Abstract
SNS-032 is a potent inhibitor of cyclin-dependent kinases (Cdk) 2, 7 and 9 that regulate the cell cycle and transcription. Our studies in indolent primary chronic lymphocytic leukemia cells demonstrated that SNS-032 inhibited transcription, diminished the anti-apoptotic protein Mcl-1, and induced apoptosis. The present study focuses on evaluating this compound in four proliferating mantle cell lymphoma (MCL) lines (Jeko-1, Granta 519, Mino and SP-53). Consistent with its action against Cdk9 and Cdk7, SNS-032 inhibited the phosphorylation of RNA pol II in all 4 lines and blocked RNA synthesis. The transcripts and protein levels of short-lived proteins decreased, including cyclin D1 and Mcl-1. Cell growth was inhibited in a concentration-dependent manner in all lines. Apoptosis was induced in JeKo-1, Mino and SP-53 cells without disrupting cell cycle distribution. However, apoptosis was limited in Granta cells; rather, there was a significant reduction of clonogenic survival. SiRNA was used to specifically knock down Mcl-1 and cyclin D1 in JeKo-1 and Granta cells. Knocking down Mcl-1 induced significant apoptosis in Jeko-1 cells but not Granta cells. Reducing cyclin D1, rather than Mcl-1 was associated with loss of clonogenic survival in Granta cells. Thus, these results indicated that MCL cell lines have distinct mechanisms sustaining their survival, and the mechanism of action SNS-032 is dependent on the biological context of an individual line.
Keywords: SNS-032, mantle cell lymphoma, Mcl-1, cyclin D1, Cdk9
Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype of non-Hodgkin’s lymphomas that constitutes 5-10% of the disease (1, 2). It is the result of a malignant transformation of B lymphocytes in the outer edge of a lymph node follicle, called the mantle zone. MCL is genetically characterized by the t(11;14)(q13;q32) translocation that juxtaposes the proto-oncogene CCND1, which encodes cyclin D1, at chromosome 11q13, to the immunoglobulin heavy chain gene at chromosome 14q32. This translocation leads to the constitutive overexpression of cyclin D1, which is not detected in normal lymphocytes. As cyclin D1 couples with cyclin dependent kinases (Cdks) and regulates the transition of cells from the G1 to S phase of the cell cycle, this overexpression was thought to contribute to uncontrolled growth of the disease (1). Despite the response rates of 50-70% with current regimens of chemotherapy and immunotherapy, the disease typically progresses after treatment. The median survival time is approximately 3 years; the 10-year survival rate is only 5-10%. Thus, MCL remains incurable with current therapeutics and awaits more effective treatment approaches (1-3).
SNS-032 (formerly BMS-387032), is a potent Cdk inhibitor for a select group of Cdks with Ki values in the nanomolar range (4). It has been evaluated both in vitro (4, 5) and in clinical trials against advanced B cell malignancies (6) and solid tumors (7). Originally selected as an inhibitor of Cdk2 (IC50 38 nM) (4), the compound was later found to be a potent inhibitor of Cdk9 (IC50 4 nM) and Cdk7 (IC50 62 nM) (4), the Cdks that regulate the initiation and elongation of transcription by phosphorylating Ser2 and Ser5 sites in the tandem repeat of RNA polymerase II (pol II) C-terminal domain (CTD), respectively. Recent investigations demonstrated the actions of SNS-032 as an inhibitor of transcription in chronic lymphocytic leukemia (CLL) cells, an indolent disease model which do not exhibit cell cycle progression (5).
In the present study, we postulated that SNS-032 would be a unique and active compound in mantle cell lymphoma, a highly proliferative disease, based on the following rationale: 1) the inhibition of transcription will eliminate the short-lived anti-apoptosis protein Mcl-1 and induce apoptosis (5). 2) Transcriptional inhibition will also reduce cyclin D1 levels which would affect proliferation. Cyclin D1 mRNA is transcriptionally up-regulated in MCL cells. However, both the mRNA and protein of cyclin D1 turn-over rapidly. Although some variation of cyclin D1 transcripts has been described, greater than 90% of cases reported have the AUUUUA sequence in the 3′-untranslated portion of the transcript that predisposes the transcript for rapid degradation (8). 3) Direct inhibition of Cdk2 by SNS-032 would inhibit cell cycle progression. 4) Inhibition of Cdk7, which together with Mat also functions as the Cdk activating kinase by phosphorylating Cdk1, 2, 4 and 6, in concert with the down regulation of cyclin D1 would block cell cycle progression. Our results in four MCL cell lines showed that the inhibition of transcription by SNS-032 causes a profound reduction of the cellular proteins. Elimination of the anti-apoptotic protein Mcl-1, rather than cyclin D1, was responsible for apoptosis in JeKo-1, Mino and SP-53 cells. In contrast, reduction of cyclin D1 and inhibition of Cdk2, may contribute to the diminished clonogenic survival in Granta 519 cells.
Materials and methods
Materials
SNS-032 was provided by Sunesis Pharmaceuticals, Inc. (South San Francisco, CA). It was prepared as a 10 mM stock solution in dimethylsulfoxide (DMSO) and stored at −20°C in small aliquots. [3H]uridine (50 Ci/mmol) was purchased from Moravek Biochemical Inc. (Brea, CA). Annexin V-FITC Apoptosis Detection Kit was purchased from BD Biosciences (San Jose, CA). Propidium iodide (PI) solution (1mg/ml), was purchased from Sigma Aldrich (St. Louis, MO).
Cell lines
The four MCL cell lines (Granta 519, Jeko-1, Mino and SP-53) (9) used in this study were kindly provided by Dr. Hesham Amin in our institution. The cells were maintained in DMEM with 20% FBS (Granta-519), RPMI 1640 with 10% FBS (JeKo-1), RPMI 1640 with 20% FBS (Mino and SP-53), respectively (10). Cell lines were authenticated by STR DNA fingerprinting using the AmpFℓSTR Identifiler kit (Applied Biosystems, Foster City, CA). The STR profiles were compared to known ATCC fingerprints (ATCC.org), to the Cell Line Integrated Molecular Authentication database (CLIMA) version 0.1.200808 (http://bioinformatics.istge.it/clima/) (11) and to the German Collection of Microorganisms and Cell Cultures database (http://www.dsmz.de/). The STR profiles of JeKo-1, Mino and Granta 519 matched known DNA fingerprints. The STR profile of SP-53 cells was unique.
Growth inhibition
MCL cells were seeded on 24 well plates at 5 × 104/ml, SNS-032 was added the next day. Cell concentrations were measured at 24, 48 and 72 hr after addition of SNS-032 by Cell and Particle Counter (Beckman Coulter, Inc. Fullerton, CA). Data were presented as percentage of control cell growth.
Quantitation of cell death
Cell death after SNS-032 treatment was evaluated by flow cytometry analysis using annexin V and PI double staining according to the manufacturer’s instruction (Becton Dickinson, Franklin Lakes, NJ). Samples were analyzed with a Becton Dickinson FACS Calibur flow cytometer (Becton Dickinson). Data acquisition and analysis were performed by the CellQuest program. Cells stained positive for either annexin V or PI were considered dead cells.
Clonogenic assay
After incubating with SNS-032 for 2 to 24 hr and washed with phosphate buffered saline (PBS), or 6 hr after transfection with the siRNAs, Granta cells were mixed with methylcellulose media (Stemcell, Vancouver, BC, Canada) with 5 mM glutamine, 1% BSA, 30% FBS and 0.5% glucose at the density of 400 cells/ml, and dispensed to 12-well plates at 1ml/well in triplicates. Colonies (aggregates composed of more than 50 cells) were counted after 14 days of culture at 37°C. Clonogenic efficiency was about 30%.
RNA synthesis
RNA synthesis was measured by quantitating incorporation of [3H]uridine into the perchloric acid-insoluble materials as described previously (12).
Immunoblot analysis
Cells were collected and lysed by sonication, and the lysates were subjected to immunoblotting as described before (12). The blots were scanned by an Odyssey Infrared Imaging system (LI-COR Biosciences, Lincoln, Nebraska) to obtain images and quantitations. The antibody to Mcl-1 (S-19) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody to PARP was from Biomol International Inc (Plymouth Meeting, PA). Antibodies for total RNA Pol II (8WG16), phosphorylated CTD at Ser2 (H5) or Ser5 (H14) were purchased from Covance Research Products, Inc (Berkeley, CA). pT821 retinoblastoma protein (Rb) and nucleophosmin (NPM) antibodies were purchased from Invitrogen (Carlsbad, CA). Antibody for cyclin D1 was from BD Biosciences (San Jose, CA) which recognize the A isoform of cyclin D1. Antibodies for pS807/811-RB, total Rb and pT199-NPM were from Cell Signaling Technology (Danvers, MA). Actin antibody was purchased from Sigma Aldrich (St. Louis, MO). Alexa Fluor® 680 goat anti-mouse IgG was purchased from Invitrogen (Carlsbad, CA). IRDye 800CW Goat Anti-rabbit IgG was from LI-COR Biosciences (Lincoln, Nebraska).
RNA isolation and real-time quantitative RT-PCR
Total cellular RNA was isolated by the RNeasy kit from Qiagen (Valencia, CA). The mRNA levels were measured by real-time quantitative RT-PCR as previously described (5). The relative gene expression was analyzed by the Comparative Ct method using 18s ribosomal RNA as endogenous control. The results were presented as the percentage of gene expression of the time-matched controls. All primers, probes and reaction mix were purchased from Applied Biosystems (Foster City, CA).
Cell cycle analysis
Exponentially growing MCL cells were incubated with SNS-032 for 24 hr. About 5×105 cells were collected, washed with ice-cold PBS and fixed in 2.5 ml 70% ethanol overnight. Fixed cells were washed twice with cold PBS before incubation in 300 μl PBS with 15 μg/ml PI and 2.5 μg/ml DNase-free RNase A (Roche, Nutley, NJ) for 30 min at 4°C. Fluorescence was measured by flow cytometry.
Small interfering RNA (siRNA) transfection
The On Target Plus Smartpool siRNAs specific of Mcl-1, cyclin D1 and c-Myc were purchased from Dharmacon Inc (Lafayette, CO). Granta 519 and Jeko-1 cells were transfected with 1.5 μg siRNA with Nucleofector 1 (Amaxa, Gaithersburg, MD) using the Cell Line Nucleofector Kit V and program D-23 for Granta 519 cells and T-02 for JeKo-1 cells. Six hr after transfection, Granta 519 cells were seeded for clonogenic assay as described above. At 24 and 48 hr after transfection, cells were collected for viability assay and immunoblotting. Transfection efficiency was measured by flow cytometry using a plasmid expressing the green fluorescent protein (Amaxa). Transfection efficiency was about 60% for both cell lines.
Statistical analysis
Statistical analysis was carried out by the student’s t test using the GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). A p-value less than 0.05 was considered to be statistically significant.
Results
SNS-032 inhibited cell proliferation and induced cell death in MCL cells
Toxicity of SNS-032 in the MCL cells was first evaluated by measuring cell growth after addition of SNS-032 (Figure 1A). SNS-032 at a range of 0.03 μM to 3 μM, induced a concentration dependent inhibition of cell growth. Although sensitivity varied, 0.3 μM SNS-032 was maximally effective in all cell lines. The IC50 values of inhibition of cell growth after a 72-hr incubation with SNS-032 were similar in JeKo-1 (0.06 +/− 0.04 μM, mean +/− SD) and Granta 519 cells (0.06 +/− 0.02 μM), followed by Mino (0.12 +/− 0.02 μM) and SP-53 cells (0.14 +/− 0.02 μM).
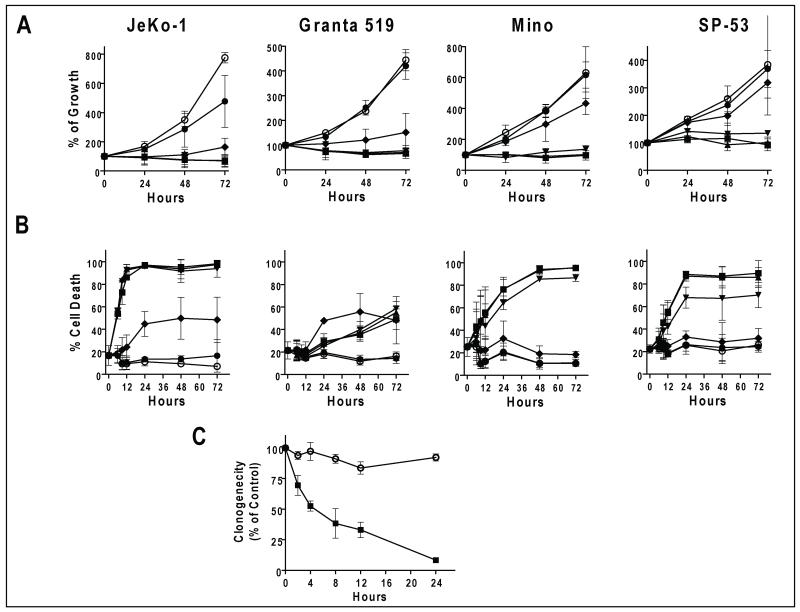
Figure 1. Toxicity of SNS-032 in the MCL cell lines.
A. SNS-032 inhibited proliferation of the four MCL cell lines. MCL cells were seeded on 24 well plates at 5 × 104/ml, SNS-032 at concentrations of 0 (○), 0.03, (●), 0.1 (◆), 0.3 (▼), 1 (▲) and 3 (■) μM were added the next day. Cell concentrations were measured at 24, 48 and 72 h after addition of SNS-032. Data (mean +/− SD) represent percentage of cell growth compared to day 1 of three independent experiments. B. SNS-032 induced apoptosis in the four MCL cell lines. MCL cells were incubated with SNS-032 at concentrations as in A for 6, 9, 12, 24, 48 and 72 h and apoptosis was measured by Annexin/PI staining. Data (mean +/− SD) represent percentage of cells stained positive for apoptosis in three independent experiments. C. SNS-032 inhibited clonogenicity of Granta 519 cells. Cells were incubated with 0.3 μM SNS-032 for indicated time before clonogenic assays. Data represent mean +/− SD of percentage of controls from three experiments each performed in triplicate.
As inhibition of cell growth represents the combined effect of blocking cell cycle progression and induction of cell death, we studied both aspects of SNS-032 in the MCL cells. Apoptosis was induced rapidly in JeKo-1 cells in as soon as 6 hr and reached a maximum at 12 hr by 0.3 μM SNS-032 (Figure 1B). The pattern was similar in Mino and SP-53 cells, except that they were relatively less sensitive. In contrast, Granta 519 cells were resistant to SNS-032-induced apoptosis. There was very little cell death at 24 hr after SNS-032, even at concentrations greater than 0.3 μM, while the same concentrations clearly inhibited cell growth (Fig. 1A). These data indicated that the major effect of SNS-032 on Granta cells is blocking proliferation. Clonogenic assays were used to further investigate the effect of SNS-032 on the cell repopulating capacity (Figure 1C). Granta 519 cells were incubated with 0.3 μM SNS-032 for 2, 4, 8, 12 and 24 hr before they were washed and seeded in semi-solid media. There was a time-dependent inhibition on clonogenicity in which 4 hr of exposure to SNS-032 resulted in 50% reduction in the number of colonies.
SNS-032 inhibited RNA synthesis and reduced the expression of short-lived oncoproteins
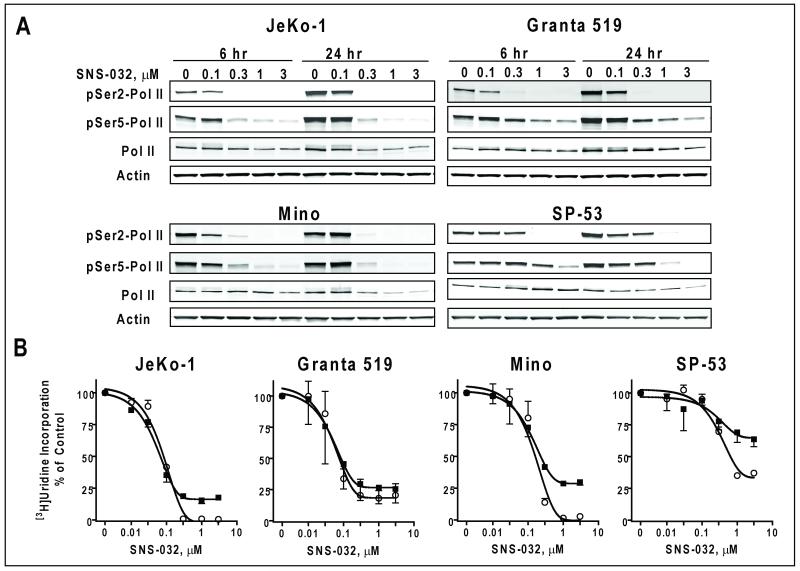
Previous studies demonstrated that transcriptional inhibition of anti-apoptotic proteins is a key mechanism for Cdk9 inhibitor-induced cell death in indolent B-cell malignancies (5, 13). To evaluate if a similar mechanism was active in proliferating MCL cells, we studied the phosphorylation status of the Pol II CTD after a 6 hr and 24 hr exposure to increasing concentrations of SNS-032 (Figure 2A). Phosphorylation of both Ser2 (Cdk9 site) and Ser5 (Cdk7 site) was inhibited by SNS-032 at 6 hr. There was less inhibition of Ser5 phosphorylation in all cell lines, consistent with a higher Ki in the inhibition of Cdk7 (62 nM) compared to Cdk9 (4 nM) (4). The inhibition of pol II phosphorylation was associated with the decrease of total RNA synthesis, measured by [3H]uridine incorporation at both 6 and 24 hr (Figure 2B). The IC50 values for inhibition of RNA synthesis after a 24-h incubation were 0.08 +/− 0.01 μM (mean +/− SD) for JeKo-1 cells, 0.08 +/− 0.02 μM for Granta 519 cells, 0.16 +/− 0.03 μM for Mino and 0.66 +/− 0.11 μM for SP-53 cells. Similar to the effect on growth inhibition, JeKo-1 and Granta 519 cells were more sensitive to SNS-032 than were Mino and SP-53 cells.
Figure 2. SNS-032 inhibited transcription in the MCL cell lines.
A. SNS-032 inhibited phosphorylation of CTD of RNA pol II in MCL cells. MCL cells were incubated with indicated concentrations of SNS-032 for 6 and 24 hr. The phosphorylation of RNA pol II at Ser2 and Ser5 sites as well as total pol II protein were studied by immunoblotting. B. SNS-032 inhibited RNA synthesis in the MCL cells, measured by [3H]uridine incorporation at 6 (○) and 24 h (■) after incubation with a series of concentrations of SNS-032, and presented as percentage of controls from three to six triplicate experiments.
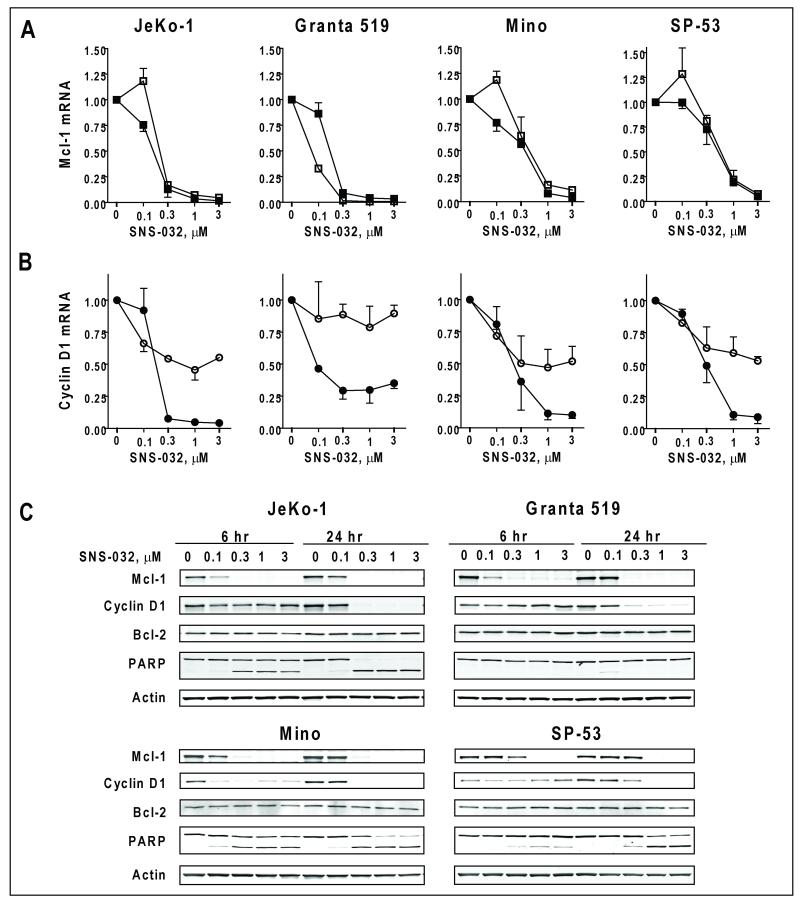
Because both Mcl-1 and cyclin D1 oncoproteins have rapid turn-over rates, we expected that their transcripts and protein levels would decrease upon the inhibition of transcription. A real-time RT-PCR analysis showed a concentration-dependent reduction of Mcl-1 mRNA by >90% at 0.3 μM for JeKo-1 and Granta 519 cells (Figure 3A). In Mino and SP-53 cells, 1 μM SNS-032 was required to achieve a 90% reduction. There was little difference in Mcl-1 mRNA level between 6 and 24 hr. Cyclin D1 mRNA was reduced moderately after 6 hr, and was further reduced at 24 hr, consistent with a relatively longer mRNA half-life (Figure 3B). The protein levels of Mcl-1 were also reduced after only 6 hr of incubation (Figure 3C) when all cell lines except Grant 519 initiated apoptosis, as indicated by cleaved PARP. Cyclin D1 protein levels were decreased in all cell lines after a 24-hr exposure to SNS-032. There was a renewed expression of Mcl-1 and cyclin D1 at 24 hr compared to 6 hr at 0.1 μM SNS-032, which did not completely inhibit transcription. This reversal may reflect a compensatory stress response under conditions of partial inhibition of transcription. Alternatively, cells with relatively low Mcl-1 may have been killed by this time, whereas those with greater levels may have survived for analysis. Pro-apoptotic proteins such as Bim and Noxa have been implicated in MCL pathogenesis (14, 15). Bim was not detected in JeKo-1, Mino and SP-53 cells likely due to homozygous deletions (14). In Granta cells, there was no change in Bim expression after SNS-032 treatment (Figure S1). We did not observe substantial changes in the level of Noxa in any of the cell lines.
Figure 3. SNS-032 reduced the expression levels of critical oncoproteins in the MCL cell lines.
A and B: SNS-032 reduced the mRNA levels of Mcl-1 and cyclin D1. mRNA levels were measured after 6 hr (open symbols) and 24 h (solid symbols) exposure of SNS-032 by real-time quantitative RT-PCR and expressed as relative levels compared to controls. C. SNS-032 reduced the protein levels of Mcl-1 and cyclin D1 and induced apoptosis.
Effect of SNS-032 on cell cycle
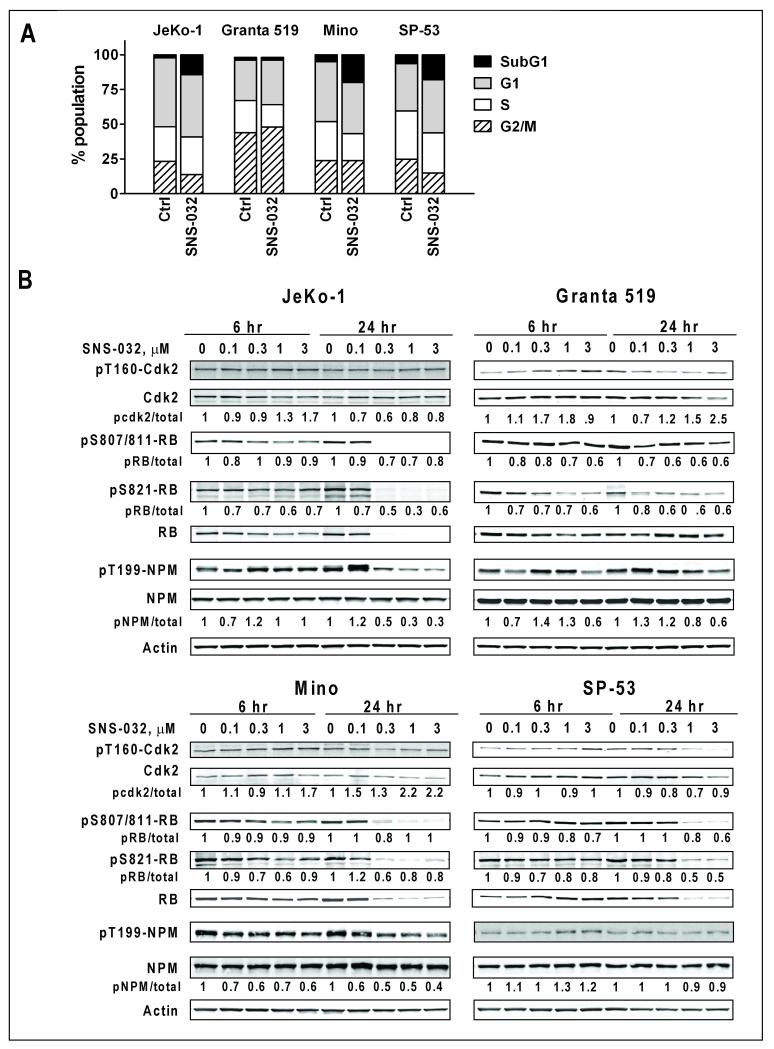
In contrast to a previous report of SNS-032-induced G2 arrest in HCT-116 cells (2), there was no significant cell cycle arrest in JeKo-1, Mino and SP-53 cells 24 hr after incubation with SNS-032 (Figure 4A). There was, however, an increase in cells with a sub-G1 DNA content, indicating dead cells. In Granta 519 cells, there was no change in the small sub-G1 population. Rather, there was a subtle increase in G1 and G2/M population and a decrease in cells in S phase. We observe substantially more annexin V positive cells than sub-G1 population in samples of the same treatment, possibly because annexin V detects an early stage of apoptosis, whereas sub-G1 cells are in late stages of cell death during which the DNA has been fragmented and substantially lost from the cells. The effect of SNS-032 on cell cycle regulating proteins and kinases were investigated by immunoblotting. First, we studied the phosphorylation of Cdk2 by the CAK activity of Cdk 7. There was no significant changes of Cdk2 phosphorylation at Thr160, indicating that the Cdk7 CAK activity was not affected by SNS-032 (Figure 4B). Next, we investigated the phosphorylation status of Rb protein. Upon phosphorylation by Cdk4/6/cyclin D, and further by Cdk2/cyclin E, Rb dissociates from E2F, which activates the expression of proteins needed for S-phase entry and progression. In Jeko-1, Mino and SP-53 cells, Rb phosphorylation on S807-811 (Cdk4/cyclin D1 substrate), as well as pT821 Rb (Cdk2/cyclin E substrate) was reduced 6 hr after incubation with SNS-032, and was further decreased after 24 hr. However, there was a coincident and profound decrease in the total Rb level, which correlated with cell death and PARP cleavage. Additional immunoblotting with the G3-245 antibody showed a caspase cleavage product p100 Rb at 6 hr after SNS-032 exposure (Figure S1), consistent with the suggestion that such processes may contribute to the decrease of Rb protein (16). On the contrary in Granta 519 cells, in which Rb protein level remained relatively stable, there was a consistent decrease in phosphorylated Rb/total Rb protein, indicating inhibition of kinase(s) responsible for Rb phosphorylation.
Figure 4. Effect of SNS-032 on cell cycle.
A: SNS-032 did not disturb the cell cycle distribution in the four MCL cell lines after incubation with 0.3 μM SNS-032 for 24 h. B. Effect of SNS-032 on Cdk2, Rb and NPM phosphorylation. Phosphorylation status were analyzed by immunoblotting with antibodies specific for the Cdk2 activation site by Cdk7 (pT160), pS807/811-Rb (Cdk4/cyclin D1 site), and pS821-Rb (Cdk2/cyclin E site), as well as pT199-NPM (Cdk2/cyclin E site). The fluorescence intensity was quantitated and normalized to level of total protein, setting values for untreated cells as 1.
NPM is a multifunctional protein involved in ribosome biosynthesis, stress response, and maintenance of genomic stability (17). Upon phosphorylation by Cdk2/cyclin E at threonine 199 in early G1 phase, NPM dissociates from centrosomes which triggers centriole separation and centrosome replication (17). After SNS-032 treatment, NPM protein appeared stable in all cell lines. However, inhibition of NPM phosphorylation was apparent in JeKo-1, Mino cells, and moderately in Granta cells, indicating loss of Cdk2 activity.
In addition to Rb, we also observed a general decrease of other cell cycle regulating proteins, such as the replication initiation protein Cdc6 and the Cdk regulator p27, in all four cell lines (Figure S1). There was no apparent change in the expression of Cdk inhibitor p21, consistent with a previous report in JeKo-1 cells after flavopiridol treatment (18). Both Rb and p27 are tumor suppressor proteins that inhibit cell cycle progression. Eliminating these proteins by SNS-032 would facilitate cell cycle transition, which counteract the inhibition of Cdks and the reduction of Cdc6. The lack of a prominent cell cycle arrest after SNS-032 treatment may be due to the simultaneous activation of these antagonistic processes.
Effect of siRNA depletion of Mcl-1 and cyclin D1 on short and long term cell survival
Reducing Mcl-1 expression was associated with SNS-032-induced cell death in CLL cells. As SNS-032 reduced the expression of both Mcl-1 and cyclin D1, siRNA was used to differentiate the contribution of each protein in cell survival and proliferation. SiRNA against Mcl-1 reduced the Mcl-1 protein level by about 80% in both JeKo-1 (Figure 5A, B) and Granta 519 cells (Figure 6A, B). There was a significant reduction of cell viability in JeKo-1 cells 24 (not shown) and 48 hr (Figure 5C) after the transfection. However, reduction of Mcl-1 had no effect on cell viability in Granta 519 cells (Figure 6C), indicating that Mcl-1 is not required for maintaining Granta cell viability. Cyclin D1 expression was reduced by its siRNA by 50% in each cell line with no change in cell viability (Figures 5C, 6C). Reducing both Mcl-1 and cyclin D1 levels in JeKo-1 cells did not induce more cell death than Mcl-1 siRNA alone. These results indicated that cyclin D1 is not a survival factor for either cell lines. However, clonogenic assays showed that reducing cyclin D1, rather than Mcl-1, was associated with a significant reduction of clonogenic survival in Granta (Figure 6D), suggesting depletion of cyclin D1 may contribute to the growth arrest and loss of clonogenicity in these cells.
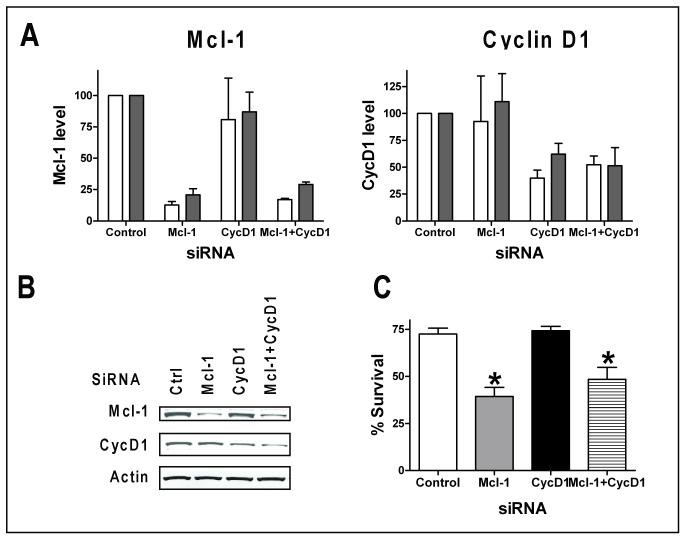
Figure 5. Effect of siRNA of Mcl-1 and cyclin D1 in JeKo-1 cells.
JeKo-1 cells were transfected with siRNAs of control siRNA (ctrl), Mcl-1, cyclin D1 (CycD1) or the combination of both siRNAs (Mcl-1+CycD1). A. Quantitation of Mcl-1 and cyclin D1 protein levels at 24 (white bars) and 48 h (grey bars) after transfection, expressed as the mean +/− SD of two independent experiments. B. A representative immunoblot of protein expression at 48 h after transfection. C. Transfection of Mcl-1 siRNA significantly reduced JeKo-1 cell survival. Cell viability was measured at 48 h after transfection by Annexin V/PI staining. Data represent mean percentage +/− SD of live cells after transfection from three independent experiments. * indicates significantly different from control siRNA transfected group. p = 0.005 for Mcl-1; p= 0.03 for Mcl-1+CycD1.
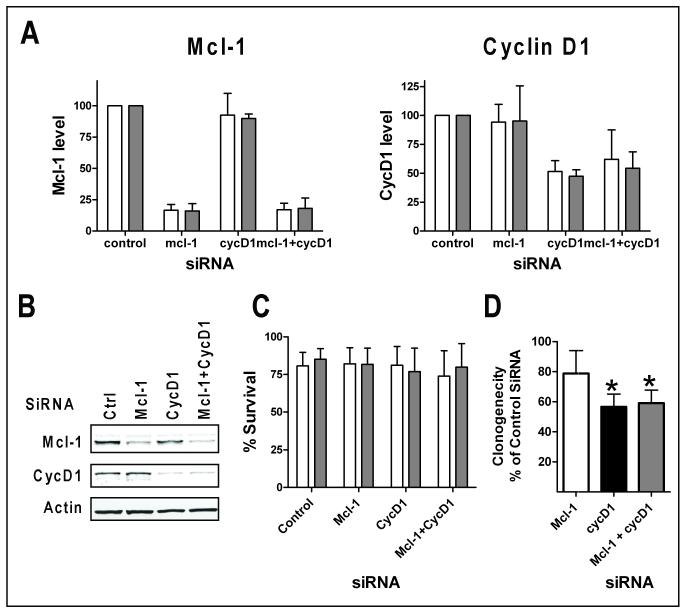
Figure 6. Effect of siRNA of Mcl-1 and cyclin D1 in Granta 519 cells.
Granta 519 cells were transfected with siRNAs of control siRNA (ctrl), Mcl-1, cyclin D1 (CycD1) or the combination of both siRNAs (Mcl-1+CycD1). A. Quantitation of Mcl-1 and cyclin D1 protein levels at 24 (white bars) and 48 h (grey bars) after transfection, expressed as the mean +/− SD of three independent experiments. B. A representative immunoblot of protein expression at 48 h after transfection. C. Neither siRNA of Mcl-1 nor cyclin D1 affected short-term survival of Granta 519 cell. Cell viability was measured at 24 (white bars) and 48 h (grey bars) after transfection by Annexin V/PI staining. Data represent mean percentage +/− SD of live cells after transfection from three independent experiments. D. Cyclin D1 siRNA significantly reduced clonogenic survival of Granta 519 cells. 6 hr after transfection, the cells were seeded onto methylcellulose media for cloning. Data represent percentage of colonies of control siRNA-transfected cells of three independent experiments performed in triplicates. * Significantly different from control siRNA-transfected group. p = 0.006 for CycD1; p= 0.009 for Mcl-1+CycD1.
Discussion
This study investigated the actions of a potent Cdk 2, 7 and 9 inhibitor, SNS-032, in MCL cell lines. Our results showed that SNS-032 was active against all four lines studied. However, its mechanism of action appears to be dependent on the biological context of the disease. In JeKo-1, Mino and SP-53 cells which are dependent on the expression of the anti-apoptosis protein Mcl-1 for survival, transcription inhibition of Mcl-1 by SNS-032 induced a rapid apoptosis without perturbing cell cycle distribution. In Granta 519 cells in which Mcl-1 is dispensable for viability, apoptosis was not induced. Rather, the long term proliferating potential was inhibited after a transient exposure to the SNS-032, associated with inhibition of Cdk2 and suppression of cyclin D1 expression. These results indicated that Cdk inhibition by SNS-032 results in a differential downstream response in different cell lines, predicated on the intrinsic properties of that line. Likely, this differential sensitivity reflects heterogeneity in MCL lines derived from different sources.
Although the MCL is genetically characterized by the t(11;14)(q13;q32) and the over-expression of cyclin D1, deregulation of cyclin D1 in transgenic mouse models is not sufficient for lymphoma development (19, 20). Thus, additional oncogenic events that either drive cell cycle progression or alter the apoptosis pathway may cooperate with cyclin D1 over-expression in lymphomagenesis. In this study, SNS-032 reduced cyclin D1 levels in all cell lines, but specifically knocking down cyclin D1 by siRNA failed to induce apoptosis in JeKo-1 or Granta 519 cells (Figures 5C, 6C). These results are consistent with the work published by Klier et al., using lentiviral shRNA-mediated knock down of cyclin D1 in MCL cell lines (21). They reported retardation of growth without induction of apoptosis, and a small shift of S phase of cells to G1 phase. The compensatory induction of cyclin D2 expression or secondary genetic events may render cyclin D1 dispensable for maintaining MCL cell survival. Thus, these data indicate that the dysregulation of cyclin D1 may contribute to proliferation, but does not offer a strong survival advantage. If so, cyclin D1 alone may not be a sufficient target for therapeutic designs.
In contrast to cyclin D1, expression of Mcl-1 was reported to be associated with blastoid/large-cell morphology and proliferative state in MCL (22). This variant of MCL, demonstrating numerous medium-to-large blast-like cells, is associated with a more aggressive clinical course. It has been suggested that the high expression of Mcl-1 may contribute to maintaining cell viability in this variant of tumors (22). The JeKo-1 cell line was derived from such a large cell variant of MCL (23). Knocking down Mcl-1 expression in JeKo-1 cells induced significant apoptosis (Figure 5C), indicating dependence for Mcl-1 expression in maintaining JeKo-1 cell survival. Although inhibition of Cdk2 was apparent in JeKo-1 cells, and many cell cycle regulating proteins, both activators and inhibitors, were significantly reduced (Figure 3B, 4B), the pronounced effect of SNS-032 was transcriptional inhibition; no cell cycle disturbance was observed. Thus in JeKo-1 cells, the rapid decrease of Mcl-1 maybe the major mechanism of action that committed the cells to apoptosis.
The Granta 519 cell line was derived from the peripheral blood of a MCL patient with stage IV high grade disease. However, this cell line is positive for Epstein-Barr virus (EBV) (24), that encodes viral proteins such as LMP-1, which has polytrophic functions to activate signal transduction and up-regulate anti-apoptotic proteins (24). Granta 519 cells over-express the anti-apoptotic protein Bcl-2 (25), which may be one of the consequences of EBV infection. Thus, Granta 519 represents a variant of MCL that has an alternative survival mechanism to sustain cell viability compared to JeKo-1 cells. Nevertheless, SNS-032 clearly inhibited Granta 519 proliferation (Figure 1A) and clonogenic survival (Figure 1C). Diminishing the cyclin D1 level may contribute to this effect, as knocking down cyclin D1 by siRNA led to significant reduction in clonogenicity (Figure 6D), as did an shRNA approach (26). There was clear reduction of the cyclin D1 protein level (Figure 3C) as well as reduced phosphorylation of Rb (Figure 4B) after incubation with SNS-032. Further, inhibition of Cdk2 activity in Granta 519 cells was evident from the reduced phosphorylation of Rb at serine 821 (Figure 4B). Thus, the combined actions of transcriptional inhibition, decreasing cyclin D1 protein, and inhibition of proliferation through Cdk2, may cause the loss of clonogenic survival in Granta 519 cells following SNS-032 exposure. Consistent with this, Cdk antagonists with inhibitory activity against both transcription and cell cycle, such as flavopiridol (27) and roscovitine (28), have shown induction of cell death in MCL cells lines (18, 29). Compared to flavopiridol and roscovitine, SNS-032 is more selective (4) and demonstrated greater potency in the inhibition of transcription and induction of apoptosis in primary CLL cells (5).
It is known that both Mcl-1 and cyclin D1 proteins contain signals that program their rapid turnover (<1 hr) (30-32). Thus, even transient inhibition of translation could lead to a reduction of these protein levels. For instance, using a fusion toxin to specifically deliver a translational inhibitor gelonin to B cells, Lyu et al. reported efficient inhibition of cell growth and induction of apoptosis in JeKo-1, Mino and SP-53 (33). Because of the labile feature of Mcl-1, gelonin may have acted similarly to SNS-032 to suppress Mcl-1 protein and induced apoptosis. A more direct approach could be to employ a inhibitor of translation, such as homoharringtonine (34), which has been shown to reduce cyclin D1 (35) and Bcr-Abl levels (12). The mTORC1 inhibitor rapamycin and its derivatives have also shown effectiveness in MCL cell lines (36, 37), and clinical trials demonstrated that these compounds have therapeutic benefit for relapsed MCL (38).
Our results demonstrated that 0.3 μM SNS-032 was the maximally effective concentration in all cell lines. This concentration was achieved at the 75 mg/m2 dose level in the phase 1 clinical trial of SNS-032 in B cell malignancies (6). Biomarker modulations, including inhibition of RNA pol II phosphorylation, marked reduction of Mcl-1 levels and induction of apoptosis was observed in CLL samples taken from the patients during the clinical trial. Because only moderate clinical activities were observed in these patients, it was not possible to evaluate the relationship between pharmacodynamics and clinical response. In addition to SNS-032, other Cdk inhibitors with similar mechanisms of action such as roscovitine, flavopiridol and SCH 727965 are also under evaluation in clinical trials in B-cell malignancies (39, 40). Thus, this in vitro study provided a rationale for clinical evaluation of these Cdk inhibitors in MCL.
In conclusion, we investigated the action of Cdk inhibitor SNS-032 in a proliferating cell model in four MCL cell lines. Our results showed that SNS-032 has inhibitory activity on both transcription and cell cycle progression. Although cytotoxicity was demonstrated in all lines, the mechanism of action was dependent on the biological context of individual cell lines. Thus, SNS-032 may be an active compound against different variants of MCL through distinct mechanisms of action.
Supplementary Material
Acknowledgements
STR DNA fingerprinting was done by the Characterized Cell Line Core at M.D. Anderson Cancer Center which is supported by Cancer Center Support Grant, P30 CA16672 from the National Cancer Institute, Department of Health and Human Service.
Work in the authors’ laboratory was supported in part by grants CA81534, CA100632, CA136411 and Cancer Center Support Grant CA16672 from the National Cancer Institute, Department of Health and Human Service and a research grant from Sunesis Pharmaceuticals, Inc., South San Francisco, CA.
Footnotes
Conflict-of-interest: W.P. received research funding from Sunesis Pharmaceuticals.
References
- 1.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR. Mantle cell lymphoma: advances in biology and therapy. Curr Opin Hematol. 2008;15:415–21. doi: 10.1097/MOH.0b013e328302c9c5. [DOI] [PubMed] [Google Scholar]
- 3.Williams ME, Densmore JJ. Biology and therapy of mantle cell lymphoma. Curr Opin Oncol. 2005;17:425–31. doi: 10.1097/01.cco.0000174039.69656.2b. [DOI] [PubMed] [Google Scholar]
- 4.Conroy A, Stockett DE, Walker D, et al. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009;64:723–32. doi: 10.1007/s00280-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Wierda WG, Chubb S, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009;113:4637–45. doi: 10.1182/blood-2008-12-190256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong WG, Chen R, Plunkett W, et al. Phase I study of SNS-032, a potent and specific Cdk2, 7 and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) J Clin Oncol. 2010;(28):3015–22. doi: 10.1200/JCO.2009.26.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath EI, Bible K, Martell RE, Adelman DC, Lorusso PM. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Invest New Drugs. 2008;26:59–65. doi: 10.1007/s10637-007-9090-3. [DOI] [PubMed] [Google Scholar]
- 8.Rimokh R, Berger F, Bastard C, et al. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood. 1994;83:3689–96. [PubMed] [Google Scholar]
- 9.Amin HM, McDonnell TJ, Medeiros LJ, et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127:424–31. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- 10.Dennison JB, Balakrishnan K, Gandhi V. Preclinical activity of 8-chloroadenosine with mantle cell lymphoma: roles of energy depletion and inhibition of DNA and RNA synthesis. Br J Haematol. 2009;147:297–307. doi: 10.1111/j.1365-2141.2009.07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano P, Manniello A, Aresu O, Armento M, Cesaro M, Parodi B. Cell Line Data Base: structure and recent improvements towards molecular authentication of human cell lines. Nucleic acids research. 2009;37:D925–32. doi: 10.1093/nar/gkn730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Gandhi V, Plunkett W. A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer research. 2006;66:10959–66. doi: 10.1158/0008-5472.CAN-06-1216. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Keating MJ, Gandhi V, Plunkett W. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–9. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagawa H, Karnan S, Suzuki R, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–58. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 16.Tan X, Wang JY. The caspase-RB connection in cell death. Trends Cell Biol. 1998;8:116–20. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- 17.Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 18.Venkataraman G, Maududi T, Ozpuyan F, et al. Induction of apoptosis and down regulation of cell cycle proteins in mantle cell lymphoma by flavopiridol treatment. Leuk Res. 2006;30:1377–84. doi: 10.1016/j.leukres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Bodrug SE, Warner BJ, Bath ML, Lindeman GJ, Harris AW, Adams JM. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–30. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovec H, Grzeschiczek A, Kowalski MB, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994;13:3487–95. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klier M, Anastasov N, Hermann A, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 22.Khoury JD, Medeiros LJ, Rassidakis GZ, McDonnell TJ, Abruzzo LV, Lai R. Expression of Mcl-1 in mantle cell lymphoma is associated with high-grade morphology, a high proliferative state, and p53 overexpression. J Pathol. 2003;199:90–7. doi: 10.1002/path.1254. [DOI] [PubMed] [Google Scholar]
- 23.Jeon HJ, Kim CW, Yoshino T, Akagi T. Establishment and characterization of a mantle cell lymphoma cell line. Br J Haematol. 1998;102:1323–6. doi: 10.1046/j.1365-2141.1998.00911.x. [DOI] [PubMed] [Google Scholar]
- 24.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph C, Steinemann D, Von Neuhoff N, et al. Molecular cytogenetic characterization of the mantle cell lymphoma cell line GRANTA-519. Cancer Genet Cytogenet. 2004;153:144–50. doi: 10.1016/j.cancergencyto.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Pscherer A, Schliwka J, Wildenberger K, et al. Antagonizing inactivated tumor suppressor genes and activated oncogenes by a versatile transgenesis system: application in mantle cell lymphoma. FASEB J. 2006;20:1188–90. doi: 10.1096/fj.05-4854fje. [DOI] [PubMed] [Google Scholar]
- 27.Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–70. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 28.Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–36. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 29.Lacrima K, Valentini A, Lambertini C, et al. In vitro activity of cyclin-dependent kinase inhibitor CYC202 (Seliciclib, R-roscovitine) in mantle cell lymphomas. Ann Oncol. 2005;16:1169–76. doi: 10.1093/annonc/mdi217. [DOI] [PubMed] [Google Scholar]
- 30.Schubert KM, Duronio V. Distinct roles for extracellular-signal-regulated protein kinase (ERK) mitogen-activated protein kinases and phosphatidylinositol 3-kinase in the regulation of Mcl-1 synthesis. Biochem J. 2001;356:473–80. doi: 10.1042/0264-6021:3560473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–84. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson B, Lahusen T, Singh S, et al. Down-regulation of cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced by flavopiridol. Cancer Res. 1999;59:4634–41. [PubMed] [Google Scholar]
- 33.Lyu MA, Cheung LH, Hittelman WN, Marks JW, Aguiar RC, Rosenblum MG. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol Cancer Ther. 2007;6:460–70. doi: 10.1158/1535-7163.MCT-06-0254. [DOI] [PubMed] [Google Scholar]
- 34.Gurel G, Blaha G, Moore PB, Steitz TA. U2504 determines the species specificity of the A-site cleft antibiotics: the structures of tiamulin, homoharringtonine, and bruceantin bound to the ribosome. J Mol Biol. 2009;389:146–56. doi: 10.1016/j.jmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng H, Yang C, Jin J, Zhou Y, Qian W. Homoharringtonine inhibits the AKT pathway and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Leuk Lymphoma. 2008;49:1954–62. doi: 10.1080/10428190802320368. [DOI] [PubMed] [Google Scholar]
- 36.Hipp S, Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90:1433–4. [PubMed] [Google Scholar]
- 37.Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–9. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 38.Ansell SM, Inwards DJ, Rowland KM, Jr., et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–14. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16:36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen R, Plunkett W. Strategy to induce apoptosis and circumvent resistance in chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2010;23:155–66. doi: 10.1016/j.beha.2010.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.