Summary
By definition central respiratory chemoreceptors (CRCs) are cells that are sensitive to changes in brain PCO2 or pH and contribute to the stimulation of breathing elicited by hypercapnia or metabolic acidosis. CO2 most likely works by lowering pH. The pertinent proton receptors have not been identified and may be ion channels. CRCs are probably neurons but may also include acid-sensitive glia and vascular cells that communicate with neurons via paracrine mechanisms. Retrotrapezoid nucleus (RTN) neurons are the most completely characterized CRCs. Their high sensitivity to CO2 in vivo presumably relies on their intrinsic acid-sensitivity, excitatory inputs from the carotid bodies and brain regions such as raphe and hypothalamus, and facilitating influences from neighboring astrocytes. RTN neurons are necessary for the respiratory network to respond to CO2 during the perinatal period and under anesthesia. In conscious adults, RTN neurons contribute to an unknown degree to the pH-dependent regulation of breathing rate, inspiratory and expiratory activity. The abnormal prenatal development of RTN neurons probably contributes to the congenital central hypoventilation syndrome. Other CRCs presumably exist but the supportive evidence is less complete. The proposed locations of these CRCs are the medullary raphe, the nucleus tractus solitarius, the ventrolateral medulla, the fastigial nucleus and the hypothalamus. Several wake-promoting systems (serotonergic and catecholaminergic neurons, orexinergic neurons) are also putative CRCs. Their contribution to central respiratory chemoreception may be behavior-dependent or vary according to the state of vigilance.
Introduction
Central respiratory chemoreception is the mechanism by which an increase in brain PCO2 stimulates breathing. The term also refers to the respiratory stimulation caused by metabolic acidosis (blood acidification at normal levels of CO2). Under normal circumstances (absence of metabolic acidosis), central respiratory chemoreception operates as a sensitive feedback that helps to maintain arterial PCO2 within a few mmHg of the steady-state (∼40 mmHg) regardless of the metabolic production of this gas and the level of vigilance (Nattie, 1999; Feldman et al., 2003; Nattie and Li, 2009). Central respiratory chemoreception normally operates in concert with peripheral chemoreceptors (Smith et al., 2006). Central respiratory chemoreception has a very slow time constant (around 50s) attributed to the time needed for brain extracellular pH to equilibrate with a change in arterial PCO2 (Ahmad and Loeschcke, 1982; Eldridge et al., 1984; Smith et al., 2006). Central respiratory chemoreception also has a very high gain. For example, in a conscious goat, a rise in brain PCO2 of approximately 2 mmHg (0.5% change from normal values) increases resting ventilation by around 50% (Pappenheimer et al., 1965) and presumably causes a reduction of no more than 0.01 pH unit in the vicinity of the central chemoreceptors (Nattie, 1999). In man at rest, ventilation approximately doubles for a 1.5 mmHg rise in alveolar (presumed arterial) PCO2 (Haldane and Priestley, 1905).
Central respiratory chemoreception also refers to the effects produced by abnormally high levels of CO2 to which mammals and man are exposed only by accident (airway blockade of some sort, including sleep apnea in man) or because of intentional administration of high levels of CO2 as is commonly done in experiments designed to study the central respiratory chemoreflex. Under such conditions, arterial PCO2 may rise by tens of mmHg and, in intact unanesthetized mammals, this rise typically produces arousal and some form of interoceptive awareness in addition to respiratory stimulation (Phillipson et al., 1977; Berthon-Jones and Sullivan, 1984; Moosavi et al., 2003). These behavioral effects and or sensations indicate that high levels of CO2 recruit neural pathways that are not normally influenced by the small variations of PCO2 that regulate breathing under physiological conditions. This fact should be taken into consideration when interpreting breathing data from animals that have been exposed to high levels of CO2. The phenomenon is not unique to the central chemoreflex. Incremental levels of stimulation of the peripheral chemoreceptors also produce a hierarchy of responses that range from simple cardiorespiratory adjustments to arousal and, finally, to behaviors denoting obvious discomfort (defense reaction, vocalizations, escape behavior) (Marshall, 1994).
At this time, the dominant theory of central respiratory chemoreception is that CO2 works via the proxy of pH, breathing stimulation derives from the simultaneous recruitment of numerous types of acid-sensitive CNS neurons (the central respiratory chemoreceptors, CRCs) and CRCs detect pH via a cell-specific combination of several acid-sensitive channels (Jiang et al., 2005; Chernov et al., 2008; Nattie and Li, 2009). As this review will indicate, this apparently straightforward summary masks an inordinate number of uncertainties.
1. Theories of central respiratory chemoreception
1.1. What is a central respiratory chemoreceptor, CRC?
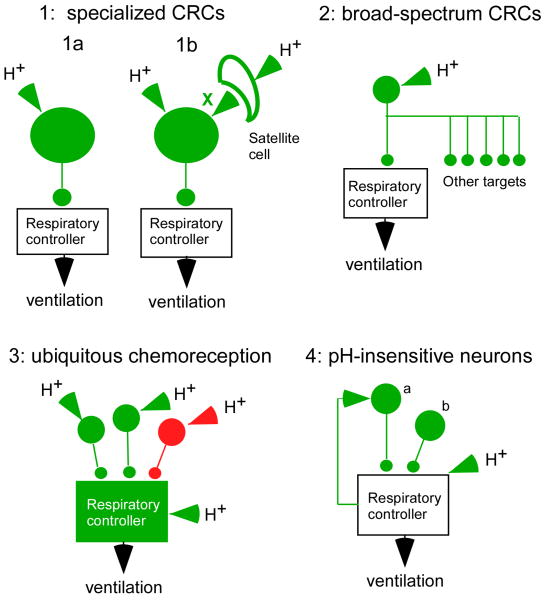
Central respiratory chemoreception is a reflex initiated by sensors located within the CNS. Like all reflexes, central respiratory chemoreception has three defining aspects: molecular (the receptors), cellular (the cells that express the receptors, a.k.a. the respiratory chemoreceptors) and integrative (the brain circuit engaged by the respiratory chemoreceptors). The first of many problems that this field of research faces is that the molecules that are presumably being sensed are protons. Protons, unlike most other intercellular signaling molecules (odorant molecules, hormones, transmitters, NO etc.) have the potential to modify the activity of countless regulatory proteins that are expressed not only by neurons but by glial cells and by blood vessels. At this time, the proton sensors that are germane to respiratory chemoreception are unidentified. Even the type of cell that expresses the pertinent proton sensors is not settled definitively (Figure 1). Many brain neurons are intrinsically sensitive to acid in vitro (Putnam et al., 2004; Nichols et al., 2008; Corcoran et al., 2009) but there is no hard evidence that this property persists in vivo or that the intrinsic effect of acid on neurons in vivo is sufficiently powerful and sensitive to account for respiratory chemoreception. In fact, serious consideration is still given to the possibility that CRCs are glial cells or possibly vascular cells that regulate the activity of surrounding neurons via paracrine mechanisms (Gourine et al., 2005). Given these uncertainties, it is premature to define a unique set of ideal criteria that a CNS cell must possess in order to qualify as a CRC. Figure 1 represents a few possibilities and an attempt will be made in this review to fit the various proposed chemoreceptors into one of these categories. Option 1 depicts the simplest concept, called here specialized CRCs, (Loeschcke, 1982). The specialized CRCs are viewed as separate from the respiratory rhythm and pattern network (the respiratory controller in Figure 1 which includes the motor neurons). These CRCs would selectively regulate the activity of the breathing network according to the level of acidity of the parenchyma that surrounds them. These specialized neurons could be intrinsically sensitive to acid (option 1a) and or could respond to the acidification of satellite non-neuronal cells (glia, vascular cells) via a paracrine mechanism (option 1b; factor X being one or several mediators). In such a case, the satellite cells would also qualify as CRCs. There could be one type or many types of specialized CRCs, an issue that is hotly debated at present. They need not all be excitatory neurons since some neurons are inhibited by acid in vitro and could increase breathing by disinhibition. Broad-spectrum CRCs (Figure 1, option 2) could be defined as pH-sensitive neurons that target multiple downstream networks in addition to the respiratory controller. As depicted in Figure 1 option 3, respiratory chemoreception could also reflect the widespread, possibly ubiquitous, acid-sensitivity of the neurons that make up the respiratory network or the ubiquitous location of the paracrine mechanism depicted in option 1b. In this view, all neurons are to some extent CRCs although the contribution of the various types of neurons to the overall pH-sensitivity of the respiratory motor outflow could be unequal and the degree to which each class of neurons contributes to the overall chemosensitivity of the respiratory outflow could vary according to the behavior or the state of consciousness of the organism. Option 4 (regulators of breathing) illustrates a scenario that is useful to consider when interpreting the literature on central respiratory chemoreception. The two hypothetical neurons that are represented regulate the activity of the breathing network but are unresponsive to acidification of the surrounding brain parenchyma. The activation of these neurons would be expected to influence the activity of the respiratory controller and its response to CO2. The destruction of such neurons would also be expected to modify the overall reflex. However, because these hypothetical neurons do not respond to changes in the surrounding pH, neither of them qualifies as a CRC.
Figure 1. what is a central respiratory chemoreceptor (CRC)?
The concept of central respiratory chemoreceptors is still being refined as more pertinent experimental evidence accumulates. Schemes 1-3 represent various CRC concepts. Scheme 4 represents neurons that should not be considered as CRCs. Specialized CRCs (option 1) could be defined as neurons that detect the concentration of protons in the surrounding brain parenchyma and drive the respiratory rhythm and pattern generating network selectively and in a graded manner according to the level of acidity. Option 1a assumes that these specialized neurons express the proton receptors. Option 1b assumes that the proton receptors reside both on these specialized neurons and on satellite cells (e.g. glia) that communicate with the neurons via a messenger X (possibly ATP). In this second case, the satellite cells would also qualify as CRCs. Broad-spectrum CRCs (option 2) could be defined as proton-sensitive neurons that modulate the activity of numerous targets besides the respiratory controller. Subsets of serotonergic neurons, the locus coeruleus and orexin neurons may be in this category. Ubiquitous chemoreception (option 3) refers to the possibility that central respiratory chemoreception could be an emergent property caused by the summation of small effects of pH almost everywhere in the respiratory network. Option 4 illustrates hypothetical examples of neurons that regulate the respiratory controller and its reactivity to protons. These neurons do not qualify as CRCs because they are not directly affected by the extracellular proton concentration.
1.2. CO2 stimulates respiration via the proxy of pH
No fundamentally new supportive evidence for this highly plausible yet unproven hypothesis has accrued in recent years (for prior discussions of this issue see (Loeschcke, 1982; Nattie, 1999; Feldman et al., 2003)). The hypothesis rests on the following evidence. Hypercapnia reduces the pH of brain extracellular fluid (ECF) with possible regional variations (Eldridge et al., 1984; Arita et al., 1989; Nattie, 2007). Metabolic acidosis also activates breathing and topical brain acidification, in particular at the level of the ventrolateral medulla, increases breathing (Loeschcke, 1982; Nattie, 1999; Feldman et al., 2003). Finally, in vitro, metabolic acidosis generally activates / inhibits neurons and depolarizes certain glial cells to an extent roughly comparable to a rise in CO2 that produces the same change in extracellular pH (Wang et al., 2002; Putnam et al., 2004; Kawai et al., 2006).
It is generally assumed that the proton-sensitive molecules responsible for respiratory chemosensitivity are ion channels and that the relevant channels are expressed by neurons. The reason is quite simply that many brainstem neurons express such pH-sensitive channels and respond to acidification in vitro under conditions of complete or semi synaptic isolation (Dean et al., 1990; Scheid et al., 2001; Mulkey et al., 2004; Corcoran et al., 2009). The interpretation of this evidence is subject to some degree of circular reasoning since putative CRCs are identified by their acid-sensitivity in vitro and the fact that neurons respond to acid in vitro is taken as evidence that central respiratory chemosensitivity operates via the effect of acid on these neurons. There are alternative theories regarding how CO2 works. One is that the proton sensors are indeed channels but that these channels are expressed by glial cells which influence neurons by releasing gliotransmitters such as ATP (Gourine et al., 2005; Spyer and Gourine, 2009) (option 1b in Figure 1). Other proposed mechanisms include pH-sensing G-protein coupled receptors (GPCRs, (Ludwig et al., 2003)), and receptors to inorganic carbon (inorganic carbon is defined as CO2 or bicarbonate) such as adenylate cyclases (Chen et al., 2000; Townsend et al., 2009). In Drosophila, CO2 detection operates within a range of CO2 concentration not much different from that present in the arterial blood of mammals and this sensory process requires the presence of two seven-transmembrane gustatory receptors (Gr21a and Gr63a) (Jones et al., 2007). Both cognate genes are required to confer CO2 response to olfactory neurons from which the original odorant receptors have been removed (Kwon et al., 2007). This evidence suggests that Gr21a and Gr63a form a heterodimeric receptor for the detection of CO2. Whether this dimer is a receptor for CO2, protons or bicarbonate is not known. No mammalian homolog of these insect receptors has been identified yet but proton-sensing GPCRs are expressed by the central nervous system of mammals (Huang et al., 2007) and could conceivably serve an equivalent function (Ludwig et al., 2003).
In vitro studies designed to determine whether internal or external acidification plays the dominant role in mediating the effects of CO2 or metabolic acidosis have produced mixed results. Raphe neurons grown in culture on a bed of glia are activated to the same degree by hypercapnic acidosis (increase in CO2 at constant bicarbonate level), isocapnic acidosis (reduced bicarbonate at constant CO2), isohydric hypercapnia (increased CO2 with lowered bicarbonate to maintain extracellular pH, pHo, constant) and by changes in pH produced in a CO2-bicarbonate-free HEPES buffer (Wang et al., 2002). Thus, the chemosensitivity of raphe serotonergic neurons in culture can occur independently of changes in pHo, PCO2 or bicarbonate. Because intracellular acidification is a common denominator of all these manipulations, pHi would seem to be the primary stimulus for chemosensitivity in serotonergic neurons in culture. Similar findings and interpretations have been reported for retrotrapezoid neurons in slices (Ritucci et al., 2005b). However, the primacy of the change in pHi for neuronal responses to acidification is not clear because the latter authors also found that the degree to which RTN neurons were activated did not correlate with the magnitude of the global intracellular acidification (Ritucci et al., 2005b). Furthermore, in other cells such as the locus coeruleus, hypercapnic acidosis is also able to produce neuronal activation when pHi is clamped (Hartzler et al., 2008). This effect may rely on the closure of a TEA-resistant potassium conductance, presumably due to TASK-1 channels (Sirois et al., 2000; Filosa and Putnam, 2003). From these studies it appears that a change in pHi can be sufficient but is not absolutely required for cells to respond to CO2.
In isolated rabbit glomus cells, an increase in PCO2 activates a Ca channel current by a mechanism that seems independent of the proton concentration inside or outside but appears to rely on the production of cAMP and protein kinase A activation (Summers et al., 2002). One interpretation is that some form of adenylyl cyclase functions as a sensor for inorganic carbon (CO2 or bicarbonate) (Chen et al., 2000; Townsend et al., 2009). Bicarbonate is very unlikely to mediate the effects of CO2 in brain because CO2-activated neurons are inhibited by increasing extracellular bicarbonate at constant CO2 level, a procedure that should also increase the intracellular concentration of this anion (Mulkey et al., 2004; Bouyer et al., 2004; Kawai et al., 2006). However, the possibility that molecular CO2 could contribute to the response to hypercapnia has not been eliminated.
Evidence that chemosensitivity could involve several proton receptors on a given neuron comes from studies in which the permeability changes elicited by metabolic acidosis (constant CO2, reduced bicarbonate) vs. hypercapnic acidosis (constant bicarbonate, elevated CO2) have been compared in the same neurons. In the neonate retrotrapezoid nucleus for example, both stimuli depolarize the cells equally after action potential blockade with tetrodotoxin but the depolarization caused by hypercapnic acidosis is cadmium-insensitive, blocked by high barium (1mM) and associated with significant increase in membrane resistance suggesting that a decrease in potassium conductance is dominant (Kawai et al., 2006). In contrast, the depolarization produced by metabolic acidosis is less barium sensitive and is associated with a smaller decrease in input resistance suggesting that it could be mediated by a mix of potassium and other channels (Kawai et al., 2006).
A concern with all these studies is that they typically rely on small numbers of neurons that are often only assumed to contribute to respiratory chemoreception in vivo. The range of CO2 or pH to which the cells are exposed is large (up to 15 % CO2; 0.6 pH units) compared to normal physiological variations (<0.1 pH unit). Neurons are typically studied in the presence of astrocytes that are also pH-responsive (Ritucci et al., 2005b) and may contribute to central respiratory chemosensitivity (Gourine et al., 2005; Spyer and Gourine, 2009). Finally, different recording conditions have been used (age, temperature, presence or absence of glia) and, more often than not, heterogeneous populations of neurons were recorded within a single study. The relevant sites of intracellular acidification are unknown. They could conceivably be membrane-associated microdomains whose pH changes are not adequately reflected by whole cell pH measurements. This possibility could account for the above mentioned discrepancies between apparent intracellular acidification and neuronal responses and for the slight differences between the effects of acidification by CO2 vs. fixed acids in vitro due to a different mode of penetration into cells. In any event, given all these uncertainties and the possibility that different cells have different mechanisms of chemosensitivity, channels whose proton sensitivity resides on either side of the neuronal membrane could potentially contribute to central chemosensitivity.
1.3. How many respiratory chemoreceptors are there?
Most investigators in the field are convinced that CO2 stimulates breathing via simultaneous effects of pH on multiple types of acid-sensitive neurons (Chernov et al., 2008; Nattie and Li, 2009). In our estimation, most putative CRCs are not characterized well enough to pass judgment on this issue.
The notion that there are many CRCs is supported by the following evidence. Lesions of several regions of the pontomedullary respiratory network attenuate the chemoreflex (e.g. (St John, 1972; Berger and Cooney, 1982)). This approach is largely of historical interest because it does not discriminate between neurons that are intrinsically pH-sensitive and those that are activated synaptically during exposure to hypercapnia. At present, the main evidence supporting a wide anatomical distribution of respiratory chemoreceptors is that topical acidification of many brainstem regions increases breathing (Nattie, 1999; Hodges et al., 2004a). Historically, topical acidification was first used at the ventral medullary surface because of its accessibility (Mitchell et al., 1963b; Mitchell, 2004). Strong breathing stimulation was caused by the application of acid-soaked material to two large regions of the ventral medullary surface, one located roughly under the facial motor nucleus and the other towards the caudal medulla (Mitchell et al., 1963b; Loeschcke, 1982; Mitchell, 2004). These responses were interpreted as evidence that CRCs were very superficially located. More recently, acidification of many brainstem or cerebellar regions with dialysis probes (NTS, RTN, ventral respiratory column, midline medulla, fastigial nucleus) was found to activate breathing to some degree and simultaneous acidification of two regions usually produced additive effects (e.g.(Solomon et al., 2000; Hodges et al., 2004a; Nattie and Li, 2008)). Of note, respiration is activated by acidifying the ventrolateral medulla anywhere from its rostral end, the RTN (Li and Nattie, 2002), to the caudal region presumed to overlay the “Loeschke area” (da Silva et al., 2010) and in-between for example within the pre-Bötzinger complex (Solomon et al., 2000). The degree of respiratory stimulation caused by acidifying any given site is typically not very large in conscious animals (∼20% increase in minute-volume) and does not vary much between regions. The small magnitude of the responses may be due to the fact that direct activation of a subset of CRCs exposes the other CRCs to a more alkaline environment due to the resulting hyperventilation and deactivates them (Dias et al., 2008). The interpretation of the evidence rests on the sensible but unverified assumption that focal tissue acidification, for example using dialysis probes, correctly reproduces the effects of respiratory or metabolic acidosis on the surrounding neurons. It has been demonstrated that the tissue surrounding a dialysis probe is indeed acidified to an appropriate degree. However, evidence that neurons located close to the dialysis probe respond identically to focal acidification and to a commensurate rise in arterial PCO2 has not been produced nor has it been shown that the neurons that are unresponsive to hypercapnia are also unaffected by local acidification.
The second argument supporting a wide distribution of central respiratory chemoreceptors is that hypercapnia increases the level of expression of the protooncogene product c-Fos in many brain regions including regions located close to the ventral medullary surface (e.g.(Sato et al., 1992)). Although, putative respiratory chemoreceptors such as retrotrapezoid neurons are among the cells that express c-Fos in animals exposed to hypercapnia (Sato et al., 1992; Fortuna et al., 2009), the c-Fos method does not distinguish between neurons that are directly pH-sensitive in vivo, neurons that are activated synaptically during exposure to hypercapnia and neurons that are directly pH-activated but play no role in breathing.
The third argument supporting a wide distribution of central respiratory chemoreceptors is that, by all measures (electrical recordings or voltage-sensitive dyes), every region of the brainstem contains a substantial proportion of neurons that respond to acid in vitro, often by a depolarization but sometimes with the opposite response (Scheid et al., 2001; Ritucci et al., 2005b; Nichols et al., 2008; Erlichman et al., 2009). This characteristic is not unique to the brainstem, however. Other brain regions, including cortex and cerebellum, contain neurons that respond to acid and CO2 either by an excitation or an inhibition, the proportion depending on the recording conditions and the magnitude of the pH change (Wang and Richerson, 2000). In the neonate brainstem preparation, the vast majority of respiratory neurons located in the ventrolateral medulla were found to respond to acidification either by a depolarization or by a hyperpolarization, even after action potential blockade with TTX (Kawai et al., 1996). The CO2-sensitivity of many respiratory neurons located in the ventral respiratory column of adult cats is attenuated by point source delivery (iontophoresis) of the purinergic receptor antagonist suramin (Thomas and Spyer, 2000). Because iontophoretic delivery of a drug can only exert spatially restricted effects, this evidence has been interpreted to suggest that pH sensitivity is local, is due to the release of ATP and is widespread in the respiratory network (Thomas and Spyer, 2000). The dubious selectivity of suramin weakens the credibility of this evidence. Finally, experiments done on cultures of brainstem neurons indicate that synaptically connected neurons are generally more strongly activated by acidification than isolated ones, suggesting that networks amplify the small effects of acid observed in a high proportion of isolated neurons (Su et al., 2007). The last pieces of evidence discussed here can be interpreted as suggesting that central respiratory chemoreception is virtually ubiquitous (scheme 3 of Figure 1).
Other evidence contradicts the notion that respiratory neurons cooperate widely and on an equal footing to the stimulation of the respiratory network by acid. This evidence is not as extensive as that which supports the opposite view but it is highly significant. In the neonate brainstem-spinal cord preparation, a.k.a. Suzue preparation (Suzue, 1984), selective genetic deletion of the retrotrapezoid nucleus eliminates the activation of the phrenic nerve outflow by CO2 but does not eliminate the ability of the brainstem to generate a regular inspiratory activity (Dubreuil et al., 2008; 2009b). Thus, the apparently ubiquitous pH sensitivity of the respiratory neurons located in the ventrolateral medulla (Kawai et al., 1996) does not produce a pH-sensitive network unless the retrotrapezoid nucleus is present. These results were obtained using late embryonic or early postnatal tissue and their interpretation may not apply later on in life. In anesthetized adult rats, the integrity of the retrotrapezoid nucleus is necessary for the respiratory network to be activated by CO2 (Takakura et al., 2008). The respiratory pathology observed in the congenital central hypoventilation syndrome (CCHS) also supports the notion that central respiratory chemosensitivity may rely on relatively few specialized neurons. In this genetic disease, central chemosensitivity is essentially absent, severe hypoventilation occurs during sleep but relatively adequate breathing persists during waking and some degree of exercise-induced hyperventilation remains, at least in the milder cases (Paton et al., 1989; 1993; Shea et al., 1993; Gozal, 1998; Amiel et al., 2003; 2009; Carroll et al., 2010). The severity of the symptoms increases according to the number of extra alanine residues in the mutated polyalanine track of Phox2b (Carroll et al., 2010). The respiratory symptoms of the disease suggest that the central respiratory controller of CCHS patients is largely intact but that the brain of these individuals lacks neurons that are either uniquely specialized in detecting CO2 or are specialized in funneling the excitatory influence of CO2 to the respiratory controller. The mouse model of the disease (Phox2b27ala/+) suggests that these missing neurons could be the ccRTN neurons (Dubreuil et al., 2008), a chemically defined subset of retrotrapezoid nucleus neurons whose properties will be extensively discussed later on.
In short, current evidence is insufficient to assert whether central respiratory chemoreception relies on just a few cells or is a property widely distributed throughout the brain. Recent animal data, reinforced by the natural experiment provided by the congenital central hypoventilation syndrome, suggest that the high gain central chemosensitivity responsible for CO2 stability under physiological conditions (pH changes of considerably less than 0.1 unit) could rely on relatively few cells, the retrotrapezoid nucleus being most critical (Guyenet, 2008; Goridis and Brunet, 2010), whereas a much larger number of cells could be implicated in the effects produced by high levels of CO2 (behavioral arousal, dyspnea or panic attacks). However, the high gain of the central chemoreflex, especially in the adult, could also be due to a network summation of individually small effects of pH on vast numbers of neurons, a concept that is generally dominant at this time (see for example (Nattie and Li, 2009)).
2. Molecular basis of central respiratory chemoreception
Acid-sensitive ion channels located on neurons or satellite cells are currently believed to be the proton sensors responsible for respiratory chemosensitivity (e.g.(Chernov et al., 2008)). However, to interpret the literature optimally, one should keep in mind that ion channels could theoretically contribute to the pH response of central chemoreceptor neurons in three distinct ways. The proton receptors could be ion channels as is currently hypothesized. A subset of pH-insensitive ion channels could be intracellular effectors of a membrane-associated proton receptor, e.g. a GPCR. Finally, the vast majority of the ion channels expressed by chemoreceptor neurons are likely to be pH-insensitive channels that shape the intrinsic properties of these cells. The presence of these channels will influence the response of the chemoreceptor neurons to acid and by way of consequence the chemoreflex. A few of these channels may be somewhat selectively expressed by central chemoreceptor neurons (TASK-2 may be an example) but most are probably not.
The channels most frequently mentioned as proton receptor candidates are considered in this section of the review. Although the hypothesis “makes sense” no channel has been shown convincingly to contribute to respiratory chemosensitivity by virtue of its proton binding ability and alternative hypotheses should still be pursued at this time.
2.1. Acid-sensitive channels
Potassium channels: 2 pore channels and others
Two-pore potassium channels (K2P channels) regulate the excitability of neurons by setting their resting membrane potential and changing their input resistance (Lesage and Lazdunski, 2000; Duprat et al., 2007; Bayliss and Barrett, 2008). The family includes around 15 subunits that have been grouped into six structurally and functionally different subclasses (Holzer, 2009). Many of these channels are activated or inhibited, in vitro at least, by relatively small deviations of extra- or intracellular pH from physiological levels. The TASK subfamily of K2P channels (TASK1, KCNK3; TASK3, KCNK9) form homo- or heterodimers and are widely expressed in brain (Lesage and Lazdunski, 2000; Talley et al., 2001; Duprat et al., 2007). They are constitutively active, voltage-insensitive, non-inactivating and inhibited by the actions of many GPCRs (serotonin type 2 receptors, TRH, alpha-1 adrenergic etc.) (Talley and Bayliss, 2002). TASK1/3 homo- or hetero-dimer current is reduced by acidification with slight variations in pH50 according to the sub-unit composition. TASK1-/-, TASK3-/- and double knock-out mice have normal ventilatory responses to hyperoxic hypercapnia (Mulkey et al., 2007b). This result suggests that neither isoform of the channel makes a critical contribution to central respiratory chemosensitivity. The fact is surprising given the very wide distribution of these channels in the ventrolateral medulla oblongata and elsewhere in the brainstem, including in motor neurons and the prediction, based on modeling, that TASK channels should make the greatest single contribution to neuronal pH sensitivity (Washburn et al., 2003; Chernov et al., 2008). Yet, in agreement with the whole animal observation, the pH-induced potassium current recorded in retrotrapezoid neurons is the same in TASK1-/-, TASK3-/- and double knock-out mice as in control mice (Mulkey et al., 2007b). It seems unlikely that the absence of TASK1/3 channels since conception could be fully compensated by the up-regulation of other acid-sensitive potassium channels in RTN with exactly the same properties (Mulkey et al., 2007b). TASK1 deletion in mice reduces the sensitivity of the carotid body to both oxygen and CO2 (Trapp et al., 2008). This observation does not demonstrate that TASK1 is the pH sensor of the carotid body but it highlights an important molecular difference between central and peripheral pH-detection.
Despite their name, TASK2 (KCNK5) channels are only distantly related to TASK 1 or 3 from an evolutionary viewpoint. They belong to the TALK subgroup of alkaline-activated K-2P channels because their pKa is 8.0 or above (Morton et al., 2005; Niemeyer et al., 2007; Bayliss and Barrett, 2008). TASK-2 mRNA levels are very low in brain (Reyes et al., 1998; Talley et al., 2001; Aller and Wisden, 2008) and, according to recent genetic evidence, the channel is expressed in only a few neuronal clusters including the retrotrapezoid neurons (Dubreuil et al., 2009a; Gestreau et al., 2010). Oddly, TASK-2-like immunoreactivity has been reported as quite extensive in brain (Gabriel et al., 2002). The selectivity of the antibody used in these studies should be verified in a TASK-2 knock-out mouse. TASK-2 channel deletion does not change the pH sensitivity of the neonate breathing network in vitro, contrary to the deletion of the RTN neurons (Dubreuil et al., 2009b; Gestreau et al., 2010) therefore TASK-2 does not seem responsible for the pH sensitivity of RTN neurons under these conditions. This result is consistent with the fact that the pKa of TASK-2 channels is far outside the physiological range on the alkaline side. Because of this characteristic, TASK-2 channel conductance is unlikely to be modulated by acidification below the normal brain pH of 7.3. The Gestreau study suggests that TASK-2 activation by hypoxia contributes to post-hypoxic respiratory depression. Given that its expression is potentially down-regulated by chronic hypoxia (Brazier et al., 2005), TASK-2 may serve to increase the excitability of RTN neurons under chronic hypoxic conditions where lowering of the CO2 set-point might be advantageous to increase oxygen intake.
TASK-2 is abundantly expressed in the kidney where it plays a major role in acid-base homeostasis (Morton et al., 2005; Bayliss and Barrett, 2008). Due to their inability to retain bicarbonate, TASK-2 KO mice are in metabolic acidosis (Warth et al., 2004). This peculiarity may account for the left shift of their breathing response to CO2 and the reduced effect of high levels of hypercapnia (Gestreau et al., 2010).
Inwardly rectifying potassium channels (Kir)
In heterologous expression systems, inwardly rectifying potassium channels (e.g. Kir1.1, Kir4.1–Kir5.1) can generate acid-sensitive currents with a pKa appropriately close to physiological pH (7.4) (Xu et al., 2000; Su et al., 2007). Kir4.1 is a primary molecular substrate of astroglial K+ transport (Neusch et al., 2006). This channel underlies most of the inwardly rectifying current of astrocytes and is essential to maintain their very hyperpolarized membrane potential (Neusch et al., 2006). Inwardly rectifying potassium channels are closed by intracellular acidification and appear to contribute to the acid sensitivity of some brainstem neurons in culture because acid sensitivity is suppressed by a concentration of barium that blocks Kir channels with some selectivity in heterologous expression systems (Su et al., 2007). The activation of mature locus coeruleus neurons by hypercapnic acidosis in slices has also been attributed to the closure of an inwardly rectifying potassium current although, in this case, intracellular protonation of a polyamine has been invoked as the cause of the reduced current (Pineda and Aghajanian, 1997). However, in native RTN neurons, the acid-sensitive potassium current is neither inwardly rectifying nor sensitive to 40 mM tetraethylammonium which excludes the contribution of Kir (Mulkey et al., 2007b).
The ASIC family of acid-sensitive channels
Acid-sensing ion channel (ASIC) subunits associate as trimers to form acid-activated channels permeable to Na+ and Ca2+ (Yermolaieva et al., 2004; Jasti et al., 2007). ASICs and transient receptor potential vanilloid-1 (TRPV1) underlie the detection of peripheral tissue acidosis by primary sensory afferents, ASICs being responsive to moderate decreases in extracellular pH whereas TRPV1 requires pH to drop below 6.0 (Holzer, 2009). ASIC1a, -2a, and −2b are the most commonly expressed subunits in the CNS, where they assemble into homo- and heterotrimeric complexes. Disrupting the ASIC1a gene (ACCN2) in mice eliminates currents evoked in vitro by pH as low as 5.0 in brain regions such as the hippocampus or the amygdala (Wemmie et al., 2002; Askwith et al., 2004). ASIC1a-/- mice have attenuated fear behavior but normal ventilatory responses to moderate levels of normoxic hypercapnia suggesting that ASIC1a is not critical for central respiratory chemosensitivity (Ziemann et al., 2009). This interpretation is also in line with evidence that acidification most often operates selectively by closing a potassium conductance in putative respiratory chemoreceptors (Dean et al., 1989; Mulkey et al., 2004; 2007b; Nichols et al., 2008).
Other acid-sensitive channels
Other acid-sensitive channels include members of the TRP family, ionotropic purinoceptors (P2X), voltage-activated potassium channels, L-type Ca2+ channels, hyperpolarization-activated cyclic nucleotide gated channels, gap junction channels, and Cl- channels (Shi et al., 1998; Vacher et al., 2008; Holzer, 2009). A large fraction of these channels are expressed in the brainstem. Their contribution to respiratory chemosensitivity is typically untested. Increased calcium current may contribute to the response of locus coeruleus neurons and the carotid body to acid (Summers et al., 2002; Filosa and Putnam, 2003).
2.2. GPCRs as pH sensors
Four structurally related G protein-coupled receptors (OGR1, GPR4, TDAG8, and G2A) originally believed to respond to lipid messengers have recently been promoted to proton detectors (Ludwig et al., 2003; Holzer, 2009). Their pH-sensitivity, like that of many ion channels, is attributed to a strategic subset of histidine residues. In vitro, the receptors are inactive at pH 7.8 and fully activated at pH 6.8. OGR1 family members (OGR1, GPR4, TDAG8, and G2A) are widely expressed in the nervous system (CNS and sensory afferents) and in many other organs or tissues (bone, vascular system). At this time, there is still no evidence that any member of the OGR1 receptor family is implicated in central respiratory chemosensitivity.
3. Contribution of glial cells to central respiratory chemosensitivity
Central respiratory chemoreception could be a multicellular process along the lines depicted in Figure 1, option 1b. In this section, we discuss the possibility that the satellite cells might be glia. There is also anatomical and functional evidence for interaction between brain blood flow and CRCs (Xie et al., 2006; Ainslie and Duffin, 2009; Lazarenko et al., 2009). This aspect of central respiratory chemoreception will not be covered here.
3.1. pH-sensitivity of glial cells
The first evidence that some brainstem glial cells are depolarized by acidification is usually traced back to the experiments of Fukuda and Honda (1975). These authors recorded intracellularly from ventral medullary surface cells located in the “intermediate chemosensitive zone” just lateral to the rootlets of the hypoglossal nerve in thin horizontal slices. Many cells located at the ventral surface i.e. less than 200 microns from the pia mater were depolarized by isocapnic acidosis (acidification at constant CO2 via bicarbonate reduction). The recorded cells were unexcitable, with notably negative membrane potential at alkaline pH and could therefore have been glia. Isohydric hypercapnia was ineffective in these cells suggesting that CO2 operated strictly via the proxy of pH and that the cells were selectively sensitive to changes in extracellular pH (Fukuda and Honda, 1975). This result is noteworthy because, in neurons, acid sensitivity is generally attributed to a change in intracellular pH (Wang et al., 2002; Ritucci et al., 2005b). If the Fukuda and Honda results are indeed representative of all glial cells, Kir4.1, the major barium-sensitive inwardly rectifying channel of astrocytes (Neusch et al., 2006) could not be responsible for astrocyte depolarization by acid since this channel is responsive to intracellular pH. More work is obviously required because of the presumed heterogeneity of the potassium channels expressed by astrocytes (Fukuda et al., 1978; Grass et al., 2004).
3.2. Amplification of CO2-induced extracellular fluid pH changes by glia
The idea that CO2-dependent changes in pH could be amplified by glial cells is based on the phenomenon called depolarization-induced alkalosis (DIA), whereby an increase in extracellular potassium and or acidification causes glial cell depolarization and the extrusion of protons from these cells (Fukuda et al., 1978; Erlichman et al., 2008). This process may be limited to a subset of astrocytes and matures a couple of weeks after birth in rodents (Erlichman et al., 2008). This mechanism, which should boost the response of central chemoreceptor neurons to a given level of PCO2, seems to be at work in the retrotrapezoid nucleus but not in the nucleus of the solitary tract (NTS) (Erlichman et al., 2008). Because it matures later in development, this phenomenon could also perhaps explain the higher acid sensitivity of RTN neurons in the adult in vivo (∼0.4 Hz per 0.01 pH unit (Guyenet et al., 2005a)) compared to the neonate in vitro at comparable temperature (∼0.17 Hz per 0.01 pH unit (Guyenet et al., 2005b)).
3.3. Acid-induced release of ATP by glial cells
Large subsets of glial cells (presumably astrocytes) are depolarized by acidification, in vitro at least (Fukuda and Honda, 1975; Fukuda et al., 1978). ATP is released by glial cells and this release is required for the intercellular propagation of calcium waves in these cells (Guthrie et al., 1999). ATP is released by hypercapnia at the ventral medullary surface in vivo (Spyer et al., 2004; Gourine et al., 2005). Topical application of P2 receptor antagonists at the ventral medullary surface reduces somewhat the respiratory response to CO2 in anesthetized rats (Gourine et al., 2005). Finally, the purinergic receptor antagonist suramin delivered by iontophoresis attenuates the activation by CO2 of various types of respiratory neurons in vivo (Thomas and Spyer, 2000).
The ATP hypothesis of central chemosensitivity faces a number of unresolved issues. As mentioned previously, suramin is not a selective purinergic antagonist. There is uncertainty concerning the cellular origin of the ATP released at the central medullary surface. For example, pia-arachnoid cells could be the source of this ATP since these cells communicate via this nucleotide very much like glial cells (Grafstein et al., 2000). More importantly, purinergic P2-receptor antagonists do not change the pH-sensitivity of RTN neurons in slices (Mulkey et al., 2004; Mulkey et al., 2006) and these drugs do not change the pH/CO2 response of the Suzue preparation, a neonate brainstem-spinal cord preparation that generates a respiratory like outflow (Lorier et al., 2004). Finally PPADS, the most commonly used non-selective P2 receptor blocker does not attenuate the acid sensitivity of brainstem cultures (Su et al., 2007). The lack of effect of P2 antagonists in these in vitro models could mean that acidification causes glial cells to release ATP only in vivo. Possible reasons include the immaturity of the brain tissue used in vitro, especially the glia (see previous section), the use of a low recording temperature and/or the absence of blood perfusion. Two findings support the possibility that ATP release by glial cells might be occurring in vivo only. Although the activation of the ccRTN neurons by acid does not depend on ATP release in vitro, these cells do express purinergic 2Y receptors (Mulkey et al., 2006). Secondly, the pH-sensitivity of these cells is roughly 2-3 times higher in vivo, a difference that could conceivably represent the contribution of ATP release from surrounding mature glial cells.
4. Central respiratory chemoreception and level of vigilance
Under normal conditions, the regulation of breathing operates within a very narrow range of PCO2 and minimal CO2 fluctuations have no effect on the level of consciousness or vigilance or on the depth of sleep. In contrast, severe hypercapnia produces arousal from sleep in animals and man (Phillipson et al., 1977; Berthon-Jones and Sullivan, 1984). In awake individuals, hypercapnia also causes an aversive form of interoceptive awareness called air hunger (Moosavi et al., 2003) and in susceptible individuals, CO2 can even trigger outright panic. In addition, rodents are capable of detecting very low levels of atmospheric CO2 and they express fear behavior when exposed to hypercapnia (Hu et al., 2007; Ziemann et al., 2009).
Studies in man demonstrated long ago that the gain of the chemoreflex is greater in awake than in sleeping subjects (reviewed in (Ainslie and Duffin, 2009)). In the awake state, breathing is also activated by CO2-independent drives collectively grouped under the name of the “waking drive to breathe” (Ainslie and Duffin, 2009). Thus, the state of vigilance influences the gain of the chemoreflex at all levels of CO2 including around the normal operating range of 40 mmHg but the normal homeostatic regulation of breathing by CO2 is evidently not caused by continual fluctuations of the state of vigilance (Kuwaki, 2008). By contrast, general CNS arousal and its associated somatic and autonomic responses presumably make a significant contribution to the breathing stimulation caused by high levels of CO2 under experimental or pathological conditions (airway obstruction or exposure to abnormally elevated levels of CO2) (Berthon-Jones and Sullivan, 1984). The chemoreflex studied in awake experimental animals is always elicited by high levels of CO2 (5% minimum), therefore this reflex presumably depends on general arousal mechanisms engaged by this stimulus, fear responses etc., in addition to the chemosensory mechanisms that intervene around physiological levels of CO2.
5. Putative respiratory chemoreceptor neurons
This section focuses on the properties of neurons that are currently believed to make a particularly important contribution to central respiratory chemoreception.
5.1. The retrotrapezoid nucleus (RTN)
The discovery of the RTN, the earliest evidence that this brain region controls breathing and may contain chemoreceptors, and the potential relationship between the RTN and the parafacial respiratory group of the neonate are well documented and outside the scope of this review (Smith et al., 1989; Nattie et al., 2001; Nattie, 2001; Li and Nattie, 2002; Feldman et al., 2003; Onimaru and Homma, 2003; Guyenet, 2008; Guyenet and Mulkey, 2010). The rest of this section focuses on a group of 2000 chemically defined neurons (in rats; ∼ 800 in mice) located within the region originally defined as the RTN (Connelly et al., 1989). These cells have a distinctive biochemical phenotype that includes the presence of Phox2b, neurokinin-1 receptors, vesicular glutamate transporter2 mRNA and TASK-2, and the lack of tyrosine hydroxylase, choline acetyl transferase, glutamic acid decarboxylase and tryptophan-hydroxylase (Mulkey et al., 2004; Stornetta et al., 2006; Takakura et al., 2008; Gestreau et al., 2010; Guyenet and Mulkey, 2010). We refer to these specific neurons as the ccRTN (chemically coded RTN) neurons (Lazarenko et al., 2009) to distinguish them from other types of neurons that may also reside in this heterogeneous region of the reticular formation. ccRTN neurons are a subset of a heterogeneous collection of neurons called the parafacial respiratory group in the neonate (Onimaru et al., 2008; Guyenet and Mulkey, 2010). The developmental lineage of the ccRTN neurons has also been worked out recently in considerable detail (Thoby-Brisson et al., 2009; Dubreuil et al., 2009b).
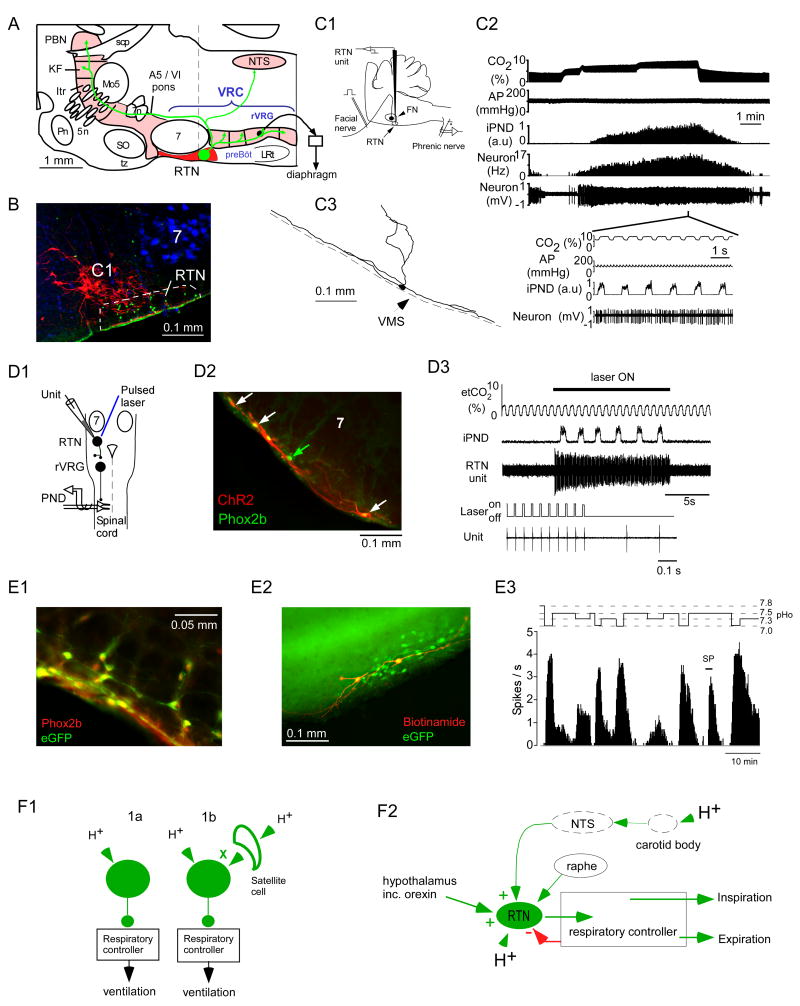
The key evidence that the ccRTN neurons are CRCs is illustrated in Figure 2. First, these neurons respond vigorously and sensitively to hypercapnia in vivo (Figure 2C) (Mulkey et al., 2004; Guyenet et al., 2005a). Under anesthesia, their discharge rate varies by an average of 2.8 Hz for a 1% change in arterial PCO2 or an estimated ∼0.5 Hz (5% of full range) per 0.01 unit change in arterial pH (Guyenet et al., 2005a). These cells are silent at low levels of CO2 and many of them have a CO2 recruitment threshold (CO2 level above which the cells are active) that is below that of the phrenic nerve and below that of the respiratory neurons of the ventral respiratory column, regardless of the anesthetic used (Figure 2C2) (Guyenet et al., 2005a; Fortuna et al., 2009). Such a characteristic is expected from neurons that drive the respiratory controller, at least the portion of this network that generates the motor outflow to the diaphragm.
Figure 2. the retrotrapezoid nucleus (RTN).
A. The RTN contains a neurochemically well-defined group of glutamatergic neurons, the ccRTN neurons (Takakura et al., 2008; Lazarenko et al., 2009) so named to distinguish them from the overlapping but phenotypically heterogeneous collection of cells called the parafacial respiratory group (Guyenet and Mulkey, 2010). The axonal projections of the ccRTN neurons in the rat are represented schematically based on the anterograde transport of the membrane bound fusion protein channelrhodopsin2-mCherry (for primary data see (Abbott et al., 2009). The scale (1 mm) is an approximation based on the rostrocaudal extent of the facial motor nucleus in the adult rat. B. transverse histological section along the plane identified by the dotted line in panel A (after (Stornetta et al., 2006)). The ccRTN neurons express the transcription factor Phox2b (nuclei in green) and lack tyrosine hydroxylase (TH, in red). The TH-ir neurons located at this level are the C1 neurons, which regulate the heart and vasomotor tone. The location of the facial motor neurons (7) is revealed by the presence of fluorogold (in blue). The scale bar is 100 microns. The ventral medullary surface is recognizable by a green edge artifact. C. CO2-sensitivity of the ccRTN neurons in an anesthetized rat in vivo. C1: experimental design for unit recording in anesthetized rats. The ccRTN neurons are located under the facial motor nucleus the lower boundary of which is identified by antidromic field potentials elicited by stimulation of the facial nerve. C2: single ccRTN neuron recorded alongside end-expiratory CO2 (top trace), arterial blood pressure (AP) and the mass activity of the phrenic nerve (iPND; a reporter of the level of activity of the respiratory controller). ccRTN neurons are robustly activated by adding CO2 to the breathing mixture. The expanded time-scale insert shows that the neuron has only a mild propensity to discharge in synchrony with the central respiratory controller. C3 is an example of one such neuron labeled in vivo with biotinamide following its physiological characterization. Note the profusion of dendrites at the ventral medullary surface (VMS) (after (Mulkey et al., 2004). D: selective stimulation of the ccRTN neurons activates breathing in vivo. D1, experimental design. A thin optical fiber is introduced in the vicinity of a group of ccRTN neurons that were selectively transfected with the channelrhodopsin2-mCherry (ChR2) fusion protein. ccRTN neurons are recorded while blue laser light is applied to activate ChR2-expressing neurons. The rat is anesthetized with isoflurane. D2, transverse section showing several superficial Phox2b-expressing ccRTN neurons that were transfected with ChR2 (m-Cherry exhibits a natural red fluorescence). The transgene was expressed almost exclusively by Phox2b-containing neurons (Phox2b immunoreactivity appears green in untransfected neurons, green arrow, and yellow in transfected cells, white arrows). The transfected neurons line the ventral medullary surface. Note that the overlying facial motor neurons (7) did not express the transgene (scale bar: 0.1 mm). D3, activation of ChR2-transfected ccRTN neurons (one of them is being simultaneously recorded) activates breathing. In this excerpt, the end-expiratory CO2 was below the apneic threshold therefore both the RTN unit and the phrenic nerve were silent prior to shining the laser light (after (Abbott et al., 2009)). During light application, the RTN unit was entrained 1 to 1 to the laser pulses (bottom two traces). E: pH sensitivity of ccRTN neurons in brain slices. E1, ccRTN neurons in a Phox2b-eGFP transgenic mouse (the B/G mouse). In this strain, GFP identifies selectively the ccRTN neurons (scale bar: 50 microns). E2, thick section (∼300 μm) through the RTN of the B/G mouse. Two ccRTN neurons were recorded in vitro and were filled with biotinamide (scale bar: 100 microns). E3, typical response of a ccRTN neuron to acidification in vitro (B/G mouse, temperature: 22°C). The cell was silent at pH 7.5. It was activated by acidification and by substance P (SP; 100 nM), the latter due to the presence of neurokinin1 receptors on the cells. Panels E1-3 after (Lazarenko et al., 2009). F1, ccRTN neurons fit the concept of specialized chemoreceptors. The molecular and cellular mechanism by which they detect surrounding protons (1a or 1b) is uncertain however. F2, a slightly more elaborate concept of how ccRTN neurons might regulate breathing. ccRTN neurons drive the breathing rate, the amplitude of inspiration and, probably active expiration. ccRTN neurons regulate breathing according to the level of acidity that surrounds the neurons but also according to the strength of the input that they receive from the carotid bodies, the raphe and the hypothalamus, including from orexinergic neurons. Distinct subsets of ccRTN neurons may drive the various respiratory motor outflows (inspiratory vs. expiratory muscles).
By definition, the activation of CRCs should stimulate breathing. The ccRTN neurons pass this essential test since their selective activation in vivo using genetically targeted channelrhodopsin2 produces a vigorous increase in breathing rate and amplitude (Figure 2D) (Abbott et al., 2009). The ccRTN neurons are therefore CO2-sensitive, histologically glutamatergic (VGLUT2 mRNA-expressing) and functionally excitatory with respect to the respiratory network. Also, the excitatory drive contributed by the ccRTN neurons is notably powerful because a large respiratory response can be produced by activating at most 15% of the cell population (Abbott et al., 2009).
Whether or not the ccRTN neurons are part of the respiratory rhythm and pattern generator is important from a conceptual standpoint. Three pieces of evidence support our contention that these neurons are not part of the rhythm and pattern generating network but neurons that simply regulate the level of activity of this circuitry according to the level of CO2/pH as depicted in Figure 2F1,2. First, the ccRTN neurons have a modest and phase-spanning respiratory entrainment at all but extreme levels of CO2 in vivo (Figure 2C2, insert) (Guyenet et al., 2005a). Second, when a subset of ccRTN neurons are activated at a constant rate of around 20 Hz using the optogenetic approach, the resulting effect on respiration is a normal rhythmic activation of the respiratory controller similar to what is observed during central chemoreceptor stimulation (Figure 2 D3) (Abbott et al., 2009). Finally, the time constant of the excitatory effects of ccRTN neurons on the rodent respiratory network is long (T1/2 ∼11 s) relative to the breathing period (1 s or less) (Abbott et al., 2009). In the aggregate, this evidence suggests that the exact pattern of discharge of the ccRTN neurons is less important than the mean rate of discharge of these cells. This characteristic is in contrast with the precisely timed phasic discharges of the neurons that are assumed to directly contribute to rhythm and pattern generation (Rybak et al., 2007).
The third essential attribute of CRCs (Figure 2F1) is pH-sensitivity which could theoretically be either intrinsic (option 1a) or due to a local autocrine process (option 1b). Proving that a neuron that responds to hypercapnia in vivo does so exclusively via such mechanisms would require deleting the proton sensor in a cell specific manner and showing that the targeted neurons no longer respond to hypercapnia in vivo. This type of evidence has not yet been produced for any candidate CRC. However, in the case of the ccRTN neurons and only in this case, it has at least been possible to show that hypercapnia in vivo remains capable of activating these neurons normally under conditions of partial synaptic blockade which silence the respiratory controller (Mulkey et al., 2004). This evidence makes the essential point that the activation of the ccRTN neurons by CO2 is not secondary to the stimulation of the respiratory controller. In brain slices, ccRTN neurons are activated by hypercapnic acidosis and metabolic acidosis even under conditions of partial synaptic blockade (blockade of glutamate GABA, glycine and purinergic transmission) and acidification produces a reduction in potassium conductance in the presence of tetrodotoxin (Mulkey et al., 2004; 2006). This evidence partially illustrated in Figure 2E indicates that the ccRTN neurons are responsive to local changes in pH. It does not prove that their acid-sensitivity is (entirely) an intrinsic property because the participation of satellite cells (glia, blood vessels) has not been ruled out (Gourine et al., 2005; Erlichman et al., 2008). In addition, there is still an unexplained mismatch between the dynamic range of the response of ccRTN neurons to acidification in vitro vs. in vivo (∼0.16 Hz - at 33 °C in slices vs. ∼ 0.5 Hz per hundredth pH unit in vivo). This discrepancy may have to do with the limitations inherent to experimentation with slices (dendritic pruning, lower temperature). It could also simply be related to the age of the animals (6-13 day-old rodents for in vitro recordings vs. young adults for in vivo experiments). Indeed rodents have notably less powerful chemoreflexes at and soon after birth than later on (Conrad et al., 2009). Finally, in vivo, the ccRTN neurons may also integrate information from additional central chemoreceptors (Figure 2F2) and their response to acidification could be enhanced by unknown factors that modify their intrinsic properties and increase the gain of their action potential response to acid-induced current.
The projection pattern of candidate CRCs is also an important consideration. Neurons that innervate selectively the respiratory controller are more likely to be specifically dedicated to central respiratory chemoreception than neurons that have projections throughout the brain, even if these projections include elements of the respiratory controller. The ccRTN neurons innervate exclusively the pontomedullary regions known to contain the respiratory controller, i.e. ventrolateral medulla, dorsal pons and ventrolateral portions of the NTS (Figure 2A) (Mulkey et al., 2004; Rosin et al., 2006; Abbott et al., 2009).
Finally, as alluded to previously, the ccRTN neurons are of special relevance to CCHS, a human disease caused by polyalanine expansion of transcription factor Phox2b (Amiel et al., 2009; Carroll et al., 2010). CCHS is a disease in which the newborn child presents with extremely depressed chemoreflexes and a severe hypoventilation that would be rapidly lethal in the absence of mechanical ventilation in all but the milder forms of the disease (Amiel et al., 2009). The mouse model of the disease (Phox2b27ala/+ strain) presents similar breathing problems at birth (Dubreuil et al., 2008; Dubreuil et al., 2009b). The only brainstem defect that has been identified so far in the Phox2b27ala/+ mouse is a massive and selective loss of the ccRTN neurons (Dubreuil et al., 2008; 2009b). This evidence suggests that the ccRTN neurons are fundamentally important for central respiratory chemoreception during the perinatal period. Because CCHS patients with 7 extra alanines in the PHOX2B sequence experience severe if not complete sleep apnea, it is possible that the ccRTN neurons are indispensable to maintain breathing automaticity during sleep. This working hypothesis has not been tested adequately in laboratory animals. The RTN region contributes to ventilation and to the central chemoreflex in unanesthetized rats regardless of the level of vigilance (Nattie and Li, 2002a). Sleep apnea was not observed in these experiments but the RTN lesions performed in these experiments were probably very partial and compensatory mechanisms are likely to have developed.
In short, the ccRTN neurons contribute a powerful excitatory drive to the respiratory controller and their firing characteristics suggest that they are not directly involved in the generation of the respiratory rhythm or the classic 3-phase respiratory pattern. Their activity is up-regulated by the level of acidity of the neuropil that surrounds them. These properties qualify the ccRTN neurons as CRCs. However, it is also clear that the level of activity of the ccRTN neurons is not solely determined by the surrounding pH (Figure 2F2). Of particular significance, ccRTN neurons are also activated by peripheral chemoreceptor stimulation (Takakura et al., 2006), they are up-regulated by inputs originating from the hypothalamus (Fortuna et al., 2009; Dias et al., 2009b), they are subject to a negative feedback from the respiratory controller (Guyenet et al., 2005a) and their activity is probably regulated by the raphe (Mulkey et al., 2007a; Dias et al., 2008). Finally, a growing amount of evidence suggests that several subsets of ccRTN CRCs may exist, which differentially regulate the breathing rate vs. the inspiratory amplitude and/or active expiration (Abdala et al., 2009; Guyenet and Mulkey, 2010). Other targets, notably cardiovascular, are also not excluded.
5.2. Serotonergic neurons and central respiratory chemosensitivity
Richerson and colleagues have proposed the following three-part theory (for a recent review see (Corcoran et al., 2009)). 1: All serotonergic neurons are CO2/pH detectors (Richerson, 2004). 2: Midbrain raphe serotonergic neurons contribute to the arousal-promoting effects of hypercapnia. 3: The lower brainstem serotonergic neurons mediate the respiratory chemoreflex or some large portion of it. The main supportive arguments, briefly summarized, are as follows (Corcoran et al., 2009). Collectively, serotonergic neurons innervate every region of the brain including the respiratory controller and they also target respiratory motor neurons. Some serotonergic neurons are acid-activated in slices. In neuronal culture, serotonergic neurons generally respond vigorously to CO2 and acid (Figure 3B). Hypercapnia increases the overflow of serotonin in the hypoglossal nucleus in vivo and experimental acidification of the midline raphe can increase breathing (Nattie and Li, 2001; Hodges et al., 2004b; 2009; Corcoran et al., 2009). Serotonergic neurons reside in close proximity to blood vessels which can be viewed as potentially advantageous for the detection of arterial CO2 (Figure 3A). Finally, genetic deletion of serotonergic neurons markedly attenuates the chemoreflex (Hodges et al., 2008).
Figure 3. serotonergic neurons and central respiratory chemosensitivity.
A, ventral view of the rat brainstem showing the location of the serotonergic neurons that reside close to the ventral medullary surface (tryptophan-hydroxylase, TpOHase, immunoreactivity in green; calibration bar: 1.5 mm). The blood vessels are red because they have been filled with a resin. The superficial serotonergic neurons are close to blood vessels and reside in regions of the ventral medullary surface assumed to contain CRCs because experimental acidification of these regions increases breathing. We added the white ovals that outline the retrotrapezoid nucleus. B, typical serotonin neuron in culture that responds briskly to extracellular acidification. A and B reprinted with slight modification from Respiration Physiology and Neurobiology, Volume 168, Corcoran et al., Medullary serotonin neurons and central CO2 chemoreception, pp. 49-58, Copyright (2009), with permission from Elsevier. C. the lateral B3 group of serotonergic neurons is insensitive to hypercapnia in anesthetized rats. C1, example of a serotonergic neuron (tryptophan-hydroxylase-immunoreactive) filled with biotinamide after electrophysiological study in vivo (calibration bar: 20 microns). C2, location of 24 biochemically identified serotonergic neurons found unresponsive to hypercapnia in vivo (red dots). These neurons were located in the lateral band of serotonergic neurons shown in panel A at the level of the RTN (pyr, pyramidal tract; RPa, raphe pallidus; TpOHase, tryptophan hydroxylase). C3 example of a single identified lateral B3 group serotonergic neuron. The neuron was unaffected by increasing end-expiratory CO2 by 5% from just below the apneic threshold. The activity of the respiratory controller, monitored at the level of the phrenic nerve (iPND) was robustly activated by CO2 as expected. C1-3 from (Mulkey et al., 2004). D, raphe obscurus serotonergic neurons driven by the respiratory controller. These recordings were obtained in a coronal slice of neonate medulla oblongata that generates a respiratory-like activity (the “breathing slice”) (Smith et al., 1991). In this slice, the activity of the residual respiratory network was monitored by the mass discharge of the hypoglossal motor neurons (integral XII). In the lower two traces the membrane potential of the serotonergic cell was deliberately hyperpolarized to reveal the excitatory drive potential synchronized with the inspiratory phase. Reproduced with permission from the Journal of Neuroscience from (Ptak et al., 2009). E, putative raphe obscurus serotonergic neurons recorded in a conscious cat. Most cells (21/27) did not respond to hypercapnia. Small subsets of putative serotonergic neurons, one of which is illustrated here, were activated by CO2 but only while the cats were awake. Reproduced with permission from the Journal of Neuroscience from (Veasey et al., 1995). F, the release of serotonin contributes to the activity of the respiratory controller in vitro. The figure shows the mass activity of the preBötzinger region of the ventral respiratory column in the “breathing slice” (ipreBot, an indication of the inspiratory phase of the breathing cycle; top trace) and an inspiratory neuron that was recorded intracellularly (lower trace). Superfusion of the slice with a serotonin receptor 2A antagonist slowed the fictive breathing rate and the amplitude of the respiratory bursts indicating that the ongoing release of serotonin was contributing to the activity of the network under these in vitro conditions. Reproduced with permission from the Journal of Neuroscience from (Pena and Ramirez, 2002). G, putative role of serotonergic neurons in central chemoreception. Left: a small subset of serotonergic neurons, the location of which needs to be clarified, may be central respiratory chemoreceptors, i.e. may be acid-sensitive in vivo and may activate the respiratory controller among other targets. Right, most serotonergic neurons recorded so far in adult mammals in vivo were not CO2-responsive. A few serotonergic neurons were CO2–activated, either because of an intrinsic acid-sensitivity or because they receive excitatory inputs from the respiratory controller. The serotonergic system at large activates the breathing network and regulates its response to CO2. RTN neurons receive an excitatory input from serotonergic cells (Mulkey et al., 2007a). In theory, this input could originate from pH-sensitive (left) or pH-insensitive serotonergic cells (right).
Serotonergic neurons are at least one order of magnitude more numerous than the ccRTN neurons (∼20,000 vs. ∼2,000, in rats) and they are extremely diverse, embryologically, anatomically and functionally (Hökfelt et al., 2000; Kocsis et al., 2006; Jensen et al., 2008; Wylie et al., 2010). Recent evidence that only a small fraction of these neurons may have properties consistent with putative CRCs is therefore not surprising. In brainstem slices from neonate rodents, serotonergic neurons are virtually insensitive to acid at birth (Corcoran et al., 2009). This insensitivity may be related to the fact that the chemoreflex is weak at birth in rodents. However, only about 18% of brainstem serotonergic neurons recorded in slices are acid-sensitive two weeks after birth when the chemoreflex is reasonably mature (Corcoran et al., 2009). In the adult in vivo, the proportion of CO2-responsive serotonergic neurons seems to be equally low and is dependent on which portion of the raphe is being considered. For example, an extremely small fraction of medullary midline raphe neurons respond consistently to activation of peripheral and central chemoreceptors in anesthetized cats (5 neurons excited and 4 inhibited by both stimuli out of a sample of 125) (Nuding et al., 2009). The interpretation of this data should be qualified because no attempt was made to determine whether these neurons were serotonergic in these studies. However, in a study on unanesthetized cats where the characterization of the serotonergic cells was convincing, if not definitive, only twenty-two percent of the putative serotonergic neurons located in the midline medullary raphe (raphe obscurus or magnus) were activated by hypercapnia (Veasey et al., 1995). One of the few putatively serotonergic neurons (6/27) that responded to hypercapnia in this study is depicted in Figure 3E. The general conclusion of these studies in conscious cats was that the activity of midline medulla serotonergic neurons correlates best with the overall motor activity of the animals (Jacobs et al., 2002). In anesthetized rats, the serotonergic neurons of the parapyramidal region, including those that are located at the ventral medullary surface in an area presumed to be “chemosensitive”, are remarkably unresponsive to even severe hypercapnia (sample: 24 chemically identified serotonergic neurons; Figure 3C) (Mulkey et al., 2004).
Nonetheless, the possibility that a small subset of serotonergic neurons are acid-sensitive is supported by evidence that in conscious rats, acidification of the raphe causes small and region-specific increases in breathing with best results produced in the rostral midline raphe (presumably r. obscurus and magnus) (Dias et al., 2008). However, also in rats, acidification of more caudal regions of the raphe obscurus that are believed to contain serotonergic neurons which innervate the respiratory region of the ventrolateral medulla (Ptak et al., 2009) produced no effect on breathing (Dias et al., 2008). The results are also apparently species specific. Midline raphe acidification increases breathing during waking but not during non-REM sleep in goats whereas, in rats, breathing stimulation is seen only during sleep (Nattie and Li, 2001; Hodges et al., 2004a). Regions of the raphe where serotonergic cells are apparently CO2 insensitive in vivo (parapyramidal region, nucleus interfascicularis hypoglossi, a.k.a. lateral B3 group) innervate sympathetic efferents (Strack et al., 1989) and primarily regulate thermogenesis and skin blood flow (Morrison, 2004).
The response of raphe obscurus serotonergic neurons to CO2 in vivo could also be due to synaptic inputs. Indeed, in unanesthetized cats, this response was state-dependent therefore unlikely to be a cell autonomous pH response (4/4 cells tested; Figure 3E) (Veasey et al., 1995). In support of this interpretation, many raphe obscurus serotonergic neurons receive respiratory phasic excitatory inputs in brain slices (Figure 3D) (Ptak et al., 2009). This observation means that the activation of a subset of serotonergic neurons by CO2 in vivo could be a consequence of the activation of the respiratory controller rather than an intrinsic sensitivity to acid. In slices, the ongoing activity of the residual respiratory network is maintained by the release of serotonin (Figure 3F) (Pena and Ramirez, 2002) which suggests that some of the serotonergic cells of the medullary raphe may participate in some feed-forward loop involving the pre-Bötzinger complex (Figure 3G, right). Finally, serotonergic neurons receive an orexinergic input which could also contribute to their activation by hypercapnia in vivo since the emotional control of breathing and the chemoreflex depends on the integrity of the orexin system (Kuwaki, 2008; Dias et al., 2009a).
To our knowledge, the hypothesis that the intrinsic pH sensitivity of serotonergic neurons (and other wake-promoting systems) is responsible for CO2-induced arousal is untested. The notion seems at odds with the fact that severe hypercapnia fails to wake humans with central congenital hypoventilation syndrome (CCHS) from sleep (Gozal, 1998; Spengler et al., 2001; Amiel et al., 2009). This genetic disease is not known to interfere with the sleep cycle per se and the PHOX2B mutation that underlies it produces no change in the number of serotonergic neurons in the mouse model of the disease (Pattyn et al., 2003; Dubreuil et al., 2008; Amiel et al., 2009).
In summary, subsets of serotonergic neurons make an important contribution to the activity and plasticity of the respiratory network (Pena and Ramirez, 2002; Richter et al., 2003; McCrimmon et al., 2008; Ptak et al., 2009). Furthermore, in the absence of serotonergic neurons or when brainstem serotonergic cells are acutely inhibited, the chemoreflex is markedly attenuated (Taylor et al., 2005; Taylor et al., 2006; Hodges et al., 2008). Yet, evidence that CO2 increases the activity of more than a small minority of the serotonergic neurons in vivo is yet to be produced and there is reason to believe that this activation could be due, in part at least, to synaptic inputs (Veasey et al., 1995; Nattie and Li, 2001; Hodges et al., 2004b; Ptak et al., 2009). Figure 3G summarizes a few of the many possibilities that could account for the role of brainstem serotonergic neurons in breathing and CO2 regulation based on current experimental evidence. One of these possibilities is that a small subset of serotonergic neurons, possibly located in the rostral medullary midline raphe, encodes pH changes in vivo and functions as CRCs.
5.3. Orexinergic neurons
These neurons, like the pontine noradrenergic neurons and the serotonergic neurons have extremely widespread projections and are wake promoting (Lu et al., 2006). Orexin-KO mice have lowered BP and breathing during the waking state (Deng et al., 2007; Kuwaki, 2008; Kuwaki et al., 2008). The orexin system targets the ventrolateral medulla and the RTN among many other regions (Kuwaki, 2008; Dias et al., 2009b). Orexin neurons are activated by acidification in vitro (Williams et al., 2007). This effect, initially attributed to TASK channel inhibition, persists in TASK1/3 KO mice and is therefore still unexplained (Williams et al., 2007; Gonzalez et al., 2009). There is no information regarding the sensitivity of orexin neurons to CO2 in vivo except for the fact that severe hypercapnia increase Fos expression in a relatively small fraction of these neurons (Sunanaga et al., 2009). The latter result could be also explained by the general arousal effect of CO2. Because of the widespread projection pattern, the orexin neurons are either broad-spectrum central respiratory chemoreceptors (Figure 1, option 2) or simply neurons that regulate breathing along the lines depicted in Figure 1, option 4.
5.4. Locus coeruleus (LC)
This major collection of pontine noradrenergic neurons has an activity level that is state-dependent and is activated by a large number of intero- and exteroceptive inputs (Aston-Jones et al., 1996; 1999). The LC is one of several groups of wake-promoting neurons that include subsets of serotonergic neurons, orexinergic neurons and histaminergic neurons. The LC innervates vast tracts of the brain from cortex to spinal cord (Aston-Jones et al., 1996; 1999). The LC is mildly activated by hypercapnia in anesthetized animals and by hypercapnic acidosis in slices (Elam et al., 1981; Ritucci et al., 2005a). The activation of LC neurons by CO2 observed in vivo could be partially or wholly due to the activation of synaptic inputs since these cells receive a massive innervation from the C1 neurons and from the orexin neurons which are activated by hypercapnia (Horvath et al., 1999; Aston-Jones et al., 2001; Card et al., 2006; Kuwaki, 2008; Sunanaga et al., 2009). Like the serotonergic neurons, LC neurons are more responsive to CO2 in culture than in slices (Johnson et al., 2008). This characteristic, as in the case of serotonergic neurons, could be due to a higher input resistance in vitro because of low ongoing synaptic activity or it could be due to the expression of different channels in culture. Lesions of LC and other NE neurons reduce the respiratory chemoreflex induced by hypercapnia (Li et al., 2008; Biancardi et al., 2008). Relatively selective lesions of the locus coeruleus (with 6-hydroxydopamine) do not modify the stimulation of breathing induced by hypoxia and a similar selectivity towards CO2-induced breathing stimulation has been consistently observed after inhibition or lesions of the serotonergic system (Taylor et al., 2005; Hodges et al., 2008; Biancardi et al., 2010). In the aggregate, these results suggest that both noradrenergic neurons and serotonergic cells contribute to the respiratory stimulation elicited by hypercapnia but not hypoxia. Given that both stimuli activate the retrotrapezoid nucleus (Takakura et al., 2006), the difference could be due to the fact that hypoxia is sedative in rodents whereas CO2 or asphyxia cause arousal. CO2 may also directly activate noradrenergic (Sirois et al., 2000; Filosa and Putnam, 2003) and a subset of serotonergic cells (see above discussion). Evidence that selective activation of the locus coeruleus neurons activates breathing in vivo has not been produced but indirect evidence suggests that the locus coeruleus has a tonic facilitatory influence on the neonate breathing network (Hilaire et al., 2004). Because of their widespread brain projections and the lack of evidence that subsets of locus coeruleus neurons target the respiratory network with any degree of selectivity, locus coeruleus neurons should provisionally be viewed as either broad-spectrum central respiratory chemoreceptors (Figure 1, option 2) or neurons that regulate breathing along the lines depicted in Figure 1, option 4, possibly in a state-dependent context.
5.5 Other putative chemoreceptors
CRCs may be located at the ventral medullary surface caudal to the retrotrapezoid nucleus, within the respiratory column, the NTS and the fastigial nucleus of the cerebellum (Nattie and Li, 2009). The raphe has been mentioned above. The presence of non-serotonergic CRCs within the region of the raphe could conceivably account for the mild breathing stimulation caused by raphe acidification (Nattie and Li, 2001; Hodges et al., 2004a). The regions of the ventral medullary surface that were originally described as chemosensitive based on the respiratory effect produced by topical acidification in cats extend from the caudal-most hypoglossal rootlets to the trapezoid body (Mitchell et al., 1963a; Schlaefke et al., 1979; Loeschcke, 1982). Only the rostral chemosensitive region also called area M can be viewed as roughly in register with the retrotrapezoid nucleus. The intermediate or caudal chemosensitive regions which overlap considerably with the serotonin rich areas of the ventral medullary surface (Figure 3A) contain tonically active neurons that are excited by injection of 100% CO2-saturated saline into the vertebral artery of anesthetized cats (Arita et al., 1988) or iontophoretic applications of acid (Ribas-Salgueiro et al., 2003). These observations are difficult to interpret because the identity, function and connectivity of these acid-sensitive cells are unknown and, in the case of the Ribas-Salgueiro paper, there is no evidence that these cells respond to hypercapnia. This general area of the brain also expresses c-Fos in animals exposed to hypercapnia (Teppema et al., 1994; 1997; Berquin et al., 2000). The NTS contains large numbers of neurons that are activated by acidification in slices and acidification of the NTS, particularly its caudal part, increases breathing in unanesthetized rats (Dean et al., 1989; 1990; Nattie and Li, 2002b). This evidence suggests that the caudal aspect of the NTS could harbor CRCs although, paradoxically, lesions of this region increase the ventilatory response to CO2 (Sinclair et al., 1985). The caudal NTS mediates the effect of peripheral chemoreceptors on breathing and on the circulation (Boscan et al., 2002). Transmission of peripheral chemoreceptor information through the NTS could conceivably be also influenced by ECF pH.
6. Contribution of peripheral chemoreceptors to respiratory chemosensitivity
The long-standing and still unanswered question is how the motor outflow of the respiratory network could achieve such a high sensitivity to CO2. Glial mechanisms that amplify the response of certain neurons to acid have been already been evoked as well as the possibility that multiple types of acid-sensitive neurons cooperate to stimulate breathing. Synaptic gain between the putative CRCs and their targets within the respiratory network has to be another critical variable but this aspect of the biology of CRCs remains essentially undocumented.
Another very important contributing factor is the input from the carotid bodies. In the absence of anesthesia, peripheral chemoreceptors provide a tonic stimulus to the central respiratory controller, even at rest and under normocapnic conditions (Smith et al., 2006; Forster et al., 2008). Surgical excision of the carotid bodies causes chronic hypoventilation, reduction of the gain of the chemoreflex gain and respiratory acidosis in awake rats, goats and man (Olson, Jr. et al., 1988; Pan et al., 1998; Dahan et al., 2007). These deficits are attenuated with the passage of time presumably because of some form of CNS plasticity. In addition, virtually all studies indicate that the ventilatory effects produced by simultaneous activation of peripheral and central chemoreceptors are at least additive (Nattie, 1999). The recent study by Day and Wilson (2009) is a very rare exception that may be explained by a ceiling effect of each stimulus applied individually in an extremely responsive preparation. In the aggregate, this evidence indicates that the central respiratory drive depends both on brain parenchymal pH and the ongoing resting activity of the carotid bodies at all times.
The ccRTN neurons are a site of convergence between peripheral and central chemoreceptor inputs (Takakura et al., 2006). The carotid body input to the RTN is via a presumably direct glutamatergic projection from the commissural portion of the NTS (Takakura et al., 2006). In theory, the loss of this glutamatergic input should elevate the CO2 recruitment threshold of the ccRTN neurons and reduce the gain of their activation by CO2. Thus, the loss of a tonic carotid body input to the RTN neurons should contribute to produce the type of respiratory deficits that have been observed after carotid body excision in man and animals (hypoventilation, reduced central chemoreflex and chronic elevation in arterial PCO2). Other sites of convergence between carotid body input and putative central chemoreceptors are suspected but are less thoroughly documented. Locus coeruleus neurons and certain serotonergic neurons for example are activated by stimulation of peripheral chemoreceptors (Elam et al., 1981; Erickson and Millhorn, 1994) but the pathway is not worked out and the question remains as to whether these neurons would respond to very small deviations in PCO2 around physiological levels.
Summary and conclusions
CO2 most likely works by changing brain pH but the existence of receptors for molecular CO2 remains a possibility. The number of potential proton receptors is large. In peripheral tissues, acidity is detected by many cell types (blood vessels, glial cells, bone, sensory afferents) through a variety of proton-sensitive molecules (mostly channels plus a few GPCRs). Most of these proteins are expressed in brain and could, in theory, mediate the pH sensitivity of CNS neurons and, more specifically, central respiratory chemosensitivity. Despite the virtually ubiquitous presence of pH-sensitive channels in neurons, in the brainstem and elsewhere, a substantial proportion of neurons do not respond in vitro to even severe (>0.2 pH units) acidification. The fact is noteworthy because it suggests that some sort of neuronal homeostasis (Bucher, 2009) may render most neurons insensitive or very weakly sensitive to the small pH fluctuations (<0.1 pH unit) that are relevant to the homeostatic regulation of CO2 via breathing.
There is uncertainty regarding which cells (neurons, glia, others) are the primary proton detectors involved in central respiratory chemosensitivity. Neurons are likely contributors but the pH-response of the few putative CRC neurons that have been examined in some detail in vitro is fairly modest and varies relatively little between various chemoreceptor candidates. A significant problem with this evidence is that the properties of neurons in slices and especially in culture may be very different from their properties in vivo. This quandary is illustrated by the specific case of the serotonergic neurons which are robustly activated by acid in culture and generally insensitive to CO2 in vivo (Figure 3). In vitro, the quasi absence of active synaptic inputs likely increases neuronal input resistance which should greatly enhance the effects of minor changes in membrane current relative to what would be expected in vivo (Destexhe et al., 2003). This phenomenon could blur the difference between the real CRCs and other cells in unpredictable ways when the cells are studied in vitro. In addition, the channel expression profile of neurons is likely to be different in culture. Finally, the pH-induced changes in conductance present in individual neurons may have no impact at the level of the respiratory network. Proof of concept was provided by the recent experiments of Dubreuil et al. (2009b) who showed that the apparently uniform acid-sensitivity of the ventral respiratory column neurons (Kawai et al., 1996) does not add up to an acid-sensitive respiratory network in the absence of the ccRTN neurons.
Evidence that the pH sensitivity of the CRCs relies on acid-sensitive channels is also weak although the hypothesis is eminently plausible. The same can be said of the theory that multiple pH-sensitive channels are involved simultaneously and that a different mix of channels contributes to the acid response of different CRCs. However, if correct, the latter possibility adds an additional level of complexity to an already challenging issue because many of the candidate proton-sensitive channels (e.g. TASK channels, calcium channels) are modulated by extracellular signals, which means that their contribution to the overall pH sensitivity of the cells could be synaptically regulated. On the other hand, the complexity of proton detection in the context of central respiratory chemoreception may also be greatly exaggerated. One or very few pH-sensitive channels could conceivably be essential for central respiratory chemoreception if they were expressed by neurons that play a pivotal role in this function. This hypothesis does not presuppose the existence of an esoteric proton detector in such cells although this possibility is not ruled out. The right anatomical connections combined with intrinsic properties that are appropriate to give these cells a robust response range to pH-induced current would be enough. If this view is correct, cell-specific deletion of an already well described and widely expressed pH-sensitive channel or receptor may provide a simultaneous answer to two of the most critical questions in this field, namely the real importance of a particular cell group to central respiratory chemoreception and the importance of a particular channel for this function. This type of approach has already been carried out to a limited extent using the retrotrapezoid nucleus as a model (Mulkey et al., 2007b; Gestreau et al., 2010) but it has not yet borne fruit. Accordingly, the issue of whether central respiratory chemosensitivity relies on one or a few specialized cell groups or is a diffuse (“emergent”) property of the respiratory controller and the many brain pathways that regulate its activity (Nattie and Li, 2009) remains open. These options are supported by very different types of evidence, none of which is incontrovertible, singly or in the aggregate. The highly sensitive response of the respiratory network to very small deviations of CO2 around the normal level could be the purview of a limited number of specialized cells with a low CO2 threshold and high response gain to acid whereas higher levels of CO2, which produce an alerting response and conscious aversive sensations, could be recruiting additional groups of cells that are responsive only to much larger deviations of brain pH. An alternate view is that the sensitive response of the respiratory network to very small deviations of CO2 is a network effect caused by the summation of very small effects of pH on a very large number of neurons (Figure 1, option c).
The retrotrapezoid nucleus contains the most thoroughly documented group of CRCs although many important aspects of the biology of these neurons are still missing. The most notable missing piece is the molecular mechanism responsible for their activation by CO2 and one can argue that the definitive demonstration that these neurons are CRCs will require deleting the proton receptor selectively from these cells. The ccRTN neurons contribute a powerful pH-modulated excitatory drive to the breathing network and their absence at birth is lethal (Dubreuil et al., 2008; Guyenet, 2008; Amiel et al., 2009; 2009b). The function of the ccRTN neurons is clearly far more complex than that of a mere pH detector. The notion that RTN is a major convergence point for the various pathways that automatically regulate breathing to maintain arterial PCO2 constant is, at present, the most sensible explanation of the respiratory deficits observed in the CCHS (Guyenet, 2008; Weese-Mayer et al., 2010; Goridis and Brunet, 2010).
The other putative CRCs are in various stages of characterization at this time. In all cases, one or more critical pieces of evidence are missing, for example evidence that these neurons respond to CO2 in vivo or that their selective activation in vivo increases breathing. The case of the serotonergic neurons is the most intriguing because of the unresolved discrepancy between their obvious importance to breathing and the chemoreflex and their general insensitivity to CO2 in vivo. One possible interpretation of the literature is that the other postulated chemosensitive regions of the brainstem regulate breathing primarily by modulating the activity of the ccRTN neurons. This notion could explain why, despite the existence of multiple other CRCs, the chemoreflex is absent when these few RTN neurons are destroyed, as is presumably the case in CCHS patients. This possibility could also explain why the sensitivity of RTN neurons to acid is lower in vitro than in vivo.
The chemoreflex is enhanced during waking compared to slow-wave sleep. This reflex is depressed by genetic deletion and other forms of insult to several major wake-promoting systems (orexinergic, serotoninergic, noradrenergic). Large increases in CO2 above the normal range produce arousal from sleep and the arousal presumably contributes to the stimulation of breathing in part by recruiting the orexinergic, serotonergic and noradrenergic neurons many of which are known to innervate and activate several components of the breathing network down to the motor neurons. How hypercapnia causes cortical / behavioral arousal is not known. One possibility is that the orexinergic, pontine serotoninergic and pontine noradrenergic neurons are directly activated by high level of CO2 in vivo. This suggestion is supported by physiological evidence obtained in vitro but there is also contrary evidence. If CO2-induced arousal and the interoceptive awareness of hypercapnia depended on an effect of CO2 on these systems, these effects of CO2 should be preserved in CCHS which is a disease that impacts the brainstem selectively and does not or at least is not known to affect the sleep cycle markedly. In fact, a remarkable feature of the disease is the loss of the interoceptive awareness of asphyxia and the inability of hypercapnia to produce arousal from sleep (Carroll et al., 2010). CO2-mediated arousal may therefore depend primarily on ascending signals from peripheral and brainstem chemoreceptors that reach the vigilance-regulating hypothalamic centers either without further processing or on efferent copies of the activity of the central respiratory controller or as a result of sensory inputs from the chest and lungs, a combination of mechanisms that may also underlie the sensation of dyspnea (Chen et al., 1992; Flume et al., 1996; Moosavi et al., 2004). Alternately, chemoreceptor-mediated arousal could be primarily triggered by the activation of the carotid body via pathways that are unrelated to those that activate breathing. As reviewed by Marshall (1994) strong carotid body stimulation also produces arousal and a constellation of behavioral effects indicative of discomfort.
Acknowledgments
This work was supported by research grants from the National Institutes of Health (HL74011 and HL 28785 to PGG).
Literature Cited
- Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins, and implications for respiratory rhythm generation. J Physiol. 2009 doi: 10.113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad HR, Loeschcke HH. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar PCO2. Pflugers Arch. 1982;395:285–292. doi: 10.1007/BF00580791. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Aller MI, Wisden W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neurosci. 2008;151:1154–1172. doi: 10.1016/j.neuroscience.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Arita H, Ichikawa K, Kuwana S, Kogo N. Possible locations of pH-dependent central chemoreceptors: intramedullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res. 1989;485:285–293. doi: 10.1016/0006-8993(89)90572-6. [DOI] [PubMed] [Google Scholar]
- Arita H, Kogo N, Ichikawa K. Locations of medullary neurons with non-phasic discharges excited by stimulation of central and/or peripheral chemoreceptors and by activation of nociceptors in cat. Brain Res. 1988;442(1):1–10. doi: 10.1016/0006-8993(88)91426-6. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol. 1982;52:131–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57:59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Biancardi V, da Silva LT, Bicego KC, Gargaglioni LH. Role of Locus coeruleus noradrenergic neurons in cardiorespiratory and thermal control during hypoxia. Respir Physiol Neurobiol. 2010;170:150–156. doi: 10.1016/j.resp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Boscan P, Pickering AE, Paton JFR. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002;87:259–266. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J Physiol. 2004;559:85–101. doi: 10.1113/jphysiol.2004.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier SP, Mason HS, Bateson AN, Kemp PJ. Cloning of the human TASK-2 (KCNK5) promoter and its regulation by chronic hypoxia. Biochem Biophys Res Commun. 2005;336:1251–1258. doi: 10.1016/j.bbrc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bucher D. Neuronal homeostasis: does form follow function or vice versa? Curr Biol. 2009;19:R64–R67. doi: 10.1016/j.cub.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Carroll MS, Patwari PP, Weese-Mayer DE. Carbon Dioxide Chemoreception and Hypoventilation Syndromes with Autonomic Dysregulation. J Appl Physiol. 2010 January 28; doi: 10.1152/japplphysiol.00004.2010. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Chen Z, Eldridge FL, Wagner PG. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol. 1992;90:99–113. doi: 10.1016/0034-5687(92)90137-l. [DOI] [PubMed] [Google Scholar]
- Chernov M, Putnam RW, Leiter JC. A computer model of mammalian central CO2 chemoreception. Adv Exp Med Biol. 2008;605:301–305. doi: 10.1007/978-0-387-73693-8_52. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva GS, Li A, Nattie E. High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke's area) increases ventilation in wakefulness. Respir Physiol Neurobiol. 2010;171:46–53. doi: 10.1016/j.resp.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neurosci. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. The orexin receptor 1 (OX(1)R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol. 2009a;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor 1 (OX1R) in the retrotrapezoid nucleus (RTN) inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009b;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with phox2b. Philos Trans R Soc Lond B Biol Sci. 2009a;364:2477–2483. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009b;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lauritzen I, Patel A, Honore E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007;30:573–580. doi: 10.1016/j.tins.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and Hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Kiley JP, Millhorn DE. Respiratory effects of carbon dioxide-induced changes of medullary extracellular fluid pH in cats. J Physiol. 1984;355:177–189. doi: 10.1113/jphysiol.1984.sp015413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce c-Fos-like immunoreactivity within catecholaminergic and serotinergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol. 2009;102:1577–1590. doi: 10.1152/jn.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv Exp Med Biol. 2008;605:317–321. doi: 10.1007/978-0-387-73693-8_55. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Flume PA, Eldridge FL, Edwards LJ, Mattison LE. Relief of the ‘air hunger’ of breathholding. A role for pulmonary stretch receptors. Respir Physiol. 1996;103:221–232. doi: 10.1016/0034-5687(95)00094-1. [DOI] [PubMed] [Google Scholar]
- Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol. 2008;605:322–326. doi: 10.1007/978-0-387-73693-8_56. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. pH-sensitive cells at ventro--lateral surface of rat medulla oblongata. Nature. 1975;256:317–318. doi: 10.1038/256317a0. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch. 1978;376:229–235. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Abdallah M, Yost CS, Winegar BD, Kindler CH. Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat central nervous system. Brain Res Mol Brain Res. 2002;98:153–163. doi: 10.1016/s0169-328x(01)00330-8. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes A, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C, Brunet JF. Central chemoreception: Lessons from mouse and human genetics. Respir Physiol Neurobiol. 2010 March 20; doi: 10.1016/j.resp.2010.03.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gozal D. Congenital central hypoventilation syndrome: an update. Pediatr Pulmonol. 1998;26:273–282. doi: 10.1002/(sici)1099-0496(199810)26:4<273::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Grafstein B, Liu S, Cotrina ML, Goldman SA, Nedergaard M. Meningeal cells can communicate with astrocytes by calcium signaling. Ann Neurol. 2000;47:18–25. [PubMed] [Google Scholar]
- Grass D, Pawlowski PG, Hirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F, Hulsmann S. Diversity of functional astroglial properties in the respiratory network. J Neurosci. 2004;24:1358–1365. doi: 10.1523/JNEUROSCI.4022-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010 Feb 25; doi: 10.1016/j.resp.2010.02.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol. 2004a;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004b;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. Journal of Chemical Neuroanatomy. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. 2009:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van den Pol AN. Hypocretin (Orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Huang CW, Tzeng JN, Chen YJ, Tsai WF, Chen CC, Sun WH. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci. 2007;36:195–210. doi: 10.1016/j.mcn.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing locus ceruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol. 2008;105:1301–1311. doi: 10.1152/japplphysiol.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem--spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci U S A. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci. 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol. 2008;605:371–376. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol. 2004;142:27–42. doi: 10.1016/j.resp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiological Reviews. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Mitchell GS, Alheid GF. Overview: the neurochemistry of respiratory control. Respir Physiol Neurobiol. 2008;164:1–2. doi: 10.1016/j.resp.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS. Back to the future: carbon dioxide chemoreceptors in the mammalian brain. Nat Neurosci. 2004;7:1288–1290. doi: 10.1038/nn1204-1288. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963a;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Severinghaus JW, Richardson JW, Massion WH. Regions of respiratory chemosensitivity on the surface of the medulla. Ann NY Acad Sci. 1963b;109:661–681. [Google Scholar]
- Moosavi SH, Banzett RB, Butler JP. Time course of air hunger mirrors the biphasic ventilatory response to hypoxia. J Appl Physiol. 2004;97:2098–2103. doi: 10.1152/japplphysiol.00056.2004. [DOI] [PubMed] [Google Scholar]
- Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol. 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci U S A. 2005;102:16102–16106. doi: 10.1073/pnas.0506870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Resp Physiol. 2001;124:179–193. doi: 10.1016/s0034-5687(00)00212-7. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Resp Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Chemoreceptors, breathing, and pH. In: Alpern RJ, Hebert SC, editors. Seldin and Giebisch's The Kidney. 1-2. Elsevier; 2007. pp. 1587–1600. [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002a;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li AH. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002b;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Neusch C, Papadopoulos N, Muller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bahr M, Richter DW, Kirchhoff F, Hulsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol. 2006;95:1843–1852. doi: 10.1152/jn.00996.2005. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol. 2008;605:348–352. doi: 10.1007/978-0-387-73693-8_61. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Gonzalez-Nilo FD, Zuniga L, Gonzalez W, Cid LP, Sepulveda FV. Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci U S A. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Shannon R, O'Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol. 1988;64:666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol. 1965;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- Paton JY, Swaminathan S, Sargent CW, Hawksworth A, Keens TG. Ventilatory response to exercise in children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1993;147:1185–1191. doi: 10.1164/ajrccm/147.5.1185. [DOI] [PubMed] [Google Scholar]
- Paton JY, Swaminathan S, Sargent CW, Keens TG. Hypoxic and hypercapnic ventilatory responses in awake children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1989;140:368–372. doi: 10.1164/ajrccm/140.2.368. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF, Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis. 1977;115:251–259. doi: 10.1164/arrd.1977.115.2.251. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neurosci. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Ribas-Salgueiro JL, Gaytan SP, Crego R, Pasaro R, Ribas J. Highly H+-sensitive neurons in the caudal ventrolateral medulla of the rat. J Physiol. 2003;549:181–194. doi: 10.1113/jphysiol.2002.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol. 2005a;289:C1094–C1104. doi: 10.1152/ajpcell.00329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005b;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Scheid P. Putnam RW, Dean JB, Ballantyne D, guest editors. Special issue: central chemosensitivity. Respir Physiol Neurobiol. 2001;129:1–278. doi: 10.1016/s0034-5687(01)00297-3. [DOI] [PubMed] [Google Scholar]
- Schlaefke ME, Kille JF, Loeschcke HH. Elimination of central chemosensitivity by coagulation of a bilateral area on the ventral medullary surface in awake cats. Pflugers Arch. 1979;378:231–241. doi: 10.1007/BF00592741. [DOI] [PubMed] [Google Scholar]
- Shea SA, Andres LP, Shannon DC, Banzett RB. Ventilatory responses to exercise in humans lacking ventilatory chemosensitivity. J Physiol. 1993;468:623–640. doi: 10.1113/jphysiol.1993.sp019792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Wang HS, Pan Z, Wymore RS, Cohen IS, McKinnon D, Dixon JE. Cloning of a mammalian elk potassium channel gene and EAG mRNA distribution in rat sympathetic ganglia. J Physiol. 1998;511(Pt 3):675–682. doi: 10.1111/j.1469-7793.1998.675bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, St JW, Bartlett D. Enhancement of respiratory response to carbon dioxide produced by lesioning caudal regions of the nucleus of the tractus solitarius. Brain Res. 1985;336:318–320. doi: 10.1016/0006-8993(85)90659-6. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei QB, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex--a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O'Neill MH. CO2 / H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Resp Physiol. 2001;129:247–255. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol. 2004;89:53–59. doi: 10.1113/expphysiol.2003.002659. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM. Respiratory tidal volume responses of cats with chronic pneumotaxic center lesions. Respir Physiol. 1972;16:92–108. doi: 10.1016/0034-5687(72)90091-6. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol. 2007;578:831–841. doi: 10.1113/jphysiol.2006.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. CO(2) and pH independently modulate L-type Ca(2+) current in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO(2) activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels - Volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Berkenbosch A, Veening JG, Olievier CN. Hypercapnia induces c-fos expression in neurons of retrotrapezoid nucleus in cats. Brain Res. 1994;635:353–356. doi: 10.1016/0006-8993(94)91462-1. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J Physiol. 2000;523:441–447. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend PD, Holliday PM, Fenyk S, Hess KC, Gray MA, Hodgson DR, Cann MJ. Stimulation of mammalian G-protein-responsive adenylyl cyclases by carbon dioxide. J Biol Chem. 2009;284:784–791. doi: 10.1074/jbc.M807239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and P(CO2) J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WA, Richerson GB. Chemosensitivity of non-respiratory rat CNS neurons in tissue culture. Brain Res. 2000;860:119–129. doi: 10.1016/s0006-8993(00)02033-3. [DOI] [PubMed] [Google Scholar]
- Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci U S A. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181:626–644. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, Fox S, Maeno H, Deneris ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Morgan BJ, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of Cerebrovascular Function on the Hypercapnic Ventilatory Response in Healthy Humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, III, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]