Summary
Purpose
Diffusion tensor imaging (DTI) studies have reported substantial white matter abnormalities in patients with temporal lobe epilepsy (TLE). However, limited data exist regarding the extent of white matter tract abnormalities, cognitive effects of these abnormalities, and relationship to clinical factors. The current study examined these issues in subjects with chronic TLE.
Methods
DTI data were obtained in 12 TLE subjects and 10 age-matched healthy controls. Voxelwise statistical analysis of fractional anisotropy (FA) was carried out using tract-based spatial statistics (TBSS). White matter integrity was correlated with cognitive performances and epilepsy-related clinical parameters.
Results
Subjects with TLE, as compared to healthy controls, demonstrated four clusters of reduced FA, in anterior temporal lobe, mesial temporal lobe, and cerebellum ipsilateral, as well as frontoparietal lobe contralateral, to the side of seizure onset. Mean FA was positively correlated with delayed memory, in anterior temporal lobe; immediate memory, in mesial temporal lobe. Lower FA values in the posterior region of corpus callosum were related to earlier age of seizure onset.
Conclusion
TLE is associated with widespread disturbances in white matter tracts and these changes have important cognitive and clinical consequences.
Keywords: temporal lobe epilepsy, diffusion tensor imaging, cognition, age of seizure onset
Introduction
Temporal lobe epilepsy (TLE) is the most common form of focal epilepsy with structural and functional abnormalities both within and distant from the ictal onset zone (Lin et al., 2007, Mueller et al., 2004). Diffusion tensor imaging (DTI) studies have reported substantial white matter abnormalities in the epileptogenic network of subjects with TLE (Arfanakis et al., 2002, Ahmadi et al., 2009, Gross et al., 2006, Focke et al., 2008, Schoenfeld et al., 1999). However, limited data exist regarding the extent of abnormal white matter tracts, the cognitive effects of these abnormalities and their relationship to epilepsy-related clinical parameters. The current study addresses these issues.
The first goal of this study is to compare whole brain DTI characteristics of individuals with chronic drug-resistant TLE with that of age-matched healthy controls. Several DTI tractography studies have found that white matter abnormalities are most pronounced in the cerebral hemisphere ipsilateral to the side of seizure onset (Lin et al., 2008, Ahmadi et al., 2009). On the other hand, whole brain voxel analyses of gray matter density and thickness as well as other DTI tractography studies have shown diffuse symmetric abnormalities (Bernasconi et al., 2004, Gross et al., 2006, Lin et al., 2007), suggesting that TLE subjects have widespread structural abnormalities, despite unilateral seizure focus. The current study uses tract-based spatial statistics (TBSS) and probabilistic tractography to define the extent of white matter derangement in TLE (Smith et al., 2007). The primary measure of white matter integrity used in this study is fractional anisotropy (FA), which is determined by the directional magnitude of water diffusion in three-dimensional space. Tightly packed white matter fascicles provide structural coherence, which result in water diffusion in a preferred direction (high FA). In contrast, white matter fascicles that have poor structural organization will allow water to diffuse more randomly (low FA). TBSS provides an automated whole brain voxel-by-voxel analysis of FA without a priori bias for a specific brain region.
The second goal of the study is to determine the degree to which abnormal white matter is related to cognitive performances. Individuals with TLE also have impairments in multiple cognitive domains including memory, executive function, language, intelligence and motor speed (Oyegbile et al., 2004). A disconnection model has been proposed to explain this diffuse cognitive profile (Catani and ffytche, 2005). In essence, TLE may disrupt widespread cortical and subcortical connections that are germane to higher cognitive processing, leading to cognitive deficits in multiple domains. The current study tests the disconnection hypothesis by correlating the strength of limbic and neocortical connections with cognitive measures in TLE and healthy controls.
The third goal of the study is to investigate the relationship between white matter integrity with epilepsy-specific clinical variables, including age of epilepsy onset, epilepsy duration, seizure frequency and anti-epileptic medication history (i.e., the number of ineffective antiepileptic medications patients experienced in their lifetime). Although several DTI studies have correlated white matter integrity with these clinical parameters, their results have been inconsistent (Schoene-Bake et al., 2009, Hermann et al., 2003).
Methods
Subjects
We recruited 12 patients with TLE (age = 37.9 ± 3.2 years, mean ± SEM, range: 20-52; Female/Male = 9/3) and 10 age-matched healthy controls (42.1 ± 3.1 years, range: 33-55; Female/Male = 4/6) for this study, using an approved protocol from our Institutional Review Board. Entry criteria for patients included ability to undergo MRI, plus definite or probable unilateral TLE. The diagnosis of TLE was confirmed in 11 patients using continuous video-EEG monitoring, demonstrating unilateral ictal temporal EEG discharges of theta frequency or higher within 30 seconds after onset and thus were classified as definite TLE (Risinger et al., 1989). One patient did not undergo video-EEG monitoring but had appropriate temporal lobe seizure semiology and predominantly left temporal interictal epileptiform discharges on EEG and thus was classified as probable TLE. In 9 patients, the diagnosis with TLE was also supported by MRI evidence of mesial temporal sclerosis and/or unilateral temporal lobe hypometabolism on fluorodeoxyglucose positron emissions tomography. Patient clinical information was obtained from chart review as well as direct patient interviews and outlined in Table 1.
Table 1.
Summary of temporal lobe epilepsy patient clinical characteristics
| Patient | Age (years) | Age of seizure onset (years) | Duration of epilepsy (years) | Initial precipitating factor | Seizure frequency (per month) | MRI (clinical) | EEG | PET | Surgery | Antiepileptic medications (Current and *Past) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 19 | 15 | None | 1 | Right temporal lobar atrophy | Right temporal | Right temporal hypometabolism | Yes | PHT, LEV |
| 2 | 52 | 1 | 51 | Febrile Seizure | 2 | Left MTS | Left temporal | Normal | No | CBZ, LEV, LTB PHT*, PB*, PRM*, TPM* |
| 3 | 45 | 36 | 9 | Head trauma | 3 | Normal | Left temporal | Left temporal hypometabolism | No | OXC, VPA, LEV*, TPM * |
| 4 | 49 | 12 | 37 | None | 0.5 | Right MTS | Right temporal | Right temporal hypometabolism | Yes | LEV, LGT, PHT*, PB*, CBZ*, PB*, GBP* |
| 5 | 39 | 21 | 18 | None | 1 | Normal | Left >>Right interictal temporal sharp waves | -- | No | LGT, LEV* |
| 6 | 28 | 2 | 26 | Febrile Seizure | 0.5 | Normal | Left temporal | Normal | No | CBZ, LGT, PB* FBM*, ZNS*, OXC* |
| 7 | 50 | 39 | 11 | None | 2 | Left MTS | Left temporal | Left temporal hypometabolism | No | PHT, TPM, LGT*, LEV* |
| 8 | 33 | 7 | 26 | None | 8 | Left MTS | Left temporal | Left temporal hypometabolism | Yes | CBZ, LEV, PHT, TPM |
| 9 | 20 | 8 | 12 | None | 3 | Normal | Left temporal | Left temporal hypometabolism | Yes | LGT, PB*, CBZ*, PHT* |
| 10 | 24 | 11 | 13 | Head trauma | 6 | Normal | Left temporal | Normal | No | LEV, OXC, CBZ*, GBP* |
| 11 | 37 | 12 | 25 | Febrile Seizure | 2 | Left MTS | Left temporal | Normal | No | LEV, OXC |
| 12 | 34 | 4 | 30 | Febrile Seizure | 8 | Left MTS | Left temporal | Left temporal hypometabolism | No | PHT, GBP, LEV |
MRI = magnetic resonance imaging; EEG = electroencephalogram; PET = positron emission tomography; MTS = mesial temporal sclerosis; PHT = phenytoin; LEV = levetiracetam; CBZ = carbamazepine, LGT = lamotrigine; PB = phenobarbital; PRM = primidone; TPM = topiramate; OXC = oxcarbazepine, VPA = valproic acid; GBP = gabapentin; FBM = felbamate; ZNS = zonisamide; -- indicates test not done.
The control subjects were recruited through responses to advertisements posted at the University of California, Irvine Medical Center and School of Medicine. Criteria for controls subjects included 1) no neurological or psychiatric diagnoses; 2) no family member with epilepsy, 3) no history of a loss of consciousness for >5 minutes, and 4) ability to undergo MRI.
MRI acquisition
MRI data was obtained using a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany) with an 8 phased array head coil. DTI data acquisition involved a single-shot echo-planar pulse sequence with parallel acquisition technique (IPAT) in conjunction with generalized autocalibrating partially parallel acquisition (GRAPPA), with the following parameters: two volumes, image matrix = 128×128, field of view = 256×256 mm, TR = 9300 ms, TE = 93 ms, slice thickness = 2.0 mm with no interslice gap, 12 non-collinear directions with a b value of 1000 s/mm.
Image analysis
Images obtained in DICOM format were converted to ANALYZE. Subsequent images were analyzed using tools from the Oxford Center for Functional MRI of the Brain (FMRIB, FSL version 3.3 http://www.fsl.ox.ac.uk/fsl). Images were corrected for eddy currents and head motion by affine coregistration to the b0 image of the first repetition using FMRIB's Diffusion Toolbox (FDT) (Jenkinson and Smith, 2001), and then skull and dura were removed (Smith, 2002). The diffusion tensor was computed at each voxel, generating images of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). Within the epilepsy patient group, MRI studies were grouped according to the side of seizure onset and compared to the healthy control group. Thus two individuals with right TLE were transformed across the midline so that comparisons with the ten subjects with left TLE could be made.
Using TBSS, voxelwise cross-subject comparisons were made between FA profiles of TLE and control subjects to identify discrete regions of white matter abnormalities (Smith et al., 2007). First, a target image was determined by aligning every subject's FA image to each other image, in order to determine the most representative subject. This target image was then normalized to MNI152 standard space using an affine transformation. All other subjects were then aligned first to the target image and then to 1×1×1 mm MNI152 space using Nonlinear Registration Toolkit [IRTK, www.doc.ic.ac.uk/~dr/software] (Rueckert et al., 1999). This process created a mean FA skeleton that represents the centers of all tracts common to the group. All individual subjects’ aligned FA data (TLE and control subjects) was projected onto the FA skeleton, and the resulting data was used for voxel-wise statistics. Specifically, in TBSS, a design matrix is generated that groups subjects into either TLE or controls and then tests for a significant difference at each voxel between the two groups. TBSS was also applied to the non-FA images generated from the diffusion tensor (i.e. MD, AD, and RD). To do this, the nonlinear registration used in the FA analysis is applied to the non-FA images. The resultant images are then projected onto the same skeleton used in the FA analysis using the same skeleton projection vectors. These skeletonised non-FA images are then used to perform the same voxel-wise statistics as FA data (see statistical analysis).
Tractography
Diffusion tensor tractography was performed for each subject to map specific white matter tracts underlying the regions of white matter abnormalities identified in TBSS. To do this, the clusters of significance found in TBSS were first transformed back into each subject's native space. These clusters were then applied as seed masks for probabilistic tractography (5000 streamline samples, curvature threshold of 0.2, step length 0.5 mm), using FMRIB's Diffusion Toolbox (FDT v2.0). Tractography proceeded by drawing multiple streamline samples through the probability density functions from each seed voxel, generating volumes in which each voxel represents the probability of connection to the seed voxel. The current model also accounts for multiple fiber orientations at each voxel (Behrens et al., 2007). Tractography results for each subject were thresholded to include only those voxels in which at least ten percent of samples were included. Individual tracts were then transformed into standard space, binarized, and then combined to give group tractography results. Visual identification of the resulting tracts was made based on an MRI-based atlas of white matter tracts (Mori S, 2005)
Neuropsychological Assessment
Neuropsychological testing was obtained in 20 subjects (10 TLE, 10 HC), as two subjects with epilepsy were non-native English speaker and thus were unable to complete the neuropsychological testing (Table 2). Components of the Repeatable Battery of Neuropsychological Status (RBANS) were used to assess immediate memory, delayed memory, visualspatial skills, attention and language in individuals with TLE and healthy controls (Table 2) (Randolph, 1998). The immediate memory index consists of a list recall, which requires each subject to learn ten words across four trials as well as a story memory recall. The delayed memory index consists of three subtests: delayed spontaneous recall, recognition memory, and delayed visual memory. The visuospatial index consists of a figure copy and line orientation subtests. The attention index measures both auditory attention (digit span forward) and visual attention (Coding). During the digit span forward subtest each subject is required to repeat back a string of numbers while during the coding subtest each subject is asked to write numbers that correlate with symbols. The language index consists of confrontational naming and semantic/category fluency (i.e. generating as many fruits and vegetables in a one minute). In addition to the RBANS, executive functioning was assessed using the Controlled Oral Word Association Test, and the Stroop Color/word Test (Strauss et al., 2006, Stroop, 1935). Measures of fine motor functioning were assessed using the Purdue Pegboard Test (Tiffin and Asher, 1948).
Table 2.
Neuropsychological performance in epilepsy patients and controls
| Domain | Test | TLE | Controls | p-value |
|---|---|---|---|---|
| Immediate memory | List recall | 78 ± 16.0 | 101.5 ± 18.2 | 0.0072 |
| Story recall | ||||
| List recall | ||||
| Delayed memory | Story recall | 84.4 ± 19.9 | 101.7 ± 13.0 | 0.0246 |
| Visual recall | ||||
| Language | Naming | 79.4 ± 25.4 | 92.7 ± 11.3 | 0.1528 |
| Categorical fluency | ||||
| Attention | Digit span | 94.2 ± 17.2 | 109.9 ± 18.9 | 0.0745 |
| Coding | ||||
| Executive | Stroop (color and word) | 43± 11.7 | 52.3 ± 11.6 | 0.1642 |
| Controlled oral word association | 30.3 ± 16.5 | 40 ± 9.9 | 0.1690 | |
| Fine motor | Purdue pegboard (right) | 20.7 ± 2.1 | 23 ± 2.5 | 0.0471 |
| Purdue pegboard (left) | 19.9 ± 1.4 | 21.7 ± 2.6 | 0.0947 | |
TLE = temporal lobe epilepsy
Statistical Analyses
The statistical method employed for voxel-wise comparison was based on a nonparametric approach using permutation tests (randomize, part of FSL). Group comparison between TLE and control subjects was performed, using two-sample t-test (age as nuisance variable), corrected for multiple comparisons, thresholded at a cluster level of t=2, and a corrected cluster size significance level of p<0.05. In an attempt to increase the power of the analysis and test the hypothesis that DTI abnormalities are more pronounced on the side of the seizure onset, we pooled data ipsilateral and contralateral to the side of seizure onset. Two right TLE subject's images were transformed across the midline plane in order maintain consistent side of seizure onset with the 10 left TLE subjects. Thus we combined data from the side of seizure onset, and also combined data from the hemispheres opposite to that of seizure onset.
For the TLE group, FA maps were generated to localize the degree to which white matter integrity was statistically linked to patients’ clinical measures. For this purpose, inter-subject voxel-wise correlation was performed between the skeletal voxel FA and each of the following clinical measures: age of epilepsy onset, epilepsy duration, seizure frequency, history of febrile seizures and the number of ineffective antiepileptic medications. The output was threshold at a cluster level of t=2, corrected for multiple comparisons (using permutation tests), with a significance level of p<0.05. In addition, region of interest analysis (ROI) was performed on brain regions where voxelwise cross-subject statistics in TBSS revealed significantly different FA values between the TLE and control group. The FA values of these ROIs were correlated with the five clinical parameters and a level of significance was set at p<0.05/5=0.01.
The mean FA value of each significant brain region was correlated with cognitive measures obtained from neuropsychological testing performed in the 10 native English-speaking TLE subjects and 10 control subjects. To reduce type 1 error, primary cognitive measures were selected based on known structural and functional relationships between white matter tracts and cognitive domains found in the literature. The PubMed database (http://www.ncbi.nlm.nih.gov/sites/entrez) was searched for functional MRI and lesional studies of white matter tracts relevant to the current study, as revealed by TBSS and DTI tractography results (Figure 1 and 2). The primary cognitive measures for temporal lobe white matter regions, which encompass frontal, temporal and limbic tracts, were immediate and delayed memory (Levine et al., 1998, Poreh et al., 2006, Gilboa et al., 2006) as well as language functions (Parker et al., 2005, Saur et al., 2008) (Bonferroni correction, significance level set at p<0.05/3=0.017). The primary cognitive measures for the cerebellum were executive function, attention, language and motor (Gottwald et al., 2004, Schmahmann and Sherman, 1998) (significance level set at p<0.05/4=0.0125). The primary cognitive measures for parietal regions, which include frontal parietal and postcentral gyrus projection tracts, were visuospatial, language and executive function (Chen et al., 2009) (Parker et al., 2005) (significance level set at p<0.05/3=0.017). A bivariate analysis (pairwise correlation) was performed, correlating the mean FA value of each white matter region with its respective primary cognitive measures, adjusting for multiple comparisons.
Figure 1.

Whole brain DTI analysis (TBSS) revealed regional FA reduction in the TLE group. The yellow/red voxels indicate brain regions where the FA was significantly reduced in TLE patients when compared to controls. The TLE patients are grouped according to the side of seizure onset (ipsi = Ipsilateral, contra = contralateral to the side of seizure onset). The group average FA skeleton (green) is projected on the mean FA and the coordinates are in MNI 152 space.
Figure 2.
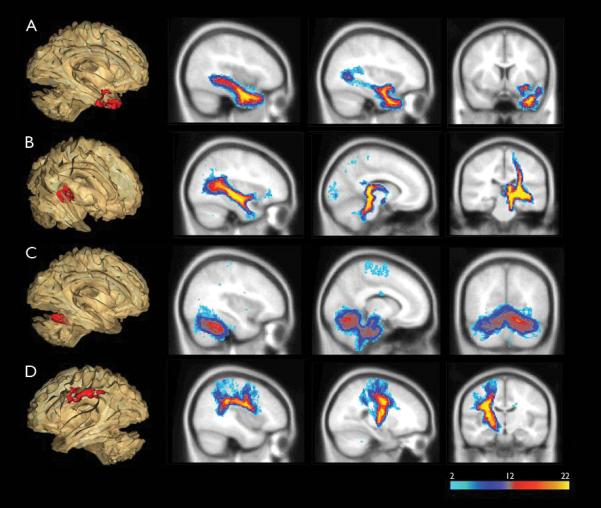
Probabilistic DTI tractography showed abnormal white matter clusters found in TBSS linked to multiple distinct white matter tracts. DTI tractography was performed on each abnormal white matter cluster found in TBSS (shown in 3D glass brain, left side of the figure). Blue color indicates regions of the tract in which few subjects have in common, while red/yellow colors shows regions in which most subjects have in common. The anterior temporal cluster (A) was part of the uncinate fasciculus and inferior longitudinal fasciculus. The mesial temporal cluster (B) was associated with fornix, inferior longitudinal fasciculus, and motor projection tracts. The cerebellum cluster (C) had projections to bilateral anterior cerebellum. Tracking of the frontal parietal cluster (D) showed arcuate fasciculus and motor projection tracts.
Results
Reduced white matter integrity in TLE subjects
The primary measure of white matter integrity in TBSS is FA. TLE subjects have reduced FA, predominately in the epileptogenic cerebral hemisphere (Figure 1). TLE subjects demonstrated four significant clusters in which FA values were reduced, when compared to healthy controls: anterior temporal lobe (p=0.04, MNI x = -41, y = 8, z = -32), posterior mesial temporal lobe (p=0.026, MNI x = -28, y = -25, z = -3), and cerebellum (p=0.045 MNI x = -23, y = -57 z = -22) ipsilateral to the side of seizure onset, as well as frontoparietal lobe (p=0.016, 834 MNI x = 32, y = -13, z = 31) contralateral to the side of seizure onset.
To investigate the microstructural mechanisms of this DTI change, the following non-FA diffusion parameters were analyzed using TBSS: MD, AD and RD. The anterior temporal lobe cluster showed increased MD (p=0.018) and increased RD (p=0.007), with no change in AD. No other brain regions showed abnormal mean or directional diffusivity.
Abnormal white matter regions correspond to distinct white matter tracts
Probabilistic tractography was performed to determine which white matter tracts were involved in the four abnormal white matter clusters identified by TBSS analysis (Figure 2). Individual tracts from both TLE and control subjects were combined to illustrate the consistency and intra-subject variability across all subjects. When tractography results were separated into TLE and controls, very similar tract profiles emerged for these two groups. White matter tracts generated were identified by reference to an MRI-based atlas of white matter tracts (Mori S, 2005). In the anterior temporal cluster, the resulting group track clearly revealed the uncinate fasciculus with projections to the anterior temporal lobe, as well as the inferior longitudinal fasciculus with projections to the posterior temporal and parietal occipital lobe. Fiber tracking of the posterior temporal cluster showed the fornix with projections to hippocampus, inferior longitudinal fasciculus, and fibers projecting to the postcentral gyrus. Bilateral anterior lobe of the cerebellum was seen with fiber tracking of the cerebellum cluster. Fiber tracking of the frontoparietal cluster showed the arcuate fasciculus, connecting the inferior frontal lobe to the parietal and posterior temporal lobe as well as the fibers projecting to the postcentral gyrus.
White matter tract integrity is related to specific cognitive functions
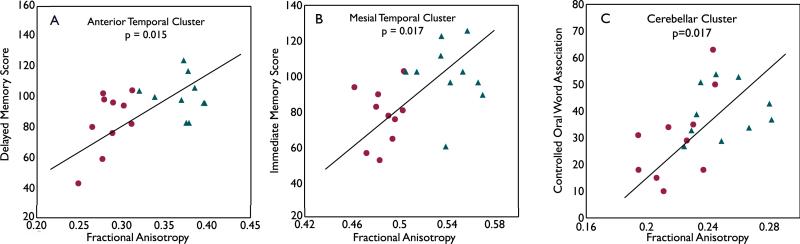
Each subject's mean FA value from the four white matter regions found in TBSS was correlated with primary cognitive measures (Table 2). Among TLE (denoted as red dots in the figure) and control subjects (denoted as blue triangles), the FA values of white matter clusters were correlated with the following domains of cognitive function: 1) the anterior temporal lobe FA was related to delayed memory score (p=0.015), 2) the mesial temporal lobe FA was linked to immediate memory score (p=0.017). The relationship between the cerebellum FA and Controlled Oral Word Association Test score, a measure of executive function approached significance at p=0.017 (Figure 3). The relationship between the anterior temporal lobe and immediate memory also approached significance at p=0.026. There were no significant correlations between the frontoparietal cluster and cognitive performances. When analyzing TLE and controlled groups separately, two correlations in the TLE group approached significance: the relationship between anterior temporal lobe FA and delayed memory (p=0.053) and cerebellum FA and Controlled Oral Word Association Test score (p=0.054). There were no significant correlations between FA and cognitive performances in the healthy control group.
Figure 3.
White matter integrity is related to specific cognitive performances. Significant positive correlations were found between FA values and cognitive performances in the following regions: the anterior temporal cluster and with delayed memory (A), mesial temporal cluster with immediate memory (B), and cerebellar cluster with Controlled Oral Word Association test score (C), a measure of executive function. TLE subjects are denoted as red dots and control subjects are denoted as blue triangles.
Corpus callosum integrity was correlated with age of seizure onset
In order to investigate the relationship between white matter integrity and clinical characteristics, regressions of FA were performed against the clinical characteristics listed in Table 1. Significant correlations (corrected for multiple comparisons) were found between the earlier age of seizure onset and reduced FA in the isthmus and splenium of the corpus callosum (permutation test, correcting for multiple comparisons, p=0.033; Figure 4). When correlating clinical parameters with FA values from the four white matter regions that were abnormal when compared to controls, the link between epilepsy duration and mesial temporal lobe FA approached significance at p<0.033.
Figure 4.
Posterior callosal FA values correlated with age of seizure onset. Voxels in red (A) showed white matter in the posterior regions (isthmus and splenium) of the corpus callosum in which reduced FA values are significantly correlated with earlier seizure onset (p = 0.033). The plot of FA of this region versus age of seizure onset (denoted as red dots) is displayed in B.
Discussion
This study aimed to define white matter deficit profiles of TLE patients and to relate these discrete regions of white matter abnormalities with cognitive performances, epilepsy-related clinical factors and histopathological findings. There were five main findings. First, TLE patients, as compared to healthy controls, demonstrated four discrete abnormal white matter regions predominately ipsilateral but also contralateral to the side of seizure onset. Second, these abnormal white matter regions corresponded to a widespread network of white matter tracts, not only in the limbic circuit (fornix), but also in frontal-temporal connections (uncinate fasciculus and arcuate fasciculus), temporal-occipital connections (inferior longitudinal fasciculus), motor projection tracts, and the cerebellum. Third, three of the four abnormal white matter regions correlated with cognitive performances germane to the specific cognitive task, such as the relationship between mesial temporal lobe white matter integrity and memory performances. Fourth, decreased white matter integrity in the posterior region of the corpus callosum was correlated with earlier age of seizure onset.
This study demonstrated that unilateral TLE was associated with white matter changes in a large network of intrahemispheric, projection, and cerebellar fiber tracts. When compared to other TBSS studies in the TLE population, this study in general showed a similar pattern of white matter abnormalities (Schoene-Bake et al., 2009, Focke et al., 2008). However, the other TBSS studies showed a greater number of abnormal white matter clusters, likely due to their larger sample size. Even in our smaller patient population, we found four significant clusters of abnormal white matter (after adjusting for multiple comparisons), suggesting that the effect size of these changes is large. A distinguishing component of our study is that we performed probabilistic tractography on all the significant regions found in TBSS analysis. The results of this analysis showed that each of these abnormal white matter clusters could affect multiple tracts linking different brain regions. For example, the anterior temporal lobe white matter cluster was part of the uncinate fasciculus as well as the inferior longitudinal fasciculus. Similarly, the mesial temporal lobe cluster was not only part of the fornix but also a segment of the posterior motor projection tract. Thus, disruption in a discrete white matter region could have more widespread downstream consequences.
In the current study, predominant white matter abnormalities were located in the ipsilateral mesial and lateral temporal lobe. In addition, TLE subjects also exhibited white matter abnormalities in the cerebellum, consistent with prior volumetric and neuropathological studies (Keller and Roberts, 2008), (Margerison and Corsellis, 1966). Cerebellar degeneration has been attributed to febrile seizures, duration of epilepsy and severity of the disease, such as the number of secondary generalized seizures (Sandok et al., 2000) (Hermann et al., 2005). In addition, phenytoin use has been associated with cerebellar atrophy and 50% of the current patient population had exposure to this medication (Lee et al., 2003, De Marcos et al., 2003).
The abnormal frontoparietal white matter region contralateral to the side of seizure onset is an unexpected finding because greater disease burden is expected on side of seizure onset, as demonstrated in previous TBSS studies (Focke et al., 2008) (Schoene-Bake et al., 2009). In the current study, we attempted to increase statistical power by combining DTI data according to the side of seizure onset. However, the right and left cerebral hemisphere white matter tracts are asymmetric in healthy subjects, likely reflecting underlying functional specialization (Barrick et al., 2007). We pooled data from the two epilepsy groups because we expect the pathological contribution from epilepsy to this asymmetry is substantially greater than normal underlying asymmetry. However, collapsing the image data across the two epilepsy groups may have added anatomical variance, making group differences in certain areas, such as the ipsilateral frontoparietal region, more difficult to detect. In fact, the ipsilateral frontoparietal white matter abnormality could be detected with a lower cluster significance threshold of p=0.24.
When considering microstructural mechanisms underlying the diffusion changes, the anterior temporal lobe showed decreased FA, increased MD, increased RD, and no change in AD. This diffusivity pattern has been associated with chronic white matter degeneration such as those seen after corpus callosotomy (Concha et al., 2006) and in chronic changes related to ischemic stroke (Thomalla et al., 2004). These diffusion abnormalities likely reflect a combination of myelin and axon loss, leading to lower membrane density and higher extracellular volume (Sen and Basser, 2005). The other three regions showed decreased FA without significant changes in other diffusion parameters. This pattern may reflect subtle white matter fiber incoherence, such as minor fiber loss, which may lead to overall decrease in FA but no net change in MD. The current study showed that FA reduction could occur without associated changes in other diffusion parameters. This observation is in line with Focke and colleagues TBSS study as well as a recent study evaluating age-related changes in white matter microstructure (Focke et al., 2008, Burzynska et al., 2009).
Our next aim was to evaluate potential cognitive consequences of abnormal white matter regions found in TBSS. Recent DTI tractography studies have linked individual tract integrity to cognitive dysfunction in TLE patients. Diehl and colleagues found that the integrity of the left uncinate fasciculus was correlated with verbal memory performances in left TLE patients (Diehl et al., 2008). McDonald and coworkers extended this finding by examining relationships between multiple white matter tracts and language and memory function in TLE patients (McDonald et al., 2008). They demonstrated that verbal memory and language performances were correlated with the integrity of multiple cortical-to-cortical association tracts as well as limbic projection tracts, especially evident in the left hemisphere. Although these studies revealed structural and functional relationships between white matter tract integrity and cognitive performances, individual tracts are outlined by placing predetermined seed points throughout the cerebral cortex and thus may be prone to a priori bias. We took a different approach in our current study, in that we examined the cognitive consequences of only those white matter regions in which TLE patient demonstrated reduced FA when compared to normal controls in the TBSS analysis. We found that three of the four abnormal clusters correlated with cognitive performances. Indeed, cognitive performances were related to anatomical derangements in connections that were germane to the specific cognitive tasks. The integrity of the mesial temporal lobe cluster, which encompasses the fornix and posterior aspects of the inferior longitudinal fasciculus, was correlated with immediate memory performances. These findings are consistent with lesional and DTI studies in which damaged fornix could lead to impaired episodic memory and transection of bilateral fornix results in severe anterograde and retrograde amnesia (Poreh et al., 2006). The inferior longitudinal fasciculus, a major connection between the temporal and occipital lobe, also plays an important role in visual memory (Tusa and Ungerleider, 1985). The integrity of the anterior temporal lobe cluster, containing the uncinate fasciculus and anterior aspects of the inferior longitudinal fasciculus, was correlated with delayed memory. The uncinate fasciculus provides connections between the anterior temporal and the inferior frontal lobes and thus is a linchpin in the retrograde memory circuitry (Markowitsch, 1995, Ebeling and von Cramon, 1992). Finally, bilateral anterior lobe cerebellar white matter integrity was correlated with performances in executive function. In addition to the cerebellum's function in motor control, converging lesional and functional MRI studies have implicated the role it plays in higher cognitive abilities, including executive function (Gottwald et al., 2004, Schmahmann and Sherman, 1998). Our findings are complementary to functional MRI studies of verbal fluency in which the cerebellum is critically involved in the network for verbal fluency (Schlosser et al., 1998, Stoodley and Schmahmann, 2009). Executive function tasks such as verbal fluency employed in this study require a cerebro-cerebellar network with reciprocal connections between the dorsolateral prefrontal neocortex, lateral parietal neocortex and neocerebellum (Schlosser et al., 1998). Taken together, these results suggest that widespread and coordinated networks are required for complex cognitive functioning. Disconnection between important cortical and subcortical regions would impair information transfer and thus contribute to cognitive impairments in TLE.
Patients with earlier onset of TLE had a greater degree of white matter abnormalities in the posterior regions of corpus callosum, suggesting that this region is particularly vulnerable to early life seizures or initial precipitating factors that led to the development of epilepsy. Our results are consistent with Hermann and colleagues’ findings, demonstrating that early-onset TLE patients (N=32) have reduced posterior callosal volume when compared to late-onset TLE and healthy controls (Hermann et al., 2003). In a larger TLE population (N=96), Weber and coworkers also showed that TLE in general was associated with reduced posterior callosal thickness, but early-onset TLE showed a greater magnitude of reduction when compared to late-onset TLE (Weber et al., 2007). The regional specificity of the callosal deficit pattern is remarkably similar across all three studies. Indeed, the areas found most affected by early-onset TLE are located in the isthmus and splenium of the corpus callosum. These callosal regions are critical for interhemispheric transfer of information by providing connections between the superior temporal lobe and posterior parietal lobe (isthmus) as well as inferior temporal lobe and occipital lobe (splenium). The differential vulnerability of the posterior callosal regions to the age of epilepsy onset corresponds well to neuropsychological findings that childhood epilepsy is associated with reduced general intellectual abilities and poorer academic performances when compared to late-onset epilepsy (Neyens et al., 1999, Schoenfeld et al., 1999).
In conclusion, the current study found that TLE is associated with widespread disturbances in white matter tracts and these changes have important cognitive consequences. Furthermore, the posterior callosal regions exhibited differential vulnerability to age of epilepsy onset. The main limitation of the current study is small sample size. TBSS group analyses were performed based on the side of seizure onset and thus were unable to differentiate whether or not DTI abnormalities and their cognitive correlates were differentially affected depending on the hemisphere of seizure onset (i.e. right versus left). In addition, the relationship between white matter integrity and cognitive performances was only significant when TLE and controlled subjects data were analyzed as an aggregate group. Further studies in larger well-matched patient samples will examine these issues.
Acknowledgement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
We thank Tallie Z. Baram, M.D., Ph.D., for encouragement and critique of this manuscript. We thank Howard L. Kim, M.D. and Barbara E. Swartz, M.D., Ph.D., for referring patients for this study. This work was supported by grants from National Institutes of Health (NIH T32 NS45540, PI: Baram, T. Z.; NIH K23 NS060993, PI: Lin, J. J.), Epilepsy Foundation Target Initiative for Mood Disorders (PI: Lin, J. J.), and the Institute for Clinical and Translational Science, School of Medicine, University of California, Irvine, with funds provided by the National Center for Research Resources, 5M01RR00827-29, U.S. Public Health Service.
References
- AHMADI ME, HAGLER DJ, JR., MCDONALD CR, TECOMA ES, IRAGUI VJ, DALE AM, HALGREN E. Side Matters: Diffusion Tensor Imaging Tractography in Left and Right Temporal Lobe Epilepsy. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARFANAKIS K, HERMANN BP, ROGERS BP, CAREW JD, SEIDENBERG M, MEYERAND ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–9. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- BARRICK TR, LAWES IN, MACKAY CE, CLARK CA. White matter pathway asymmetry underlies functional lateralization. Cereb Cortex. 2007;17:591–8. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- BEHRENS TE, BERG HJ, JBABDI S, RUSHWORTH MF, WOOLRICH MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNASCONI N, DUCHESNE S, JANKE A, LERCH J, COLLINS DL, BERNASCONI A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–23. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- BURZYNSKA AZ, PREUSCHHOF C, BACKMAN L, NYBERG L, LI SC, LINDENBERGER U, HEEKEREN HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- CATANI M, FFYTCHE DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- CHEN TF, CHEN YF, CHENG TW, HUA MS, LIU HM, CHIU MJ. Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONCHA L, GROSS DW, WHEATLEY BM, BEAULIEU C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–9. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- DE MARCOS FA, GHIZONI E, KOBAYASHI E, LI LM, CENDES F. Cerebellar volume and long-term use of phenytoin. Seizure. 2003;12:312–5. doi: 10.1016/s1059-1311(02)00267-4. [DOI] [PubMed] [Google Scholar]
- DIEHL B, BUSCH RM, DUNCAN JS, PIAO Z, TKACH J, LUDERS HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- EBELING U, VON CRAMON D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–8. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- FOCKE NK, YOGARAJAH M, BONELLI SB, BARTLETT PA, SYMMS MR, DUNCAN JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–37. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- GILBOA A, WINOCUR G, ROSENBAUM RS, POREH A, GAO F, BLACK SE, WESTMACOTT R, MOSCOVITCH M. Hippocampal contributions to recollection in retrograde and anterograde amnesia. Hippocampus. 2006;16:966–80. doi: 10.1002/hipo.20226. [DOI] [PubMed] [Google Scholar]
- GOTTWALD B, WILDE B, MIHAJLOVIC Z, MEHDORN HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–31. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS DW, CONCHA L, BEAULIEU C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–3. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- HERMANN B, HANSEN R, SEIDENBERG M, MAGNOTTA V, O'LEARY D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- HERMANN BP, BAYLESS K, HANSEN R, PARRISH J, SEIDENBERG M. Cerebellar atrophy in temporal lobe epilepsy. Epilepsy Behav. 2005;7:279–87. doi: 10.1016/j.yebeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- JENKINSON M, SMITH S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- KELLER SS, ROBERTS N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–57. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- LEE SK, MORI S, KIM DJ, KIM SY, CHU M, HEO K, LEE BI, KIM DI. Diffusion tensor MRI and fiber tractography of cerebellar atrophy in phenytoin users. Epilepsia. 2003;44:1536–40. doi: 10.1111/j.0013-9580.2003.43502.x. [DOI] [PubMed] [Google Scholar]
- LEVINE B, BLACK SE, CABEZA R, SINDEN M, MCINTOSH AR, TOTH JP, TULVING E, STUSS DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121(Pt 10):1951–73. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- LIN JJ, RILEY JD, JURANEK J, CRAMER SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–70. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- LIN JJ, SALAMON N, LEE AD, DUTTON RA, GEAGA JA, HAYASHI KM, LUDERS E, TOGA AW, ENGEL J, JR., THOMPSON PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- MARGERISON JH, CORSELLIS JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- MARKOWITSCH HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Res Brain Res Rev. 1995;21:117–27. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- MCDONALD CR, AHMADI ME, HAGLER DJ, TECOMA ES, IRAGUI VJ, GHARAPETIAN L, DALE AM, HALGREN E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIS WS, NAGAE-POETSCHER LM, VAN ZIJL PCM. MRI atlas of human white matter. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- MUELLER SG, LAXER KD, CASHDOLLAR N, FLENNIKEN DL, MATSON GB, WEINER MW. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia. 2004;45:355–66. doi: 10.1111/j.0013-9580.2004.27603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEYENS LG, ALDENKAMP AP, MEINARDI HM. Prospective follow-up of intellectual development in children with a recent onset of epilepsy. Epilepsy Res. 1999;34:85–90. doi: 10.1016/s0920-1211(98)00118-1. [DOI] [PubMed] [Google Scholar]
- OYEGBILE TO, DOW C, JONES J, BELL B, RUTECKI P, SHETH R, SEIDENBERG M, HERMANN BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- PARKER GJ, LUZZI S, ALEXANDER DC, WHEELER-KINGSHOTT CA, CICCARELLI O, LAMBON RALPH MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- POREH A, WINOCUR G, MOSCOVITCH M, BACKON M, GOSHEN E, RAM Z, FELDMAN Z. Anterograde and retrograde amnesia in a person with bilateral fornix lesions following removal of a colloid cyst. Neuropsychologia. 2006;44:2241–8. doi: 10.1016/j.neuropsychologia.2006.05.020. [DOI] [PubMed] [Google Scholar]
- RANDOLPH C. Repeatable Battery for the Assessment of Neuropsychological Status. The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- RISINGER MW, ENGEL J, JR., VAN NESS PC, HENRY TR, CRANDALL PH. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989;39:1288–93. doi: 10.1212/wnl.39.10.1288. [DOI] [PubMed] [Google Scholar]
- RUECKERT D, SONODA LI, HAYES C, HILL DL, LEACH MO, HAWKES DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- SANDOK EK, O'BRIEN TJ, JACK CR, SO EL. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia. 2000;41:1315–20. doi: 10.1111/j.1528-1157.2000.tb04611.x. [DOI] [PubMed] [Google Scholar]
- SAUR D, KREHER BW, SCHNELL S, KUMMERER D, KELLMEYER P, VRY MS, UMAROVA R, MUSSO M, GLAUCHE V, ABEL S, HUBER W, RIJNTJES M, HENNIG J, WEILLER C. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLOSSER R, HUTCHINSON M, JOSEFFER S, RUSINEK H, SAARIMAKI A, STEVENSON J, DEWEY SL, BRODIE JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64:492–8. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMAHMANN JD, SHERMAN JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- SCHOENE-BAKE JC, FABER J, TRAUTNER P, KAADEN S, TITTGEMEYER M, ELGER CE, WEBER B. Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage. 2009;46:569–76. doi: 10.1016/j.neuroimage.2009.03.013. [DOI] [PubMed] [Google Scholar]
- SCHOENFELD J, SEIDENBERG M, WOODARD A, HECOX K, INGLESE C, MACK K, HERMANN B. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- SEN PN, BASSER PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–38. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH SM, JOHANSEN-BERG H, JENKINSON M, RUECKERT D, NICHOLS TE, MILLER KL, ROBSON MD, JONES DK, KLEIN JC, BARTSCH AJ, BEHRENS TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- STOODLEY CJ, SCHMAHMANN JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- STRAUSS E, SHERMAN EMS, SPREEN O. A compendium of neuropsychological tests : administration, norms, and commentary. Oxford University Press; Oxford ; New York: 2006. [Google Scholar]
- STROOP JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- THOMALLA G, GLAUCHE V, KOCH MA, BEAULIEU C, WEILLER C, ROTHER J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- TIFFIN J, ASHER EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–47. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- TUSA RJ, UNGERLEIDER LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985;18:583–91. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- WEBER B, LUDERS E, FABER J, RICHTER S, QUESADA CM, URBACH H, THOMPSON PM, TOGA AW, ELGER CE, HELMSTAEDTER C. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–54. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]