PubMed] [Google Scholar]
- 5.Kardos P, Keenan J. Tackling COPD: a multi-component disease driven by inflammation. MedGenMed. 2006;8:54. [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 7.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal. 2008;10:799–811. doi: 10.1089/ars.2007.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth M. Pathogenesis of COPD. Part III. Inflammation in COPD. Int J Tuberc Lung Dis. 2008;12:375–380. [PubMed] [Google Scholar]
- 9.Yawn BP, Kaplan A. Co-morbidities in people with COPD: a result of multiple diseases, or multiple manifestations of smoking and reactive inflammation= Prim Care Respir J. 2008;17:199–205. doi: 10.3132/pcrj.2008.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziesche R, Petkov V, Mosgoller W, Block LH. Regulation of human endothelial nitric oxide synthase by hypoxia and inflammation in human pulmonary arteries–implications for the therapy of pulmonary hypertension in COPD patients. Acta Anaesthesiol Scand Suppl. 1996;109:97–98. [PubMed] [Google Scholar]
- 11.Herget J, Palecek F, Preclik P, Cermakova M, Vizek M, Petrovicka M. Pulmonary hypertension induced by repeated pulmonary inflammation in the rat. J Appl Physiol. 1981;51:755–761. doi: 10.1152/jappl.1981.51.3.755. [DOI] [PubMed] [Google Scholar]
- 12.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H677–690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 15.Voelkel NF, Tuder R. Interleukin-1 receptor antagonist inhibits pulmonary hypertension induced by inflammation. Ann N Y Acad Sci. 1994;725:104–109. doi: 10.1111/j.1749-6632.1994.tb39794.x. [DOI] [PubMed] [Google Scholar]
- 16.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Stenmark KR. Circulating mono-nuclear cells with a dual, macrophage-fibroblast phenotype contribute robustly to hypoxia-induced pulmonary adventitial remodeling. Chest. 2005;128:583S–584S. doi: 10.1378/chest.128.6_suppl.583S. [DOI] [PubMed] [Google Scholar]
- 17.Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol. 2005;98:715–721. doi: 10.1152/japplphysiol.00840.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–1164. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 19.Imaizumi T, Matsumiya T, Fujimoto K, Okamoto K, Cui X, Ohtaki U, Hidemi, Yoshida, Satoh K. Interferon-gamma stimulates the expression of CX3CL1/fractalkine in cultured human endothelial cells. Tohoku J Exp Med. 2000;192:127–139. doi: 10.1620/tjem.192.127. [DOI] [PubMed] [Google Scholar]
- 20.Harrison JK, Jiang Y, Wees EA, Salafranca MN, Liang HX, Feng L, Belardinelli L. Inflammatory agents regulate in vivo expression of frac-talkine in endothelial cells of the rat heart. J Leukoc Biol. 1999;66:937–944. doi: 10.1002/jlb.66.6.937. [DOI] [PubMed] [Google Scholar]
- 21.Manes TD, Pober JS. Antigen presentation by human microvascular endothelial cells triggers ICAM-1-dependenttransendothelial protrusion by, and fractalkine-dependent transendo-thelial migration of, effector memory CD4+ T cells. J Immunol. 2008;180:8386–8392. doi: 10.4049/jimmunol.180.12.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moatti D, Vele O, Nemerson Y. Induction of fractalkine by endothelial cells under shear stress. Blood Coagul Fibrinolysis. 2004;15:197. doi: 10.1097/00001721-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Schulz C, Schafer A, Stolla M, Kerstan S, Lorenz M, von Bruhl ML, Schiemann M, Bauersachs J, Gloe T, Busch DH, Gawaz M, Massberg S. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: a critical role for P-selectin expressed on activated platelets. Circulation. 2007;116:764–773. doi: 10.1161/CIRCULATIONAHA.107.695189. [DOI] [PubMed] [Google Scholar]
- 24.Cambien B, Pomeranz M, Schmid-Antomarchi H, Millet MA, Breittmayer V, Rossi B, Schmid-Alliana A. Signal transduction pathways involved in soluble fractalkine-induced monocytic cell adhesion. Blood. 2001;97:2031–2037. doi: 10.1182/blood.v97.7.2031. [DOI] [PubMed] [Google Scholar]
- 25.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H. CX3C-chemokine, frac-talkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–4320. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- 27.Green SR, Han KH, Chen Y, Almazan F, Charo IF, Miller YI, Quehenberger O. The CC chemokine MCP-1 stimulates surface expression of CX3CR1 and enhances the adhesion of monocytes to fractalkine/CX3CL1 via p38 MAPK. J Immunol. 2006;176:7412–7420. doi: 10.4049/jimmunol.176.12.7412. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Chen T, Wang B, Zhang M, An H, Guo Z, Yu Y, Qin Z, Cao X. Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol Lett. 2003;89:1–7. doi: 10.1016/s0165-2478(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 29.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa M, Nakajima T, Matsumoto K, Okada N, Tsukidate T, Iida M, Otori N, Haruna S, Moriyama H, Imai T, Saito H. TNF-alpha and IL-4 regulate expression of fractalkine (CX3CL1) as a membrane-anchored proadhe-sive protein and soluble chemotactic peptide on human fibroblasts. FEBS Lett. 2004;561:105–110. doi: 10.1016/S0014-5793(04)00132-2. [DOI] [PubMed] [Google Scholar]
- 31.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 32.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metal-loproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 33.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 34.Le Gall SM, Bobe P, Reiss K, Horiuchi K, Niu XD, Lundell D, Gibb DR, Conrad D, Saftig P, Blobel CP. ADAMs 10 and 17 Represent Differentially Regulated Components of a General Shedding Machinery for Membrane Proteins such as TGF{alpha}, L-Selectin and TNF{alpha} Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflamm Res. 2002;51:83–84. doi: 10.1007/BF02684007. [DOI] [PubMed] [Google Scholar]
- 36.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 37.Moss ML, Jin SL, Becherer JD, Bickett DM, Burkhart W, Chen WJ, Hassler D, Leesnitzer MT, McGeehan G, Milla M, Moyer M, Rocque W, Seaton T, Schoenen F, Warner J, Willard D. Structural features and biochemical properties of TNF-alpha converting enzyme (TACE) J Neuroimmunol. 1997;72:127–129. doi: 10.1016/s0165-5728(96)00180-4. [DOI] [PubMed] [Google Scholar]
- 38.Schulte A, Schulz B, Andrzejewski MG, Hundhausen C, Mletzko S, Achilles J, Reiss K, Paliga K, Weber C, John SR, Ludwig A. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by alpha- and gamma-secretases. Biochem Biophys Res Commun. 2007;358:233–240. doi: 10.1016/j.bbrc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 39.McComb JG, Ranganathan M, Liu XH, Pilewski JM, Ray P, Watkins SC, Choi AM, Lee JS. CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. Am J Pathol. 2008;173:949–961. doi: 10.2353/ajpath.2008.071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Wei H, Wang H, Tian Z. Involvement of interaction between Fractalkine and CX3CR1 in cytotoxicity of natural killer cells against tumor cells. Oncol Rep. 2006;15:485–488. [PubMed] [Google Scholar]
- 41.Raoul W, Feumi C, Keller N, Lavalette S, Houssier M, Behar-Cohen F, Combadiere C, Sennlaub F. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008;40:115–119. doi: 10.1159/000119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanchi C, Zoja C, Morigi M, Valsecchi F, Liu XY, Rottoli D, Locatelli M, Buelli S, Pezzotta A, Mapelli P, Geelen J, Remuzzi G, Hawiger J. Fractalkine and CX3CR1 mediate leukocyte capture by endothelium in response to Shiga toxin. J Immunol. 2008;181:1460–1469. doi: 10.4049/jimmunol.181.2.1460. [DOI] [PubMed] [Google Scholar]
- 43.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 44.Gevrey JC, Isaac BM, Cox D. Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine) J Immunol. 2005;175:3737–3745. doi: 10.4049/jimmunol.175.6.3737. [DOI] [PubMed] [Google Scholar]
- 45.Lucas AD, Bursill C, Guzik TJ, Sadowski J, Channon KM, Greaves DR. Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1) Circulation. 2003;108:2498–2504. doi: 10.1161/01.CIR.0000097119.57756.EF. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Underwood MJ, Hsin MK, Liu XC, He GW. Dysfunction of pulmonary vascular endothelium in chronic obstructive pulmonary disease: basic considerations for future drug development. Curr Drug Metab. 2008;9:661–667. doi: 10.2174/138920008785821684. [DOI] [PubMed] [Google Scholar]
- 47.Peinado VI, Barbera JA, Abate P, Ramirez J, Roca J, Santos S, Rodriguez-Roisin R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1605–1611. doi: 10.1164/ajrccm.159.5.9807059. [DOI] [PubMed] [Google Scholar]
- 48.Brantly ML, Paul LD, Miller BH, Falk RT, Wu M, Crystal RG. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;138:327–336. doi: 10.1164/ajrccm/138.2.327. [DOI] [PubMed] [Google Scholar]
- 49.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endo-thelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- 50.Michiels C, Arnould T, Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim Biophys Acta. 2000;1497:1–10. doi: 10.1016/s0167-4889(00)00041-0. [DOI] [PubMed] [Google Scholar]
- 51.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 52.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2004;101:14895–14900. doi: 10.1073/pnas.0401168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, Boichot E. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol. 2008;154:1206–1215. doi: 10.1038/bjp.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 55.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med. 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 56.Preston IR. Clinical perspective of hypoxia-mediated pulmonary hypertension. Antioxid RedoxSignal. 2007;9:711–721. doi: 10.1089/ars.2007.1587. [DOI] [PubMed] [Google Scholar]
- 57.Raguso CA, Guinot SL, Janssens JP, Kayser B, Pichard C. Chronic hypoxia: common traits between chronic obstructive pulmonary disease and altitude. Curr Opin Clin Nutr Metab Care. 2004;7:411–417. doi: 10.1097/01.mco.0000134372.78438.09. [DOI] [PubMed] [Google Scholar]
- 58.Pierson DJ. Pathophysiology and clinical effects of chronic hypoxia. Respir Care. 2000;45:39–51. discussion 51–33. [PubMed] [Google Scholar]
- 59.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Godot V, Capel F, Adnot S, Eddahibi S, Mazmanian M, Fadel E, Herve P, Simonneau G, Emilie D, Humbert M. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J. 2007;29:937–943. doi: 10.1183/09031936.00104706. [DOI] [PubMed] [Google Scholar]
- 60.Stievano L, Piovan E, Amadori A. C and CX3C chemokines: cell sources and physiopa-thological implications. Crit Rev Immunol. 2004;24:205–228. doi: 10.1615/critrevimmunol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- 61.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 62.Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, Shim J, Nam KB, Choi KJ, Kim YH, Han KH. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc Res. 2008;78:333–340. doi: 10.1093/cvr/cvm067. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa M, Sato S, Echigo T, Hamaguchi Y, Yasui M, Takehara K. Up regulated expression of fractalkine/CX3CL1 and CX3CR1 in patients with systemic sclerosis. Ann Rheum Dis. 2005;64:21–28. doi: 10.1136/ard.2003.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marasini B, Cossutta R, Selmi C, Pozzi MR, Gardinali M, Massarotti M, Erario M, Battaglioli L, Biondi ML. Polymorphism of the fractalkine receptor CX3CR1 and systemic sclerosis -associated pulmonary arterial hypertension. Clin Dev Immunol. 2005;12:275–279. doi: 10.1080/17402520500303297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dragomir E, Manduteanu I, Calin M, Gan AM, Stan D, Koenen RR, Weber C, Simionescu M. High glucose conditions induce upregulation of fractalkine and monocyte chemotactic protein-1 in human smooth muscle cells. Thromb Haemost. 2008;100:1155–1165. [PubMed] [Google Scholar]
- 66.Fujimoto K, Imaizumi T, Yoshida H, Takanashi S, Okumura K, Satoh K. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mono-nuclear cell adherence. Am J Respir Cell Mol Biol. 2001;25:233–238. doi: 10.1165/ajrcmb.25.2.4275. [DOI] [PubMed] [Google Scholar]
- 67.Hatakeyama M, Imaizumi T, Tamo W, Yamashita K, Yoshida H, Fukuda I, Satoh K. Heparin inhibits IFN-gamma-induced fractalkine/CX3CL1 expression in human endothelial cells. Inflammation. 2004;28:7–13. doi: 10.1023/b:ifla.0000014706.49598.78. [DOI] [PubMed] [Google Scholar]
- 68.Manduteanu I, Dragomir E, Calin M, Pirvulescu M, Gan AM, Stan D, Simionescu M. Resistin up-regulates fractalkine expression in human endothelial cells: lack of additive effect with TNF-alpha. Biochem Biophys Res Commun. 2009;381:96–101. doi: 10.1016/j.bbrc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 70.Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- 71.Liu P, Patil S, Rojas M, Fong AM, Smyth SS, Patel DD. CX3CR1 deficiency confers protection from intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2006;26:2056–2062. doi: 10.1161/01.ATV.0000234947.47788.8c. [DOI] [PubMed] [Google Scholar]
- 72.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci. 2009;14:3128–3144. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudolph TK, Rudolph V, Baldus S. Contribution of myeloperoxidase to smoking-dependent vascular inflammation. Proc Am Thorac Soc. 2008;5:820–823. doi: 10.1513/pats.200807-063TH. [DOI] [PubMed] [Google Scholar]
- 74.Yong T, Zheng MQ, Linthicum DS. Nicotine induces leukocyte rolling and adhesion in the cerebral microcirculation of the mouse. J Neuroimmunol. 1997;80:158–164. doi: 10.1016/s0165-5728(97)00151-3. [DOI] [PubMed] [Google Scholar]
- 75.Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–1633. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- 76.Lehr HA, Frei B, Arfors KE. Vitamin C prevents cigarette smoke-induced leukocyte aggregation and adhesion to endothelium in vivo. Proc Natl Acad Sci U S A. 1994;91:7688–7692. doi: 10.1073/pnas.91.16.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalra VK, Ying Y, Deemer K, Natarajan R, Nadler JL, Coates TD. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol. 1994;160:154–162. doi: 10.1002/jcp.1041600118. [DOI] [PubMed] [Google Scholar]
- 78.Rao SP, Sikora L, Hosseinkhani MR, Pinkerton KE, Sriramarao P. Exposure to environmental tobacco smoke induces angiogenesis and leukocyte trafficking in lung microvessels. Exp Lung Res. 2009;35:119–135. doi: 10.1080/01902140802449729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 81.Brozyna S, Ahern J, Hodge G, Nairn J, Holmes M, Reynolds PN, Hodge S. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. Copd. 2009;6:4–16. doi: 10.1080/15412550902724164. [DOI] [PubMed] [Google Scholar]
- 82.Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2006;27:546–553. doi: 10.1016/j.tips.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Panina-Bordignon P, D'Ambrosio D. Chemokines and their receptors in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2003;9:104–110. doi: 10.1097/00063198-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Sabroe I, Lloyd CM, Whyte MK, Dower SK, Williams TJ, Pease JE. Chemokines, innate and adaptive immunity, and respiratory disease. Eur Respir J. 2002;19:350–355. doi: 10.1183/09031936.02.00253602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicolls MR, Voelkel NF. Hypoxia and the lung: beyond hypoxic vasoconstriction. Antioxid Redox Signal. 2007;9:741–743. doi: 10.1089/ars.2007.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J. 1995;8:2129–2138. doi: 10.1183/09031936.95.08122129. [DOI] [PubMed] [Google Scholar]
- 87.Schlichting CL, Schareck WD, Weis M. Renal ischemia-reperfusion injury: new implications of dendritic cell-endothelial cell interactions. Transplant Proc. 2006;38:670–673. doi: 10.1016/j.transproceed.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 88.Kokura S, Wolf RE, Yoshikawa T, Ichikawa H, Granger DN, Aw TY. Endothelial cells exposed to anoxia/reoxygenation are hyperadhesive to T-lymphocytes: kinetics and molecular mechanisms. Microcirculation. 2000;7:13–23. [PubMed] [Google Scholar]
- 89.Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- 90.Maurus CF, Schneider MK, Schmidt D, Zund G, Seebach JD. Activation of human microvascular endothelial cells with TNF-alpha and hypoxia/reoxygenation enhances NK-cell adhesion, but not NK-Cytotoxicity. Transplantation. 2006;81:1204–1211. doi: 10.1097/01.tp.0000205175.53938.bd. [DOI] [PubMed] [Google Scholar]
- 91.Humbert M. Update in pulmonary arterial hypertension 2007. Am J Respir Crit Care Med. 2008;177:574–579. doi: 10.1164/rccm.200801-029UP. [DOI] [PubMed] [Google Scholar]
- 92.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 94.Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24:34–40. doi: 10.1161/01.ATV.0000095360.62479.1F. [DOI] [PubMed] [Google Scholar]
- 95.Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M, Varesio L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 96.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dichmann S, Herouy Y, Purlis D, Rheinen H, Gebicke-Harter P, Norgauer J. Fractalkine induces chemotaxis and actin polymerization in human dendritic cells. Inflamm Res. 2001;50:529–533. doi: 10.1007/PL00000230. [DOI] [PubMed] [Google Scholar]
- 98.Nevo I, Sagi-Assif O, Meshel T, Ben-Baruch A, Johrer K, Greil R, Trejo LE, Kharenko O, Feinmesser M, Yron I, Witz IP. The involvement of the fractalkine receptor in the transmigration of neuroblastoma cells through bone-marrow endothelial cells. Cancer Lett. 2009;273:127–139. doi: 10.1016/j.canlet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 99.Popovic M, Laumonnier Y, Burysek L, Syrovets T, Simmet T. Thrombin-induced expression of endothelial CX3CL1 potentiates monocyte CCL2 production and transendothelial migration. J Leukoc Biol. 2008;84:215–223. doi: 10.1189/jlb.0907652. [DOI] [PubMed] [Google Scholar]
- 100.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 101.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 102.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR) Novartis Found Symp. 2002;248:171–176. [PubMed] [Google Scholar]
- 103.Fraticelli P, Sironi M, Bianchi G, D'Ambrosio D, Albanesi C, Stoppacciaro A, Chieppa M, Allavena P, Ruco L, Girolomoni G, Sinigaglia F, Vecchi A, Mantovani A. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K, et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93:1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buras JA, Reenstra WR. Endothelial-neutrophil interactions during ischemia and reperfusion injury: basic mechanisms of hyper-baric oxygen. Neurol Res. 2007;29:127–131. doi: 10.1179/016164107X174147. [DOI] [PubMed] [Google Scholar]
- 107.Matsumiya T, Imaizumi T, Fujimoto K, Cui X, Shibata T, Tamo W, Kumagai M, Tanji K, Yoshida H, Kimura H, Satoh K. Soluble inter-leukin-6 receptor alpha inhibits the cytokine-Induced fractalkine/CX3CL1 expression in human vascular endothelial cells in culture. Exp Cell Res. 2001;269:35–41. doi: 10.1006/excr.2001.5300. [DOI] [PubMed] [Google Scholar]
- 108.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The trans-membrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–866. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ollivier V, Faure S, Tarantino N, Chollet-Martin S, Deterre P, Combadiere C, de Prost D. Fractalkine/CX3CL1 production by human aortic smooth muscle cells impairs monocyte procoagulant and inflammatory responses. Cytokine. 2003;21:303–311. doi: 10.1016/s1043-4666(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 110.Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–149. doi: 10.1097/00041433-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 111.Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Liu F, Melby PC. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J. 2003;373:547–558. doi: 10.1042/BJ20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen YM, Tu CJ, Hung KY, Wu KD, Tsai TJ, Hsieh BS. Inhibition by pentoxifylline of TNF-alpha-stimulated fractalkine production in vascular smooth muscle cells: evidence for mediation by NF-kappa B down-regulation. Br J Pharmacol. 2003;138:950–958. doi: 10.1038/sj.bjp.0705088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ludwig A, Berkhout T, Moores K, Groot P, Chapman G. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metallopro-teinase activity. J Immunol. 2002;168:604–612. doi: 10.4049/jimmunol.168.2.604. [DOI] [PubMed] [Google Scholar]
- 114.Sukkar MB, Issa R, Xie S, Oltmanns U, Newton R, Chung KF. Fractalkine/CX3CL1 production by human airway smooth muscle cells: induction by IFN-gamma and TNF-alpha and regulation by TGF-beta and corticosteroids. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1230–1240. doi: 10.1152/ajplung.00014.2004. [DOI] [PubMed] [Google Scholar]
- 115.Fahy OL, Coates NJ, McColl SR. Inhibition of cytokine-induced fractalkine production by bacterial invasion of human-dermal fibroblasts. Lab Invest. 2003;83:721–730. doi: 10.1097/01.lab.0000069518.49544.b8. [DOI] [PubMed] [Google Scholar]
- 116.Klosowska K, Volin MV, Huynh N, Chong KK, Halloran MM, Woods JM. Fractalkine functions as a chemoattractant for osteoarthritis synovial fibroblasts and stimulates phosphory-lation of mitogen-activated protein kinases and Akt. Clin Exp Immunol. 2009;156:312–319. doi: 10.1111/j.1365-2249.2009.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2007;56:3215–3225. doi: 10.1002/art.22919. [DOI] [PubMed] [Google Scholar]
- 118.Apostolakis S, Krambovitis E, Vlata Z, Kochiadakis GE, Baritaki S, Spandidos DA. CX3CR1 receptor is up-regulated in monocytes of coronary artery diseased patients: impact of pre-inflammatory stimuli and renin-angiotensin system modulators. Thromb Res. 2007;121:387–395. doi: 10.1016/j.thromres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 119.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 120.Foussat A, Coulomb-L'Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30:87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 121.Kobayashi T, Okamoto S, Iwakami Y, Nakazawa A, Hisamatsu T, Chinen H, Kamada N, Imai T, Goto H, Hibi T. Exclusive increase of CX3CR1+CD28-CD4+ T cells in inflammatory bowel disease and their recruitment as intraepithelial lymphocytes. Inflamm Bowel Dis. 2007;13:837–846. doi: 10.1002/ibd.20113. [DOI] [PubMed] [Google Scholar]
- 122.Muehlhoefer A, Saubermann LJ, Gu X, Luedtke-Heckenkamp K, Xavier R, Blumberg RS, Podolsky DK, MacDermott RP, Reinecker HC. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164:3368–3376. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- 123.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 124.del Rio ML, Rodriguez-Barbosa JI, Bolter J, Ballmaier M, Dittrich-Breiholz O, Kracht M, Jung S, Forster R. CX3CR1+ c-kit+ bone marrow cells give rise to CD103+ and CD103- dendritic cells with distinct functional properties. J Immunol. 2008;181:6178–6188. doi: 10.4049/jimmunol.181.9.6178. [DOI] [PubMed] [Google Scholar]
- 125.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikuchi T, Andarini S, Xin H, Gomi K, Tokue Y, Saijo Y, Honjo T, Watanabe A, Nukiwa T. Involvement of fractalkine/CX3CL1 expression by dendritic cells in the enhancement of host immunity against Legionella pneumophila. Infect Immun. 2005;73:5350–5357. doi: 10.1128/IAI.73.9.5350-5357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 128.Papadopoulos EJ, Sassetti C, Saeki H, Yamada N, Kawamura T, Fitzhugh DJ, Saraf MA, Schall T, Blauvelt A, Rosen SD, Hwang ST. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur J Immunol. 1999;29:2551–2559. doi: 10.1002/(SICI)1521-4141(199908)29:08<2551::AID-IMMU2551>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]