Abstract
Others have shown that exposing oocytes to high levels of NH3/NH4+ (10–20 mM) causes a paradoxical fall in intracellular pH (pHi), whereas low levels (e.g., 0.5 mM) cause little pHi change. Here we monitored pHi and extra-cellular surface pH (pHS) while exposing oocytes to 5 or 0.5 mM NH3/NH4+. We confirm that 5 mM NH3/NH4+ causes a paradoxical pHi fall (−ΔpHi ≅ 0.2), but also observe an abrupt pHS fall (−ΔpHS ≅ 0.2)—indicative of NH3 influx—followed by a slow decay. Reducing [NH3/NH4+] to 0.5 mM minimizes pHi changes but maintains pHS changes at a reduced magnitude. Expressing AmtB (bacterial Rh homologue) exaggerates −ΔpHS at both NH3/NH4+ levels. During removal of 0.5 or 5 mM NH3/NH4+, failure of pHS to markedly overshoot bulk extracellular pH implies little NH3 efflux and, thus, little free cytosolic NH3/NH4+. A new analysis of the effects of NH3 vs. NH4+ fluxes on pHS and pHi indicates that (a) NH3 rather than NH4+ fluxes dominate pHi and pHS changes and (b) oocytes dispose of most incoming NH3. NMR studies of oocytes exposed to 15N-labeled NH3/NH4+ show no significant formation of glutamine but substantial NH3/NH4+ accumulation in what is likely an acid intracellular compartment. In conclusion, parallel measurements of pHi and pHS demonstrate that NH3 flows across the plasma membrane and provide new insights into how a protein molecule in the plasma membrane—AmtB—enhances the flux of a gas across a biological membrane.
Keywords: NH3 permeability, Surface pH measurement, Xenopus oocytes, AmtB
The movement of NH3 across cell membranes is important for several physiological processes, including nitrogen metabolism by the liver and acid-base transport by the kidney. In 1897, Overton—monitoring the precipitation of tannins in the algae Spirogyra—demonstrated that it is NH3 rather than NH4+ that readily crosses the cell membrane. Later, Warburg (1922), Harvey (1911), and Jacobs (1922) reached similar conclusions working on a variety of preparations and using different approaches to show that the influx of NH3 produces a rise in internal pH (pHi). More recently, work with pH-sensitive microelectrodes by Boron and De Weer (1976b) on squid giant axons demonstrated that, although the influx of the weak base NH3 causes a large and rapid increase in pHi, the lower influx of the weak acid NH4+ produces a slow fall in pHi during the “plateau phase” of the NH3/NH4+ exposure. Moreover, this influx of NH4+ during the exposure to extracellular NH3/NH4+ leads to large undershoot of the original pHi, once the NH3/NH4+ is removed from the extracellular solution. This effect is the basis of the widely used “ammonium prepulse” technique that they introduced (Boron and De Weer 1976a, 1976b).
Aickin and Thomas (1977) subsequently showed that the uptake of NH4+ can be so powerful in mammalian skeletal muscle that the alkalinizing effect of NH3 entry is barely detectable. Kikeri et al. (1989) made similar observations when introducing NH3/NH4+ into the lumen of the renal thick ascending limb. Waisbren et al. (1994) were the first to definitively identify a gas-impermeable membrane, showing that neither NH3 nor NH4+ (nor CO2 nor HCO3−) could cross the apical membranes of gastric gland cells.
In small-diameter Xenopus oocytes (Keicher and Meech 1994), an exposure to 20 mM extracellular NH3/NH4+ produces the classic biphasic rise in pHi, followed by a fall. However, in large-diameter oocytes (Burckhardt and Frömter 1992; Keicher and Meech 1994), an exposure to 20 mM NH3/NH4+ causes a paradoxical fall in pHi and a strong positive shift in membrane potential (Vm). To explain the above effect, Burckhardt and Frömter hypothesized that NH4+ enters via a nonselective cation channel, dissociates to form NH3 + H+, possibly followed by sequestration of NH3 in lipid stores. This model calls for negligible NH3 permeability and accounts for the then-available data. Keicher and Meech found that the NH3/NH4+-induced fall in pHi requires extracellular Cl− and proposed that the fall in pHi is due in part to an Na/K/Cl cotransporter that carries NH4+ in place of K+. However, the acidification was only slightly inhibited by Na+ removal or by bumetanide. Moreover, Na/NH4/Cl cotransport would not account for the positive shift in Vm, which was not abolished by Cl− removal. Note that, in the Keicher-Meech model, the pHi decrease would require that the NH4+ influx be accompanied by an NH3 efflux, as outlined originally by Boron and De Weer, and lead to a buildup of cytosolic NH4+. However, if the oocytes could mediate NH3 efflux, then NH3/NH4+ removal would lead to a rapid decrease in pHi, which is not observed. Thus, although the Keicher-Meech model has interesting features, it cannot account for all of the then-available observations.
Later, Bakouh et al. (2006) confirmed that Xenopus oocytes exposed to relatively high levels of NH3/NH4+ (i.e., 10 mM) exhibit the fall in pHi noted above, but also found that oocytes exposed to only 0.5 mM NH3/NH4+ exhibited no change in pHi and no substantial induced inward current (related to net entry of positive charge, namely, NH4+) (Bakouh et al. 2004, 2006). The above results are consistent with the hypothesis that the NH3/NH4+-induced fall in pHi and positive shift in Vm are due to a low-affinity NH4+- uptake mechanism that is, to some extent, Cl− dependent and that is largely inoperative at 0.5 mM NH3/NH4+.
More recently, it has become clear that the AmtB/Rh family of membrane proteins can serve as a conduit for NH3/NH4+. The crystal structures of several prokaryotic family members are consistent with the idea that NH3 passes through pores in each of the three monomers of the homotrimer (Fabiny et al. 1991; Soupene et al. 2002; Khademi et al. 2004; Zheng et al. 2004; Andrade et al. 2005; Khademi and Stroud 2006; Conroy et al. 2007). In Xenopus oocytes expressing RhCG, Bakouh et al. (2004, 2006) found that an exposure to 0.5 mM NH3/NH4+ produced a rapid, small, and short-lived alkalinization that was followed by a slow, small, and sustained acidification. These authors proposed that the transient pHi increase reflects the influx of NH3, whereas the subsequent pHi decrease reflects NH4+ influx in concert with NH3 efflux— all fluxes mediated by RhCG. However, as noted in connection with the Keicher-Meech data, if RhCG could mediate NH3 efflux—and if appreciable NH4+ accumulated inside the oocyte during the preceding NH3/NH4+ exposure—then NH3/NH4+ removal should have led to a large and rapid pHi decrease, which was not observed.
The purpose of the present work was to elucidate the movements of NH3 vs. NH4+ across the plasma membrane—and the potential role of Amt in mediating these fluxes—using the oocyte as a model system. An ancillary goal, necessary to verify our interpretation of the data, was to explore the unusual handling of NH3/NH4+ by the oocyte. Our approach was to extend to NH3 fluxes a technique that we introduced earlier to assess CO2 fluxes across the oocyte membrane (Endeward et al. 2006; Musa-Aziz et al. 2009). In the earlier work, we pushed a blunt pH microelectrode against the oocyte membrane and found that an exposure to CO2/HCO3− causes a transient rise in surface pH (pHS). First observed in 1984 by De Hemptinne and Huguenin (1984), who worked on rat soleus muscle, this rise in pH occurs as CO2 uptake causes the depletion of CO2 at the cell surface, leading to the reaction HCO3−+H+ → CO2 + H2O. In 1986, Chesler (1986) found that exposing lamprey neurons to the weak base NH3 causes a transient decrease in extracellular pH. We hypothesized that a hitherto unseen flux of NH3 into the oocyte would deplete NH3 at the extracellular surface. This depletion would lead to the reaction NH4+ → NH3 + H+, which would lower pHS and also partially replenish surface NH3 (Fig. 1). We found that exposures to both 5 and 0.5 mM elicited transient pHS decreases, and that these decreases were augmented by the expression of AmtB. The exposure to 5 mM NH3/NH4+ also caused a paradoxical fall in pHi; thus, in this case, the pH fell on both sides of the membrane. We have developed a model that allows us to predict the direction in which fluxes of NH3 and NH4+ would affect pH on both sides of the membrane. Guided by this model and our new pHS data, we conclude that (a) earlier models of how 20 mM NH3/NH4+ affects oocyte pHi are incomplete; (b) in oocytes exposed to 5 or 0.5 mM NH3/NH4+, the influx of NH3 produces the dominant effects on pHS (though we cannot rule out substantial NH4+ fluxes); (c) oocytes metabolize or sequester incoming NH3; and (d) AmtB enhances permeability to NH3 more than that to NH4+.
Fig. 1.
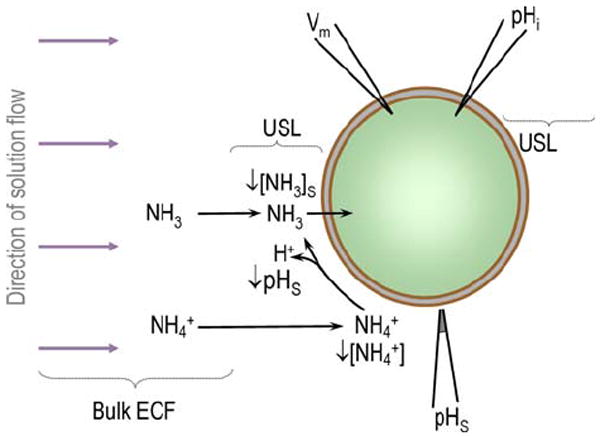
Model of an oocyte exposed to NH3/NH4+. The large purple arrows indicate the directon of bulk solution flow. USL unstirred layer; Bulk ECF bulk extracellular fluid
Methods
Expression in Xenopus Oocytes
cRNA Synthesis
As described elsewhere (Musa-Aziz et al. 2009), we used AmtB that we had tagged at the C terminus with EGFP (enhanced green fluorescent protein). We then used NotI to linearize AmtB cDNA constructs in pGH19, purified linearized cDNA using the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA), transcribed capped cRNA using the T7 mMessage mMachine kit (Ambion, Austin, TX), and, finally, purified and concentrated cRNA using the RNeasy MinElute RNA Cleanup Kit (QIAGEN). We determined the RNA concentration using ultraviolet absorbance and assessed quality using gel electrophoresis.
Xenopus Oocyte Isolation
We surgically removed ovaries from anesthetized frogs, separated the oocytes using a collagenase treatment (Romero et al. 1998; Toye et al. 2006), selected Stage V–VI oocytes, and stored them until use at 18°C in OR3 medium supplemented with 500 U of penicillin and 500 U of streptomycin.
Microinjection of cRNAs
One day after isolation, we injected oocytes with either 25 ng of cRNA encoding AmtB-EGFP cRNA (50 nl of a 0.5 ng/nl cRNA solution) or 50 nl of sterile water (Ambion, Austin, TX) in the case of control (“H2O”) oocytes. We stored the oocytes 4–6 days after injection for use in experiments. We verified delivery of EGFP-tagged AmtB to a region near the plasma membrane using a 96-well plate reader (BMG Labtechnologies, Inc., Durham, NC) to assess whole-oocyte fluorescence (Toye et al. 2006).
Solutions
The nominally CO2/HCO3−-free ND96 solution contained (mM): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES. We titrated the solution to pH 7.50 using NaOH. The osmolality of all solutions was 200 mosmol/kg H2O. We made the solution containing 5 mM NH3/NH4+ in ND96 by replacing 5 mM NaCl with 5 mM NH4Cl. We made 0.5 mM NH3/NH4+ in ND96 by diluting the 5 mM NH3/NH4+ solution 1:10 with ND96 solution.
Electrophysiological Measurements
Chamber
Oocytes were placed in plastic perfusion chamber with a channel 3 mm wide × 30 mm long, and constantly superfused at a flow of 3 ml/min. Perfusing solutions were delivered from plastic syringes and Tygon tubing using syringe pumps (Harvard Apparatus, South Natick, MA). Switching between solutions was performed by pneumatically operated valves (Clippard Instrument Laboratory, Cincinnati, OH). All experiments were performed at room temperature (~ 22°C).
Measurement of Intracellular pH (pHi)
Our approach was similar to that detailed previously (Toye et al. 2006; Lu et al. 2006; Parker et al. 2008). Briefly, we impaled the oocyte with two microelectrodes, one for measuring membrane potential (connected to a model 725 two-electrode oocyte voltage-clamp amplifier; Warner Instruments Corp., Hamden, CT) and the other for measuring pHi (connected to a model FD223 high-impedance electrometer; World Precision Instruments, Sarasota, FL). Both electrodes had tip diameters of ~ 1 μm. The pH microelectrode was of the liquid membrane style (proton cocktail no. 95293; Fluka Chemical Corp., Ronkonkoma, NY). The analog subtraction of the Vm-electrode signal from the pH-electrode signal produced the voltage due to pHi. We acquired and analyzed data by computer, using software written in-house. We calibrated the pHi/Vm in the chamber with pH standards at pH 6.0 and 8.0 (to determine the slope), followed by a single-point calibration with the standard ND96 solution (pH 7.50) in the chamber with the oocyte present, just before impalement. The mean spontaneous initial Vm in this study was −39 ± 2 mV (n = 30).
Measurement of Surface pH (pHS)
The microelectrode for measuring pHS (connected to a FD223 electrometer; World Precision Instruments) had a tip diameter of 15 μm and was of the same liquid-membrane design as the pHi microelectrode. The external reference electrode for the Vm and pHS measurements was a calomel half-cell (connected to a model 750 electrometer; World Precision Instruments) contacting a 3 M KCl-filled micropipette, which in turn contacted the fluid in the chamber. The analog subtraction of the calomel-electrode signal from the pHS-electrode signal produced the signal due to pHS. The analog subtraction of the calomel-electrode signal from the Vm-electrode signal produced the signal due to Vm. The voltage-clamp amplifier generated the virtual ground via a Ag/AgCl half cell (connected to the ISense input) contacting a second 3 M KCl-filled micro-electrode, the tip of which we positioned close to the oocyte. We used an ultrafine micromanipulator (model MPC-200 system; Sutter Instrument Co., Novato, CA) to position the pHS-electrode tip at the surface of the oocyte, and then to advance it ~ 40 μm further, whereupon we observed a slight dimple in the membrane. Periodically, we withdrew the electrode 300 μm from the surface of the oocyte for recalibration in the bulk extracellular fluid (pH 7.50). The fluid bathing the oocyte flowed at 3 ml/min. Relative to the flowing solution, the tip of the pHS microelectrode was just in the “shadow” of the oocyte (Fig. 1).
Analysis of pH Data
Initial dpHi/dt
We computed the initial rate of change of pHi (dpHi/dt)— produced by introducing 5 mM NH3/NH4+—by determining the line of best fit to the steepest part of the pHi vs. time record.
Maximum pHS Spike Heights
We used the following approach to compute the maximum magnitude (i.e., “spike height” or ΔpHS) of the pHS transient elicited by applying 5 or 0.5 mM extracellular NH3/NH4+. We determined the initial pHS—that is, before application of NH3/NH4+—by comparing the pHS-electrode voltage signal when the electrode tip was at the oocyte surface with the voltage signal obtained when the tip was in the bulk extracellular fluid (assumed pH = 7.50). We determined the maximum pHS during the NH3/NH4+ exposure by comparing the voltage signal (at a time corresponding to the extreme pHS value) when the electrode tip was at the oocyte surface with the voltage signal obtained a few minutes later, when the tip was in the bulk extracellular fluid (assumed pH = 7.50). The ΔpHS was the algebraic difference between the extreme and the initial pH values, one based on a calibration in an ordinary ND96 solution and the other based on a separate calibration in the NH3/NH4+- containing solution. We performed separate calibrations of the pHS electrode in the plain and NH3/NH4+-containing ND96 solutions to minimize potential errors in the event that NH3/NH4+ affects the proton cocktail.
NMR Spectroscopy
Measurements of Metabolites with 1H-[13C]NMR
Twenty oocytes were incubated with 0.5 mM 15NH3/15NH4+, washed extensively, and homogenized with ethanol to extract water-soluble metabolites. A small quantity of [2-13C]glycine (30 nmol) was added as an internal concentration reference and to control for potential sample losses during the extraction procedure. After centrifugation of the homogenate, the supernatant was lyophilized and resuspended in 10% D2O buffer containing a small amount of 3-(trimethylsilyl)-[2,2,3,3-d4]- propionate(Na+) (TSP) as a chemical-shift reference. 1H-[13C]NMR spectra were acquired fully relaxed at 11.7 T (Patel et al. 2005), using a high-resolution Bruker Avance NMR spectrometer operating at 500.13 MHz (1H) and 125.7 MHz (13C). Spectral data were acquired fully relaxed with a sweep width of 6009 Hz, 8192 data points, interscan delay of 20 s, and 128 scans. Free-induction decays were zero filled, exponential filtered (LB = 0.5 Hz), and Fourier transformed. Metabolite peak intensities were measured by integration and referenced to 13C-labeled glycine with correction for differences in the number of hydrogen atoms. The glycine signal was determined to be 100% enriched with 13C, indicating no significant overlap with endogenous signals.
Measurements of 15NH3/15NH4+ with 1H-15N HSQC
Eighty oocytes were incubated with 15NH3/15NH4+ for 30 min, followed by extensive washing with 15N-free/NH3/NH4+-free buffer. The washed oocytes were cooled on ice and homogenized, yielding ~0.4 ml of supernatant after centrifugation. The medium was then acidified with 50 μl of 2.5 M HCl, 50 μl of D2O was added for field-frequency lock, and samples were loaded into 5-mm NMR tubes. NMR spectra were obtained at 5°C to minimize the exchange of hydrogen atoms between ammonia and water. NMR experiments were performed at 11.7 T using a high-resolution Bruker Avance NMR spectrometer operating at 500.13 MHz (1H) and 50.683 MHz (15N). Measurements of total 15NH3/15NH4+ concentrations in oocytes were determined with the Heteronuclear (1H-15N) Single Quantum Coherence (HSQC) NMR spectroscopy technique (Grzesiek and Bax 1993; Kanamori et al. 1995). HSQC is well suited for detection of nuclei (e.g., 15N) with a low gyromagnetic ratio (γ). Because γ for 15N is ~10 times lower than for 1H, the sensitivity for ammonia-nitrogen detection is enhanced by a factor of (γH/γN)5/2 ≈ 306. Water suppression was achieved using the Water Suppression by Gradient-Tailored Excitation (WATERGATE) technique (Piotto et al. 1992). 15N decoupling was obtained using Waltz16 (Shaka et al. 1983). Free-induction decays were acquired with a spectral width of 6009 Hz, 8192 data points, interscan interval of 10 s, and 128 scans. All spectra were zero filled to 65,536 points. The total ammonium (NH3 + NH4+) concentrations in oocytes were determined by comparing the integrated intensities of the ammonia 1H resonance to the incubation medium containing 0.5 mM 15NH3/15NH4+, using the same pulse acquisition conditions, and assuming that oocytes are spheres of 1.2-mm diameter and contain 40% water by weight. 1H-15N HSQC spectra of oocytes and their incubation medium were acquired in a paired manner.
Statistics
We present data as mean ± SE. To compare the difference between two means, we performed a Student’s t-test (two tails). To compare more than two means, we performed a one-way ANOVA and a Student-Newman-Keuls (SNK) multiple comparison, using KaleidaGraph (Version 4; Synergy Software). We considered p < 0.05 to be significant.
Results
Figure 1 summarizes how the influx of NH3 would affect pHS. As NH3 enters the cell, [NH3] near the external surface of the membrane—[NH3]S—falls. The replenishment of the lost NH3 near membrane surface occurs by two routes. First, NH3 diffuses from the bulk extracellular fluid to approach the membrane, an effect that we cannot detect. Second, NH4+ near the membrane dissociates to form both NH3 as well as the H+ that we detect as a fall in pHS. As the concentration of NH3 inside the cell ([NH3]i) rises, the influx of NH3 slows, and pHS relaxes toward the pH of the bulk extracellular fluid (pHBulk). In Fig. 1, we assume that only NH3 enters the cell. If only NH4+ enters, the reverse set of events would occur and pHS would rise. In the Discussion, we consider the situation in which both NH3 and NH4+ enter.
We explored the model in Fig. 1 by exposing oocytes to 5 or 0.5 mM NH3/NH4+. Figures 2 and 3 show representative experiments at these two levels of NH3/NH4+, and Fig. 4 summarizes the mean values for these experiments.
Fig. 2.
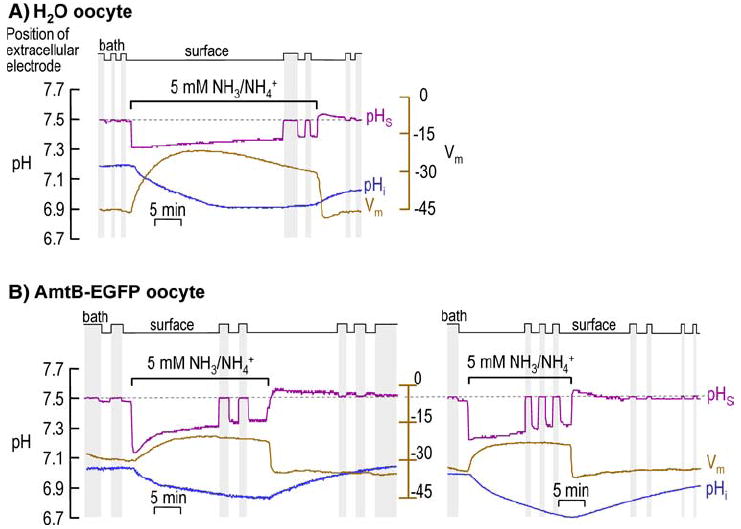
Intracellular pH (pHi) and surface (pHS) records during application and removal of 5 mM NH3/NH4+. a H2O-injected oocyte. b AmtB EGFP-tagged expressing oocytes. At the indicated times, we switched the extracellular solution from ND96 to 5 mM NH3/NH4+ and then back again. b shows two representative experiments: on the left, pHS at first decays rapidly and then more slowly, whereas on the right, pHS decays slowly throughout the NH3/NH4+ exposure. In both a and b, the purple record is pHS, the blue record is pHi, and the brown record is Vm. The vertical gray bands represent periods during which the pHS electrode was moved to the bulk ECF for calibration (fixed pH of 7.50). In H2O oocytes, mean pHi was 7.10 ± 0.04 before and 6.84 ± 0.06 during exposure to 5 mM NH3/NH4+ (n = 6; p = 0.005); the comparable mean Vm values were −43 ± 2 and − 25 ± 2 mV (n = 6; p = 0.0002). In AmtB oocytes, the mean pHi was 7.14 ± 0.04 before and 6.98 ± 0.06 during exposure to 5 mM NH3/NH4+ (n = 8; p = 0.037); the comparable mean Vm values were −38 ± 4 and −26 ± 2 (n = 8; p = 0.010)
Fig. 3.
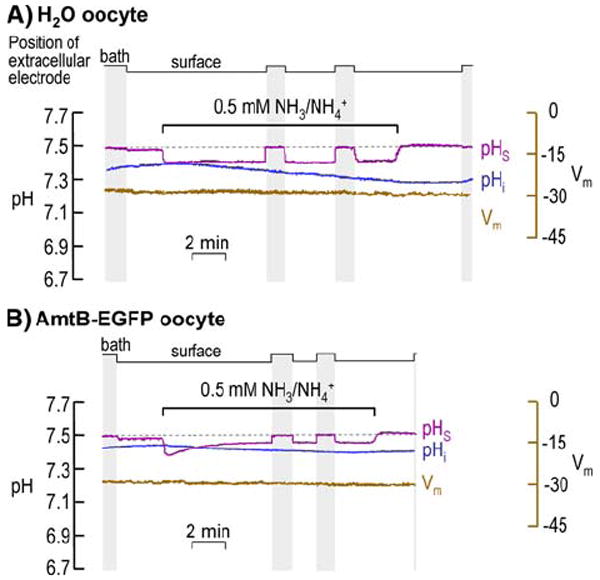
Intracellular pH (pHi) and surface (pHS) records during application and removal of 0.5 mM NH3/NH4+. a AmtB EGFP-tagged expressing oocyte. b H2O-injected oocyte. At the indicated times, we switched the extracellular solution from ND96 to 0.5 mM NH3/NH4+ and then back again. In both a and b, the purple record is pHS, the blue record is pHi, and the brown record is Vm. The vertical gray bands represent periods during which the pHS electrode was moved to the bulk ECF for calibration (fixed pH of 7.50). In H2O oocytes, the mean pHi was 7.21 ± 0.05 before and 7.17 ± 0.05 during exposure to 0.5 mM NH3/NH4+ (n = 8; p = 0.61); the comparable mean Vm values were −44 ± 2 and -40 ± 2 mV (n = 8; p = 0.14). In AmtB oocytes, the mean pHi was 7.24 ± 0.05 before and 7.21 ± 0.05 during exposure to 0.5 mM NH3/NH4+ (n = 8; p = 0.52); the comparable mean Vm values were −31 ± 4 and −30 ± 4 (n = 8; p = 0.87)
Fig. 4.
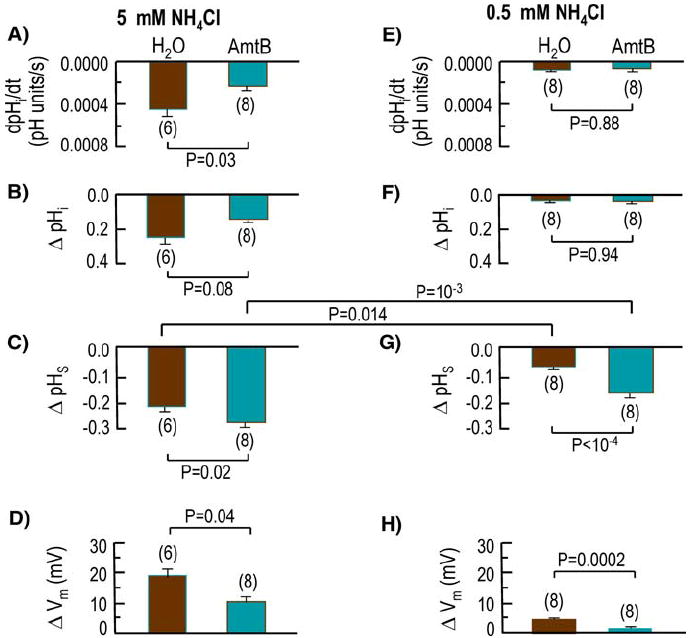
Summary of the mean initial rate of pHi decrease (dpHi/dt) and magnitude of the NH3-induced change in steady-state pHi (ΔpHi), ΔpHS, and Vm for larger groups of experiments like those in Figs. 2 and 3
Effects of 5 mM NH3/NH4+
Figure 2 shows the results of representative experiments on oocytes exposed to a relatively high level of NH3/NH4+, 5 mM.
H2O-Injected Control oocyte
Figure 2a refers to a control, H2O-injected oocyte. Here— as previously reported by others for 20 mM NH3/NH4+ (Burckhardt and Frömter 1992; Keicher and Meech 1994)—the addition of 5 mM NH3/NH4+ to the extracellular fluid causes a moderately slow but sustained fall in pHi. Simultaneously, pHS abruptly falls by ~0.2 and then slowly decays (i.e., rises toward pHBulk). The initial fall in pHS indicates that the influx of NH3 (as opposed to NH4+) is dominant with respect to effects on pHS. Note that, at a time when pHi has reached a stable value, pHS is still substantially lower than pHBulk, indicating that NH3 is still entering the cell. Applying 5 mM NH3/NH4+ also causes a very slowly developing depolarization that reaches a peak after nearly 15 min—not the instantaneous depolarization expected of a pre-existing NH4+ channel—that slowly decays by nearly 9 mV.
The removal of the NH3/NH4+ leads to a slow recovery of pHi that is incomplete over the time frame of this experiment. The removal of NH3/NH4+ causes pHS to overshoot pHBulk by a small amount (0.048 ± 0.004; n = 5), and then to decay slowly. The presence of an overshoot implies that—regarding effects on pHS—a small efflux of NH3 dominates over any efflux of NH4+. This exiting NH3 presumably arises from a small amount of intracellular NH4+ that dissociated to form H+ (which would lower pHi) and NH3 (which would exit the cell). The short duration of the overshoot implies that the efflux was of short duration (i.e., that the pool of intracellular NH4+ was small). If intracellular NH4+ had accumulated to a substantial degree during the NH3/NH4+exposure, then the removal of NH3/NH4+ should have led to an abrupt fall in pHi (which we did not observe) and a pHS overshoot whose magnitude should have matched that of the initial NH3/NH4+-induced fall in pHS (i.e., ~0.2). Removing NH3/NH4+ also causes Vm to undershoot its initial level by ~1 mV and then to relax to the pre- NH3/NH4+ value. Note that the speed of the repolarization during NH3/NH4+ removal is much faster than the depolarization during NH3/NH4+ application, but still slower than the speed of the pHS increase during NH3/NH4+ removal.
Oocyte Expressing AmtB Tagged with EGFP
Figure 2b shows two experiments on oocytes expressing AmtB-EGFP. At the left, the switch to 5 mM NH3/NH4+ causes a fall in pHi, though one that is slower than for the H2O oocyte. On the other hand, the maximal fall in surface pH (which we refer to as −ΔpHS) is substantially larger for the AmtB-EGFP oocyte than for the H2O-injected oocyte, implying that the relative uptake of NH3 over NH4+ is higher in the AmtB oocyte. In the oocyte at the left in Fig. 2b, this shift in pHS at first decays rapidly and then more slowly. In the oocyte at the right in Fig. 2b, the fall in pHS decays very slowly throughout the NH3/NH4+ exposure. In both AmtB oocytes—as was true for the H2O oocyte—pHS fails to decay fully to pHBulk during the NH3/NH4+ exposure. The Vm changes induced by application and removal of NH3/NH4+ are similar to, but smaller than, those for H2O oocytes.
The removal of NH3/NH4+ produces about the same effects in the AmtB oocyte as in the H2O oocyte (mean overshoot in AmtB oocytes, 0.037 ± 0.004; n = 6).
Summary of AmtB-EGFP Versus H2O
As summarized on the left side in Fig. 4 for a larger number of experiments, the effect of AmtB vs. H2O is statistically significant for the initial rate of pHi decrease (dpHi/dt) elicited by NH3/NH4+ (Fig. 4a). The slower fall of pHi in the case of AmtB oocytes is consistent with the hypothesis that, regarding effects on pHi (a) AmtB acts by predominantly enhancing NH3 vs. NH4+ influx (b) the enhanced influx of NH3 to some extent promotes the reaction NH3 + H+ → NH4+ in the cytosol, and (c) the consumption of cytosolic H+ slows the NH3/NH4+-induced fall in pHi.
The effect of expressing AmtB did not reach statistical significance regarding the NH3/NH4+-induced ΔpHi (Fig. 4b). However, AmtB oocytes had a greater −ΔpHS (Fig. 4c), indicating a greater relative influx of NH3 and thus supporting the hypothesis in the previous paragraph. Finally, the AmtB oocytes had a smaller ΔVm (Fig. 4d).
Effects of 0.5 mM NH3/NH4+
Figure 3 shows representative data from a pair of oocytes exposed to a relatively low level of NH3/NH4+, 0.5 mM.
H2O-Injected Control Oocyte
As shown in Fig. 3a for a H2O-injected oocyte, the switch to 0.5 mM NH3/NH4+ causes a slow and very slight fall in pHi. Although pHS abruptly falls, −ΔpHS is substantially lower than for H2O oocytes exposed to the much higher level of NH3/NH4+ (i.e., 5 mM; see Fig. 2a). Nevertheless, regarding effects on pHS, the effect of NH3 influx (over NH4+ influx) is clearly dominant. The Vm changes caused by application and removal of 0.5 mM NH3/NH4+ are much smaller than those in the experiments with 5 mM NH3/NH4+.
The removal of NH3/NH4+ evoked virtually no recovery of pHi in Fig. 3a, although some oocytes exhibited a slight pHi recovery. In Fig. 3a, pHS rose to a value ~0.02 higher than pHBulk. The mean pHS overshoot was 0.006 ± 0.003 (n = 8; 0.006 vs. 0; p = 0.28). These data indicate that— regarding effects on pHS—the efflux of NH3 is negligible relative to that of NH4+.
Oocyte Expressing AmtB Tagged with EGFP
Figure 3b shows that, with an oocyte expressing AmtB-EGFP, the switch to 0.5 mM NH3/NH4+ causes little change in pHi. The maximal fall in pHS is much larger for the AmtB-EGFP oocyte than for the H2O oocyte, though both values are much smaller than for their counterparts in exposures to 5 mM NH3/NH4+. The Vm change is minimal.
The removal of NH3/NH4+ from AmtB-EGFP oocytes (Fig. 3b) evoked virtually the same response as in H2O oocytes. The mean pHS overshoot was 0.014 ± 0.006 (n = 8; 0.014 vs. 0; p = 0.30), indicating that—regarding effects on pHS—the efflux of NH3 is negligible relative to that of NH4+.
Summary of AmtB-EGFP Versus H2O
As summarized on the right side in Fig. 4, the effect of AmtB vs. H2O for an exposure to 0.5 mM NH3/NH4+ follows essentially the same pattern as for an exposure to 5 mM, except that the difference for AmtB vs. H2O is not statistically significant for dpHi/dt (Fig. 4e). The dpHi/dt for H2O oocytes is significantly different from zero (p = 0.05) but not for AmtB oocytes (p = 0.26). As summarized in Fig. 4f, the ΔpHi was not different from zero for H2O oocytes (0.038 ± 0.010; n = 8; p = 0.15) but was significantly—but not substantially different—for AmtB oocytes (0.040 ± 0.005; n = 8; p = 0.01). As was the case for the exposures to 5 mM NH3/NH4+, the AmtB oocytes also had a higher −ΔpHS (Fig. 4g), indicating a greater relative NH3 influx. As also was the case for the 5 mM exposures, the ΔVm was lower for the AmtB oocytes (Fig. 4h).
Comparing ΔpHS data for 5 mM vs. 0.5 mM (Fig. 4c vs. g), we see that the difference (always higher for the experiments with 5 mM NH3/NH4+) is statistically significant both for H2O oocytes and for AmtB oocytes.
NMR Studies of Oocytes Exposed to 15N-Labeled 0.5 mM NH3/NH4+
A paradox in our results is that AmtB increases −ΔpHS (Fig. 4g)—indicating that AmtB enhances the influx of NH3—and yet has no significant effect on the initial dpHi/dt (Fig. 4e) or ΔpHi (Fig. 4f). Moreover, removing extra-cellular 0.5 mM NH3/NH4+ produces little pHS overshoot beyond pHBulk (Fig. 3a, b), indicating little efflux of NH3. The most straightforward explanation for these data is that the oocyte somehow disposes of most incoming NH3 by either metabolizing it to a neutral product or sequestering it. We test this hypothesis in the next two sections. Note that, a priori, we can rule out the possibility that the oocyte metabolizes or sequesters NH4+ (as opposed to NH3). If the oocyte first converted the entering NH3 to NH4+ (NH3 + H+ → NH4+) and then metabolized that NH4+ to a neutral product(s), the result would be an increase in pHi, which we would have easily observed in experiments with 0.5 mM NH3/NH4+.
Assessment of Glutamine Formation
The leading candidate for the metabolism of NH3 to a neutral product would be the conversion of glutamate to glutamine via glutamine synthetase: Glu− + MgATP= + NH3 → Gln + MgADP− + H2PO4−.
To test this hypothesis, we incubated noninjected oocytes for 10 min either in ND96 alone (for sham incubation) or in ND96 plus 0.5 mM 15NH3/15NH4+. We then homogenized the oocytes with ethanol to extract water-soluble metabolites and subjected the extract to 1H-[13C] NMR spectroscopy (Fig. 5). The extract from oocytes incubated in 15NH3/15NH4+ exhibited a very low Gln signal ([Gln] ≅ 1.1 mM) vs. the shams (undetectable Gln). RhAG, a component of the erythrocyte Rh complex, has a higher NH3/CO2 permeability ratio than AmtB (Endeward et al. 2006; Musa-Aziz et al. 2009), and RhAG-expressing oocytes (like AmtB oocytes) exhibit high ΔpHS values and very small pHi changes when exposed to 0.5 mM NH3/NH4+. However, extracts from RhAG oocytes exposed to 0.5 mM 15NH3/15NH4+ have undetectable Gln (not shown).
Fig. 5.
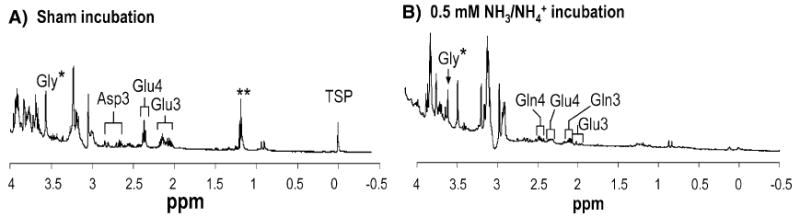
1H-NMR spectra of ethanol extracts of oocytes. a Sham incubation (i.e., oocytes not exposed to NH3/NH4+). b Oocytes incubated with 0.5 mM 15NH3/15NH4+ for 10 min. Each spectrum represents the extract of 20 oocytes. TSP, 3-trimethylsilyl-[2,2,3,3-d4]-propionate(Na+), was added as a chemical-shift reference (0.0 ppm). *Glycine was added during oocyte extraction to serve as an internal concentration standard and to control for potential metabolite losses. **Residual ethanol contaminant remaining after extraction. Estimated concentrations of metabolites in control noninjected oocytes not exposed to NH3/NH4+. (A) Glutamate (Glu): 6.48 mM. Estimated metabolite concentrations after incubation with 15NH3/15NH4+ for 10 min: Glu: H2O, 1.69 mM. Glutamine (Gln): 1.1 mM. Glu and Gln methylene protons (H4 and H3) and lactate methyl protons (H3) are depicted. Glu H4 and H3 were observed in control noninjected oocytes incubated with 15NH3/15NH4+
1H-15N-HSQC spectra (acquired as in Fig. 6 from a separate batch of RhAG oocytes incubated with 0.5 mM 15NH4Cl) showed no significant 15N-enrichment of amides in the metabolites, although we only examined Gln and a few other likely metabolites. Although we cannot rule out the possibility that oocytes sequester NH3 via unknown metabolic pathways, our 1H-[13C]NMR and 1H-15N-HSQC data rule out substantial conversion of NH3 to Gln and provide no evidence for other pathways.
Fig. 6.
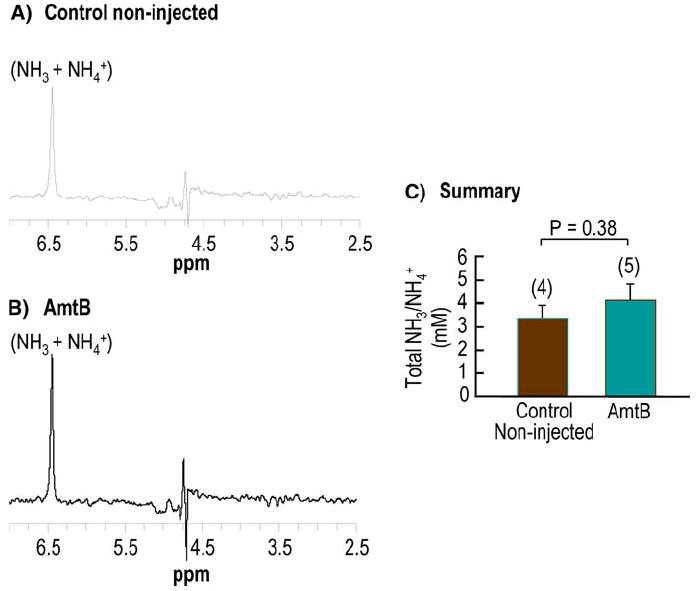
Representative 1H-15N HSQC spectra of Xenopus oocytes incubated with 15NH3/15NH4+. a Control noninjected oocytes. b AmtB oocytes. Total ammonia appears as a single resonance at ~6.5 ppm (relative to water, assigned to 4.7 ppm). Spectral intensities were scaled relative to the intensity of 0.5 mM 15N-ammonia in the incubation medium of each sample acquired with the same pulse parameter settings. The residual water signal artifact appears at 4.7 ppm. c Total ammonia (NH3 + NH4+) concentration in control noninjected and in AmtB oocytes. Values are mean ± SE, with numbers of oocyte samples for each group in parentheses. The statistical comparisons were made using unpaired two-tailed t-tests. The experimental error was large due to fast evaporation of the ammonia in both the standard solution and the extracts
NH3/NH4+ Accumulation in Oocyte Water
To determine if oocytes accumulate NH3/NH4+ per se, we incubated noninjected control oocytes or AmtB oocytes in ND96 containing 0.5 mM 15NH3/15NH4+ for 30 min. After the incubation, we washed the oocytes with a NH3-free ND96 solution and, following homogenization and centrifugation, measured the total 15NH3 + NH4+ of the supernatant using 1H-15N HSQC (Fig. 6a, b). We found that 15NH3/15NH4+—which reflects only NH3/NH4+ taken up during the incubation—was ~30% higher in AmtB oocytes (4.15 mM computed for total oocyte H2O) than in control noninjected oocytes (3.33 mM). Because pHi was ~7.40 in these experiments—and pHo was 7.50 and [NH3/NH4+]o was 0.5 mM—we can conclude that if NH3 fully equilibrated across the cell membrane, cytosolic [NH3/NH4+] (i.e., the concentration in the water in direct contact with the inner surface of the plasma membrane) could only have been 0.5 mM × 10(7.50-7.40) ≅ 0.6 mM. Note that even this level of cytosolic NH3/NH4+ would have led—upon the removal of extracellular NH3/NH4+—to a substantial fall in pHi and a large transient overshoot of pHS, neither of which we observed. Thus, we conclude that cytosolic [NH3/NH4+] must have been substantially lower than 0.6 mM. The most straightforward explanation for the extremely high level of intracellular NH3/NH4+ that we actually observed in the NMR experiments (i.e., for the 3+ – 4+ mM) is that the vast majority of intracellular NH3/NH4+ was, in fact, confined to an acidic intracellular compartment.
Discussion
Overview
The pHi data in the present paper confirm the earlier observation that an exposure of a control Xenopus oocyte (i.e., not heterologously expressing other proteins) to a relatively high level of NH3/NH4+ leads to a paradoxical intracellular acidification (Burckhardt and Frömter 1992; Keicher and Meech 1994). Earlier investigators had also found that an exposure to a relatively low level of NH3/NH4+ leads to a negligible pHi change (Bakouh et al. 2004). We now confirm both observations in H2O oocytes (Fig. 4b and f, respectively). In addition, we make the novel observation that the expression of AmtB slows the pHi fall elicited by an exposure to 5 mM NH3/NH4+ (Fig. 4a), consistent with the hypothesis that AmtB predominantly promotes the influx of NH3 over NH4+ (regarding effects on pHi). However, we believe that the most important contributions of the present study are (a) the simultaneous application of pHS and pHi measurements, which provide new insights into how Xenopus oocytes handle exposures to NH3/NH4+, and (b) novel pHS data that show that AmtB enhances NH3 fluxes during exposures to both 5 mM and 0.5 mM NH3/NH4+.
Evidence for Unusual NH3/NH4+ Handling by Xenopus Oocytes
Regardless of whether the concentration is 5 or 0.5 mM, exposing an oocyte to NH3/NH4+ causes an initial pHS decrease, the explanation for which is straightforward: the influx of NH3 causes a depletion of NH3 at the extracellular surface of the oocyte, leading to the reaction NH4+ → NH3 + H+. However, five other observations indicate that the handling of NH3 by Xenopus oocytes is unusual compared to that by other cells studied thus far.
First, when one exposes an oocyte to a relatively high level of NH3/NH4+, pHi paradoxically falls. Almost all other cells—the exceptions being membranes that mediate either a massive influx of NH4+ (Aickin and Thomas 1977; Kikeri et al. 1989) or no apparent flux of either NH3 or NH4+ (Waisbren et al. 1994; Singh et al. 1995)—exhibit a rapid pHi increase, due to the influx and protonation of NH3 (Roos and Boron 1981). Working on oocytes, previous investigators had observed NH3/NH4+-induced pHi decreases when introducing 10–20 mM NH3/NH4+ (Burckhardt and Frömter 1992; Keicher and Meech 1994; Bakouh et al. 2006). In the present paper, we observed similar, though smaller pHi decreases—with both H2O-injected controls and AmtB oocytes—when introducing 5 mM NH3/NH4+ (Fig. 2). This paradoxical fall in pHi implies that the rate of intracellular acid loading is higher than that of acid extrusion. We examine potential explanations below, under “Models of NH3/NH4+ Handling by Xenopus Oocytes: ‘High’ [NH3/NH4+]o.”
Second, when one exposes an oocyte to a relatively low level (e.g., 0.5 mM) of NH3/NH4+, pHi either falls very slowly, and by a small amount (Figs. 3, 4e, f; H2O bars), or does not change significantly (Fig. 4e, f; AmtB bars). These observations on H2O oocytes confirm earlier work (Bakouh et al. 2004, 2006).
Third, after an exposure to relatively high levels of NH3/NH4+—10 to 20 mM in the case of others (Burckhardt and Frömter 1992; Keicher and Meech 1994; Bakouh et al. 2004, 2006) or 5 mM in the present work (Fig. 2)—the removal of extracellular NH3/NH4+ causes pHi to increase slowly, rather than to decrease rapidly (due to NH3 efflux followed by the intracellular reaction NH4+ → NH3 + H+), as has been observed by numerous investigators working on most other cell types.
Fourth, although introducing NH3/NH4+ produces an abrupt fall in pHS—indicative of NH3 influx—the subsequent decay in pHS is generally very slow, regardless of whether the oocytes are exposed to 5 or 0.5 mM NH3/NH4+. In H2O-injected oocytes (Figs. 2a, 3a), the decay is monotonic and extremely slow throughout. In AmtB oocytes, the decay in pHS is sometimes fast at first, but then extremely slow (see Fig. 2b, left, and Fig. 3b). In some other experiments on AmtB oocytes, even this rapid phase of pHS decay is absent (Fig. 2b, right). In neither case does pHS return to the pHBulk value of 7.50 during the course of an NH3/NH4+ exposure lasting ~15 min or more. Thus, the decay in pHS in these experiments with NH3 is decidedly slower than the rapid decay in pHS in oocytes exposed to CO2 (Endeward et al. 2006)—a decay that is complete in ~5 min. These observations are consistent with the hypothesis that—unlike CO2 (which equilibrates across the membrane in a few minutes)—NH3 is still far from equilibrated across the plasma membrane and continues to enter the oocyte for a prolonged period (Figs. 2, 3). A corollary is that the oocyte somehow maintains a relatively low level of NH3 near the inner surface of the cell membrane.
Fifth, although one would expect that removing extracellular NH3/NH4+ would cause pHS to increase abruptly and overshoot the initial pHS (i.e., near the pHBulk of 7.50)—by an amount that approximates the magnitude of the pHS decay during the preceding NH3/NH4+ exposure—in fact we observe little or no pHS overshoot (Figs. 2, 3). Moreover, the expression of AmtB (vs. the injection of H2O) increases the magnitudes of the rapid pHS changes but does not produce a larger overshoot. These results imply that the NH3 efflux from the oocyte is small, consistent with the hypothesis that (1) the pathways for NH3 movement across the oocyte membrane are highly rectified (i.e., favoring influx over efflux), or (2) very little NH4+ is present in the cytosol near the plasma membrane at the end of an NH3/NH4+ exposure. Thus, upon removal of extracellular NH3/NH4+, very little free, cytosolic NH4+ is available for the reaction NH4+ → H+ + NH3. This reaction is necessary not only for the pHi to fall, but also to generate the NH3 that subsequently exits and produces the pHS overshoot. Moreover, a limited cytosolic NH4+ would minimize the outwardly directed diffusion potential for NH4+, and minimize a rebound hyperpolarization upon removal of extracellular NH3/NH4+. Because we know of no examples of a rectifying of a gas flux through either lipid or a protein channel, option 2 is the simplest explanation, and would account for the observations that removal of extracellular NH3/NH4+ fails to cause (a) a fall in oocyte pHi (b) a large pHS overshoot, and (c) a rebound hyperpolarization. Instead, NH3/NH4+ removal merely eliminates the processes that cause pHS to fall and Vm to become more positive, causing these parameters simply to return to their pre-NH3/NH4+ values.
Models of NH3/NH4+ Handling by Xenopus Oocytes: “Low” [NH3/NH4+]o
In the section following this one, we consider potential explanations for the pHi and pHS data obtained with the exposure to and withdrawal of relatively high NH3/NH4+ levels (e.g., 5 mM), with their attendant large and paradoxical pHi changes. In this section, we examine experiments with relatively low NH3/NH4+ levels (e.g., 0.5 mM), where the paradoxical pHi changes—mediated by a process with a low affinity for NH3 and/or NH4+—are minimal. We consider five models of NH3 handling by Xenopus oocytes (Fig. 7) that could account for why an exposure to NH3/NH4+ would cause virtually no change in pHi.
Fig. 7.
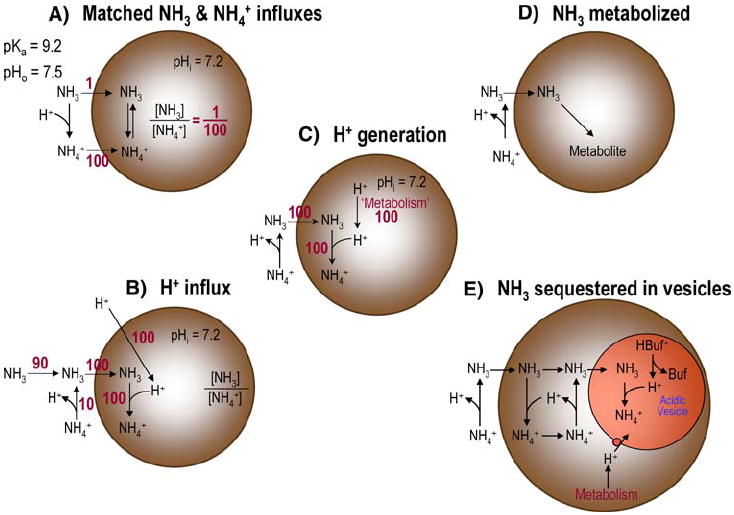
Models of NH3/NH4+ handling by Xenopus oocytes
Model A: Is the pHi Effect of NH3 Influx Perfectly Matched by the pHi Effect of NH4+ Influx (Fig. 7a)
In order for the influxes of NH3 and NH4+ to cause no change in pHi, the influx of NH3 (JNH3) and the influx of NH4+ (JNH4+) would have to be in the ratio 10(pHi–pKa), where pHi is the initial pHi and pKa refers to the intracellular equilibrium NH4+ ⇄ NH3+H+. To make this point more clearly, we imagine that the oocyte’s cytosol contains a tiny amount of NH3/NH4+ that is equilibrated at the initial pHi. If we assume that the pKa of this reaction is 9.2, and, for the sake of simplicity, that the pHi is 7.2, then
| (1) |
Thus, if JNH3/JNH4+ also were 1/100, then the parallel influxes of NH3 and NH4+ would not disturb the ratio [NH3]i/[NH4+]i and hence would not disturb the NH3/NH4+ equilibrium or pHi. (In Fig. 7a, we assume an NH3 influx of 100 arbitrary units.) We now define the virtual JNH3/JNH4+ ratio that would produce no change in the actual pHi:
| (2) |
In our example (JNH3/JNH4+)Null is 1/100. To be more general, we can define (JNH3/JNH4+)Null in terms of the pH on either side of the membrane:
| (3) |
Because solutions on opposite sides of the membrane will generally have different pH values, they will also have different requirements for (JNH3/JNH4+)Null, which—as we see below—can have interesting consequences.
We can also define the virtual value of pH that—given the actual fluxes of NH3 and NH4+—would produce no pH change in the compartment under consideration. Starting with an expression analogous to Eq. 3 and solving for pH, we have
| (4) |
This equation has the form of the familiar Henderson-Hasselbalch equation, except that here we replace concentrations with fluxes. If the JNH3/JNH4+ ratio were 1/100 and pKa were 9.2, then pHNull would be
| (5) |
If the actual pHi were 7.2 (i.e., the same as pHNull in this example), the NH3/NH4+ fluxes would not alter pHi. However, because the pH of the bulk extracellular fluid is 7.5, this same JNH3/JNH4+ ratio of 1/100 would necessarily alter pHS. To determine the direction of the effect on pHS, we return to Eq. 3. For the NH3/NH4+ fluxes to produce no change in pHS (where the initial pHS = pHBulk = 7.50),
| (6) |
To produce the observed decrease in pHS, as in Fig. 1, the fluxes would have to be in a ratio >1/50 (or 2/100). However, if JNH3/JNH4+ were only 1/100—the value necessary to stabilize pHi—the influx of NH3 would be too low (or the influx of NH4+ would be too high) to stabilize pHS, and the following reaction on the extracellular surface of the oocyte would replenish some of the lost NH4+: NH3 + H+ + NH4+ (Fig. 7a). Thus, if the JNH3/JNH4+ ratio were positioned to stabilize pHi, pHS would rise, rather than fall as actually observed. Obviously, JNH3/JNH4+ cannot simultaneously be 1/100 (to explain the stability of pHi) and >2/100 (to explain the fall in pHS). Therefore, no combination of NH3 influx and NH4+ influx—regardless of mechanism (e.g., influx through a Na/K/Cl cotransporter or a channel)—can simultaneously account, by itself, for the pHi and pHS data. Thus, we can rule out model A for experiments at 0.5 mM NH3/NH4+. Below, we see that a similar line of reasoning rules out NH4+ influx as a potential mechanism for the paradoxical fall of pHi in 5 mM NH3/NH4+.
Table 1 summarizes the directions of the expected pH changes for the three possible conditions: pH < pHNull, pH = pHNull, and pH > pHNull.1 These pH vs. pHNull relationships are mirrored by corresponding JNH3/JNH4+ vs. (JNH3/JNH4+)Null relationships. Notice that, in the case of inequalities, the directions of the pH changes are opposite on the side from which the NH3 and NH4+ exit vs. the side which NH3 and NH4+ enter.
Table 1.
Predicted pH changes
| Condition | Effect on compartment from which NH3 and NH3/NH4+ leave | Effect on compartment that NH3 and NH3/NH4+ enter |
|---|---|---|
| pH < pHNull … that is, JNH3/JNH4+ > (JNH3/JNH4+)Null | ↓pH | ↑pH |
| pH = pHNull … that is, JNH3/JNH4+ = (JNH3/JNH4+)Null | △̸pH | △̸pH |
| pH > pHNull … that is, JNH3/JNH4+ < (JNH3/JNH4+)Null | ↑pH | ↓pH |
Note. ↓pH, decrease in pH; ↑pH, increase in pH; △̸pH, no pH change in this compartment
Note that the model that we developed here only allows us to conclude that a NH3/NH4+-induced fall in pHS in our experiments is associated with a JNH3/JNH4+ that is >1/50. We cannot rule out an influx of NH4+. In fact, a hypothetical influx of NH4+ could be 49-fold greater than the influx of NH3, and yet pHS still would fall. On the other hand, a large absolute influx of NH4+ would slow the fall in pHS and reduce the magnitude of −pHS. Such kinetic issues can only be addressed by a model that computes the time course of pHS.
Model B: Is the Influx of NH3 Perfectly Matched by an Influx of H+ (Fig. 7b)
Even if NH4+ did not enter the oocyte, pHi would be stable if JNH3 were matched by a comparable JH+. Each entering NH3 would undergo the reaction NH3 + H+ → NH4+, and the lost cytosolic H+ would be replenished by an influx of H+. In Fig. 7b, we assume a JH+ of 100 arbitrary units and a JNH3 of 100, and we also assume that the NH3 disappearing from the extracellular surface of the cell would be replenished by an NH3 diffusion from the bulk extracellular fluid (ECF) of 90 and a contribution of 10 from the extracellular reaction NH4+ → H+ + NH3. Because this model results in a net consumption of H+ at the extracellular surface (100 – 10 = 90 in this example), pHS would rise rather than fall as we observe. Moreover, this model would predict an enormous production of intracellular NH4+, which would produce cell swelling; we observe no tendency for the oocyte to swell. Finally, the massive accumulation of intracellular NH4+ would, upon removal of extracellular NH3/NH4+, lead to a substantial decline in pHi and overshoot of pHS (i.e., increase beyond the pHBulk value of 7.50), neither of which we observe. In this analysis, we have assumed no flux of NH4+. To the extent that NH4+ entered the oocyte, we would require less H+ influx to produce no pHi change. However, any substantial H+ influx would be inconsistent with our pHS data, and we have already seen in our analysis of model A that NH4+ influx cannot account for our data. It is theoretically possible that the oocyte could export NH4+, thereby avoiding swelling. However, the electrochemical gradient for NH4+ is inward, and in any case pHS would rise (rather than fall, as we observe). Therefore, model B is incorrect.
Model C: Is the Influx of NH3 Perfectly Matched by the Metabolic Production of H+ (Fig. 7c)
This analysis is similar to that for model B except that we can ignore the incorrect predictions that stem from H+ influx across the plasma membrane. Nevertheless, we are left with the massive accumulation of intracellular NH4+, which would lead to cell swelling and—upon NH3/NH4+ removal—a large pHi decline and a large pHS overshoot, neither of which we observe.
Model D: Is Virtually all Entering NH3 Metabolized to a Neutral Product(s) (Fig. 7d)
If an amido-transferase reaction (e.g., the conversion of glutamate to glutamine) consumed the entering NH3, pHi would not change. Moreover, NH4+ would not accumulate inside the cell during the NH3/NH4+ exposure. Thus following the withdrawal of extracellular NH3/NH4+, pHi would not fall and pHS would not overshoot pHBulk. To test model D, we attempted to detect Gln by both 1H-[13C] (Fig. 5) and 1H-15N-HSQC NMR spectroscopy, but observed little Gln or other likely NH3 metabolite. These data, combined with our demonstration of substantial NH3/NH4+ accumulation (Fig. 6)—presumably in acidic intracellular vesicles—make it unlikely that the oocyte performs substantial conversion of NH3 by metabolic processes.
Model E: Is Virtually all Entering NH3 Sequestered in an Acidic Subcompartment as NH4+ (Fig. 7e)
In this model, a small fraction of entering NH3 equilibrates with H+ to produce NH4+ in a cytoplasmic microenvironment immediately below the surface of the plasma membrane. Both the NH3 and the NH4+ would diffuse slightly deeper into the oocyte, where NH3 enters an acidic vesicle and becomes trapped as NH4+. To test this hypothesis, we used 1H-15N HSQC NMR spectroscopy to measure total intercellular NH3/NH4+, finding values (Fig. 6) that were far too high to represent cytosolic NH3/NH4+. Thus, we conclude that the vast majority of intracellular NH3/NH4+ must be trapped as NH4+ in vesicles. Indeed, oocytes contain copious yolk granules or platelets (50% of oocyte volume), containing yolk proteins (80% of total cell proteins), and having a pH of ~5.6 (Fagotto and Maxfield 1994a, 1994b; Fagotto 1995). By microscopy, a layer of vesicles begins within ~10 μm of the plasma membrane (Lu et al. 2006).
We predict that—at least during brief experiments such as ours—NH3/NH4+ levels would rise only modestly in the small subcompartment between the membrane and the vesicles, and relatively little of the entering NH3 could escape the aforementioned vesicles to penetrate deeper into the oocyte. The influx of NH3 into the vesicle would lead to a fall in [NH3] at the vesicle surface, favoring the reaction NH4+ → NH3 + H+, which would minimize pHi changes. It is possible that a high intravesicular buffering power (provided by abundant yolk proteins) could, by itself, sufficiently stabilize intravesicular pH over the course of our experiments. In addition, vesicular H+ pumps could replenish intravesicular H+, with cytosolic metabolism providing the necessary H+, again tending to stabilize cytosolic pH.
The NH4+-trapping hypothesis would account for all key, paradoxical findings dealing with NH3. (1) The extracellular addition of 0.5 mM NH3/NH4+ produces a pHi change of virtually nil (the conversion of incoming NH3 to NH4+ occurs in a subcompartment, not in the cytosol). (2) The fall in pHS produced by the extracellular addition of NH3/NH4+ relaxes very slowly (NH3 continues to enter the subcompartment, and perhaps deeper into the oocyte, for many tens of minutes). (3) The extracellular removal of NH3/NH4+ causes virtually no fall in pHi (virtually no NH4+ is present in the cytosol). And (4) the extracellular removal of NH3/NH4+ produces little or no overshoot of pHS (because little NH3/NH4+ is present free in the cytosol near the plasma membrane, the efflux of NH3 is very low).
Models of NH3/NH4+ Handling by Xenopus Oocytes: “High” [NH3/NH4+]o
Compared to the analysis of data from experiments with 0.5 mM NH3/NH4+, that of data from experiments with 5 mM NH3/NH4+ is complicated by the paradoxical fall in pHi (Fig. 2), which creates the added paradox that pH falls on both sides of the membrane.
Model A: Does the Influx of NH4+ Cause the Paradoxical Fall in pHi?
Previous investigators favored the hypothesis that the paradoxical fall in pHi is due to the influx of NH4+—via either an Na/K/Cl cotransporter (Keicher and Meech 1994) or a nonselective cation channel (Burckhardt and Frömter 1992)—followed by the cytosolic reaction NH4+ → NH3 + H+. The channel hypothesis, in particular, requires that the oocyte dispose of the newly formed NH3—by either NH3 efflux or intracellular NH3 metabolism or sequestration. Our pHS data (see Fig. 2) demonstrate a net movement of NH3 into the cell, ruling out the first NH3-disposal option. In the introductory section, we noted limitations of the Na/K/Cl-cotransporter hypothesis. Moreover, although we agree that a NH4+ conductance is a reasonable explanation for the depolarization triggered by the exposure to NH3/NH4+, two arguments will lead us to conclude here that the hypothesized NH4+ entry via a nonselective cation channel is not a viable explanation for the paradoxical fall in pHi. When we apply 5 mM NH3/NH4+, the speed of the pHi decrease (an index of the hypothesized NH4+ influx) is maximal early on and then gradually wanes as pHi stabilizes (see Fig. 2). On the other hand, the positive shift in Vm is small early on and slowly reaches a maximal value over nearly 15 min. Thus, if the depolarization is an index of permeability to NH4+, we conclude that the NH4+ conductance develops far too slowly to account for the decrease in pHi2.
Finally, using the same logic as we did for model A in the previous section (see Fig. 7a), we can conclude that it is impossible for the NH3/NH4+-influx ratio to be—at the same time—low enough to cause pHi to fall and yet high enough to cause pHS to fall. For example, if pHi is 7.2 and pKa is 9.2, then (JNH3/JNH4+)Null would be 1/100. Thus, for the influx of NH4+ to lower pHi, JNH3/JNH4+ would have to be <1/100. However, as we saw earlier, for the influxes of NH3/NH4+ to lower pHS (JNH3/JNH4+)Null would have to be >2/100. Because (JNH3/JNH4+)Null cannot simultaneously be <1/100 and >2/100, the influx of NH4+—regardless of mechanism—cannot account for the paradoxical fall in pHi.
We might note that, at least in theory, it would be possible for the parallel influxes of NH3 and NH4+ to cause both pHi and pHS to fall. As noted in Table 1, in the compartment that NH3 and NH4+ enter, the pH would fall if pH >pHNull. As we have just seen, a fall in pHS from an initial value of 7.5 requires that JNH3/JNH4+ be >2/100; let us assume a JNH3/JNH4+ of 10/100. For this ratio, pHNull would be 8.2. Thus, if the initial pHi were >8.2, a JNH3/JNH4+ of 10/100 would cause pHi to fall along with pHS. (Of course, because the actual initial pHi is far less than 8.2, this explanation is not valid for our data.) Using similar logic, we could predict conditions in which the parallel influxes of NH3 and NH4+ would raise both pHi and pHS.
Model B: Does the Influx of H+ Causes the Paradoxical Fall in pHi?
As noted in the previous section’s model B, in our analysis of data for 0.5 mM NH3/NH4+, even an H+ influx sufficient to stabilize pHi in the face of an NH3 influx (see Fig. 7b) would lead to a rise—rather than the observed fall—in pHS. Because an even greater H+ influx would be needed to produce a net fall in pHi—and such a greater H+ influx would cause an even greater increase in pHS—we can rule out the H+-influx model.
Model C: Does the Intracellular Release or Production of H+ Cause the Paradoxical Fall in pHi?
As noted in the previous section’s model C (see Fig. 7c), the intracellular generation of H+—by itself—during the NH3/NH4+ exposure would lead to NH4+ accumulation in the cytosol and thus cell swelling (not observed). During NH3/NH4+ withdrawal, the accumulated NH4+ would dissociate to produce H+ (we observed no fall in pHi) and NH3, which would exit the cell (we observed no substantial pHS overshoot). However, if H+ generation occurred in parallel with trapping—in acidic intracellular vesicles—of nearly all incoming NH3, then cytosolic H+ production would promote accumulation of NH4+ in vesicles rather than in the cytosol. Thus, this hybrid H+-generation/vesicular NH4+-trapping model (see Fig. 7e) would account for our data with 5 mM NH3/NH4+.
Model D: Does the Closing of NH4+-Permeable Channels Cause pHi to Rise Following Withdrawal of High Levels of NH3/NH4+?
The only explanation offered by previous investigators for the observed rise in pHi with NH3/NH4+ removal is that the sudden repolarization of the oocyte membrane would close nonselective cation channels and thus allow a slow efflux of accumulated cytosolic NH4+, leading to a sluggish pHi recovery (Burckhardt and Frömter 1992). Presumably, the release of previously sequestered NH3 would lead to the cytosolic reaction NH3 + H+ → NH4+, which would lead to a slow rise in pHi. One argument against the channel model is that, as already noted, our data3 indicate that [NH4+] in the cytosol near the inner surface of the plasma membrane must be very low, and thus NH4+ efflux could not produce a sustained pHi increase. Consistent with this idea, following the removal of NH3/NH4+, the Vm undershoot is small and short-lived (Fig. 2).4 A much stronger argument flows from our new analysis of (JNH3/JNH4+)Null values. For a hypothetical efflux of NH4+ to cause a rise in pHi—starting from an initial pHi of, say, 6.9—the absolute value of (JNH3/JNH4+)Null would have to be <[NH3]i/[NH4+]i = 10(6.9–9.2) = 0.5/100 (see Eq. 3 and Table 1). On the other hand, even though pHS does not exhibit a substantial overshoot of its initial value, the small pHS overshoot that we do observe indicates that |JNH3/JNH4+| would have to be >10(7.5–9.2) = 2/100 (see Table 1). Because |JNH3/JNH4+| cannot simultaneously be <0.5/100 and >2/100, we can conclude that an NH4+ efflux, regardless of mechanism, cannot account for the slow pHi increase.
Model E: Might Endogenous Na–H Exchange Cause pHi to Rise Following Withdrawal of High Levels of NH3/NH4+?
After an intracellular acid load induced by exposure to CO2 or butyric acid, Xenopus oocytes not heterologously expressing acid-base transporters have very low rates of acid extrusion (Romero et al. 1997; Grichtchenko et al. 2001; Piermarini et al. 2007). Thus, the pHi recovery occurs only after removal of NH3/NH4+. Furthermore, by analogy to the argument made in the previous section’s point B (see Fig. 7b), the extrusion of H+ would have produced a fall in pHS, rather than the small overshoot that we observed (Fig. 2). Thus, acid extrusion by an endogenous transporter also cannot account for the pHi recovery.
Model F: Might the Activation (or Inactivation) of Cytosolic H+ Production Cause the Fall (or rise) in pHi Caused by the Application (or Removal) of Extracellular NH3/NH4+?
Although we can rule out NH4+ or H+ uptake as an explanation for the paradoxical pHi decrease caused by the application of 5 mM NH3/NH4+ (and presumably higher concentrations as well)—and NH4+ or H+ efflux as an explanation for the paradoxical pHi increase caused by removal of high levels of NH3/NH4+—we have no definitive explanation for either pHi change. Others have proposed that members of the Rh family function as NH3/NH4+ sensors in determining the choice of slug vs. culmination in Dictyostelium discoideum (Kirsten et al. 2005, 2008; Singleton et al. 2006). We propose that oocytes have a low-affinity “sensor” that responds, directly or indirectly, to extracellular NH3/NH4+. This oocyte sensor could be either at the outer surface of the plasma membrane or somewhere reasonably close to the inner surface, but ultimately must act in two ways. (a) The sensor triggers the production of cytosolic H+—perhaps by a metabolic pathway. The influx of NH3 would temper the fall in pHi and lead to the formation of some NH4+, which also would temper the depolarization by reducing the inwardly directed NH4+ diffusion potential. Indeed, AmtB reduces the depolarization (compare AmtB vs. H2O bars in Fig. 4d and h). However, ultimately, the overwhelming majority of the incoming NH3 must be trapped as NH4+ in a presumably acidic intracellular compartment. (b) The oocyte sensor triggers the slow activation of a channel permeable to NH4+. We have no data to address the issue of whether this activation requires the attendant fall in pHi, or whether the putative increase in NH4+ permeability and the observed fall in pHi are totally independent. Note that NH4+ need not enter in order to depolarize the cell. We suggest that removal of extracellular NH3/NH4+ reverses this production, leading to consumption of H+ and thus the recovery of pHi. Because the pHi recovery begins so soon after NH3/NH4+ removal, we suggest that the NH3/NH4+ sensor faces the extracellular fluid.
Vm Changes
As noted earlier, the slowly developing depolarization that develops in the presence of NH3/NH4+ could reflect permeability to NH4+, as suggested by others (Burckhardt and Frömter 1992). It is interesting to note that, at both 5 mM and 0.5 mM NH3/NH4+, expression of AmtB substantially reduced the NH3/NH4+-induced depolarization (Fig. 4d and h) without significantly affecting pHi (Fig. 4b and f). We hypothesize that the additional influx of NH3 through AmtB leads to the formation of modest amounts of NH4+ immediately beneath the plasma membrane, reducing the diffusion gradient for NH4+.
Possible Benefits of the Oocyte’s Unusual Handling of NH3/NH4+
An intriguing question that remains is why the oocyte should handle NH3 in such an unusual manner. One possibility is that the oocyte’s responses to extracellular NH3/NH4+ are an adaptation that protects the cell—and perhaps, more importantly, its developmental program— from the appearance of NH3 in pond water that contains decaying organic matter and thus NH3/NH4+. Levels up to at least 0.5 mM NH3/NH4+ cause no discernible changes in pHi, and even much higher levels lead, at most, to modest, slow, and fully reversible pHi changes. In a more typical response, an exposure to NH3/NH4+ might mimic the rise in pHi caused by the fertilization of a Xenopus oocyte (Webb and Nuccitelli 1981; Nuccitelli et al. 1981). The Xenopus oocyte seems to be particularly adept at avoiding NH3/NH4+-induced pHi increases.
Acknowledgments
This work was supported by grants from the Office of Naval Research (1N000140810532 to W·F.B.) and the National Institutes of Health (NINDS 1 P30-NS052519 to K.L.B.). At Yale University, we thank Duncan Wong for computer support. We thank Mark D. Parker for helpful discussions, Dale Huffman for engineering assistance, and Charleen Bertolini for administrative support.
Footnotes
In our analysis, we assume that NH3 and NH4+ move in the same direction. If they should move in opposite directions, then pH would always fall on the side of the membrane toward which NH4+ moves, and would always rise on the opposite side.
We cannot rule out the possibility that the NH4+ conductance is immediately high, but that other conductances—also initially high— gradually decline to allow Vm to approach ENH4+.
The removal of NH3/NH4+ causes neither a fall in pHi nor a rise in pHS that substantially overshoots the initial value.
Assuming that the NH4+-conductive pathway remained activate and that substantial NH4+ were present in the cytosol, the removal of extracellular NH4+ would create a diffusion potential that would drive Vm to well below the pre-NH3/NH4+ value. Instead, we observed a Vm minimal undershoot that decayed rapidly, presumably reflecting either NH4+ efflux per se, or NH3 efflux followed by the cytosolic reaction NH4+ → NH3 + H+. We have no data that bear on the decay of the presumed NH4+ conductance.
Contributor Information
Raif Musa-Aziz, Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Physiology & Biophysics, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA, raif.aziz@case.edu.
Lihong Jiang, Department of Diagnostic Radiology and Magnetic Resonance Research Center, Yale University School of Medicine, New Haven, CT 06520, USA.
Li-Ming Chen, Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06520, USA.
Kevin L. Behar, Department of Psychiatry and Magnetic Resonance Research Center, Yale University School of Medicine, New Haven, CT 06520, USA
Walter F. Boron, Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06520, USA Department of Physiology & Biophysics, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA, walter.boron@case.edu.
References
- Aickin CC, Thomas RC. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol (Lond) 1977;273:295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade SL, Dickmanns A, Ficner R, Einsle O. Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc Natl Acad Sci USA. 2005;102:14994–14999. doi: 10.1073/pnas.0506254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Chérif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G. The challenge of understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus Clin Biol. 2006;13:139–146. doi: 10.1016/j.tracli.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3− blocked by cyanide. Nature. 1976a;259:240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol. 1976b;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt BC, Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch. 1992;420:83–86. doi: 10.1007/BF00378645. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation of intracellular pH in reticulospinal neurones of the lamprey, Petromyzon Marinus. J Physiol (Lond) 1986;381:241–261. doi: 10.1113/jphysiol.1986.sp016325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy MJ, Durand A, Lupo D, Li XD, Bullough PA, Winkler FK, Merrick M. The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc Natl Acad Sci USA. 2007;104:1213–1218. doi: 10.1073/pnas.0610348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hemptinne A, Huguenin F. The influence of muscle respiration and glycolysis on surface and intracellular pH in fibres of the rat soleus. J Physiol. 1984;347:581–592. doi: 10.1113/jphysiol.1984.sp015084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Musa-Aziz R, Cooper GJ, Chen L, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that Aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J. 2006;20:1974–1981. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- Fabiny JM, Jayakumar A, Chinault AC, Barnes EM., Jr Ammonium transport in Escherichia coli: localization and nucleotide sequence of the amtA gene. J Gen Microbiol. 1991;137(Pt 4):983–989. doi: 10.1099/00221287-137-4-983. [DOI] [PubMed] [Google Scholar]
- Fagotto F. Regulation of yolk degradation, or how to make sleepy lysosomes. J Cell Sci. 1995;108(Pt 12):3645–3647. doi: 10.1242/jcs.108.12.3645. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Maxfield FR. Changes in yolk platelet pH during Xenopus laevis development correlate with yolk utilization. A quantitative confocal microscopy study. J Cell Sci. 1994a;107:3325–3337. doi: 10.1242/jcs.107.12.3325. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Maxfield FR. Yolk platelets in Xenopus oocytes maintain an acidic internal pH which may be essential for sodium accumulation. J Cell Biol. 1994b;125:1047–1056. doi: 10.1083/jcb.125.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Bax A. The importance of not saturating H2O in protein NMR - application to sensitivity enhancement and NOE measurements. J Am Chem Soc. 1993;115:12593–12594. [Google Scholar]
- Harvey EN. Studies on the permeability of cells. J Exp Zool. 1911;10:507–556. [Google Scholar]
- Jacobs MH. The influence of ammonium salts on cell reaction. J Gen Physiol. 1922;5:181–188. doi: 10.1085/jgp.5.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Tropp J. Selective, in vivo observation of [5-N-15]glutamine amide protons in rat-brain by H-1-N-15 heteronuclear multiple-quantum-coherence transfer NMR. J Magnet Reson Ser B. 1995;107:107–115. doi: 10.1006/jmrb.1995.1066. [DOI] [PubMed] [Google Scholar]
- Keicher E, Meech R. Endogenous Na+ -K+ (or NH4+)-2Cl−cotransport in Rana oocytes; anomalous effect of external NH4 + on pHi. J Physiol. 1994;475:45–57. doi: 10.1113/jphysiol.1994.sp020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi S, Stroud RM. The Amt/MEP/Rh family: structure of AmtB and the mechanism of ammonia gas conduction. Physiology (Bethesda) 2006;21:419–429. doi: 10.1152/physiol.00051.2005. [DOI] [PubMed] [Google Scholar]
- Khademi S, O’Connell J, Remis J, Robles-Colmenares Y, Miericke LJW, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: Structure of AmtB at 1.35 angstrom. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989;339:478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Kirsten JH, Xiong Y, Dunbar AJ, Rai M, Singleton CK. Ammonium transporter C of Dictyostelium discoideum is required for correct prestalk gene expression and for regulating the choice between slug migration and culmination. Dev Biol. 2005;287:146–156. doi: 10.1016/j.ydbio.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Kirsten JH, Xiong Y, Davis CT, Singleton CK. Subcellular localization of ammonium transporters in Dictyostelium discoideum. BMC Cell Biol. 2008;9:71. doi: 10.1186/1471-2121-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen L, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB and RhAG. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0813231106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R, Webb DJ, Lagier ST, Matson GB. 31P NMR reveals increased intracellular pH after fertilization in Xenopus eggs. Proc Natl Acad Sci USA. 1981;78:4421–4425. doi: 10.1073/pnas.78.7.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton E. Über die osmotischen Eigenschaften der Zelle in ihrer Bedeutung fur die Toxicologie und Pharmacologie. Z Phys Chem. 1897;22:189–209. [Google Scholar]
- Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl–self-exchange activity. J Biol Chem. 2008;283:12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3 cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol. 2007;292:C2032–C2045. doi: 10.1152/ajpcell.00544.2006. [DOI] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR-spectroscopy of aqueous solutions. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+/HCO3− cotransporter from rat kidney. Am J Physiol. 1998;274:F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Shaka AJ, Keeler J, Frenkiel T, Freeman R. An improved sequence for broad-band decoupling—waltz–16. J Magnet Reson. 1983;52:335–338. [Google Scholar]
- Singh SK, Binder HJ, Geibel JP, Boron WF. An apical permeability barrier to NH3/NH4+ in isolated, perfused colonic crypts. Proc Natl Acad Sci USA. 1995;92:11573–11577. doi: 10.1073/pnas.92.25.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton CK, Kirsten JH, Dinsmore CJ. Function of ammonium transporter A in the initiation of culmination of development in Dictyostelium discoideum. Eukaryot Cell. 2006;5:991–996. doi: 10.1128/EC.00058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Lee H, Kustu S. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc Natl Acad Sci USA. 2002;99:3926–3931. doi: 10.1073/pnas.062043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature. 1994;368:332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- Warburg EJ. Studies on carbonic acid compounds and hydrogen ion activities in blood and salt solutions. A contribution to the theory of the equation of Lawrence J. Henderson and K.A. Hasselbalch. Biochem Z. 1922;16:153–340. doi: 10.1042/bj0160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Nuccitelli R. Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J Cell Biol. 1981;91:562–567. doi: 10.1083/jcb.91.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]


