Abstract
We show that the dual 5-α reductase enzyme inhibitor dutasteride prevents enhanced growth of both benign and malignant prostate cell lines, incubated with physiologic to supraphysiologic doses of testosterone. Using androgen-sensitive benign BPH-1 cells, LNCaP cancer cells, their derivative C4-2 cells, or Dunning rat cancer cells, we subjected 30,000 cells/well to concomitant treatment with 10-9, 10-8, or 10-7 M testosterone in the presence of low (0.25 μM) or high (1.0 μM) doses of dutasteride. Both low- and high-dose dutasteride abrogated testosterone-stimulated growth of all 4 cell lines. If the in vitro data mimic conditions in men undergoing testosterone replacement, concomitant dutasteride use might make testosterone safe for men with benign prostatic hypertrophy, latent prostate cancer and perhaps even aggressive prostate cancer. Testosterone might also be used to prevent the rare anti-androgen side effects of dutasteride when used for benign prostatic hypertrophy and baldness. Further clinical investigation is indicated.
Keywords: Prostate cancer, BPH, testosterone, dutasteride
Introduction
Testosterone supplementation has proven benefits in improving muscle weakness, insulin resistance, osteoporosis, erectile dysfunction, erythrocytopenia, and cognition in men with hypogonadism or even low endogenous testosterone, but there is concern that androgen therapy may accelerate the growth of previously undetected, indolent prostate cancer (PC) [1]. Even though epidemiologic data are equivocal about actual increased risk of PC from androgen replacement [1,2], slow-growing latent androgen sensitive PCs are a common finding reported at autopsy in 29% or 39% - 56% of men, and longitudinal epidemiologic studies strongly correlate high endogenous testosterone with subsequent risk of prostate cancer [3-5]. There is also a risk of testosterone replacement worsening benign prostatic hypertrophy (BPH) and obstructive uropathy.
In the present study, we tested the premise that 5-α reductase enzyme inhibitors might ultimately allow safer androgen therapy, by preventing testosterone-driven growth of PC cells and benign prostatic epithelium. Inhibitors of 5-a reductase enzyme are currently used for treatment of BPH, blocking conversion of testosterone to dihydrotestosterone, which provides the main stimulation of androgen receptors. Dihydrotestosterone binds to androgen receptors on prostate cells with 5-10 fold higher affinity than testosterone does. There are two isoforms of 5-a reductase enzyme, type I and II. Type II is dominant in prostate, but type I is also present in prostate tissue and importantly, is upregu-lated in many cancers. Dutasteride (Avodart), used for our experiments, blocks both type I and II 5-α reductase.
Materials and methods
Cell lines
Four different prostate cell lines were employed, to model different clinical scenarios. First, we used the androgen-sensitive prostate cancer cell line LNCaP, originally derived from aspiration biopsy of a lymph node metastatic lesion from a 50-year-old man. These are slow-growing cells (with a doubling time of 60 hours) that represent a good model of indolent prostate cancer which might be activated by androgen therapy, especially since LNCaP has stable levels of androgen receptor (AR). Secondly, we used the C4-2 cell line, which was produced by co-injecting the original LNCaP cells with a human osteosarcoma cell line MS [6]. We chose C4-2 because it is more aggressive and is capable of producing colonies in soft agar in absence of serum, but, importantly, expresses a much lower level of AR than parental LNCaP. Thirdly, to represent a cell line that is highly metastatic and anaplastic yet still androgen-sensitive, unlike the commonly used PC-3 and DU145, we chose to test Dunning-R3327 AT6.1 cells [7]. Finally, to investigate the possible effect of dutasteride on testosterone-promoted benign hyperplasia, we used benign BPH-1 prostate cells.
LNCaP prostate cancer cells and benign BPH-1 cells, were from American Type Culture Collection (Manasas, VA). The C4-2 cells were a gift of Dr. Adrie vanBokhoven (Univ. of Colorado Health Sciences Center), and the Dunning-R3327 AT6.1 cells were a gift from Dr. Allen Gao, University of California-Davis School of Medicine). Culture medium for cell lines other than C4-2 was RPMI 1640 (Invitrogen, Carlsbad, CA) with 10% fetal calf serum and antibiotics. C4-2 cells were grown in T-medium (Invitrogen) with 10% serum and antibiotics. For cell set-up, cells in a flask were trypsinized, serum-containing medium was added to neutralize trypsin, cells were resuspended in basal medium, counted after dilution with Trypan blue by grid method [8] and plated at 30,000/well of 6-well plates. Cells were grown in a 5% CO2 incubator at 37°C.
Cell treatment and counts
The normal physiologic level of testosterone in young men is roughly 20 × 10-9 M (10.4 –36.4 nmol/liter) [9]. Our cells were subjected to concomitant treatment with 10-9, 10-8, or 10-7 M testosterone (Calbiochem, San Diego) dissolved in methanol, thus providing a range of testosterone concentrations going from sub- to supra-physiologic. The cells were also incubated in the presence of low (0.25 μM) or high (1.0 μM) doses of dutasteride (Avodart) dissolved in DMSO (Glaxo Smith Kline, Research Triangle Park, NC) or in DMSO alone. After growth intervals of 4 days (Dunning cells), 5 days (BPH-1 cells), or 7 days (LNCaP and C4-2), cells from each treatment were harvested with trypsin and diluted with Trypan blue. Cells were pipetted up and down so that clumps were not seen microscopically. Using a hemacytometer with cover glass, the cells in 10 (of 18) squares were counted to estimate cells per microliter. To estimate total cells, the cells per microliter were multiplied by the volume of medium in which they were suspended. At least two repeat experiments of each combination of doses were performed. Treatment effects were analyzed by t-test, with significance set at p ≤0.05.
Results and Discussion
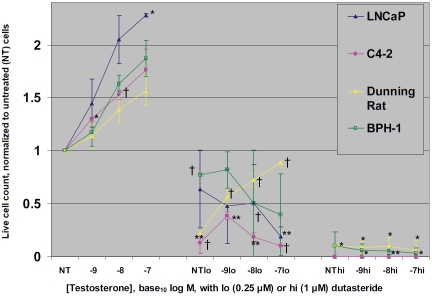
With testosterone alone (Figure 1), a dose-response relationship of cell growth was noted, with the most significant increases occurring in LNCaP and C4-2. At the lower dose of dutasteride, the dose-response relationship of cell growth to increasing testosterone was abolished, except in the Dunning cells. Growth of all cell lines was significantly inhibited, although BPH-1 cells’ response was significant with dutasteride alone, but not with the added testosterone. At the higher dose of dutasteride, minimal numbers of BPH-1 and Dunning cells survived at the end of the experiment, with abolished dose-response to testosterone. The LNCaP and the derived C4-2 cells did not survive the high dose in countable numbers.
Figure 1.
The growth rate of Dunning cells (4 days), BPH-1 (5 days), or LNCaP or C4-2 (both 7 days) cells is shown in response to testosterone doses of 10-9, 10-8, or 10-7 M, ± low (0.25 μM) or high (1.0 μM) dose dutasteride. Counts of treated cells were normalized to those receiving no treatment (NT). Compared to untreated counterparts, † indicates p≤ 0.05; * indicates p≤ 0.01; ** indicates p≤ 0.001.
Thus, even the lowest 0.25 μM dutasteride dose significantly abrogated growth of androgen-sensitive prostate cancer, and 1 μM dose significantly abrogated BPH-1 cell growth as well. This indicates dutasteride could have widespread application for protecting men receiving testosterone replacement from worsening of benign prostatic hypertrophy or activation of latent androgen-sensitive prostate cancer. However, it is also possible that lowering of dihydro-testosterone might select for prostate cancer clones with enhanced androgen receptor signaling [10], counteracting dutasteride's benefits. Thus, in vivo studies and clinical trials are required to verify the efficacy of dutasteride concomitant with testosterone as not increasing the risk of clinically significant prostate cancer. Furthermore, testosterone might also be employed to obviate rare anti-androgen side effects of 5-α reductase inhibitors used for benign prostatic hypertrophy and baldness, and this possibility also could be pursued through in vivo studies and clinical trials.
Acknowledgments
This work was supported by Department of Defense Prostate Cancer Research Program, Grant W81XWH-07-1-300 to K.A.I. We thank Dr. Kathleen C. Torkko for statistical support.
References
- 1.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. TherClin Risk Manag. 2009;5:427–48. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgentaler A. Rapidly shifting concepts regarding androgens and prostate cancer. Scientific World J. 2009;9:685–90. doi: 10.1100/tsw.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delongchamps NB, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, Jumbelic M, Haas GP. Evaluation of prostatitis in autopsied prostates–is chronic inflammation more associated with benign prostatic hyperplasia or cancer= J Urol. 2008;179:1736–40. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Eur Urol. 2005;48:739–44. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Stamatiou K, Alevizos A, Agapitos E, Sofras F. Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcinoma-tous lesions in Greek male population: an autopsy study. Prostate. 2006;66:1319–28. doi: 10.1002/pros.20339. [DOI] [PubMed] [Google Scholar]
- 6.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 7.Gao AC, Lou W, Ichikawa T, Denmeade SR, Barrett JC, Isaacs JT. Suppression of the tumori-genicity of prostatic cancer cells by gene(s) located on human chromosome 19p13.1-13.2. Prostate. 1999;38:46–54. doi: 10.1002/(sici)1097-0045(19990101)38:1<46::aid-pros6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Iczkowski KA, Omara-Opyene AL, Kulkarni TR, Pansara M, Shah GV. Paracrine calcitonin in prostate cancer is linked to CD44 variant expression and invasion. Anticancer Res. 2005;25:2075–83. [PubMed] [Google Scholar]
- 9.Wang C, Swerdloff R, Kipnes M, Matsumoto AM, Dobs AS, Cunningham G, Katznelson L, Weber TJ, Friedman TC, Snyder P, Levine HL. New testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3821–9. doi: 10.1210/jc.2003-031866. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Kim J. Molecular profiles of finasteride effects on prostate carcinogenesis. Cancer Prev Res (Phila Pa) 2009;2:518–24. doi: 10.1158/1940-6207.CAPR-08-0241. [DOI] [PubMed] [Google Scholar]