Abstract
Anorexia Nervosa (AN) is a disorder of self-starvation characterized by decreased meal size and food intake. While it is possible that reduced food intake in AN reflects an excess of inhibitory factors, e.g., cognitive inhibition related to fear of weight gain or abnormal postingestive negative feedback, it is also possible that decreased intake reflects diminished orosensory stimulation of food intake. This has been difficult to test directly because the amount of food ingested during a test meal by patients with AN reflects an integration of orosensory excitatory, and cognitive, learned, and postingestive inhibitory controls of eating. To begin to dissociate these controls, we adapted the modified sham feeding technique (MSF) to measure the intake of a series of sweetened solutions in the absence of postingestive stimulation. Subjects with AN (n=24) and normal controls (NC, n=10) were randomly presented with cherry Kool Aid® solutions sweetened with five concentrations of aspartame (0, 0.01, 0.03, 0.08 and 0.28%) in a closed opaque container fitted with a straw. They were instructed to sip as much as they wanted of the solution during 15 1-minute trials and to spit the fluid out into another opaque container. Subjects with AN sipped less unsweetened solution than NC (p<0.05). Because this difference appeared to account completely for the smaller intakes of sweetened solutions by AN, responsiveness of intake to sweet taste per se was not different in AN and NC. Since MSF eliminated postingestive and presumably cognitive inhibitory controls, and the orosensory response to sweet taste was not different in AN than NC, we conclude that decreased intake by AN subjects under these conditions reflects the increased inhibition characteristic of this disorder that is presumably learned, with a possible contribution of decreased potency of orosensory stimulation by the sipped solutions.
Keywords: Modified sham feeding, anorexia nervosa, bulimia nervosa, sip and spit, eating disorders, orosensory stimuli, hedonics, sweet solutions, aspartame, artificial sweetener, inhibitory control of food intake, liking, wanting, sweetness
Introduction
Anorexia Nervosa (AN) is a disorder of unclear etiology characterized by self-starvation and fear of fatness (1). There are two clinical subtypes: patients with Restricting Subtype fast and/or engage in excessive exercise to maintain low body weight; other individuals with AN also engage in self-induced vomiting, laxative abuse, or other behaviors in attempt to compensate for caloric consumption, and sometimes engage in binge eating (Binge Eating-Purging Subtype).
The long-term outcome of AN is poor – AN is associated with a strikingly high mortality rate, and high risk of chronic illness (2;3). Approximately one-third of individuals with AN ultimately develop Bulimia Nervosa (BN), a related eating disorder characterized by episodic binge eating with purging, typically in the setting of normal body weight (4). While numerous physiological and behavioral abnormalities are observed, many of which are likely to be starvation-related (5), mechanisms maintaining abnormal eating in AN (and BN) remain unclear.
Laboratory test meals are usually smaller in AN than in controls (6–11), but under some test conditions they are equal (12) or even larger (13). Test meals in AN are lower in energy density (7), last longer (12), and contain more pauses and short eating bouts (12) compared with meals of women with BN and those without an eating disorder. Patients with AN also show greater variability in caloric intake compared with non-eating disordered controls (12).
Test meals activate both orosensory and postingestive controls of eating. Thus, they provide no specific information about orosensory controls acting alone. To address this problem we adapted the modified sham feeding technique (MSF; e.g.(14–18)). This technique requires subjects to ingest food stimuli into the mouth and then spit them out without swallowing them. Solutions sweetened with aspartame and providing no calories were used to minimize cognitive inhibitory controls of eating that may be activated by caloric stimuli in people with eating disorders (19).
We have used the MSF technique to assess the orosensory control of intake of a series of sweetened solutions in the absence of postingestive stimulation in women without eating disorders (20) and more recently used the MSF technique to measure the intake of five solutions, one unsweetened and four sweetened with different concentrations of aspartame, in 11 women with BN and 10 healthy control women (21). Intake of each solution, measured for one minute of MSF, was followed immediately by self reports of the perceived intensities of sweetness, liking, and wanting of that solution using visual analogue scales. Women with BN sipped significantly more of the unsweetened and sweetened solutions than women without eating disorders, despite equivalent self reports of perceived sweetness, liking, and wanting.
The current study extends the MSF paradigm to 24 women with AN. It was our hypothesis, based on evidence that at least a proportion of AN patients consume large quantities of non-caloric, artificially sweetened beverages ((22), e.g., liters of “diet” beverages per day), and engage in chewing and spitting behavior (23), arguably “naturalistic” forms of sham feeding, that this sip-and-spit model of MSF providing sweet taste stimulation in the absence of calories would elicit larger intakes in patients with AN than controls.
Methods
Twenty-six women with Anorexia Nervosa (as defined in DSM-IV (24)), with or without meeting the criterion for amenorrhea (25), were recruited from the surrounding community, university, and medical center by flyers, newspaper, and internet advertisements or referred by treatment providers to the inpatient research hospital at the New York State Psychiatric Institute. Consecutive eligible inpatients were approached for study participation. All women were between 16–40 years old. Six met criteria for Restricting Subtype. Twenty met criteria for Binge-Eating/Purging Subtype; 12 reported binge-eating episodes and eight reported purging only.
Table 1 shows baseline data including age, body mass index (BMI), illness duration, weight suppression (difference between lifetime maximum and lifetime minimum weights), self-reported frequency of binge-eating and/or purging (when applicable), and total scores on the Eating Disorder Examination (EDE, Version 16.0 (26) for 7 subjects and Version 12 (27) for 18 subjects) and Beck Depression Inventory (BDI (28)). The Eating Disorder Examination is a semi-structured diagnostic interview that quantifies psychological and behavioral symptoms of eating disorders and the Beck Depression Inventory is a 21-question, self-report survey of depressive symptomatology. Scores on the EDE and BDI were unavailable for one patient with AN.
Table 1.
Clinical and Demographic Characteristics of Subjects
| Subject Group | Age (years) | BMI (kg/m2) | Illness Duration (years) | Weight Suppression (pounds) | EDE Total score | BDI Total score | Weekly servings gum (pieces) | Weekly 12-oz servings diet drink | Weekly Artificial Sweetener Packets |
|---|---|---|---|---|---|---|---|---|---|
| Anorexia Nervosa (AN, N=24) | 25.21 (1.08) | 16.08 (0.25) | 8.3 (1.4) | 41.31a (3.38) | 3.68b (0.29) | 27.19c (2.26) | 28.68d (6.6) | 34.42e (11.51) | 52.86f (19.05) |
| Normal Control (NC, N=10) | 26.70 (1.4) | 21.01 (0.66) | N/A | 8.17 (1.82) | 0.16 (0.04) | 1.40 (0.54) | 3.41 (1.56) | 2.63 (1.25) | 3.13 (2.58) |
Data for age and BMI are mean ±(SE) from 24 AN and 10 NC subjects. Data from NC subjects were previously published(Klein, Schebendach et al. 2009). Data for illness duration, weight suppression, EDI and BDI are from 23 AN and 10 NC subjects; data for weekly servings of artificially sweetened products are from 22 AN and 9 NC subjects.
Weight Suppression = Difference between lifetime maximum and current body weight.
EDE = Eating Disorder Examination; BDI = Beck Depression Inventory. Weekly servings of diet products based on subject recall of prior 4 weeks’ use. Comparisons are made between AN pooled group and NC participants.
significantly greater weight suppression compared with controls, p<0.001.
significantly higher EDE score compared with controls, p<0.001.
significantly higher BDI score compared with controls, p<0.001.
significantly more reported chewing gum compared with controls, p=0.001.
significantly more reported diet beverages compared with controls, p=0.012.
significantly more reported sweetener packets compared with controls, p=0.017.
Self-reported average weekly use in the preceding 4 weeks of selected artificially sweetened products including chewing gum, “diet” beverages, and packets of artificial sweetener, was obtained on pencil-and-paper forms from all participants, as previously described (22). Information about use of artificially sweetened products was unavailable for two participants with AN.
AN subjects had a significantly larger body weight suppression, higher BDI scores, and higher EDE scores than NC subjects (Table 1).
All patients were hospitalized on the General Clinical Research Unit at the New York State Psychiatric Unit receiving treatment for AN at the time of their participation in this study. Treatment involves a behaviorally oriented program aimed at normalizing eating behavior and restoring weight to a BMI of approximately 19.5 kg/m2. The study was conducted within the first 10 days of hospitalization for all participants. The primary treatment goal during this time was weight stabilization. Patients were prescribed a caloric intake of approximately 1800–2200 kcal per day at the time of participation in the study and were receiving no psychotropic medication. Meals were consumed under staff supervision with the expectation of 100% completion, and purging was minimized by close observation following meals.
As in our previously published protocol for BN subjects and control women (21), AN subjects were told they were participating in a study designed to test the response of people with and without eating disorders to the taste of food without swallowing it. The experimental procedure was conducted over a single one-hour period in the early afternoon, three hours after eating a standardized breakfast (English muffin, pat of butter, 6 oz apple juice; approximately 300 kcal). Subjects were instructed to sip the solution from the container on their left and to spit it into the container on their right, without holding it in their mouths, swishing it around, or swallowing it. Subjects were told that the rate at which they sipped and spit was entirely up to them and that there was no requirement or expectation for them to sip all of the solution presented.
Prior to the experimental session, subjects were asked to rate visual analogue scales (VASs) after tasting and spitting out a small sample of each of the five experimental solutions. These VASs consisted of pencil-and-paper responses to the following questions: “How HUNGRY are you right now?” “How much do you want to EAT right now?” “How much do you want to BINGE right now?” “How much do you want to VOMIT right now?” and “How ANXIOUS do you feel right now?”.
A 10-cm horizontal line, anchored at either end by “Not at all” and “Extremely” was beneath each question. Subjects were asked to indicate their answers to these questions by placing a vertical mark along the horizontal line to estimate their experiences. Each set of VAS’s for a given solution was on a separate sheet of paper; this insured that subjects did not have access to their previous responses. Responses were measured to the nearest millimeter using a centimeter ruler.
Prior to beginning the experimental session, subjects completed an additional series of VASs to rate their perceived hunger, desire to eat, desire to binge, desire to vomit and anxiety. Subjects were also given a one-minute training session during which they practiced the sipping and spitting technique using water.
Following the training session, subjects were given access to each of 15 solutions that they were instructed to sip and spit for one minute. Two L of each solution were prepared fresh 18–24 hours prior to each experimental day. Solutions were refrigerated until 9 am of the experimental day and removed 120 minutes prior to the experiment and were presented in three sets, each containing five solutions of different aspartame concentrations (0, 0.145, 0.3, 0.75, and 2.8 g/L, or 0, 0.01, 0.03, 0.08 and 0.28% wt/wt, respectively) in distilled water, flavored with a constant concentration of cherry Kool Aid® (1.902 grams per Liter). Aspartame concentrations were selected to match sweetness intensity of sucrose solutions in the concentrations of 0, 2.5, 5, 10 and 20% wt/wt. These were concentrations of sucrose we employed in our original MSF study conducted with women without eating disorders (20) that produced a dose-dependent increase in intake with the modal peak intake at 10% sucrose. Notably 9–10% sucrose is the concentration preferred by normal individuals in hedonic studies (e.g., (29)) and used in most commercially available sugar-sweetened carbonated beverages (30). We included a concentration of greater sweetness intensity (20%) because at least one group has found people with eating disorders to prefer a 15% sucrose solution (29). Aspartame was substituted for sucrose after pilot testing showed patients with BN to report extreme concern about caloric consumption and limit their intake of sucrose solutions (unpublished data).
Kool Aid® (purchased in 13 oz (3.6g) packets from NetGrocer.com) was added to make the solutions more palatable and more comparable to beverages commonly consumed in the U.S. Aspartame (Ajinomoto USA, Inc., Paramus, NJ), a low-calorie sweetener, was used to sweeten the solutions because in pilot testing prior to our recent study (21), BN women limited their intake due to concerns about the caloric content of sucrose solutions despite instructions not to swallow. All subjects were specifically informed that the experimental solutions contained no sugar and no calories.
The five flavored solutions (14.4–15.6 °C) in each group were presented in random order. Nineteen hundred mL of each solution (100 mL were drawn off from the two L to provide samples for the taste test) were presented in an identical, opaque, unmarked, closed container that prevented visualization of the volume of the solution during the one-minute test. Identical containers were used to collect the liquid spit out.
Subjects sipped solutions through a straw and spit the oral contents out immediately into a funnel in the top of the spit container. Subjects were observed by experimenters using a LorexTM four-channel closed-circuit observation system (Strategic Vista International, Inc., Baltimore, MD) and were signaled to start and stop sipping and spitting by a doorbell tone. Signaling was performed by an observer, who monitored the time using a digital timer.
There was a one-minute interval between presentations of solutions. During this interval subjects used VASs to report their perceived intensities of sweetness, wanting, and liking of the sipped solution, as well as their anxiety and desire to eat, binge, and vomit. Then they rinsed their mouths with a solution consisting of baking soda dissolved in distilled water (23.7g per 1000 ml distilled H2O).
The sip and spit containers were weighed before and after each minute of MSF to the nearest 0.1 gram using an Acculab L-Series 7200 scale (Acculab, Edgewater, NY). The grams sipped or spit was the difference in the weight of the containers before and after each trial.
After the entire test was completed, subjects were debriefed and asked about their expectations of the experimental hypotheses, ability to comply with the experimental instructions, and experience of the procedure.
All of the experimental procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
Statistical Analysis
Statistical analyses were performed using SPSS version 16.0 (Chicago, IL). Visual inspection of the data revealed two participants with AN to have intake in excess of three SD of the mean of other AN. Both of these subjects endorsed a history of purging behavior and one also described binge-eating episodes. Both were indistinguishable from the other AN subjects on other experimental assessments with the exception of lower self-reported anxiety (see Discussion). These outliers’ data were excluded from analyses.
Student’s T-test was used to assess statistical significance of differences between intake measures among subtypes of AN. No significant differences were found between Binge-Purge and Restricting subtypes. Furthermore, separation of AN subjects by the presence of binge-eating and comparison of Binge-Purge subjects with Purging and Restricting subjects combined also revealed no significant difference in intake. Thus, data from all AN subjects were pooled in subsequent comparisons.
Data obtained from AN subjects were compared with data obtained from non-eating disordered control women (Normal Control, NC) previously reported (21). These data were collected during a period that overlapped the collection of data from AN and the methods were identical except that NC were not hospitalized and they received payment for their participation in the study.
Multi-variate ANOVA (MANOVA) with repeated measures was used to analyze intake and VAS measures of the perceived intensities of sweetness, liking, and wanting, in addition to self-reported hunger, desire to eat, desire to binge, desire to vomit, and anxiety, as a function of trial (1, 2 or 3) and aspartame concentration, using diagnostic group (AN vs NC) as the between-group variable. Significant treatment effects were analyzed post hoc by the Least Significant Difference (LSD) Test. Differences were considered significant when p<0.05. Greenhouse-Geisser correction was performed on all MANOVA results to correct for dependence among observations within subjects. Independent samples t-test was used to compare intake. Student’s T-test was used to assess mean differences in clinical and demographic measures between groups. When applicable, Cohen’s d was calculated as the difference between group means divided by pooled standard deviations.
Separate Pearson correlation coefficients were calculated for the relationships between intake and VAS ratings of hunger, desire to eat, desire to binge, desire to vomit, and anxiety obtained at baseline (prior to sipping and spitting first flavored solution), as well as clinical measures including subject age, BMI, weight suppression, self-reported use of artificially sweetened products in the preceding month, and, for AN participants, duration of the eating disorder, EDE and BDI scores. Because of significant group differences on several of these measures, correlation among clinical and behavioral measures were determined separately in AN and NC.
Results
Solution Intake
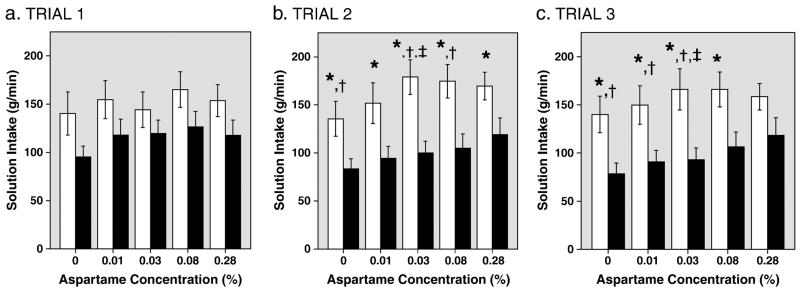
AN subjects ingested significantly less of the solutions than NC subjects during MSF (F[1, 32]=5.76, p=0.02; Figures 1a–c). Totaled across the three trials, AN subjects sipped significantly less than NC subjects at concentrations of 0%, 0.01%, 0.03% and 0.08% aspartame (p values all < 0.05) and showed a trend towards less intake at the highest (0.28%) aspartame concentration (p=0.06; Cohen’s d=−0.65).
Figure 1.
Fig. 1a–c. Intake of Aspartame Solutions in NC and AN Subjects. Bars show mean (±1 S.E.) intake by normal controls (blank bars) and AN patients (black bars) at each of five concentrations of aspartame in solution, over each of three trials.
*Significantly less intake by AN patients than controls, p<0.05.
†Significantly less intake within AN subjects compared with Trial 1, p<0.05.
‡Significantly greater intake within NC subjects compared with Trial 1, p<0.05.
Intake in MSF was also a function of sweetener concentration (MANOVA, F[1.94, 61.99]=5.51, p=0.007). Post-hoc analysis revealed that the significant effect of concentration was attributable to differences between the unsweetened solution and all the other solutions, and between the 0.01% aspartame solution and the 0.08% solution (p values <0.05).
To further assess whether the effect of sweetener on intake was a function of aspartame concentration, MANOVA was repeated excluding the unsweetened solution. Analysis of intake as a function of sweetness and trial among the four sweetened solutions showed no main effect of concentration in pooled subjects or in AN or NC subjects.
The interaction between diagnostic group and trial was significant (F[1.15, 36.83]=4.15, p=0.04. Total solution intake was smaller in the second and third trials than in the first in AN, but not in NC. There was no significant difference in mean total intake of AN subjects between trials 2 and 3 (p=0.194 by paired t-tests). The decreased intake in the second trial compared with the first was attributable to decreased intake of 0%, 0.03% 0.08% solutions (paired t-tests, p <0.05%). The decreased intake in the third trial compared with trial 1 was due to significantly smaller intakes of 0%, 0.01%, and 0.03% (p <0.05).
The only significant difference in NC across trials was that intake of the 0.03% solution was larger in the second and third trials compared to the first trial (p=0.01 and p=0.044, respectively).
Note that the mean amount of the unsweetened solution ingested by the AN group was less than that of the NC group in all 3 trials and this difference was statistically significant in trials 2 and 3 (p < 0.05; Fig 1a–c). To determine if the decreased intake of the unsweetened solution accounted for the decreased intakes of the aspartame solutions, two transformations were performed on the intake data: first, the difference between the intake of each sweetened solution and the intake of the unsweetened solution was calculated for the four aspartame solutions. Second, intake of each of the sweetened solutions was calculated as the percent difference from the intake of unsweetened solution. Separate Multivariate ANOVAs with repeated measures were performed on each of these transformed data sets, using the four solution concentrations and three trials as within-group variables and diagnostic group as the between-group variable. No significant effects of trial, concentration, or diagnostic group were found on data transformed in either way: for difference, F[1.68, 53.76]=0.78, p=0.44 for trials; F[1.79, 57.26]=1.49, p=0.23 for concentration; and F[1,32]=0.005, p=0.94 for diagnostic group; for percent difference, F[1.34, 42.88]=0.34, p=0.63 for trials; F[1.07, 34.11]=0.74, p=0.40 for concentration; and F[1,32]=0.33, p=0.57 for diagnostic group. Furthermore, the average slope of intake as a function of sweetener concentration in the AN group also did not differ significantly from that in the NC group (p=0.54; d=0.27).
To investigate the possibility that AN subjects ingested less because they swallowed more and the larger amount swallowed decreased intake through postingestive negative-feedback effects, we measured the difference between amount spit and amount sipped. There was no significant difference in grams difference or grams difference as a percentage of total intake in AN and NC subjects (Table 2). On average, subjects with AN spit out more of the solution than they sipped in: mean difference between solution spit and sipped across AN subjects as percent of total solution sipped was 0.85%. One AN subject who apparently swallowed 11.9% of the solution sipped had total intake that exceeded the mean of other AN participants (2286.0g, compared with 1534.9g), and that did not decrease over trials. This suggests that the swallowed solution did not limit intake through postingestive negative feedback.
Table 2.
Quantity of Solution Sipped versus Spit by Subject Group
| Subject Group | Aspartame Concentration in Solution | Solution Sipped (g/3 min ±SE) | Solution Spit (g/3 min ±SE) | Spit-Sipped (g/3min±SE) | Difference as Percent of Sipped (%) |
|---|---|---|---|---|---|
| Anorexia Nervosa (N=24) | 0% | 257.3 (30.9) | 259.2 (31.0) | 1.90 (1.23) | 1.89 (1.19) |
| 0.01% | 303.4 (36.2) | 304.6 (36.6) | 1.23 (1.46) | 0.45 (0.77) | |
| 0.03% | 312.5 (36.5) | 312.3 (36.6) | −0.22 (2.68) | 0.48 (1.09) | |
| 0.08% | 337.9 (43.1) | 332.8 (42.1) | −5.02 (4.97) | 0.11 (1.31) | |
| 0.28% | 355.2 (49.8) | 352.5 (48.5) | −2.68 (6.44) | 2.16 (1.84) | |
| Total | 1566.2 (182.7) g/15 min | 1561.4 (181.6) g/15 min | −4.8 (14.9) | 0.85 (1.06) | |
| Normal Control (N=10) | 0% | 415.9 (54.4) | 417.7 (53.4) | 1.82 (2.53) | 0.89 (0.62) |
| 0.01% | 456.3 (57.9) | 458.4 (56.9) | 2.11 (2.69) | 0.88 (0.61) | |
| 0.03% | 489.3 (56) | 492.2 (54.7) | 2.95 (3.10) | 0.97 (0.61) | |
| 0.08% | 505.7 (50.9) | 509.4 (50.1) | 3.76 (3.06) | 0.97 (0.58) | |
| 0.28% | 481.8 (40.1) | 485.9 (39.4) | 4.16 (2.75) | 1.06 (0.60) | |
| Total | 2348.9 (249.7) g/15 min | 2363.6 (244.4) g/15 min | 14.8 (14) | 0.94 (0.59) |
Data are solution sipped and spit in grams ± 1 SE at each aspartame concentration in solution, for AN and NC subjects. Difference between solution spit and sipped is tabulated and expressed as a percentage of total solution sipped at each concentration. Negative value for spit-sipped suggests solution swallowed.
VAS Ratings for Liking, Wanting, and Sweetness During MSF
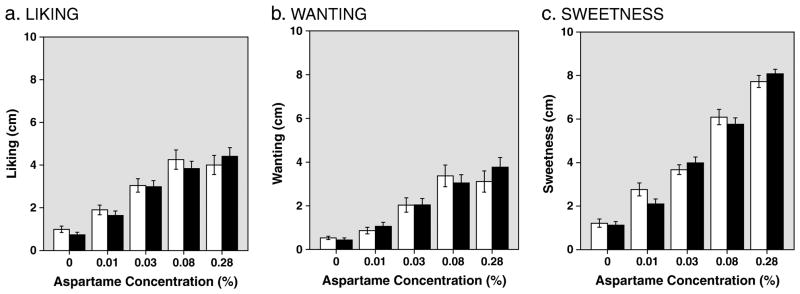
Perceived liking, wanting, and sweetness increased significantly as a function of aspartame concentration (Figures 2a–2c; MANOVA with repeated measures: liking: F[1.89,60.56]=20.05, p<0.001; wanting: F[1.85,59.16]=15.64, p<0.001; and sweetness: F[2.65,84.97]=131.63, p<0.001). There were no significant effects of diagnostic group (F[1,32]=0.05, p=0.82 for liking; F[1,32]=0.02, p=0.88 for wanting; F[1,32]=0.04, p=0.86 for sweetness) or trial (F[1.76, 56.34]=0.74, p=0.46 for liking; F[1.77, 56.55]=1.28, p=0.28 for wanting; F[1.51, 48.37]=0.10, p=0.86 for sweetness).
Figure 2.
Fig. 2a–c. Self-reported Liking, Wanting, and Sweetness of Solutions by Aspartame Concentration, Averaged Across 3 Trials. Data are mean (±1 S.E) VAS ratings for normal controls (blank bars) and AN patients (black bars) of Liking (Fig. 2a), Wanting (2b) and Sweetness (2c) of solutions by aspartame concentration, averaged across three trials.
Post-hoc analyses conducted on the above MANOVA (i.e., with diagnostic groups combined) of reported liking revealed significant increases among the 0.01, 0.03, and 0.08% aspartame solutions compared to the unsweetened solution and compared with each other (p ≤ 0.005), with a trend toward increased liking of the 0.28% compared with the 0.03% aspartame solutions (p=0.052). There was no significant difference in liking between the 0.08 and 0.28% solutions (p=0.70).
Self-reported wanting increased across 0.01%, 0.03%, and 0.08% solutions compared with the unsweetened solution and with each other (p<0.05). There was no significant difference between 0.08% and 0.28% aspartame solutions. In contrast, post-hoc analysis of perceived sweetness increased significantly across all of the aspartame solutions compared with the unsweetened solution and with each other (p< 0.001).
The concentration of aspartame that elicited the largest intake varied among AN and NC subjects. Table 3 shows the concentration of maximal intake totaled across the three trials in subjects with AN and NC. Across trials, minor variance was observed: the modal concentration of maximal intake among NC subjects in trial 1 and 2 was the 0.08% aspartame solution and in trial 3 was the 0.03% solution. For AN subjects the modal concentration of maximal intake was the 0.08% solution in trial 1, and the 0.28% solution in trials 2 and 3.
Table 3.
Number of Subjects Showing Maximal Intake and Self-Reported Liking, Wanting and Sweetness of Test Solutions
| Subject Group | Aspartame Concentration in Solution | Solution of Maximal Intake, #(%) of Subjects | Solution of Maximal Liking, #(%) of Subjects | Solution of Maximal Wanting*, #(%) of Subjects | Solution of Maximal Sweetness, #(%) of Subjects |
|---|---|---|---|---|---|
| AN (N=24) | 0% | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 0.01% | 5 (21) | 1 (4) | 2 (9) | 0 (0) | |
| 0.03% | 6 (25) | 5 (21) | 4* (18) | 0 (0) | |
| 0.08% | 6 (25) | 4 (17) | 3* (14) | 1 (4) | |
| 0.28% | 7 (29) | 14 (58) | 13 (59) | 23 (96) | |
| NC (N=10) | 0% | 0 (0) | 0 (0) | 1 (10) | 0 (0) |
| 0.01% | 2 (20) | 0 (0) | 2 (20) | 0 (0) | |
| 0.03% | 1 (10) | 2 (20) | 0 (0) | 0 (0) | |
| 0.08% | 5 (50) | 5 (50) | 4 (40) | 0 (0) | |
| 0.28% | 2 (20) | 3 (30) | 3 (30) | 10 (100) |
Numbers indicate the number and percentage of subjects in each diagnostic group showing maximal intake and self-reported liking, wanting and sweetness for each of the five solutions, arranged in order of increasing aspartame concentration. Intake was determined by summing intake of each solution across the three trials and self-report measures were determined by averaging VAS measure of each variable across the three trials.
One subject with AN reported identical maximal wanting values to the 0.03% and the 0.08% solutions. The remaining AN subject reported zero values for wanting of all solutions and is not included in this table. Data from NC subjects have been previously published (1).
Reference List
Klein D, Schebendach J, Brown A, Smith G, Walsh BT. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol Behav 2009;96:44–50.
The concentrations of aspartame that elicited the greatest reported liking and wanting similarly varied among subjects in both groups. Table 3 shows the concentration of maximal liking and wanting of solutions averaged over the three trials. Across trials, the greatest proportion of NC subjects reported maximal liking of the 0.08% solution in trials 1 and 2, and for the 0.28% solution in trial 3. The greatest proportion of AN subjects reported maximal liking for the sweetest (0.28%) solution in all three trials. Maximal wanting ratings showed the identical pattern.
Relationship Between Intake and Self-Reported Liking, Wanting and Sweetness As previously described (21), correlations among solution intake and VAS ratings of liking, wanting and sweetness varied among control participants (and BN patients). Similarly, significant variability was found among AN participants: correlation between intake and liking ranged from r= −0.60 to 0.98, with mean r=0.54, SEM=0.78; correlation between intake and wanting ranged r= −0.63 to 0.98; mean r=0.45 ± 0.45; correlation between intake and sweetness ranged from r=−0.93 to 0.99, mean r=0.31 ± 0.12. Correlation between wanting and sweetness (r=−0.80 to 0.99, mean r=0.69 ± 0.10) was similar to that between liking and sweetness (r=−0.85 to 1.0, mean 0.72 ± 0.10). Mean correlation between wanting and liking was higher, at r=0.88 ± 0.05, with a range from −0.18 to 0.99. None of these mean Pearson correlation coefficients or p values differed significantly from those of controls.
VAS Ratings for Hunger, Desire to Eat, Desire to Binge, Desire to Vomit, and Anxiety Prior to and During MSF
At baseline, subjects with AN reported significantly more anxiety, more desire to binge, more desire to vomit, and less desire to eat than controls (Table 4). Although AN subjects reported less hunger than NC, the difference was not statistically significant.
Table 4.
Baseline and Inter-Trial VAS Ratings of Subjects
| Subject Group | Hunger | Desire to Eat | Desire to Binge | Desire to Vomit | Anxiety | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Inter-Trial | Baseline | Inter-Trial | Baseline | Inter-Trial | Baseline | Inter-Trial | Baseline | Inter-Trial | |
| AN (N=24*) | 3.95 (0.69) | 3.22 (0.64) | 3.40 (0.70)a | 2.76 (0.52)b | 1.56 (0.43)c | 1.60 (0.41)d | 1.26 (0.36)e | 2.07 (0.49)f,† | 3.60 (0.58)g | 3.69 (0.52)h |
| NC (N=10) | 5.40 (0.62) | 5.16 (0.89) | 5.79 (0.61) | 5.02 (0.95) | 0.21 (0.05) | 0.24 (0.08) | 0.19 (0.06) | 0.18 (0.04) | 1.16 (0.55) | 0.69 (0.25) |
Baseline data are mean ±(SE) cm of VAS ratings obtained immediately prior to sipping and spitting test solutions. One AN subject did not provide baseline data and these data are from n=23 AN subjects only. Data from NC subjects have previously been published(1). Inter-trial data are mean ±(SE) cm of VAS ratings obtained immediately after sipping and spitting solutions. VAS measures are averaged over 15 trials. Comparisons between groups were made via independent samples t-test. Comparisons within group between baseline and inter-trial VASs were made via paired samples t-test (for n=23 subjects).
significantly lower self-reported basline desire to eat in AN patients compared with controls, p=0.016.
significantly lower self-reported inter-trial desire to eat in AN patients compared with controls, p=0.032.
significantly greater self-reported baseline desire to binge in AN patients compared with controls, p=0.005.
significantly greater self-reported inter-trial desire to binge in AN patients compared with controls, p=0.003.
significantly greater self-reported baseline desire to vomit in AN patients compared with controls, p=0.008.
significantly greater self-reported inter-trial desire to vomit in AN patients compared with controls, p=0.001.
significantly greater self-reported baseline anxiety in AN patients compared with controls, p=0.005.
significantly greater self-reported inter-trial anxiety in AN patients compared with controls, p<0.001.
significantly greater self-reported inter-trial desire to vomit in AN patients compared with baseline desire to vomit, p=0.014.
During MSF, there was no effect of trial, concentration or diagnosis on the ratings of hunger (MANOVA F[1.48,47.27]=0.28, p=0.69, F[1.12, 35.75]=0.30, p=0.67, F[1,32]=2.86, p=0.10, respectively). AN ratings for desire to eat were significantly smaller than NC ratings (F[1,32]=5.038, p=0.03), but neither trial nor concentration had a significant effect (F[1.44, 46.18]=1.12, p=0.32; F[1.92, 61.54]=0.57, p=0.56, respectively). AN ratings for desire to binge, desire to vomit, and anxiety were all significantly larger than NC ratings (F’s ≥ 4.616, p’s ≤ 0.04)). Trials and concentration had no significant effects on these ratings. Table 4 shows the inter-trial ratings of these VAS measures among AN and NC subjects averaged across 15 trials.
Repeated measures ANOVA was also performed within AN patients using concentration and trial as within-group factors. No effect of trial or concentration was found for hunger, desire to eat, desire to binge, or anxiety. A significant effect of trial was found for desire to vomit (F[1.63, 37.46]=4.72, p=0.02), but no concentration effect was observed. Post-hoc tests showed that increased desire to vomit occurred in trials 2 and 3 compared to trial 1 (p’s 0.01 and 0.04, respectively); no difference was observed between trials 2 and 3 (p=0.88).
Debriefing after the test revealed no difficulties complying with the procedure and no adverse reactions. Expectations of experimental hypotheses were obtained by interview in 24 of 26 AN subjects. Four reported having “no idea;” six speculated we were testing some component of sweet or general taste perception; nine thought we were assessing whether sweet taste exposure had effects on psychological parameters, such as the desire to binge and purge, appetite, and anxiety; six speculated we were assessing liking of sweet tastes and whether this differed by diagnosis or subtype of AN; one believed the solutions contained “some sort of medication;” three subjects spontaneously referred to solutions as “bitter,” and two as “salty,” while the remainder described them as “sour” and/or “sweet.” Finally, one subject likened the procedure to chewing and spitting out food, a behavior that occurs not uncommonly among eating disordered patients (23). Three subjects said we might be assessing sip volume and one of these specifically speculated that we were weighing the solutions.
Concern about calories in the solutions was voiced by some participants, but did not appear to influence results: of the patients who expressed any concern, the one who did so most clearly consumed a total of 2311g solution (compared with the AN group average of 1566g). Another participant reported that she “started to worry about sugar… then ‘let it go’”; her total intake was 1725g. A third indicated she had fleeting thoughts about calories; when probed further, she stated her behavior wouldn’t have been any different if she could have been absolutely certain the solutions were non-caloric. Her total intake was 187.4g, which was on the low end of the group range, but not the smallest (78g was the smallest total intake of the AN group, from a patient who did not report any concerns about calories in the solutions). While it is possible that additional patients had concerns about calories that they did not share with experimenters, we cannot conclude based upon our debriefing that fear of calories influenced the results of this study.
Discussion
The major result of this experiment is that participants with AN sipped approximately 33% less of the solutions than NC during MSF. This result contradicts our hypothesis that AN would sip more than NC. This demonstrates for the first time that orosensory stimulation by unsweetened and sweetened Kool Aid solutions without postingestive stimulation is sufficient for expression of the analogue of the eating behavioral phenotype characteristic of anorexia nervosa (restricting subtype). It also demonstrates that postingestive negative feedback or other visceral abnormalities produced by swallowed food (31–34) are not necessary for the expression of decreased intake. Given the absence of postingestive negative feedback during MSF, the significantly smaller intake in AN is either due to decreased potency of orosensory stimulation by the sipped solutions or to inhibition of the central processing of orosensory excitatory input (35;36).
The possibility that AN subjects have decreased peripheral orosensory stimulation is supported by reports of hypogeusia including elevated detection and recognition thresholds (37–41) and decreased perceived intensities (39) of sour and bitter stimuli. The psychophysical responses to sweet and salty stimuli were less impaired or normal (42–45). Decreased number of fungiform papillas in AN, but not in BN, may contribute to the hypogeusia (46). The hypogeusia of sour stimuli may be relevant to our results because the decreased intake of AN is accounted for by the decreased intake of the unsweetened Kool Aid which has a sour flavor and the increased intake of sweetened solutions in AN was not different from NC. Unfortunately, we did not specifically assess for hypogeusia in the current study.
Note that patients with BN can have similar taste abnormalities as AN (39), but BN sipped significantly more unsweetened Kool Aid than NC in MSF (21). Thus, if decreased peripheral orosensory stimulation contributes to decreased intake in MSF among AN patients, it is probably not the only factor determining intake in this paradigm.
Increased central inhibition of the processing of orosensory stimulation during MSF could also contribute to the decreased intake of AN. Presumably this is a learned inhibition related to the cognitive or psychological aspects of fear of fatness and drive for thinness. The effect of starvation itself and its associated neurobiological sequelae may also be involved (5). Recent electroencephalographic (47;48) and fMRI (49) reports of abnormal central responses to taste stimuli in AN are consistent with all of these possibilities. Further experiments are required to determine their relative contributions to decreased intake in AN during MSF.
The intake of participants with AN decreased significantly more in the second and third trials than in the first. In contrast, the intake in NC did not change significantly across the three trials. The reason for this effect of repetitive trials in AN is not clear.
Self reports during MSF
Despite the large differences in intake between AN and NC, self-reports of liking, wanting, or sweetness made immediately after each minute of MSF did not differ between AN and NC. A small number of prior psychophysical studies assessed hedonic responses of patients with AN to sweet/fat solutions, using a range of sucrose concentrations added to a dairy base with a range of fat concentrations. These studies demonstrated disliking of fattier food stimuli (42;44;45) and a preference for higher sucrose:fat ratio in AN compared to NC. Comparison of their results with sucrose with ours is difficult because they used a dairy base and a Likert scale instead of an unsweetened Kool Aid and a VAS scale.
More relevant to our results is the study by Eiber and colleagues (19). They assessed liking of water-based solutions across a range of sucrose concentrations (0–40%) in patients with eating disorders including AN. Because hedonic ratings were higher when solutions were spit out rather than swallowed, they concluded that fear of caloric consumption affected hedonic ratings in this population. This problem was apparently not present in our experiments because AN did not swallow significant volumes of the solution (Table 2) and their ratings of liking the solutions were not significantly different from NC (Figure 2a).
The discrepancy between intake (different across subject groups) and self-reported wanting of solutions (not significantly different) is itself notable given the likelihood that behavior in this MSF paradigm reflects wanting of solutions, and suggests that AN patients may use this scale in particular differently than individuals without an eating disorder.
That assessment of sweetness intensity increased in such an orderly fashion in AN subjects and did not differ from NC is consistent with the results of Sunday and Halmi (45). Thus, the lack of effect of concentration of aspartame on intake by participants with AN and NC during MSF cannot be attributed to an inability to detect increasing sweetness intensity of the aspartame solutions.
Other VAS measures more clearly distinguished participants with AN and NC. Desire to vomit, interestingly, was endorsed to a greater extent by AN subjects prior to the test and increased during the test (Table 5). This measure did not differ significantly among the subtypes of AN; thus, it does not appear to reflect prior history of vomiting. The increasing self-reported desire to vomit during MSF trials in AN is consistent with learned inhibition of intake and conditioned aversion to food stimuli.
TABLE 5.
Comparison of AN and BN Subjects
| Measure | AN (n=24) | BN (n=11) | P value for difference between AN and BN |
|---|---|---|---|
| INTAKE | |||
| Total (grams, ±SE) | 1566.2 (182.7) | 3431.6 (389.4) | <0.001 |
| Trial Effect (Unsweetened Solution) | Yes* | Yes (Trend)** | |
| Unsweetened Solution, grams | 257.3 (30.9) | 630.3 (78.9) | <0.001 |
| Sweetest (0.28%) Solution, grams | 355.2 (49.8) | 737.4 (88.3) | <0.001 |
| Difference (S-U)†, grams | 35.70 (37.92) | 32.63 (65.72) | 0.887 |
| VAS (averaged across all solutions, cm ±SE) | |||
| Liking | 2.72 (0.29) | 3.30 (0.48) | 0.284 |
| Wanting | 2.07 (0.33) | 2.66 (0.52) | 0.330 |
| Sweetness | 4.21 (0.23) | 4.52 (0.27) | 0.429 |
| Hunger | 3.22 (0.64) | 5.40 (0.57) | 0.041 |
| Desire to Eat | 2.76 (0.52) | 5.10 (0.75) | 0.016 |
| Desire to Binge | 1.60 (0.41) | 2.26 (0.86) | 0.437 |
| Desire to Vomit | 2.07 (0.49) | 1.33 (0.8) | 0.420 |
| Anxiety | 3.69 (0.52) | 3.45 (0.82) | 0.800 |
| Baseline | |||
| BMI | 16.08 (0.25) | 22.46 (0.83) | <0.001 |
| EDE, total score | 3.69 (0.29) | 4.07 (0.26) | 0.333 |
| BDI | 27.19 (2.26) | 18.50 (1.90) | 0.006 |
| VAS: Hunger | 3.95 (0.69) | 5.61 (0.45) | 0.052 |
| VAS: Desire to Eat | 3.40 (0.69) | 5.87 (0.49) | 0.007 |
| VAS: Anxiety | 3.60 (0.58) | 4.56 (0.89)a | 0.376 |
| Self-reported Low-Calorie Sweetener Use | |||
| “Diet” Drinks (weekly 12-oz serving equivs) | 34.40 (11.5) | 18.70 (5.6) | 0.402 |
| Sweetener Packets (packets weekly) | 52.9 (19.0) | 29.0 (13.6) | 0.431 |
| Gum (pieces per week) | 28.7 (6.6) | 33.1 (15.3) | 0.759 |
Intake and VAS data are from 24 AN and 11 BN subjects. Baseline clinical and baseline VAS data are from 23 AN and 10 BN subjects; self-reported sweetener data are from 22 AN and 10 BN subjects. Baseline and sweetener use data from BN subjects previously published (Klein, Schebendach et al. 2009)).
Comparison of Inter-trial VAS ratings and Baseline VAS ratings within groups was made via paired-samples t-tests.
Intake of unsweetened solution Trial 1>Trial 2 within AN subjects, p=0.019, Trial 1>Trial 3, p=0.039.
Intake of unsweetened solution Trial 1<Trial 2 within BN subjects, p=0.093.
Intake Difference between sweetest solution and unsweetened solution averaged over Trials 1–3.
Baseline anxiety significantly higher than inter-trial anxiety in BN group, p=0.023.
Pretest measures
Pretest measures including clinical history differed as expected between AN and NC subjects groups, as did pretest ratings of self-reported anxiety (greater in AN subjects) and desire to eat (lower in AN subjects, though without a difference in hunger). When intake measures (total intake across all trials and solutions) were compared with clinical and baseline VAS measures among AN subjects, no association was found between intake and BMI, age, duration of eating disorder, weight suppression, EDE, weekly servings of gum, diet beverages or sweetener packets, or baseline VAS measures of hunger, desire to eat, desire to binge, desire to vomit, or anxiety.
There was, however, a significant inverse correlation between intake and BDI (r = −0.534, p = 0.009): higher ratings of depression at the time of hospitalization predicted lower intake in AN (data not shown). This might suggest a role for anhedonia in decreased intake by AN, but there was no significant correlation between depression scores and reports of liking or wanting of any test solution (e.g., correlation between BDI and average liking across solutions was −0.02, p=0.94). Depression scores were also not related to decreasing intake across trials (T3 intake – T1 intake; r=0.11, p=0.62).
Comparison with BN subjects
Compared with data collected from women with BN tested under identical conditions (21), AN subjects consumed approximately 54% less than those with BN (Table 5). In both studies, the difference in intake from controls appeared to be attributable to a difference in the intake of the unsweetened solution.
Subjects with AN decreased intake over trials while subjects with BN increased intake from trial 1 to trial 2. For total solution intake, subjects with BN showed T2>T1, t[df=10]= −3.93, p=0.003; T3>T1 t[10]= −2.26, p=0.048. Comparisons of individual solutions were not statistically significant.
In contrast to the large difference in intake between subjects with AN and those with BN, their VAS ratings of liking, wanting and sweetness obtained immediately after ingestion of each solution were not significantly different. Among the other self reports after each solution, hunger and the desire to eat were significantly less in AN than in BN, but anxiety and the desire to binge or vomit were not significantly different in AN and BN (Table 5). That hunger and the desire to eat were less in AN than BN correlates with the smaller intake in AN than BN, but the equivalent reports of anxiety and the desire to binge or vomit contrasts with the large difference of intake. Thus, if these self reports which were significantly larger than NC are contributing to the opposite differences in intake of subjects with AN and BN from NC, their contribution must be to amplify an underlying proclivity for hyperphagia in BN and hypophagia in AN.
Study Limitations
The current study has several limitations including the lack of assessment of thirst and perceived intensity of sourness of the unsweetened solution, the small sample size of NC and patients with the Restricting Subtype of AN, the narrow range of aspartame concentrations, our use of visual analogue scales (50;51), and the lack of information about taster status (52). Furthermore, most of our analyses of the effect of increasing sweetener concentration make the assumption that the orosensory effect of increasing sweetness concentration is simply additive to the gustatory properties of the unsweetened solution. Additionally, evidence suggests that a sip-and-spit MSF paradigm does not elicit cephalic phase response in the way that a chew-and-spit MSF paradigm does in healthy individuals (53), suggesting that limited conclusions can be drawn about any physiological implications of the behavioral differences observed in this study.
Our exclusion of two outliers also represents a study limitation, as it is unclear why these two individuals with AN exhibited such strikingly high intake while others did not. Their behavior in the experiment otherwise was not atypical: one individual showed essentially orderly increases in intake with increased sweetener concentration with maximal intake of the 0.28% solution, and the other did not; both showed decreased intake over trials as did other AN participants, and ratings of solution wanting and sweetness by each of these participants were within the range of other AN patients. The outlier whose intake peaked at the 0.28% solution reported the highest liking of this solution (10 cm on the 10 cm VAS scale) of any participants for any solution, but not by far (several other patients provided ratings above 9.5 cm).
Clinical characteristics that distinguished these outliers from non-outliers include slightly lower BMIs (mean=14.8 for each, versus 16.1, SD=1.2, for other participants); higher EDE scores (5.28 and 5.29, vs 3.69±1.39), and lower self-reported anxiety before and throughout the procedure (baseline anxiety ratings per VAS 0.3 and 0.7, vs 3.60±2.80). One patient engaged in binge-eating and purging and was noted by clinical staff to have reported “dreams about binge-eating and purging” and intense “cravings” for this behavior around the time this study was conducted. The other participant reported purging only and denied binge-eating; however she described use of 2L of diet soda and 50 packets of artificial sweetener per day prior to hospital admission. Thus these individuals may have been more ill by some measures than non-outliers; however, none of the characteristics that distinguished them from the group were variables that correlated with intake among remaining subjects. The extremely low anxiety during the procedure in these two individuals however is consistent with the above speculation that anxiety may serve to amplify the proclivity in AN patients to restrict food intake. This possibility requires further investigation.
Despite these limitations, this MSF paradigm offers the advantage of being an objective assessment of an eating-related behavior that minimizes the concerns of caloric ingestion and can be conducted in its entirety within a one-hour period. Several aspects of MSF behavior are open to exploration, including baseline intake, incremental responsiveness to increasing concentrations of sweetness, intake over trials; the effect of nutritional recovery, and the effects of treatments specifically aimed at normalizing eating rate (e.g., (54).
In conclusion, the reduced intake among women with AN compared with controls in this study, in light of our previous finding of increased intake among women with BN compared with the same controls, demonstrates that orosensory stimulation during MSF under our conditions is sufficient to produce the analogues of the eating behavioral phenotypes characteristic of these two eating disorders. This confirms the heuristic value of MSF and supports its validity and utility.
Acknowledgments
This work was supported by grants from the NIH (MH071285, PI: Klein, and MH079397, PI: Walsh). We would like to thank co-Investigators on the Translational Grant, colleagues in the Eating Disorders Research Unit and General Clinical Research Unit, and study participants for their contributions to this research. This work was presented in part at the Columbia University Appetitive Behavior Seminar, March, 2008 and at the 16th Annual Meeting of the Society for the Study of Ingestive Behavior in Paris, France, July, 2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiol Behav. 2004;81:359–74. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194:10–7. doi: 10.1192/bjp.bp.108.054742. [DOI] [PubMed] [Google Scholar]
- 3.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284–93. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 4.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–50. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodersten P, Nergardh R, Bergh C, Zandian M, Scheurink A. Behavioral neuroendocrinology and treatment of anorexia nervosa. Frontiers in Neuroendocrinology. 2008;29:445–62. doi: 10.1016/j.yfrne.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Sysko R, Walsh BT, Schebendach J, Wilson GT. Eating behavior among women with anorexia nervosa. Am J Clin Nutr. 2005;82:296–301. doi: 10.1093/ajcn.82.2.296. [DOI] [PubMed] [Google Scholar]
- 7.Rolls BJ, Andersen AE, Moran TH, McNeils AL, Baier HC, Fedoroff IC. Food intake, hunger, and satiety after preloads in women with eating disorders. Am J Clin Nutr. 1992;55:1093–103. doi: 10.1093/ajcn/55.6.1093. [DOI] [PubMed] [Google Scholar]
- 8.Gwirtsman HE, Kaye WH, Curtis SR, Lyter LM. Energy intake and dietary macronutrient content in women with anorexia nervosa and volunteers. Journal of the Americal Dietetic Association. 1989;89:54–7. [PubMed] [Google Scholar]
- 9.Hetherington M, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiology Behav. 1991;50:101–8. doi: 10.1016/0031-9384(91)90505-i. [DOI] [PubMed] [Google Scholar]
- 10.Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatry. 1994;10:696–702. doi: 10.1016/0006-3223(94)91179-7. [DOI] [PubMed] [Google Scholar]
- 11.Zandian M, Ioannis I, Bergh C, Sodersten P. Cause and treatment of anorexia nervosa. Physiol Behav. 2007;92:283–90. doi: 10.1016/j.physbeh.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Sunday SR, Halmi KA. Micro– and macroanalyses of patterns within a meal in anorexia and bulimia nervosa. Appetite. 1996;26:21–36. doi: 10.1006/appe.1996.0002. [DOI] [PubMed] [Google Scholar]
- 13.Owen WP, Halmi KA, Gibbs J, Smith GP. Satiety responses in eating disorders. J Psychiatr Res. 1985;19:279–84. doi: 10.1016/0022-3956(85)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–13. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- 15.Richardson CT, Walsh JH, Cooper KA, Feldman M, Fordtran JS. Studies on the role of cephalic-vagal stimulation in the acid secretory response to eating in normal human subjects. J Clin Invest. 1977;60:435–41. doi: 10.1172/JCI108793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman M, Richardson CT. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology. 1986;90:428–33. doi: 10.1016/0016-5085(86)90943-1. [DOI] [PubMed] [Google Scholar]
- 17.Helman CA. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. Gastroenterology. 1986;90:428–33. [PubMed] [Google Scholar]
- 18.Smeets AJ, Westerterp–Plantenga MS. Oral exposure and sensory–specific satiety. Physiol Behav. 2006;89:281–6. doi: 10.1016/j.physbeh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Eiber R, Berlin I, de Brettes B, Foulon C, Guelfi JD. Hedonic response to sucrose solutions and the fear of weight gain in patients with eating disorders. Psychiatry Res. 2002;113:173–80. doi: 10.1016/s0165-1781(02)00232-9. [DOI] [PubMed] [Google Scholar]
- 20.Klein DA, Schebendach JS, Devlin MJ, Smith GP, Walsh BT. Intake, sweetness and liking during modified sham feeding of sucrose solutions. Physiol Behav. 2006;87:602–6. doi: 10.1016/j.physbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Klein D, Schebendach J, Brown A, Smith G, Walsh BT. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol Behav. 2009;96:44–50. doi: 10.1016/j.physbeh.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein DA, Boudreau GS, Devlin MJ, Walsh BT. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord. 2006;39:341–5. doi: 10.1002/eat.20260. [DOI] [PubMed] [Google Scholar]
- 23.Guarda AS, Coughlin JW, Cummings M, et al. Chewing and spitting in eating disorders and its relationship to binge eating. Eat Behav. 2004;5:231–9. doi: 10.1016/j.eatbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. 1994. [Google Scholar]
- 25.Mitchell JE, Cook-Myers T, Wonderlich SA. Diagnostic criteria for anorexia nervosa: Looking ahead to DSM-V. Int J Eat Disord. 2005;37:595–7. doi: 10.1002/eat.20125. [DOI] [PubMed] [Google Scholar]
- 26.Fairburn CG, Cooper Z, O'Connor ME. Eating Disorder Examination. In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: Guilford Press; 2008. pp. 265–308. [Google Scholar]
- 27.Fairburn CG, Cooper PJ. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press; 1993. The Eating Disorder Examination; pp. 317–60. [Google Scholar]
- 28.Steer RA, Beck AT. Manual for the Beck Depression Inventory. San Antonio: Psychological Corporation; 1993. Beck Depression Inventory (BDI) pp. 100–3. [Google Scholar]
- 29.Drewnowski A, Bellisle F, Aimez P, Remy B. Taste and bulimia. Physiol Behav. 1987;41:621–6. doi: 10.1016/0031-9384(87)90320-9. [DOI] [PubMed] [Google Scholar]
- 30.Nutrient Data Laboratory, Beltsville Human Nutrition Research Center. USDA Database for the Added Sugars Content of Selected Foods. Release 1. Beltsville, MD: U.S. Department of Agriculture; 2006. [Google Scholar]
- 31.Bruch H. Anorexia nervosa and its differential diagnosis. J Neur Trans. 1966;141:555–64. [Google Scholar]
- 32.Garfinkel PE. Perception of hunger and satiety in anorexia nervosa. Psychol Med. 1974;4:309–15. doi: 10.1017/s0033291700042999. [DOI] [PubMed] [Google Scholar]
- 33.Halmi KA, Sunday SR. Temporal patterns of hunger and fullness ratings and related cognitions in anorexia and bulimia. Appetite. 1991;16:219–37. doi: 10.1016/0195-6663(91)90060-6. [DOI] [PubMed] [Google Scholar]
- 34.Robinson PH. Perceptivity and paraperceptivity during measurement of gastric emptying in anorexia and bulimia nervosa. Brit J Psychiatry. 1989;154:400–5. doi: 10.1192/bjp.154.3.400. [DOI] [PubMed] [Google Scholar]
- 35.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–6. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 36.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–20. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 37.Aschenbrenner K, Schoze N, Joraschky P, Hummel T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J Psych Res. 2008;43:129–37. doi: 10.1016/j.jpsychires.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Casper RC, Kirschner B, Sandstead HH, Jacob RA, Davis JM. An evaluation of trace metals, vitamins, and taste function in anorexia nervosa. Am J Clin Nutr. 1980;33:1801–8. doi: 10.1093/ajcn/33.8.1801. [DOI] [PubMed] [Google Scholar]
- 39.Jirik-Babb P, Katz JL. Impairment of taste perception in anorexia nervosa and bulimia. Int J Eat Disord. 1988;7:353–60. [Google Scholar]
- 40.Nakai Y, Kinoshita F, Koh T, Tsujii S, Tsukada T. Taste function in patients with anorexia nervosa and bulimia nervosa. Int J Eating Disord. 1987;6:257–65. [Google Scholar]
- 41.Nozoe S-I, Masuda A, Naruo T, Soejima Y, Nagai N, Hiromitsu T. Changes in taste responsiveness in patients with anorexia nervosa during behavior therapy. Physiol Behav. 1996;59:549–53. doi: 10.1016/0031-9384(95)02105-1. [DOI] [PubMed] [Google Scholar]
- 42.Drewnowski A, Halmi KA, Pierce B, Gibbs J, Smith GP. Taste and eating disorders. Am J Clin Nutr. 1987;46:442–50. doi: 10.1093/ajcn/46.3.442. [DOI] [PubMed] [Google Scholar]
- 43.Lacey JH, Stanley PA, Crutchfield M, Crisp AH. Sucrose sensitivity in anorexia nervosa. J Psychosom Res. 1977;21:17–21. doi: 10.1016/0022-3999(77)90021-6. [DOI] [PubMed] [Google Scholar]
- 44.Simon Y, Bellisle F, Monneuse M, Samuel-LaJeunesse B, Drewnowski A. Taste responsiveness in anorexia nervosa. Br J Psychiatry. 1993;162:244–6. doi: 10.1192/bjp.162.2.244. [DOI] [PubMed] [Google Scholar]
- 45.Sunday SR, Halmi KA. Taste perceptions and hedonics in eating disorders. Physiol Behav. 1990;48:587–94. doi: 10.1016/0031-9384(90)90196-b. [DOI] [PubMed] [Google Scholar]
- 46.Wockel L, Jacob A, Holtmann M, Poustka F. Reduced number of taste papillae in patients with eating disorders. J Neural Transm. 2008;115:537–44. doi: 10.1007/s00702-007-0845-y. [DOI] [PubMed] [Google Scholar]
- 47.Toth E, Kondakor I, Tury F, Gati A, Weisz J, Molnar M. Nonlinear and linear EEG complexity changes caused by gustatory stimuli in anorexia nervosa. Int J Psychophysiol. 2004;51:253–60. doi: 10.1016/j.ijpsycho.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Toth E, Tury F, Gati A, Weisz J, Kondakor I, Molnar M. Effects of sweet and bitter gustatory stimuli in anorexia nervosa on EEG frequency spectra. Int J Psychophysiol. 2004;52:285–90. doi: 10.1016/j.ijpsycho.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Wagner A, Aizenstein H, Mazurkewicz L, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neurospsychopharmacol. 2008;33:513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 50.Bartoshuk LM, Duffy VB, Hayes JE, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–48. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartoshuk LM, Fast K, Snyder DJ. Differences in Our Sensory Worlds: Invalid Comparisons with Labeled Scales. Current Directions in Psychological Science. 2005;14:122–5. [Google Scholar]
- 52.Lucchina LA, Curtis OF5, Putnam P, Drewnowski A, Prutkin JM, Bartoshuk LM. Psychophysical measurement of 6-n-propylthiouracil (PROP) taste perception. Ann N Y Acad Sci. 1998:816–9. doi: 10.1111/j.1749-6632.1998.tb10666.x. [DOI] [PubMed] [Google Scholar]
- 53.Teff KL. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav. 2010;99:317–23. doi: 10.1016/j.physbeh.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergh C, Brodin U, Lindberg G, Sodersten P. Randomized controlled trial of a treatment for anorexia and bulimia nervosa. Proc Natl Acad Sci U S A. 2002;99:9486–91. doi: 10.1073/pnas.142284799. [DOI] [PMC free article] [PubMed] [Google Scholar]